Impact of Docosahexaenoic Acid on Gene Expression during Osteoclastogenesis in Vitro—A Comprehensive Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mouse Bone Marrow Macrophage (BMM) Culture and the Induction of Osteoclast Formation
2.2. Tartrate-Resistant Acid Phosphatase (TRAP) Staining
2.3. Gene Expression Analysis Using Agilent Whole Mouse Genome Oligo Microarrays
2.4. Quantification of mRNA by Real-Time PCR
2.5. Data Analyses
| Gene | Primer sequence (5′–3′) positions in mRNA | Product size (bp) |
|---|---|---|
| GAPDH | F:TACAGCAACAGGGTGGTGGAC | 233 |
| R:GTGGGTGCAGCGAACTTTATT | ||
| DC-STAMP | F:AAAACCCTTGGGCTGTTCTT | 399 |
| R:CTTCGCATGCAGGTATTCAA | ||
| Tspan7 | F:TGTAATCCTGTTACAGGTTGTGTTG | 147 |
| R:CCACACTCACTTTTAAATTGATCTGATG | ||
| Siglec-15 | F:TACTTCTGCCGCGTGGAGTT | 114 |
| R:CAGCACCGAGATGTTGACGA | ||
| Mst1r | F:CACGACCCACCTTCAGAGCCCTAGT | 300 |
| R:TTGTCCTAGGCCCAGAGGCAGCTTG |
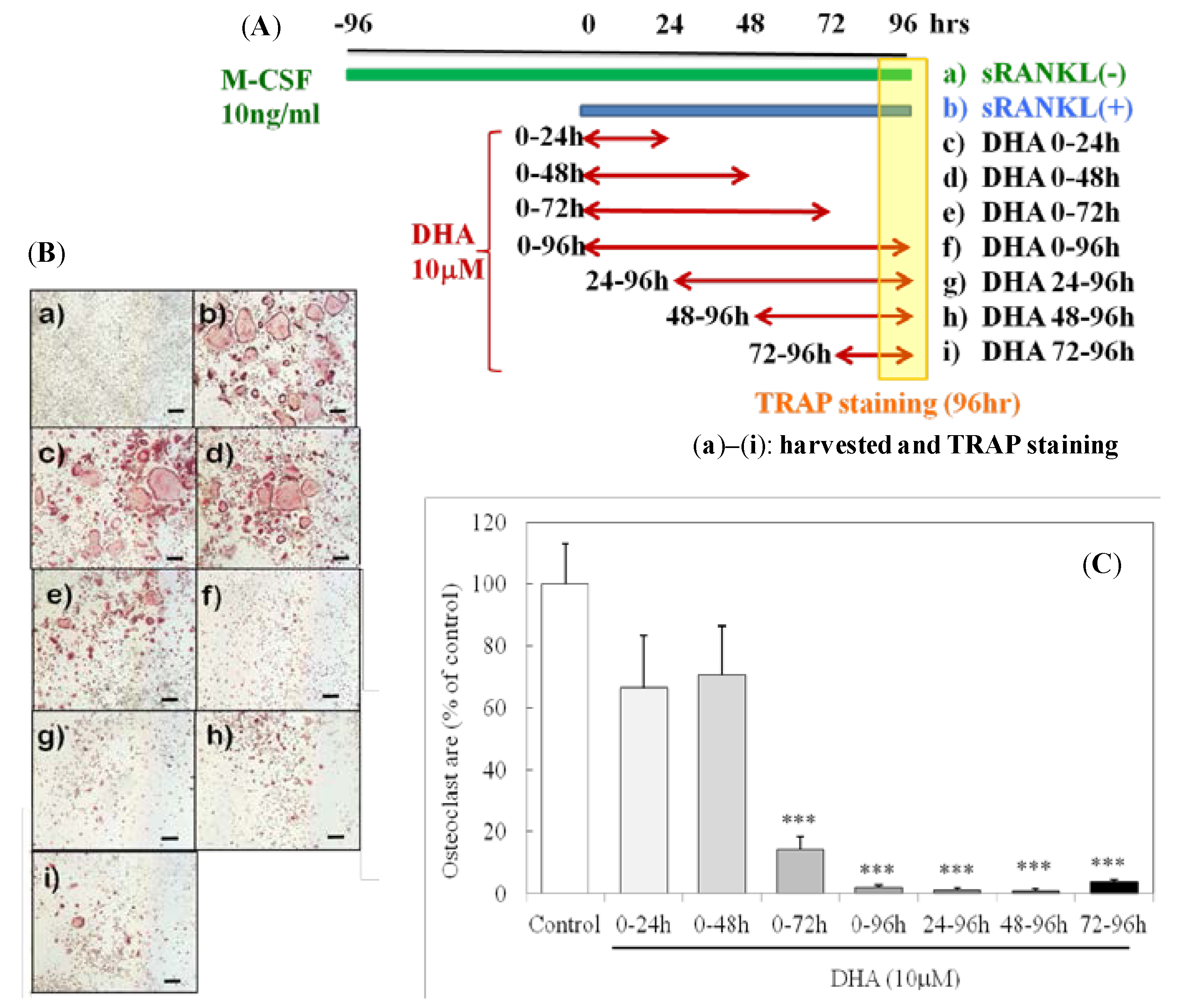
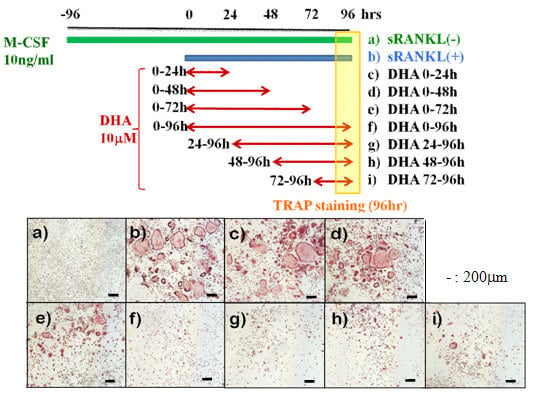
3. Results
3.1. Stage-Dependent Effect of DHA on sRANKL-Induced Osteoclastogenesis
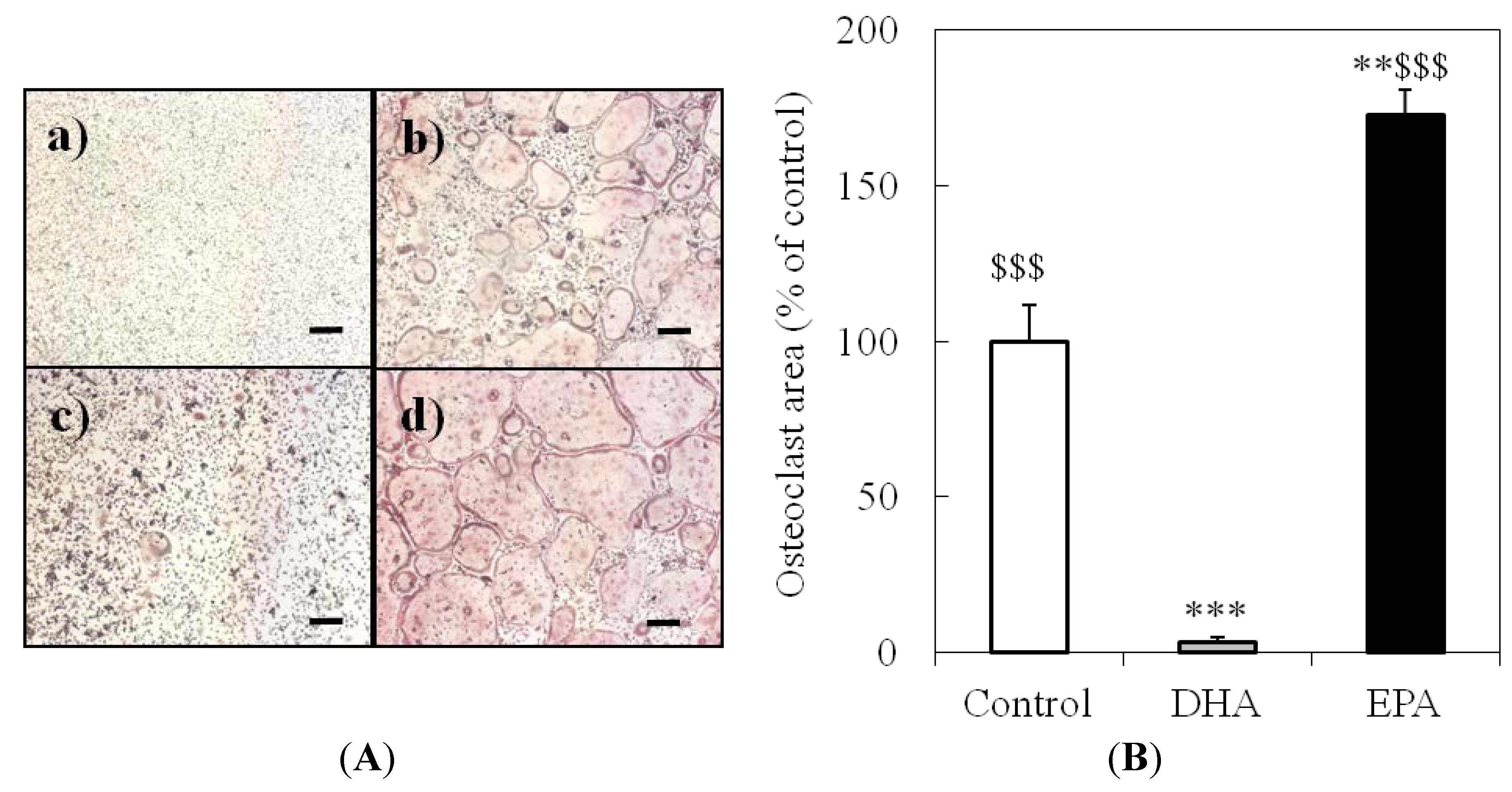
3.2. Effect of DHA and EPA on Osteoclast Formation

3.3. Gene Expression Profiles of BMMs Cultured with or without sRANKL in the Presence or Absence of DHA
| Ontology Term | Gene | Fold change downregulated by DHA |
