Application of Coenzyme Q10 for Accelerating Soft Tissue Wound Healing after Tooth Extraction in Rats
Abstract
:1. Introduction
2. Experimental Section
2.1. Animals and Diets
2.2. Pilot Study
2.3. Experimental Design
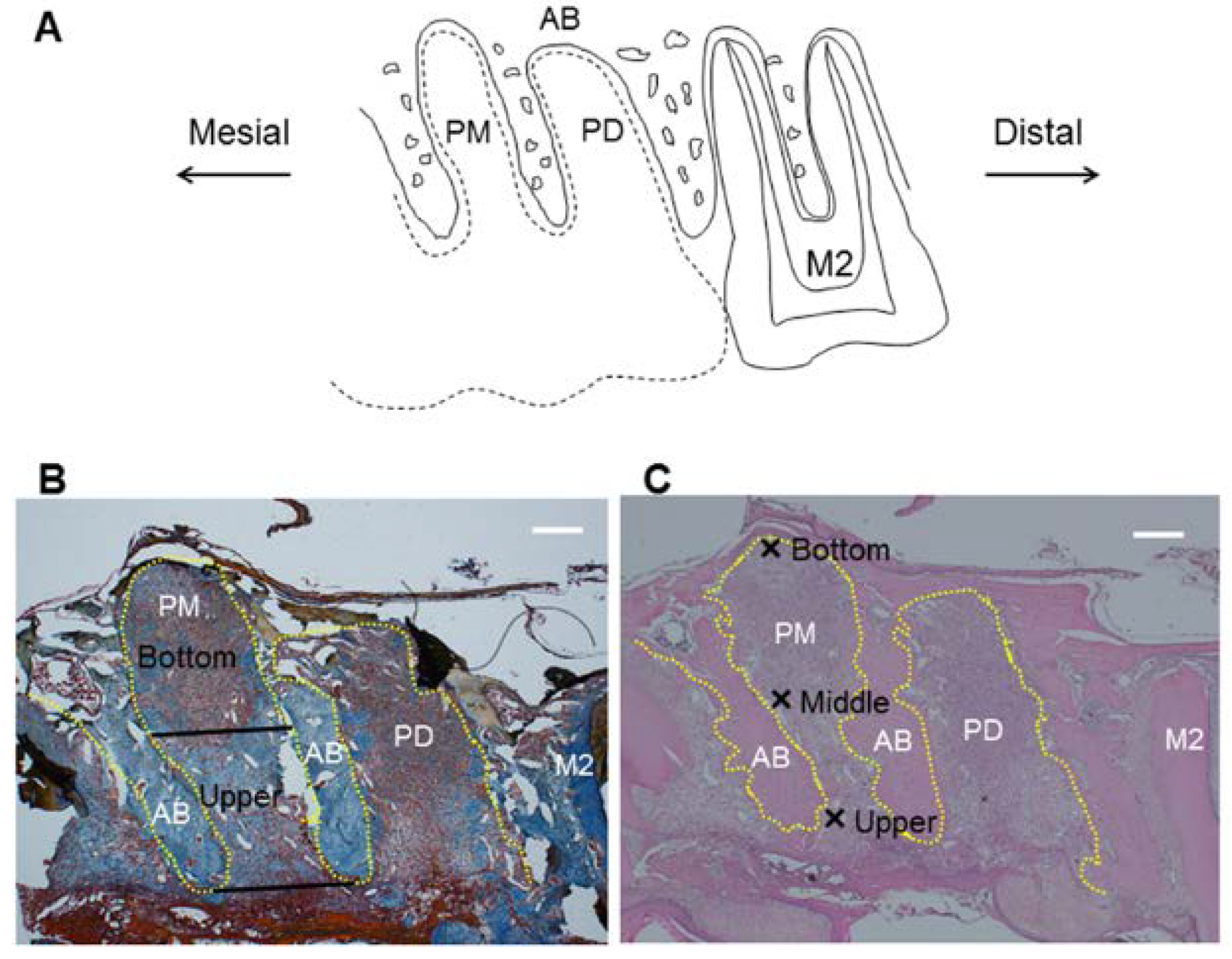
2.4. Histological Analysis
2.5. Real-Time RT-PCR
| Primer | Forward (5′–3′) | Reverse (5′–3′) | Length (bp) | Accession No. |
|---|---|---|---|---|
| MMP-3 | TGGGAAGCCAGTGGAAATG | CCATGCAATGGGTAGGATGAG | 81 | NM_133523 |
| TIMP-1 | CTGAGAAGGGCTACCAGAGC | GTCATCGAGACCCCAAGGTA | 88 | NM_053819 |
| NF-κB | CACTCTCTTTTTGGAGGT | TGGATATAAGGCTTTACG | 206 | NM_199267 |
| IL-1β | CACCTCTCAAGCAGAGCACAGA | ACGGGTTCCATGGTGAAGTC | 81 | NM_031512 |
| TNF-α | TGGGCTCATACCAGGGCTTGAG | CGTCAGCCGATTTGCCATTTC | 116 | NM_012675 |
| HO-1 | GGTGTCCAGGGAAGGCTTTA | GGGGCATAGACTGGGTTCTG | 105 | NM_012580.2 |
| β-actin | TGTTGCCCTAGACTTCGAGCA | GGACCCAGGAAGGAAGGCT | 155 | NM_007393 |
2.6. Micro Computed Tomography Assessment of Maxillae
2.7. Statistical Analysis
3. Results
3.1. Pilot Study
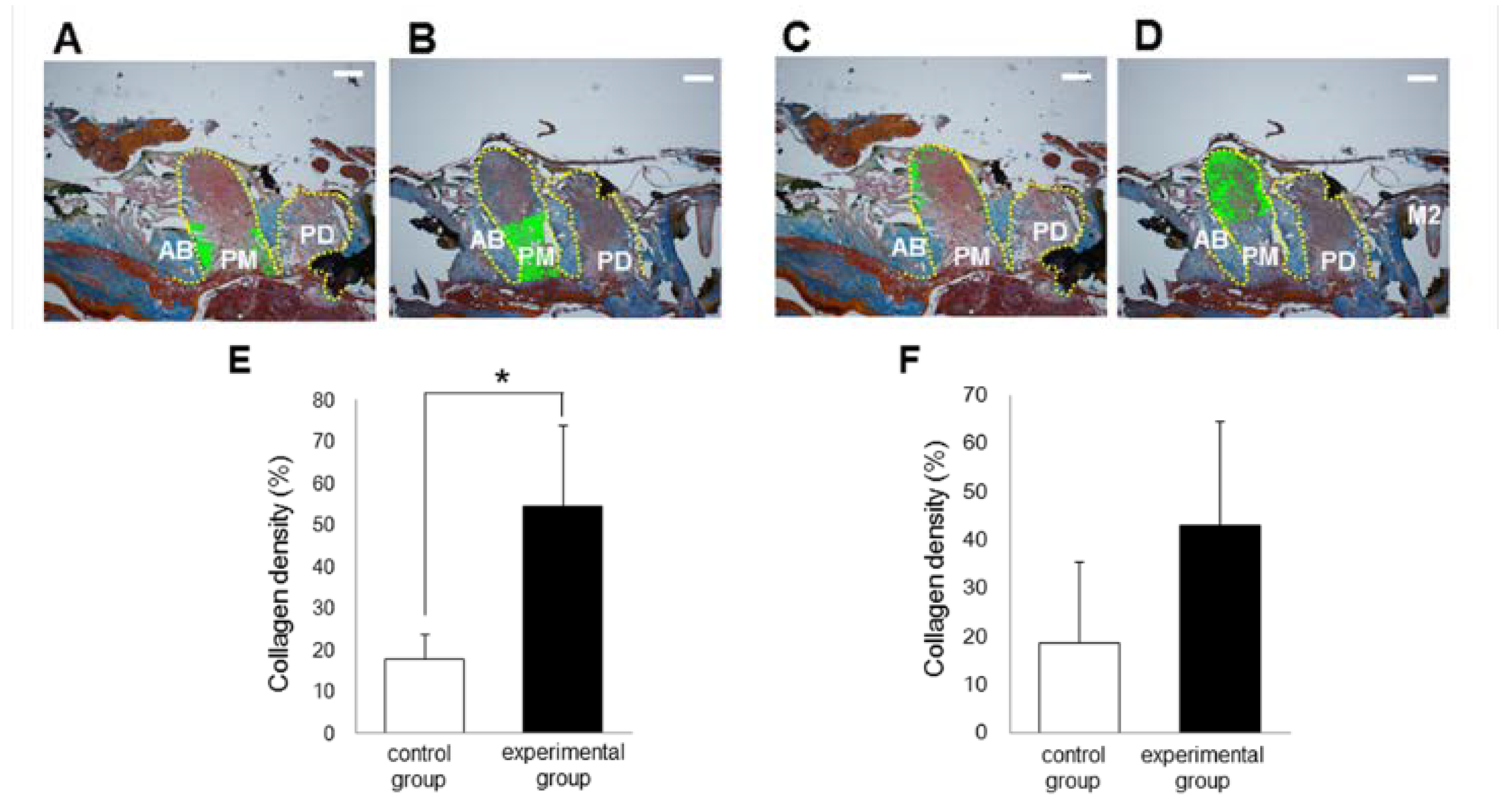
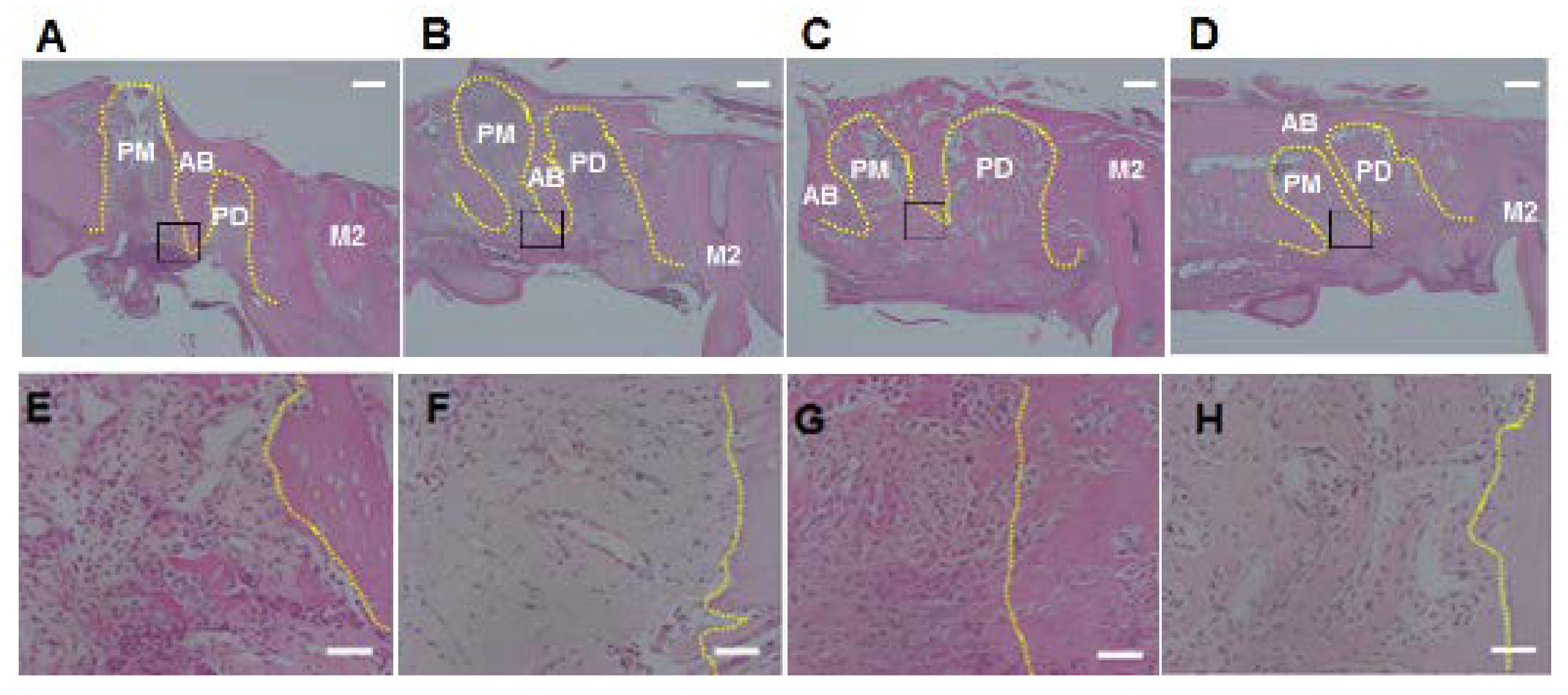
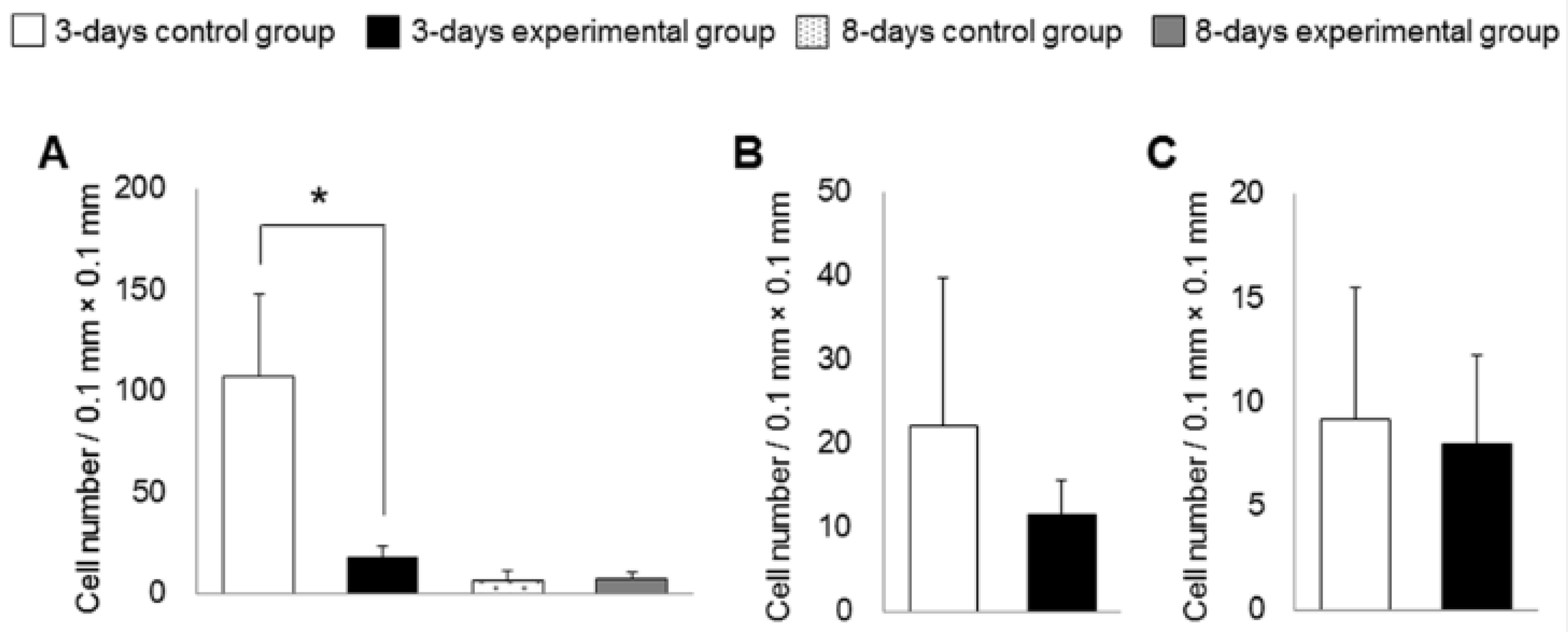
3.2. Histological Analysis
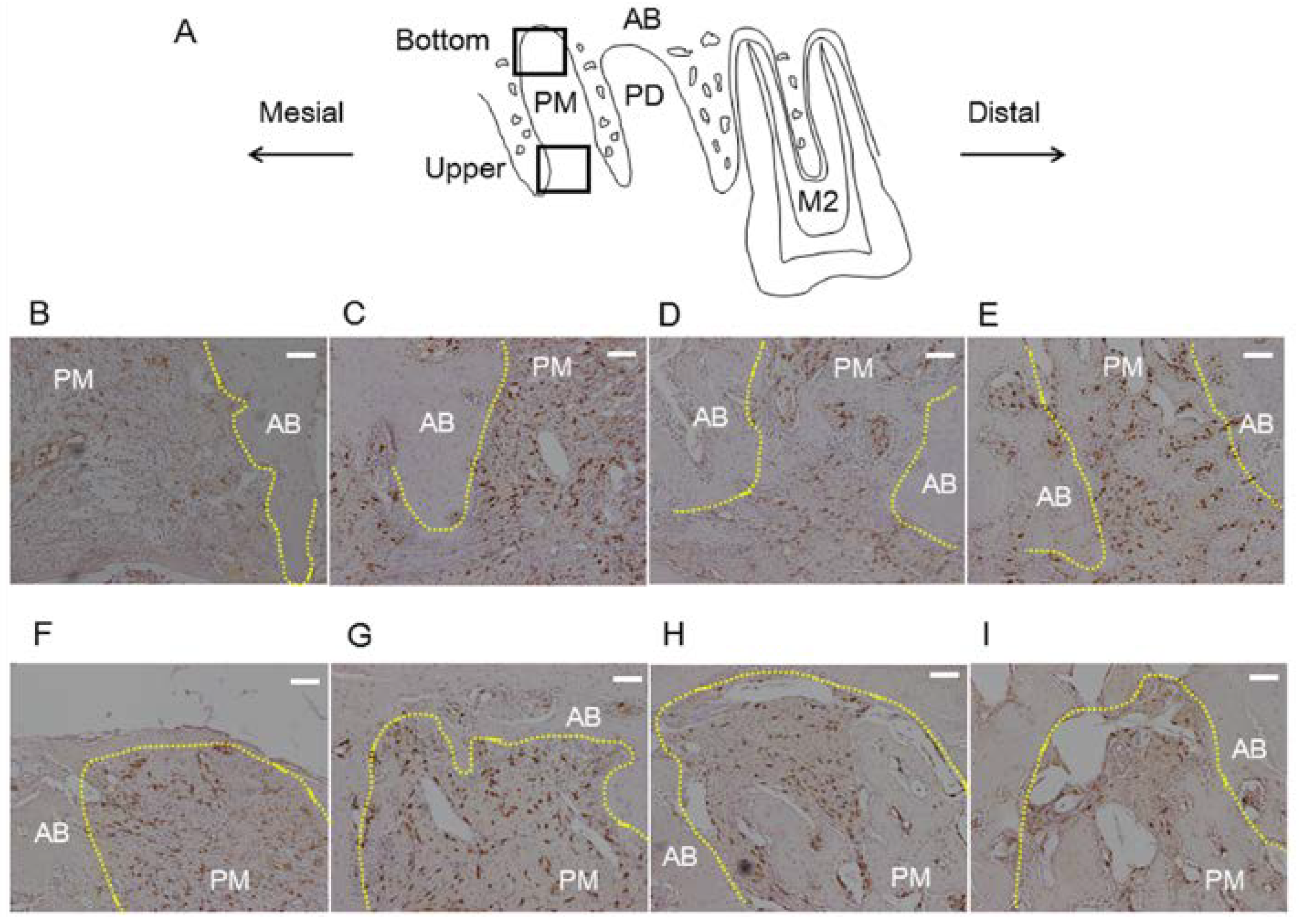
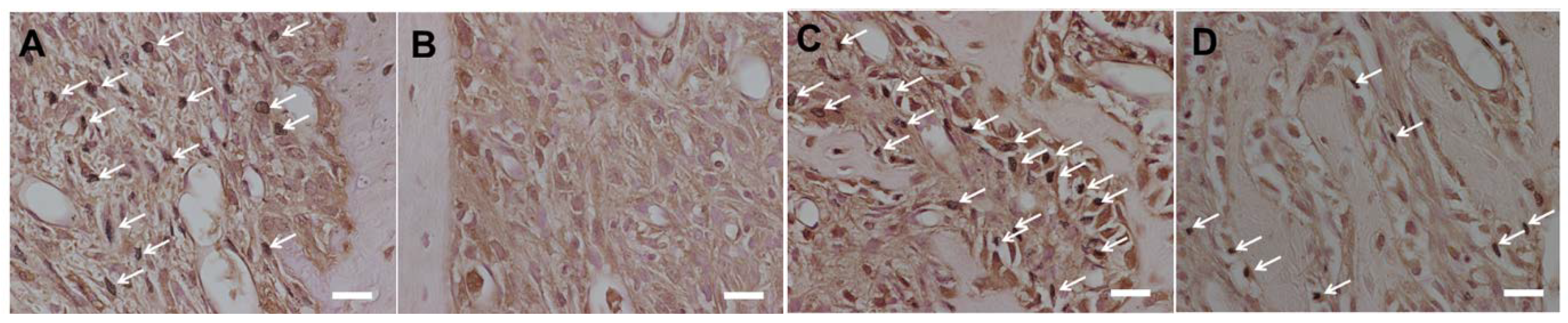
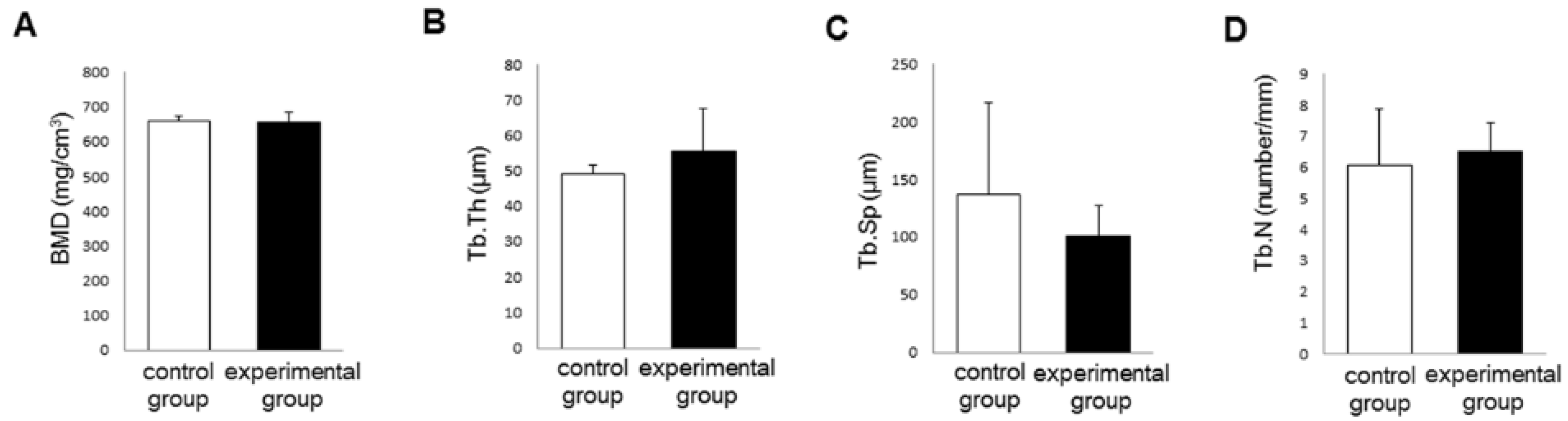
3.3. Bone Morphogenetic Changes
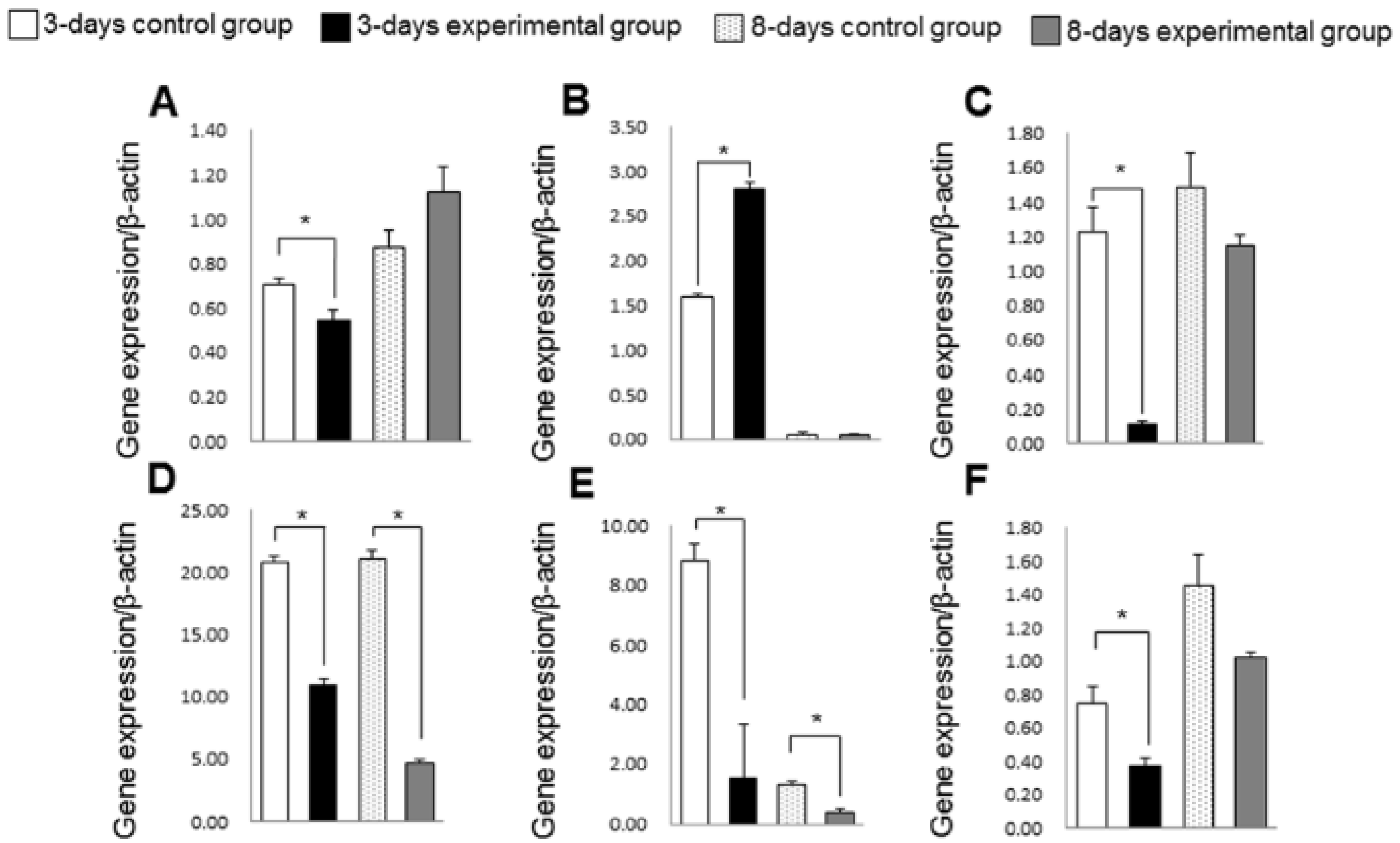
