The Role of Sodium-Dependent Glucose Transporter 1 and Glucose Transporter 2 in the Absorption of Cyanidin-3-O-β-Glucoside in Caco-2 Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Transport Studies
2.5. RNA Interference
2.6. Western Blotting Analysis
2.7. Quantification of SGLT1 and GLUT2 mRNA
2.8. Absorption of Cy-3-G after RNA Interference
2.9. LCMS Analysis of Cy-3-G Content
2.10. Statistical Analysis
3. Results
3.1. Cytotoxicity of Cy-3-G to Caco-2 Cells
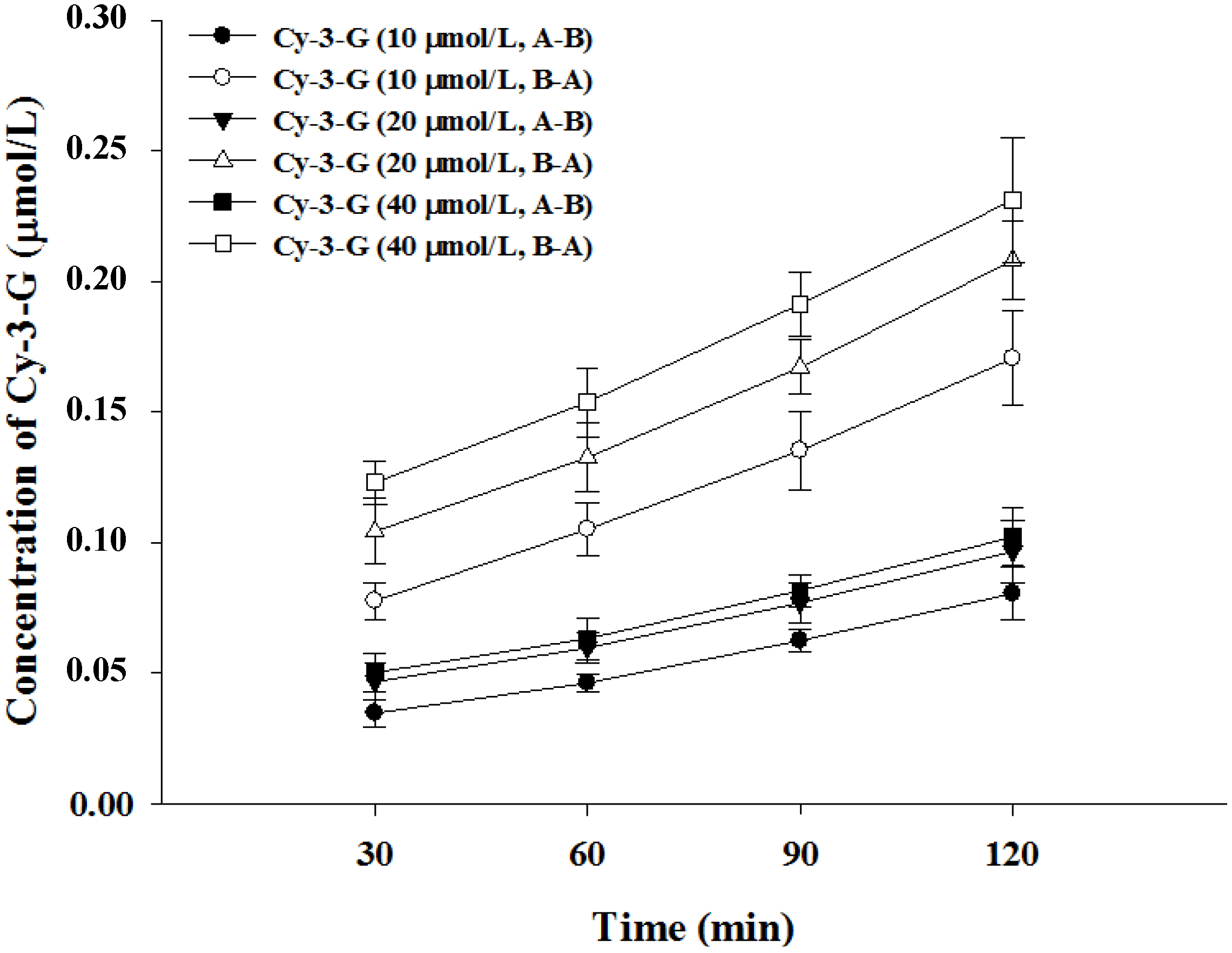
3.2. Transport of Cy-3-G in Caco-2 Cell Monolayer
| Cy-3-G (μmol/L) | Papp (×10−7 cm/s) | Efflux Ratio | Transport Efficiency (%) | |
|---|---|---|---|---|
| B → A | A → B | |||
| 10 | 10.57 ± 1.12 | 14.96 ± 1.88 | 0.71 | 2.41 |
| 20 | 6.45 ± 0.46 | 8.98 ± 1.12 | 0.72 | 1.45 |
| 40 | 3.58 ± 0.37 | 4.75 ± 0.51 | 0.75 | 0.76 |
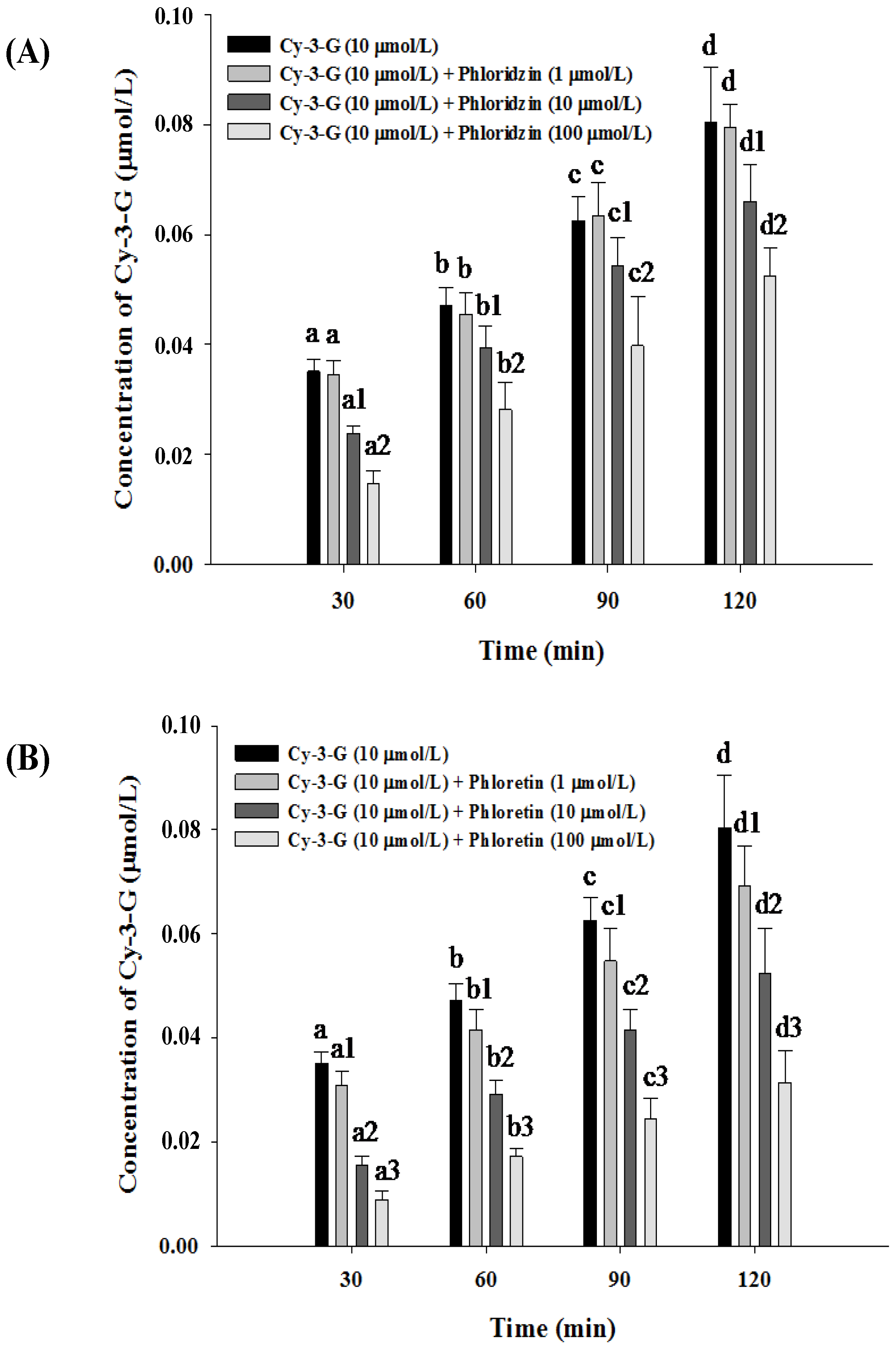
3.3. Absorption of Cy-3-G in the Presence of either Phloridzin or Phloretin
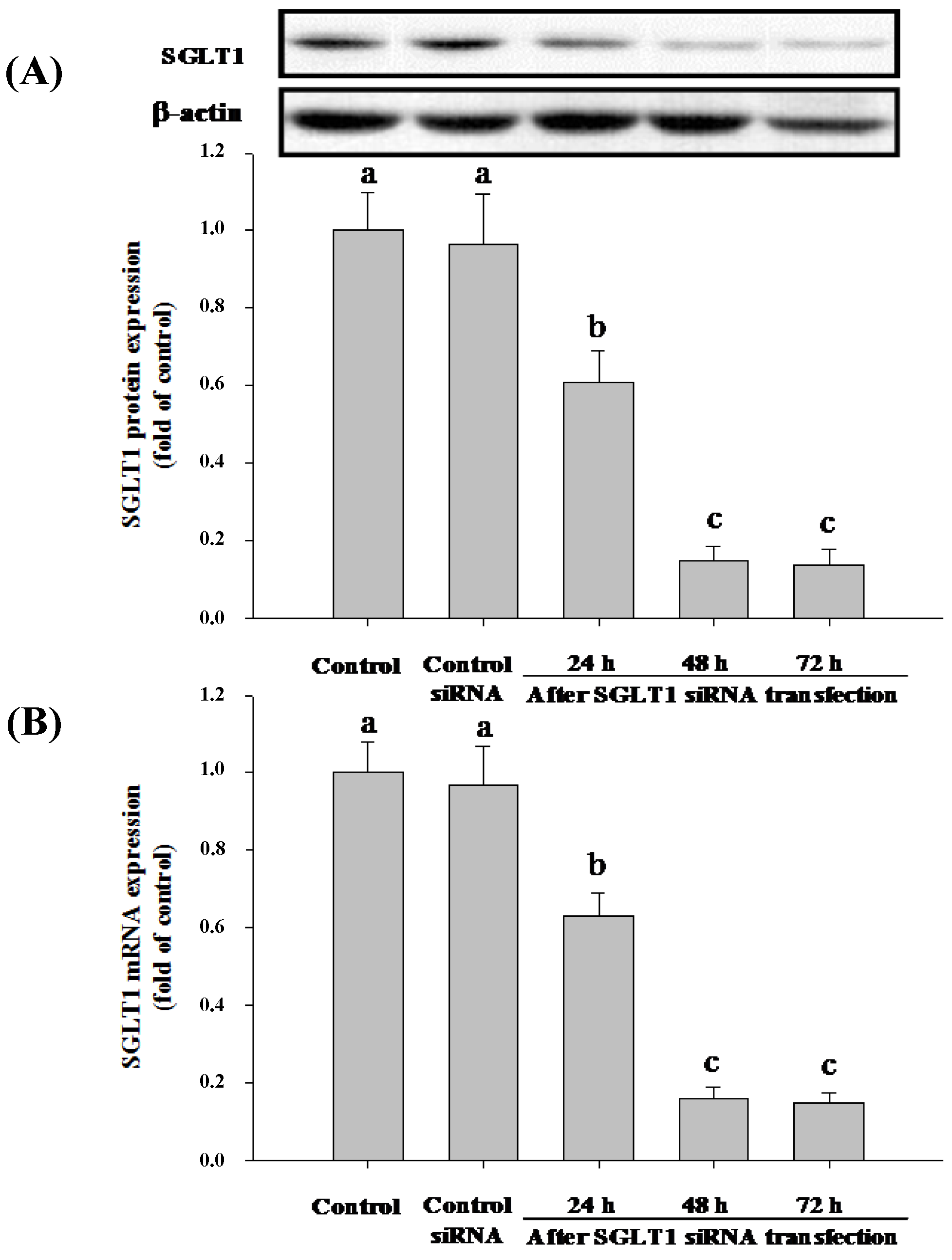
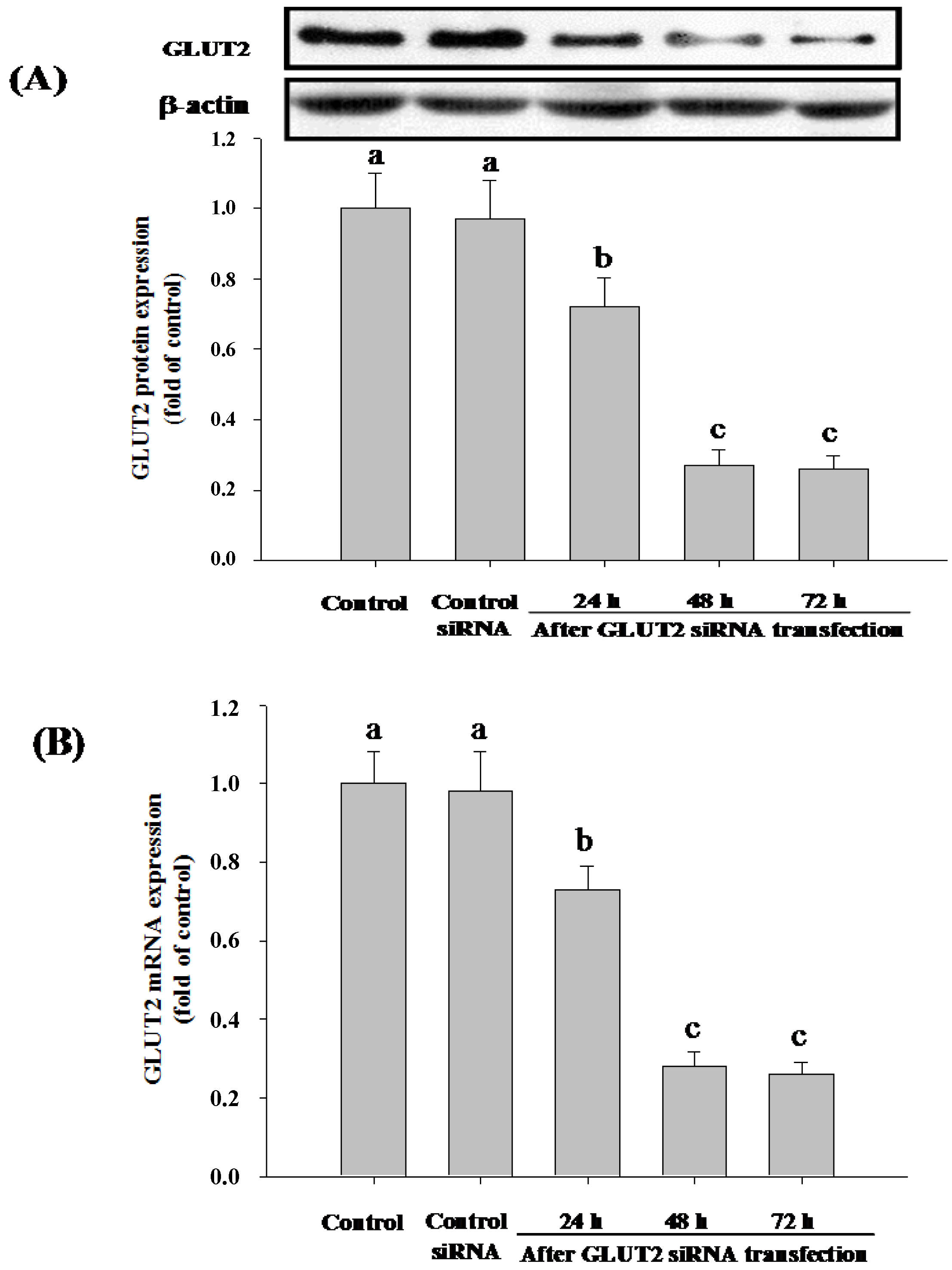
3.4. SGLT1 and GLUT2 Involve the Absorption of Cy-3-G in Caco-2 Cells
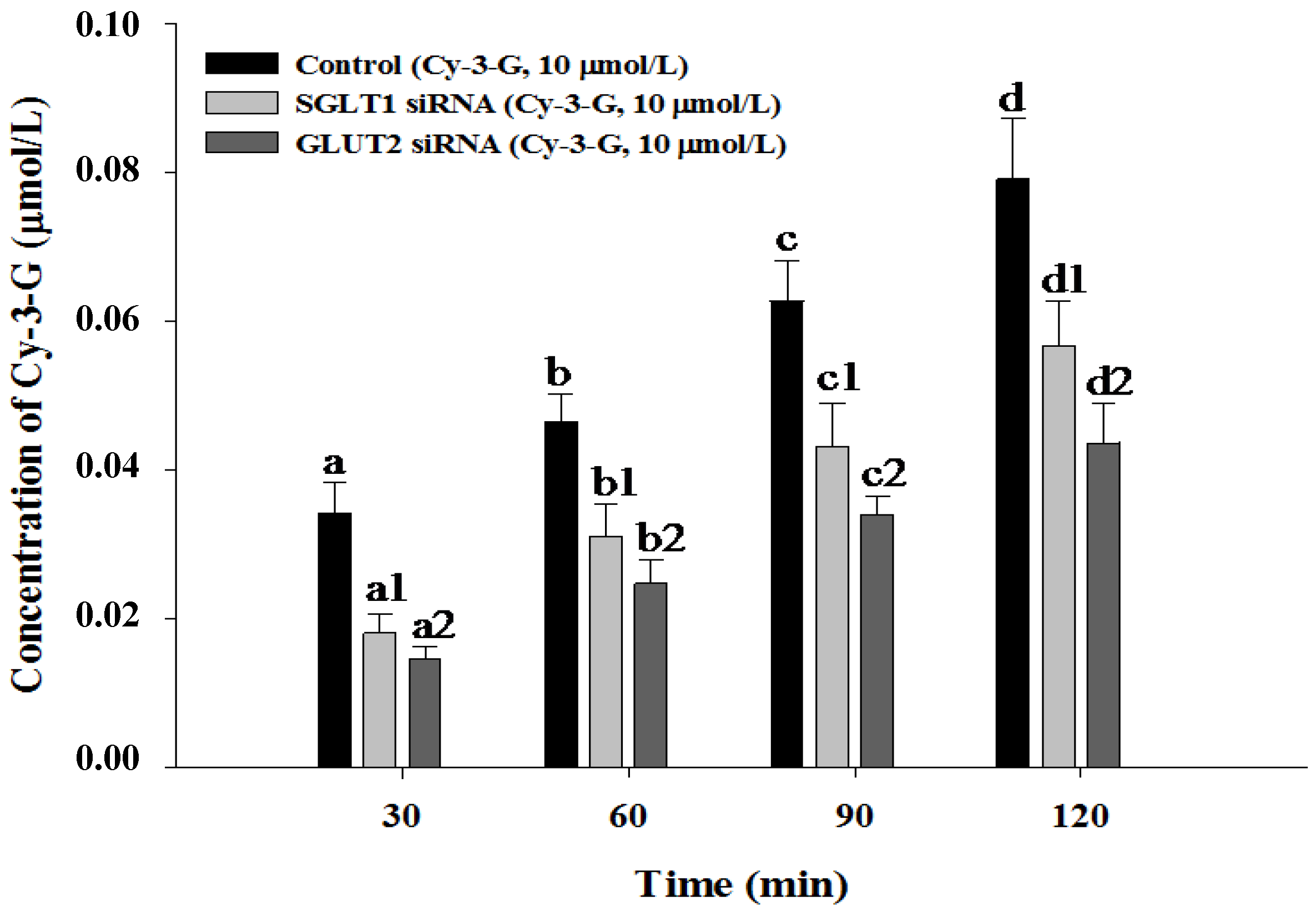
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, M.W.; Zhang, R.F.; Zhang, F.X.; Liu, R.H. Phenolic profiles and antioxidant activity of black rice bran of different commercially available varieties. J. Agric. Food Chem. 2010, 58, 7580–7587. