Association between Lutein and Zeaxanthin Status and the Risk of Cataract: A Meta-Analysis
Abstract
:1. Introduction
2. Experimental Section
2.1. Search Strategy
2.2. Study Selection
2.3. Data Abstraction and Quality Assessment
2.4. Statistical Analyses
3. Results
3.1. Literature Search
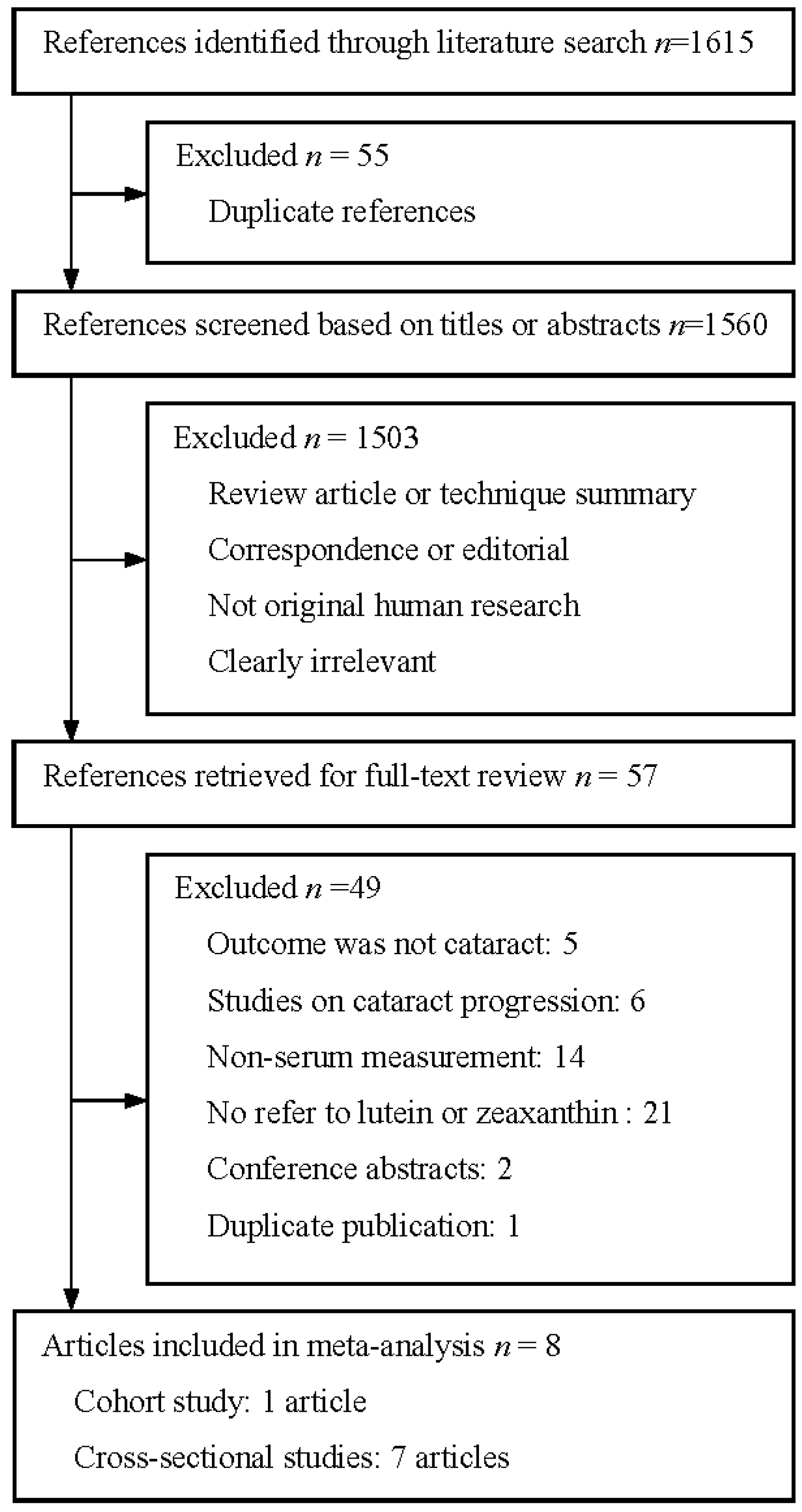
3.2. Study Characteristics
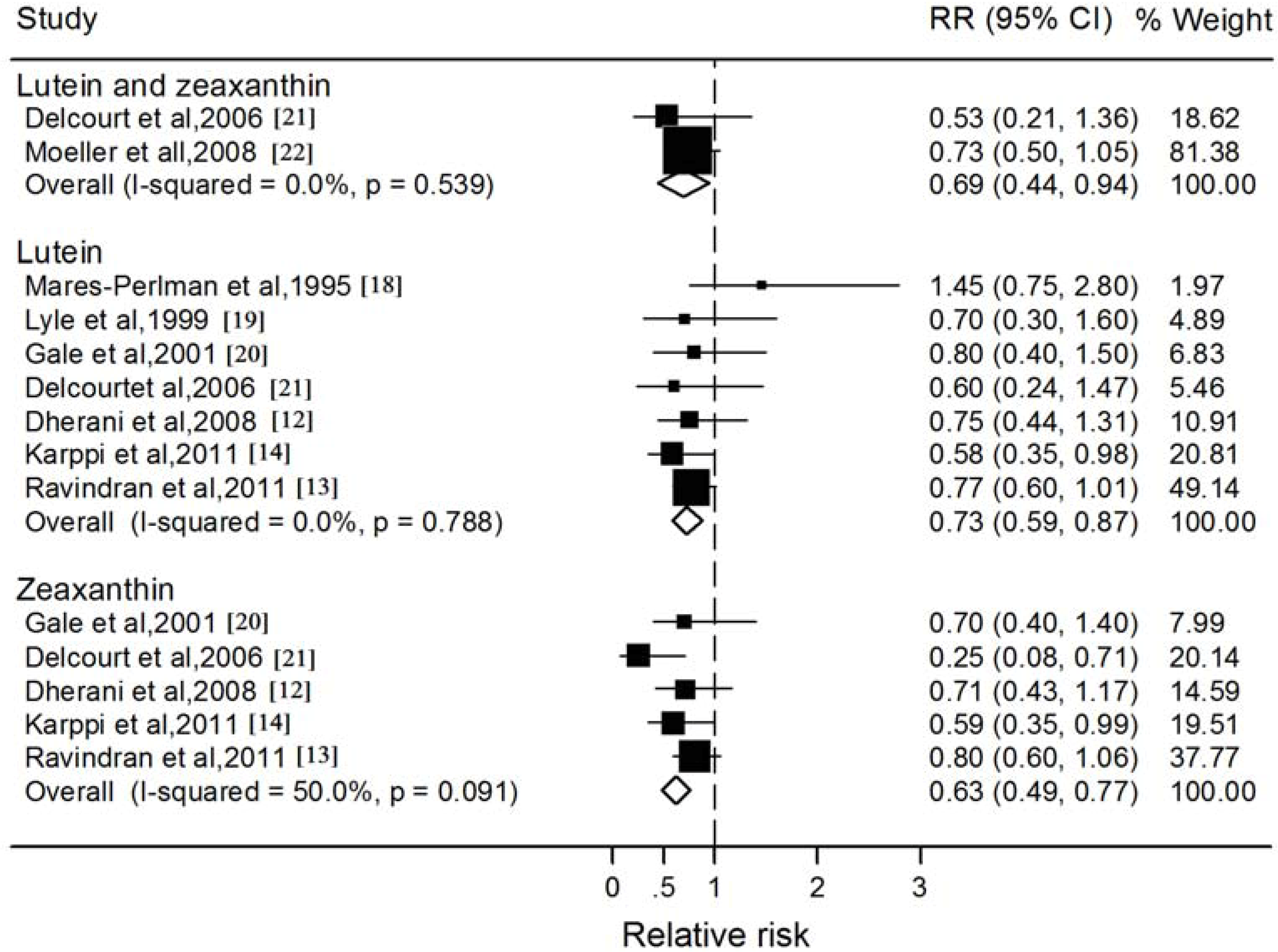
3.3. Blood Lutein and Zeaxanthin and Nuclear Cataract
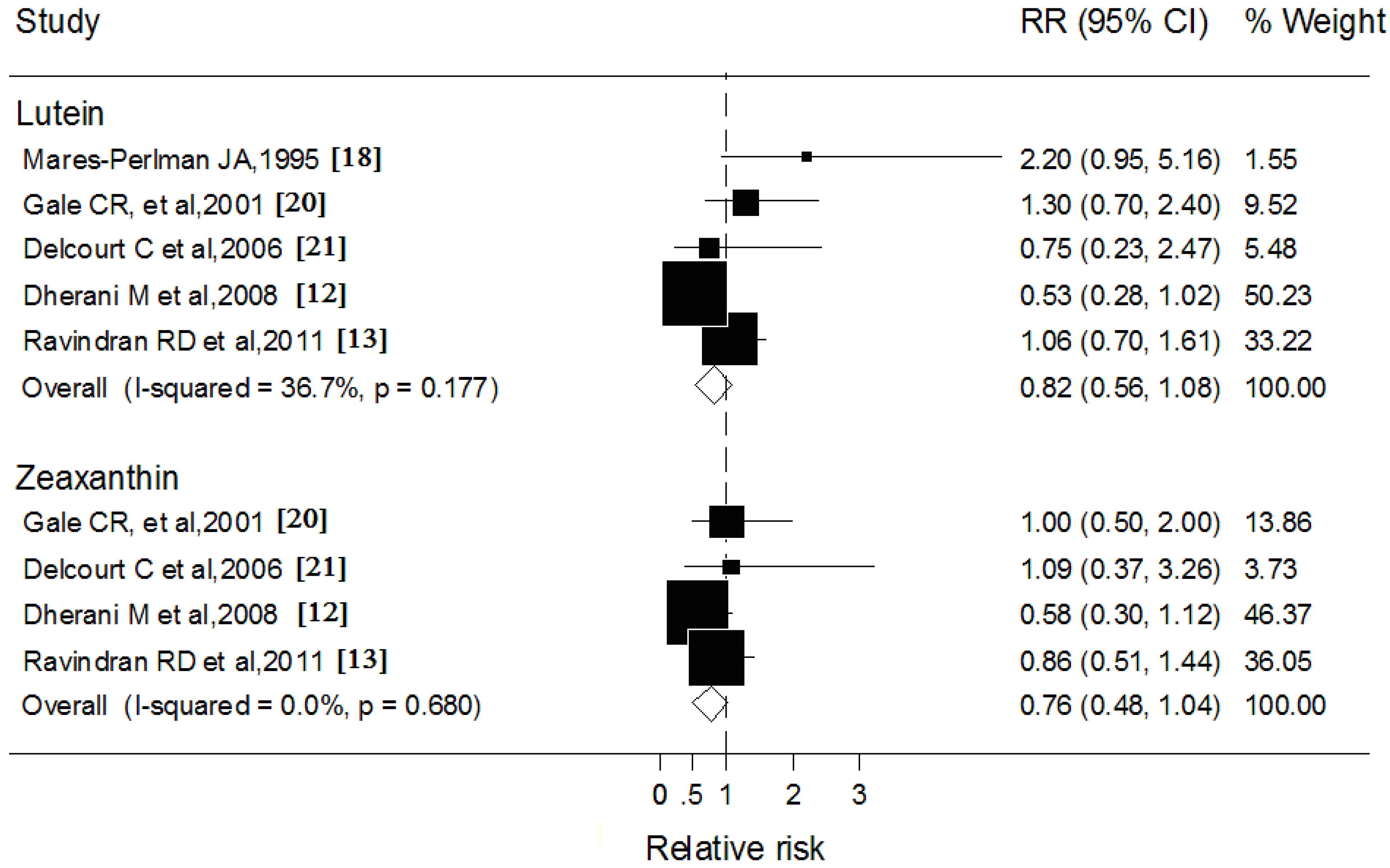
3.4. Blood Lutein and Zeaxanthin and Cortical Cataract
| Source, Year | Study Participants | Blood Assessment | Classification Criteria | Diagnosis Method | Controlled Variable |
|---|---|---|---|---|---|
| Mares-Perlman et al., 1995 [18] | 400 Americans aged 50–86 years in USA | HPLC | WCGS | Lens photography | Age, gender, smoking, alcohol intake and serum cholesterol |
| Lyle et al., 1999 [19] | 252 Americans aged over 50 years in USA | HPLC | WCGS | Lens Photograpuy | Age, smoking, alcohol intake, BMI and serum cholesterol |
| Gale et al., 2001 [20] | 372 Englishmen aged 66–75 years in England | HPLC | LOCS III | Slit lamp | Age, gender, smoking, alcohol intake, BMI, social class, serum cholesterol, glycosylated hemoglobin and steroids use |
| Delcourt et al., 2006 [21] | 815 Mediterranean people aged over 60 in France | HPLC | LOCS III | Slit lamp | Age, gender, smoking, education, iris color, plasma glutathione peroxidase, cardiovascular disease, diabetes, oral corticosteroids, cancer, and exposure to sunlight, etc. |