3.4. Gene Expression of Inflammation, Oxidative Stress and Collagen Turnover Markers
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sarsour, E.H.; Kumar, M.G.; Chaudhuri, L.; Kalen, A.L.; Goswami, P.C. Redox control of the cell cycle in health and disease. Antioxid. Redox Signal. 2009, 11, 2985–3011. [Google Scholar] [CrossRef]
- Sen, C.K.; Roy, S. Redox signals in wound healing. Biochim. Biophys. Acta 2008, 1780, 1348–1361. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Kant, V.; Gopal, A.; Pathak, N.N.; Kumar, P.; Tandan, S.K.; Kumar, D. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int. Immunopharmacol. 2014, 20, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Tortosa, M.C.; Granados, S.; Ramirez-Tortosa, C.L.; Ochoa, J.J.; Camacho, P.; García-Valdés, L.; Battino, M.; Quiles, J.L. Oxidative stress status in liver mitochondria and lymphocyte DNA damage of atherosclerotic rabbits supplemented with water soluble coenzyme Q10. Biofactors 2008, 32, 263–273. [Google Scholar] [CrossRef]
- Mezawa, M.; Takemoto, M.; Onishi, S.; Ishibashi, R.; Ishikawa, T.; Yamaga, M.; Fujimoto, M.; Okabe, E.; He, P.; Kobayashi, K.; et al. The reduced form of coenzyme Q10 improves glycemic control in patients with type 2 diabetes: An open label pilot study. Biofactors 2012, 38, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Battino, M.; Fato, R.; Parenti-Castelli, G.; Lenaz, G. Coenzyme Q can control the efficiency of oxidative phosphorylation. Int. J. Tissue React. 1990, 12, 137–144. [Google Scholar] [PubMed]