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Chang, Y.C.; Booren, A.M.; Gray, J.I.; de Witt, D.L. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J. Nat. Prod. 1999, 62, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary cyanidin 3-O-â-d-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [PubMed]
- Kowalczyk, E.; Krzesinski, P.; Fijalkowski, P.; Blaszczyk, J.; Kowalski, J. The use of anthocyanins in the treatment of cardiovascular diseases. Pol. Merkur. Lekarski. 2005, 19, 108–110. [Google Scholar] [PubMed]
- Yi, L.; Chen, C.Y.; Jin, X.; Mi, M.T.; Yu, B.; Chang, H.; Ling, W.H.; Zhang, T. Structural requirements of anthocyanins in relation to inhibition of endothelial injury induced by oxidized low-density lipoprotein and correlation with radical scavenging activity. FEBS. Lett. 2010, 584, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.H.; Cheng, Q.X.; Ma, J.; Wang, T. Red and black rice decrease atherosclerotic plaque formation and increase antioxidant status in rabbits. J. Nutr. 2001, 131, 1421–1426. [Google Scholar] [PubMed]
- Qin, Y.; Xia, M.; Ma, J.; Hao, Y.; Liu, J.; Mou, H.; Cao, L.; Ling, W. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009, 90, 485–492. [Google Scholar] [PubMed]