| Moeller et al., 2008 [22] | 1802 Americans aged 50–79 years in USA | HPLC | ETDRS | Lens photography | Age, smoking, BMI, iris color, physical activity, family history, multivitamin use, hormone replacement and pulse pressure |
| Dherani et al., 2008 [12] | 1112 North Indians aged over 50 years in India | HPLC | LOCS II | Lens photography | Age, gender, smoking, BMI, and systolic blood pressure |
| Ravindran et al., 2011 [13] | 5638 Indians aged over 60 years in India | HPLC | LOCS III | Lens photography | Age, gender, smoking, BMI, mid upper arm circumference, diastolic blood pressure, outdoor exposure, diabetes, current fuels, socioeconomic status, other antioxidants and study center |
| Karppi et al., 2011 [14] | 1689 Eastern Finnish aged 61–80 years in Finland | HPLC | Not mentioned | Medical records | Age, gender, BMI, smoking, alcohol intake, examination year, serum LDL-C, HDL-C, education, corticosteroids use, history of diabetes and hypertension with current use of antihypertensive medication |
| Subgroup | Numbers of Group | Pooled RR (95% CI) | P Value | |
|---|---|---|---|---|
| Heterogeneity | Meta-Regression | |||
| Lutein | ||||
| Study design | ||||
| Cross-sectional studies | 6 | 0.73 (0.59, 0.88) | 0.68 | 0.92 |
| Cohort studies | 1 | 0.70 (0.30, 1.60) | NA | |
| Country of origin | ||||
| USA | 2 | 0.92 (0.37, 1.46) | 0.23 | |
| Europe | 3 | 0.63 (0.38, 0.88) | 0.79 | 0.54 |
| India | 2 | 0.77 (0.58, 0.95) | 0.94 | |
| Mean age | ||||
| ≤65 year | 4 | 0.75 (0.58, 0.92) | 0.96 | 0.71 |
| >65 year | 3 | 0.69 (0.44, 0.95) | 0.26 | |
| Method of diagnosis | ||||
| Lens Photography | 5 | 0.77 (0.60, 0.94) | 0.73 | |
| Slit lamp | 1 | 0.80 (0.40, 1.50) | NA | 0.57 |
| Medical records | 1 | 0.58 (0.35, 0.98) | NA | |