- Lenaz, G.; Battino, M.; Castelluccio, C.; Fato, R.; Cavazzoni, M.; Rauchova, H.; Bovina, C.; Formiggini, G.; Parenti-Castelli, G. Studies on the role of ubiquinone in the control of the mitochondrial respiratory chain. Free Radic. Res. Commun. 1990, 8, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.S.; Song, H.S.; Kim, H.R.; Park, T.W.; Kim, T.D.; Cho, B.J.; Kim, C.J.; Sim, S.S. Effect of coenzyme Q10 on cutaneous healing in skin-incised mice. Arch. Pharm. Res. 2009, 32, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Akamatsu, M.; Machigashira, M.; Hara, Y.; Sakagami, R.; Hirofuji, T.; Hamachi, T.; Maeda, K.; Yokota, M.; Kido, J.; et al. FGF-2 stimulates periodontal regeneration: Results of a multi-center randomized clinical trial. J. Dent. Res. 2011, 90, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Shirakata, Y.; Taniyama, K.; Yoshimoto, T.; Miyamoto, M.; Takeuchi, N.; Matsuyama, T.; Noguchi, K. Regenerative effect of basic fibroblast growth factor on periodontal healing in two-wall intrabony defects in dogs. J. Clin. Periodontol. 2010, 37, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, Y.; Terashima, H.; Takedachi, M.; Maeda, K.; Nakamura, T.; Sawada, K.; Kobashi, M.; Awata, T.; Oohara, H.; Kawahara, T.; et al. Fibroblast growth factor-2 stimulates directed migration of periodontal ligament cells via PI3K/AKT signaling and CD44/hyaluronan interaction. J. Cell Physiol. 2011, 226, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, T.; Tomofuji, T.; Ekuni, D.; Azuma, T.; Endo, Y.; Kasuyama, K.; Machida, T.; Morita, M. Anti-aging effects of co-enzyme Q10 on periodontal tissues. J. Dent. Res. 2013, 92, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Kasuyama, K.; Tomofuji, T.; Ekuni, D.; Azuma, T.; Irie, K.; Endo, Y.; Morita, M. Effects of topical application of inorganic polyphosphate on tissue remodeling in rat inflamed gingiva. J. Periodontal Res. 2012, 47, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Ekuni, D.; Tomofuji, T.; Tamaki, N.; Sanbe, T.; Azuma, T.; Yamanaka, R.; Yamamoto, T.; Watanabe, T. Mechanical stimulation of gingiva reduces plasma 8-OHdG level in rat periodontitis. Arch. Oral Biol. 2008, 53, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Kusano, H.; Tomofuji, T.; Azuma, T.; Sakamoto, T.; Yamamoto, T.; Watanabe, T. Proliferative response of gingival cells to ultrasonic and/or vibration toothbrushes. Am. J. Dent. 2006, 19, 7–10. [Google Scholar] [PubMed]