- Wang, D.; Zou, T.; Yang, Y.; Yan, X.; Ling, W. Cyanidin-3-O-β-glucoside with the aid of its metabolite protocatechuic acid, reduces monocyte infiltration in apolipoprotein E-deficient mice. Biochem. Pharmacol. 2011, 82, 713–719. [Google Scholar] [PubMed]
- Miyazaki, K.; Makino, K.; Iwadate, E.; Deguchi, Y.; Ishikawa, F. Anthocyanins from purple sweet potato Ipomoea batatas cultivar ayamurasaki suppress the development of atherosclerotic lesions and both enhancements of oxidative stress and soluble vascular cell adhesion molecule-1 in apolipoprotein E-deficient mice. J. Agric. Food Chem. 2008, 56, 11485–11492. [Google Scholar] [CrossRef] [PubMed]
- Hassimotto, N.M.; Genovese, M.I.; Lajolo, F.M. Absorption and metabolism of cyanidin-3-glucoside and cyanidin-3-rutinoside extracted from wild mulberry (Morus nigra L.) in rats. Nutr. Res. 2008, 28, 198–207. [Google Scholar] [CrossRef]
- Wu, X.; Pittman, H.E., 3rd; Prior, R.L. Pelargonidin is absorbed and metabolized differently than cyanidin after marionberry consumption in pigs. J. Nutr. 2004, 134, 2603–2610. [Google Scholar]
- Wu, X.; Cao, G.; Prior, R.L. Absorption and metabolism of anthocyanins in elderly women after consumption of elderberry or blueberry. J. Nutr. 2002, 132, 1865–1871. [Google Scholar] [PubMed]