| Classification criteria | ||||
| LOCS | 4 | 0.76 (0.59, 0.93) | 0.96 | |
| WCGS | 2 | 0.92 (0.37, 1.46) | 0.23 | 0.50 |
| Not mentioned | 1 | 0.58 (0.35, 0.98) | NA | |
| Zeaxanthin | ||||
| Country of origin | ||||
| Europe | 3 | 0.47 (0.26, 0.67) | 0.20 | 0.03 |
| India | 2 | 0.78 (0.58, 0.97) | 0.69 | |
| Mean age | ||||
| ≤65 year | 3 | 0.63 (0.46, 0.80) | 0.02 | 0.97 |
| >65 year | 2 | 0.62 (0.35, 0.89) | 0.72 | |
| Method of diagnosis | ||||
| Lens Photography | 3 | 0.63 (0.46, 0.80) | 0.02 | |
| Slit lamp | 1 | 0.70 (0.20, 1.20) | NA | 0.94 |
| Medical records | 1 | 0.59 (0.35, 0.99) | NA | |
| Classification criteria | ||||
| LOCS | 4 | 0.64 (0.48, 0.79) | 0.05 | 0.80 |
| Not mentioned | 1 | 0.59 (0.35, 0.99) | NA | |
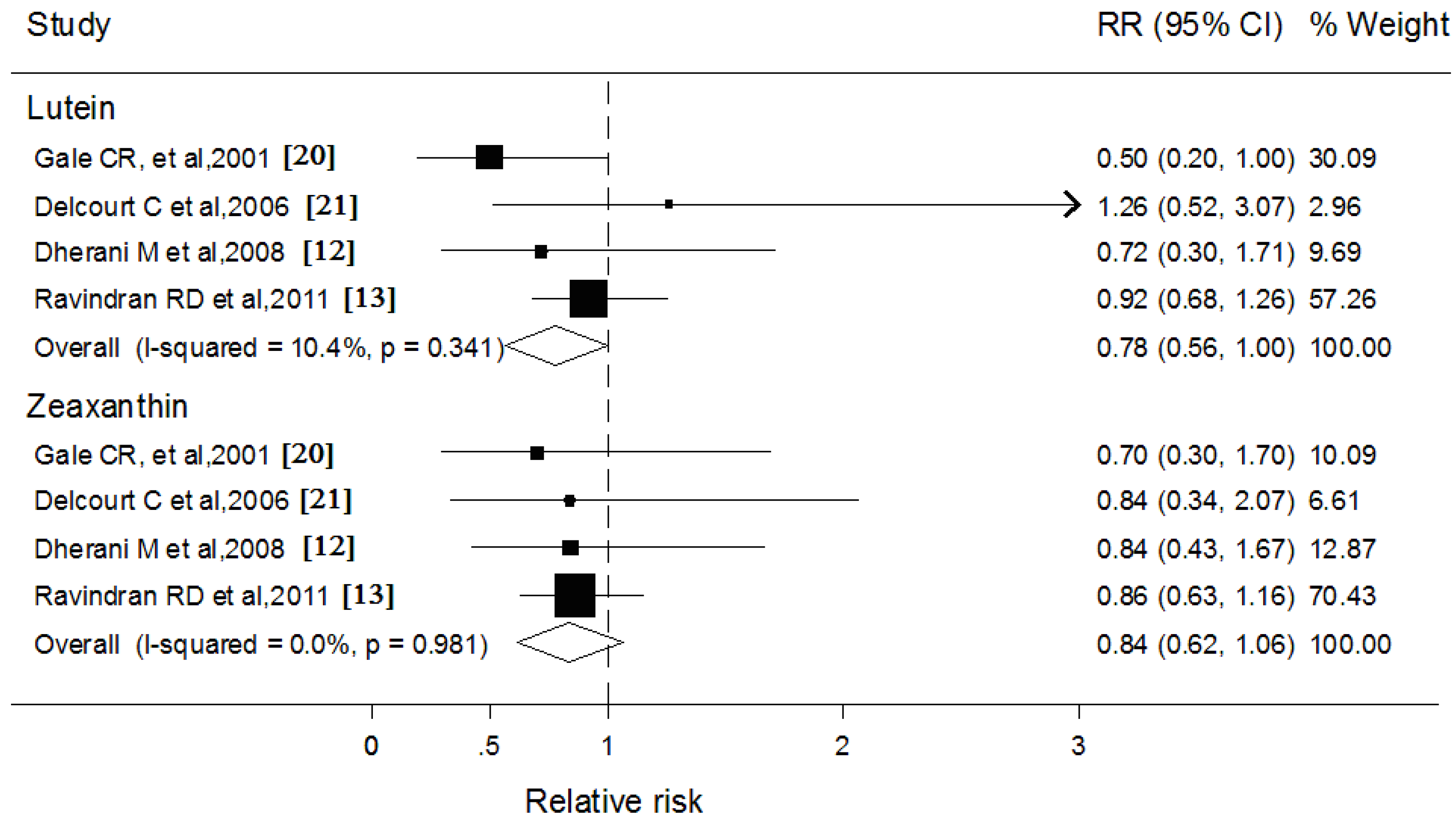
3.5. Blood Lutein and Zeaxanthin and Subcapsular Cataract
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Cabot, F.; Saad, A.; McAlinden, C.; Haddad, N.M.; Grise-Dulac, A.; Gatinel, D. Objective assessment of crystalline lens opacity level by measuring ocular light scattering with a double-pass system. Am. J. Ophthalmol. 2013, 155, 629–635. [Google Scholar] [CrossRef]
- Stevens, G.A.; White, R.A.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; Pesudovs, K.; Resnikoff, S.; et al. Global prevalence of vision impairment and blindness: Magnitude and temporal trends, 1990–2010. Ophthalmology 2013, 120, 2377–2384. [Google Scholar] [CrossRef]
- Congdon, N.; O’Colmain, B.; Klaver, C.C.; Klein, R.; Muñoz, B.; Friedman, D.S.; Kempen, J.; Tayle, H.R.; Mitchell, P. Causes and prevalence of visual impairment among adults in the United States. Arch. Ophthalmol. 2004, 122, 477–485. [Google Scholar] [CrossRef]