- Endo, Y.; Tomofuji, T.; Ekuni, D.; Azuma, T.; Irie, K.; Kasuyama, K.; Morita, M. Preventive effects of trehalose on osteoclast differentiation in rat periodontitis model. J. Clin. Periodontol. 2013, 40, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sanbe, T.; Tomofuji, T.; Ekuni, D.; Azuma, T.; Tamaki, N.; Yamamoto, T. Oral administration of vitamin C prevents alveolar bone resorption induced by high dietary cholesterol in rats. J. Periodontol. 2007, 78, 2165–2170. [Google Scholar] [CrossRef] [PubMed]
- Steiner, G.G.; Francis, W.; Burrell, R.; Kallet, M.P.; Steiner, D.M.; Macias, R. The healing socket and socket regeneration. Compend. Contin. Educ. Dent. 2008, 29, 114–124. [Google Scholar] [PubMed]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-κB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.U.; Wesche, H. Summary and comparison of the signaling mechanisms of the Toll/interleukin-1 receptor family. Biochim. Biophys. Acta 2002, 1592, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Devin, A.; Cook, A.; Lin, Y.; Rodriguez, Y.; Kelliher, M.; Liu, Z. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity 2000, 12, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Alcocer-Gómez, E.; de Miguel, M.; Culic, O.; Carrión, A.M.; Alvarez-Suarez, J.M.; Bullón, P.; Battino, M.; Fernández-Rodríguez, A.; Sánchez-Alcazar, J.A. Can coenzyme Q10 improve clinical and molecular parameters in fibromyalgia? Antioxid. Redox Signal. 2013, 19, 1356–1361. [Google Scholar] [CrossRef]