- McGhie, T.K.; Ainge, G.D.; Barnett, L.E.; Cooney, J.M.; Jensen, D.J. Anthocyanin glycosides from berry fruit are absorbed and excreted unmetabolized by both humans and rats. J. Agric. Food Chem. 2003, 51, 4539–4548. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Muccitelli, H.U.; Sanchez-Moreno, C.; Prior, R.L. Anthocyanins are absorbed in glycated forms in elderly women: A pharmacokinetic study. Am. J. Clin. Nutr. 2001, 73, 920–926. [Google Scholar] [PubMed]
- Felgines, C.; Talavera, S.; Texier, O.; Besson, C.; Fogliano, V.; Lamaison, J.L.; la Fauci, L.; Galvano, G.; Remesy, C.; Galvano, F. Absorption and metabolism of red orange juice anthocyanins in rats. Br. J. Nutr. 2006, 95, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.; Kay, C.D.; Cottrell, T.; Holub, B.J. Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects. J. Agric. Food Chem. 2002, 50, 7731–7737. [Google Scholar] [CrossRef] [PubMed]
- Talavera, S.; Felgines, C.; Texier, O.; Besson, C.; Manach, C.; Lamaison, J.L.; Remesy, C. Anthocyanins are efficiently absorbed from the small intestine in rats. J. Nutr. 2004, 134, 2275–2279. [Google Scholar] [PubMed]
- Passamonti, S.; Vrhovsek, U.; Mattivi, F. The interaction of anthocyanins with bilitranslocase. Biochem. Biophys. Res. Commun. 2002, 296, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.B.; Wang, M.; Gan, R.Y.; Ling, W.H. Optimization of ultrasound-assisted extraction of anthocyanins from mulberry, using response surface methodology. Int. J. Mol. Sci. 2011, 12, 3006–3017. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.R.; Gochenaur, K. Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. J. Appl. Physiol. 2006, 100, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.C.; Chiou, H.L.; Chen, P.N.; Yang, S.F.; Hsieh, Y.S. Silibinin inhibits the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Mol. Carcinog. 2004, 40, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ling, W.; Wang, Q.; Liu, C.; Hu, Y.; Xia, M. Cyanidin 3-glucoside protects 3T3-L1 adipocytes against H2O2- or TNF-á-induced insulin resistance by inhibiting c-Jun NH2-terminal kinase activation. Biochem. Pharmacol. 2008, 75, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Bitsch, I.; Janssen, M.; Netzel, M.; Strass, G.; Frank, T. Bioavailability of anthocyanidin-3-glycosides following consumption of elderberry extract and blackcurrant juice. Int. J. Clin. Pharmacol. Ther. 2004, 42, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M.; Takano, H.; Takahashi, K.; Sasaki, Y.F.; Yamazoe, Y. Suppression of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-induced DNA damage in rat colon after grapefruit juice intake. Cancer Lett. 2002, 183, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [PubMed]
- Walsh, K.R.; Failla, M.L. Transport and metabolism of equol by Caco-2 human intestinal cells. J. Agric. Food Chem. 2009, 57, 8297–8302. [Google Scholar] [CrossRef] [PubMed]