|---|---|---|
| GO:0030316 | Calcr | 0.43 |
| Dcstamp | 0.44 | |
| osteoclast differentiation | Nfatc1 | 0.47 |
| Ostm1 | 0.45 | |
| GO:0072674 | Gpr55 | 0.36 |
| multinuclear osteoclast differentiation | ||
| GO:0036035 | Dcstamp | 0.44 |
| osteoclast development | ||
| GO:0045672 | Car2 | 0.49 |
| positive regulation of osteoclast differentiation | Itgb3 | 0.50 |
| GO:2001204 | Siglec-15 | 0.35 |
| regulation of osteoclast development | ||
| GO:0045670 | Esrra | 0.48 |
| regulation of osteoclast differentiation |
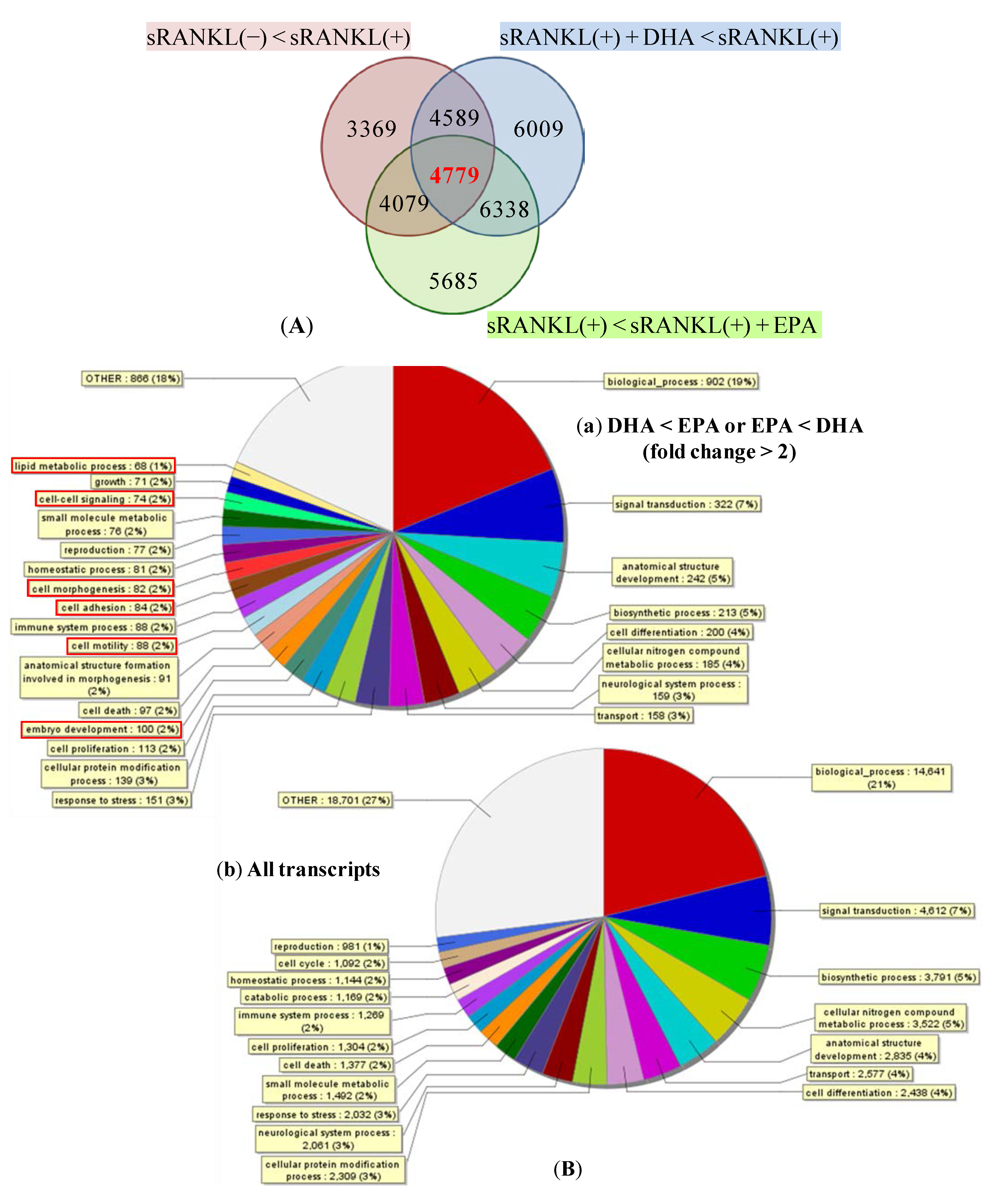
3.4. Gene ontology (GO) Enrichment on Biological Process Ontology