- Rao, G.N.; Khanna, R.; Payal, A. The global burden of cataract. Curr. Opin. Ophthalmol. 2011, 22, 4–9. [Google Scholar] [CrossRef]
- Andley, U.P. Effects of α-crystallin on lens cell function and cataract pathology. Curr. Mol. Med. 2009, 9, 887–892. [Google Scholar] [CrossRef]
- Ma, L.; Lin, X.M. Effects of lutein and zeaxanthin on aspects of eye health. J. Sci. Food Agric. 2010, 90, 2–12. [Google Scholar] [CrossRef]
- Nolan, J.M.; O’Reilly, P.; Loughman, J.; Stack, J.; Loane, E.; Connolly, E.; Beatty, S. Augmentation of macular pigment following implantation of blue light-filtering intraocular lenses at the time of cataract surgery. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4777–4785. [Google Scholar] [CrossRef]
- Lakshminarayana, R.; Aruna, G.; Sathisha, U.V.; Dharmesh, S.M.; Baskaran, V. Structural elucidation of possible lutein oxidation products mediated through peroxyl radical inducer 2,2′-Azobis (2-methylpropionamidine) dihydrochloride: Antioxidant and cytotoxic influence of oxidized lutein in HeLa cells. Chem. Biol. Interact. 2013, 203, 448–455. [Google Scholar] [CrossRef]
- Christen, W.G.; Liu, S.; Glynn, R.J.; Gaziano, J.M.; Buring, J.E. Dietary carotenoids, vitamins C and E, and risk of cataract in women: A prospective study. Arch. Ophthalmol. 2008, 126, 102–109. [Google Scholar] [CrossRef]
- Yeum, K.J.; Russell, R.M. Carotenoid bioavailability and bioconversion. Annu. Rev. Nutr. 2002, 22, 483–504. [Google Scholar] [CrossRef]
- Von Lintig, J. Metabolism of carotenoids and retinoids related to vision. J. Biol. Chem. 2012, 287, 1627–1634. [Google Scholar] [CrossRef]