- Walgren, R.A.; Lin, J.T.; Kinne, R.K.; Walle, T. Cellular uptake of dietary flavonoid quercetin 4’-beta-glucoside by sodium-dependent glucose transporter SGLT1. J. Pharmacol. Exp. Ther. 2000, 294, 837–843. [Google Scholar] [PubMed]
- Dyer, J.; Wood, I.S.; Palejwala, A.; Ellis, A.; Shirazi-Beechey, S.P. Expression of monosaccharide transporters in intestine of diabetic humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G241–G248. [Google Scholar]
- Kellett, G.L.; Brot-Laroche, E. Apical GLUT2: A major pathway of intestinal sugar absorption. Diabetes 2005, 54, 3056–3062. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB. J. 2007, 21, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Mailleau, C.; Capeau, J.; Brahimi-Horn, M.C. Interrelationship between the Na+/glucose cotransporter and CFTR in Caco-2 cells: Relevance to cystic fibrosis. J. Cell Physiol. 1998, 176, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Milbury, P.E.; Cao, G.; Prior, R.L.; Blumberg, J. Bioavailablility of elderberry anthocyanins. Mech. Ageing Dev. 2002, 123, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Mulleder, U.; Murkovic, M.; Pfannhauser, W. Urinary excretion of cyanidin glycosides. J. Biochem. Biophys. Methods 2002, 53, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.C.; McGhie, T.K.; Reynolds, G.W.; Hendriks, W.H. The flavonol quercetin-3-glucoside inhibits cyanidin-3-glucoside absorption in vitro. J. Agric. Food Chem. 2006, 54, 4913–4920. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, T.-B.; Feng, D.; Song, G.; Li, H.-W.; Tang, H.-W.; Ling, W.-H. The Role of Sodium-Dependent Glucose Transporter 1 and Glucose Transporter 2 in the Absorption of Cyanidin-3-O-β-Glucoside in Caco-2 Cells. Nutrients 2014, 6, 4165-4177. https://doi.org/10.3390/nu6104165
Zou T-B, Feng D, Song G, Li H-W, Tang H-W, Ling W-H. The Role of Sodium-Dependent Glucose Transporter 1 and Glucose Transporter 2 in the Absorption of Cyanidin-3-O-β-Glucoside in Caco-2 Cells. Nutrients. 2014; 6(10):4165-4177. https://doi.org/10.3390/nu6104165
Chicago/Turabian StyleZou, Tang-Bin, Dan Feng, Gang Song, Hua-Wen Li, Huan-Wen Tang, and Wen-Hua Ling. 2014. "The Role of Sodium-Dependent Glucose Transporter 1 and Glucose Transporter 2 in the Absorption of Cyanidin-3-O-β-Glucoside in Caco-2 Cells" Nutrients 6, no. 10: 4165-4177. https://doi.org/10.3390/nu6104165
APA StyleZou, T.-B., Feng, D., Song, G., Li, H.-W., Tang, H.-W., & Ling, W.-H. (2014). The Role of Sodium-Dependent Glucose Transporter 1 and Glucose Transporter 2 in the Absorption of Cyanidin-3-O-β-Glucoside in Caco-2 Cells. Nutrients, 6(10), 4165-4177. https://doi.org/10.3390/nu6104165