- Cordero, M.D.; Alcocer-Gómez, E.; Culic, O.; Carrión, A.M.; de Miguel, M.; Díaz-Parrado, E.; Pérez-Villegas, E.M.; Bullón, P.; Battino, M.; Sánchez-Alcazar, J.A. NLRP3 inflammasome is activated in fibromyalgia: The effect of coenzyme Q10. Antioxid. Redox Signal. 2014, 20, 1169–1180. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [PubMed]
- Ejeil, A.L.; Igondjo-Tchen, S.; Ghomrasseni, S.; Pellat, B.; Godeau, G.; Gogly, B. Expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in healthy and diseased human gingiva. J. Periodontol. 2003, 74, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Ko, W.K.; Jung, M.S.; Kim, J.H.; Lee, W.J.; Park, K.S.; Heo, J.K.; Bang, J.B.; Kwon, I.K. Coenzyme Q10 regulates osteoclast and osteoblast differentiation. J. Food Sci. 2013, 78, 785–891. [Google Scholar] [CrossRef]
- Hanioka, T.; Tanaka, M.; Ojima, M.; Shizukuishi, S.; Folkers, K. Effect of topical application of coenzyme Q10 on adult periodontitis. Mol. Aspects Med. 1994, 15, 241–248. [Google Scholar] [CrossRef]
- Bullon, P.; Quiles, J.L.; Morillo, J.M.; Rubini, C.; Goteri, G.; Granados-Principal, S.; Battino, M.; Ramirez-Tortosa, M. Gingival vascular damage in atherosclerotic rabbits: Hydroxytyrosol and squalene benefits. Food Chem. Toxicol. 2009, 7, 2327–2331. [Google Scholar] [CrossRef]