- Dherani, M.; Murthy, G.V.; Gupta, S.K.; Young, I.S.; Maraini, G.; Camparini, M.; Price, G.M.; John, N.; Chakravarthy, U.; Fletcher, A.E. Blood levels of vitamin C, carotenoids and retinol are inversely associated with cataract in a North Indian population. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3328–3335. [Google Scholar] [CrossRef]
- Ravindran, R.D.; Vashist, P.; Gupta, S.K.; Young, I.S.; Maraini, G.; Camparini, M.; Jayanthi, R.; John, N.; Fitzpatrick, K.E.; Chakravarthy, U.; et al. Inverse association of vitamin C with cataract in older people in India. Ophthalmology 2011, 118, 1958–1965. [Google Scholar] [CrossRef]
- Karppi, J.; Laukkanen, J.A.; Kurl, S. Plasma lutein and zeaxanthin and the risk of age-related nuclear cataract among the elderly Finnish population. Br. J. Nutr. 2012, 108, 148–154. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Mares-Perlman, J.A.; Brady, W.E.; Klein, B.E.; Klein, R.; Palta, M.; Bowen, P.; Stacewiczsapuntzakis, M. Serum carotenoids and tocopherols and severity of nuclear and cortical opacities. Investig. Ophthalmol. Vis. Sci. 1995, 36, 276–288. [Google Scholar]
- Lyle, B.J.; Mares-Perlman, J.A.; Klein, B.E.; Klein, R.; Palta, M.; Bowen, P.E.; Greger, J.L. Serum carotenoids and tocopherols and incidence of age-related nuclear cataract. Am. J. Clin. Nutr. 1999, 69, 272–277. [Google Scholar]
- Gale, C.R.; Hall, N.F.; Phillips, D.I.; Martyn, C.N. Plasma antioxidant vitamins and carotenoids and age-related cataract. Ophthalmology 2001, 108, 1992–1998. [Google Scholar] [CrossRef]
- Delcourt, C.; Carrière, I.; Delage, M.; Barberger-Gateau, P.; Schalch, W. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: The POLA Study. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2329–2335. [Google Scholar] [CrossRef]
- Moeller, S.M.; Voland, R.; Tinker, L.; Blodi, B.A.; Klein, M.L.; Gehrs, K.M.; Johnson, E.J.; Snodderly, D.M.; Wallace, R.B.; Chappell, R.J.; et al. Associations between age-related nuclear cataract and lutein and zeaxanthin in the diet and serum in the Carotenoids in the Age-Related Eye Disease Study, an Ancillary Study of the Women’s Health Initiative. Arch. Ophthalmol. 2008, 126, 354–364. [Google Scholar] [CrossRef]
- Chitchumroonchokchai, C.; Bomser, J.A.; Glamm, J.E.; Failla, M.L. Xanthophylls and α-tocopherol decrease UVB-induced lipid peroxidation and stress signaling in human lens epithelial cells. J. Nutr. 2004, 134, 3225–3232. [Google Scholar]
- Gao, S.; Qin, T.; Liu, Z.; Caceres, M.A.; Ronchi, C.F.; Chen, C.Y.; Yeum, K.J.; Tayler, A.; Blumberg, J.B.; Liu, Y.; et al. Lutein and zeaxanthin supplementation reduces H2O2-induced oxidative damage in human lens epithelial cells. Mol. Vis. 2011, 17, 3180–3190. [Google Scholar]
- Kijlstra, A.; Tian, Y.; Kelly, E.R.; Berendschot, T.T. Lutein: More than just a filter for blue light. Prog. Retin. Eye Res. 2012, 31, 303–315. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Wisniewska, A.; Widomska, J. Location of macular xanthophylls in the most vulnerable regions of photoreceptor outer-segment membranes. Arch. Biochem. Biophys. 2010, 504, 61–66. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Khachik, F.; Carvalho, L.S.; Muir, G.J.; Zhao, D.Y.; Katz, N.B. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp. Eye Res. 2001, 72, 215–223. [Google Scholar] [CrossRef]