- Ramirez-Tortosa, M.C.; Quiles, J.L.; Battino, M.; Granados, S.; Morillo, J.M.; Bompadre, S.; Newman, H.N.; Bullon, P. Periodontitis is associated with altered plasma fatty acids and cardiovascular risk markers. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 33–39. [Google Scholar] [CrossRef]
- Bullon, P.; Battino, M.; Varela-Lopez, A.; Perez-Lopez, P.; Granados-Principal, S.; Ramirez-Tortosa, M.C.; Ochoa, J.J.; Cordero, M.D.; Gonzalez-Alonso, A.; Ramirez-Tortosa, C.L.; et al. Diets based on virgin olive oil or fish oil but not on sunflower oil prevent age-related alveolar bone resorption by mitochondrial-related mechanisms. PLoS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Sculley, D.V. Periodontal disease: Modulation of the inflammatory cascade by dietary n-3 polyunsaturated fatty acids. J. Periodontal Res. 2014, 49, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L. Potential mechanisms underpinning the nutritional modulation of periodontal inflammation. J. Am. Dent. Assoc. 2009, 140, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Kuroshima, S.; Kovacic, B.L.; Kozloff, K.M.; McCauley, L.K.; Yamashita, J. Intra-oral PTH administration promotes tooth extraction socket healing. J. Dent. Res. 2013, 92, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.I.; Altman, M.K.; Vanegas, S.M.; Franz, S.E.; Bassit, A.C.; Wronski, T.J. Effects of alendronate on bone healing after tooth extraction in rats. Oral Dis. 2010, 16, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.M.; Silva, G.A.; Lima, M.F.; Calliari, M.V.; Almeida, A.P.; Alves, J.B.; Ferreira, A.J. Sodium hyaluronate accelerates the healing process in tooth sockets of rats. Arch. Oral Biol. 2008, 53, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoneda, T.; Tomofuji, T.; Kawabata, Y.; Ekuni, D.; Azuma, T.; Kataoka, K.; Kunitomo, M.; Morita, M. Application of Coenzyme Q10 for Accelerating Soft Tissue Wound Healing after Tooth Extraction in Rats. Nutrients 2014, 6, 5756-5769. https://doi.org/10.3390/nu6125756
Yoneda T, Tomofuji T, Kawabata Y, Ekuni D, Azuma T, Kataoka K, Kunitomo M, Morita M. Application of Coenzyme Q10 for Accelerating Soft Tissue Wound Healing after Tooth Extraction in Rats. Nutrients. 2014; 6(12):5756-5769. https://doi.org/10.3390/nu6125756
Chicago/Turabian StyleYoneda, Toshiki, Takaaki Tomofuji, Yuya Kawabata, Daisuke Ekuni, Tetsuji Azuma, Kota Kataoka, Muneyoshi Kunitomo, and Manabu Morita. 2014. "Application of Coenzyme Q10 for Accelerating Soft Tissue Wound Healing after Tooth Extraction in Rats" Nutrients 6, no. 12: 5756-5769. https://doi.org/10.3390/nu6125756
APA StyleYoneda, T., Tomofuji, T., Kawabata, Y., Ekuni, D., Azuma, T., Kataoka, K., Kunitomo, M., & Morita, M. (2014). Application of Coenzyme Q10 for Accelerating Soft Tissue Wound Healing after Tooth Extraction in Rats. Nutrients, 6(12), 5756-5769. https://doi.org/10.3390/nu6125756