- Landrum, J.T.; Bone, R.A. Lutein, zeaxanthin, and the macular pigment. Arch. Biochem. Biophys. 2001, 385, 28–40. [Google Scholar] [CrossRef]
- Sujak, A.; Gabrielska, J.; Grudzinski, W.; Borc, R.; Mazurek, P.; Gruszecki, W.I. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: The structural aspects. Arch. Biochem. Biophys. 1999, 371, 301–307. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Lott, M.G.; Marcus, D.M. The macular xanthophylls. Surv. Ophthalmol. 2005, 50, 183–193. [Google Scholar] [CrossRef]
- During, A.; Doraiswamy, S.; Harrison, E.H. Xanthophylls are preferentially taken up compared with β-carotene by retinal cells via a SRBI-dependent mechanism. J. Lipid Res. 2008, 49, 1715–1724. [Google Scholar] [CrossRef]
- O’Neill, M.E.; Carroll, Y.; Corridan, B.; Olmedilla, B.; Granado, F.; Blanco, I.; van den Berg, H.; Hininger, I.; Rousell, A.M.; Chopra, M.; et al. A European carotenoid database to assess carotenoid intakes and its use in a five-country comparative study. Br. J. Nutr. 2001, 85, 499–507. [Google Scholar] [CrossRef]
- Storey, P.; Munoz, B.; Friedman, D.; West, S. Racial differences in lens opacity incidence and progression: The Salisbury Eye Evaluation (SEE) Study. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3010–3018. [Google Scholar] [CrossRef]
- Beebe, D.C.; Holekamp, N.M.; Shui, Y.B. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res. 2010, 44, 155–165. [Google Scholar] [CrossRef]
- Lim, J.; Li, L.; Jacobs, M.D.; Kistler, J.; Donaldson, P.J. Mapping of glutathione and its precursor amino acids reveals a role for GLYT2 in glycine uptake in the lens core. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5142–5151. [Google Scholar] [CrossRef]
- Xing, K.Y.; Lou, M.F. Effect of age on the thioltransferase (glutaredoxin) and thioredoxin systems in the human lens. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6598–6604. [Google Scholar] [CrossRef]
- Koo, E.; Chang, J.R.; Agrón, E.; Clemons, T.E.; Sperduto, R.D.; Ferris, F.L.; Chew, E.Y. Ten-year incidence rates of age-related cataract in the Age-Related Eye Disease Study (AREDS): AREDS report no. 33. Ophthalmic Epidemiol. 2013, 20, 71–81. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, X.-H.; Yu, R.-B.; Liu, R.; Hao, Z.-X.; Han, C.-C.; Zhu, Z.-H.; Ma, L. Association between Lutein and Zeaxanthin Status and the Risk of Cataract: A Meta-Analysis. Nutrients 2014, 6, 452-465. https://doi.org/10.3390/nu6010452
Liu X-H, Yu R-B, Liu R, Hao Z-X, Han C-C, Zhu Z-H, Ma L. Association between Lutein and Zeaxanthin Status and the Risk of Cataract: A Meta-Analysis. Nutrients. 2014; 6(1):452-465. https://doi.org/10.3390/nu6010452
Chicago/Turabian StyleLiu, Xiao-Hong, Rong-Bin Yu, Rong Liu, Zhen-Xuan Hao, Cheng-Cheng Han, Zhong-Hai Zhu, and Le Ma. 2014. "Association between Lutein and Zeaxanthin Status and the Risk of Cataract: A Meta-Analysis" Nutrients 6, no. 1: 452-465. https://doi.org/10.3390/nu6010452
APA StyleLiu, X.-H., Yu, R.-B., Liu, R., Hao, Z.-X., Han, C.-C., Zhu, Z.-H., & Ma, L. (2014). Association between Lutein and Zeaxanthin Status and the Risk of Cataract: A Meta-Analysis. Nutrients, 6(1), 452-465. https://doi.org/10.3390/nu6010452