3.5. Effect of DHA and EPA on Osteoclast Differentiation-Related Genes
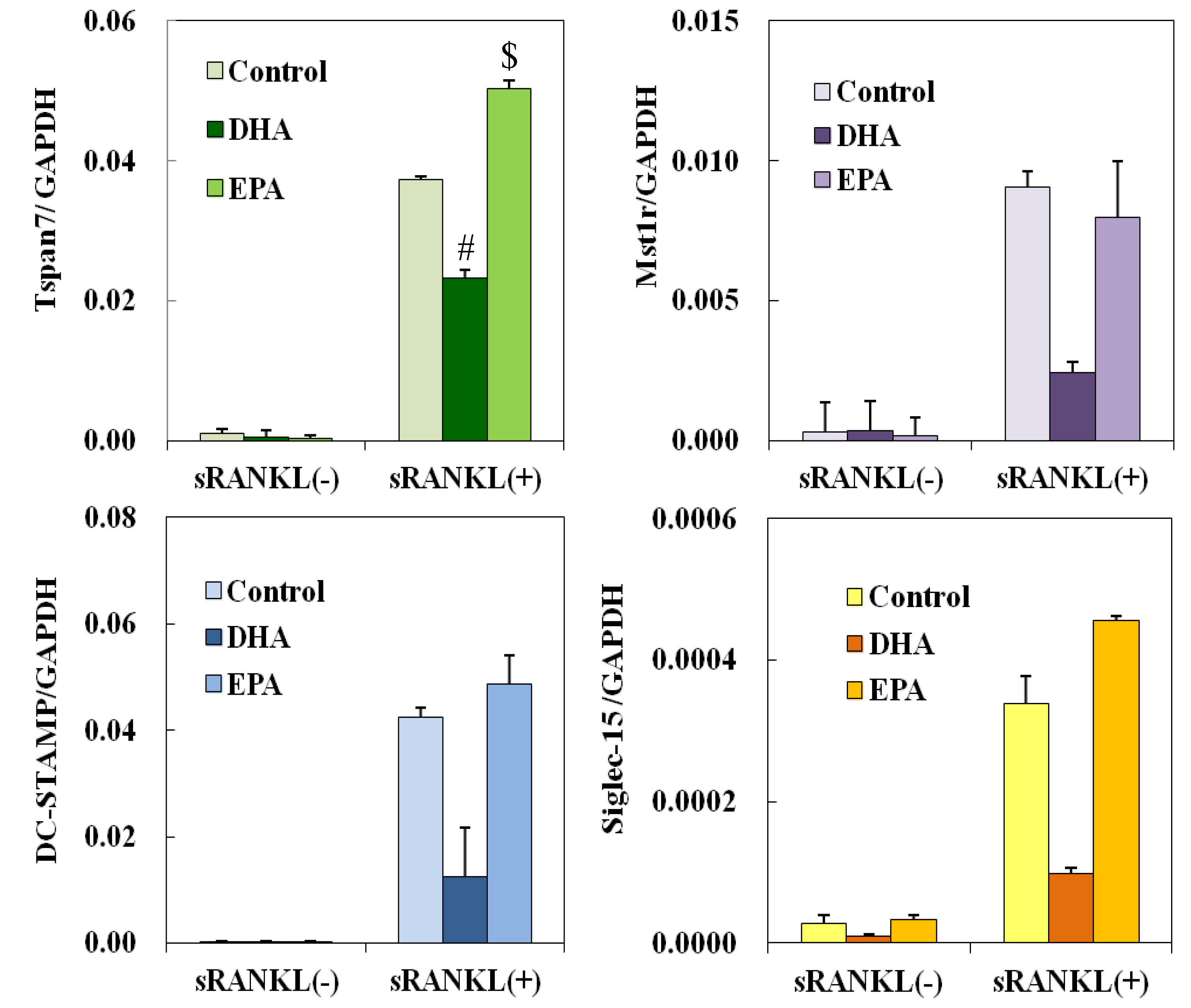
4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Högström, M.; Nordström, P.; Nordström, A. N-3 fatty acids are positively associated with peak bone mineral density and bone accrual in healthy men: The NO2 study. Am. J. Clin. Nutr. 2007, 85, 803–807. [Google Scholar]
- Farina, E.K.; Kiel, D.P.; Roubenoff, R.; Schaefer, E.J.; Cupples, L.A.; Tucker, K.L. Plasma phosphatidylcholine concentrations of polyunsaturated fatty acids are differentially associated with hip bone mineral density and hip fracture in older adults: The framingham osteoporosis study. J. Bone Miner. Res. 2012, 27, 1222–1230. [Google Scholar] [CrossRef]
- Figueredo, C.M.; Martinez, G.L.; Koury, J.C.; Fischer, R.G.; Gustafsson, A. Serum levels of long-chain polyunsaturated fatty acids in patients with periodontal disease. J. Periodontol. 2013, 84, 675–682. [Google Scholar] [CrossRef]
- Dunstan, J.A.; Mori, T.A.; Barden, A.; Beilin, L.J.; Taylor, A.L.; Holt, P.G.; Prescott, S.L. Fish oil supplementation in pregnancy modifies neonatal alergen-specific immune responses and clinical outcomes in infants at high risk of atopy: A randomized, controlled trial. J. Allergy Clin. Immunol. 2003, 112, 1178–1184. [Google Scholar] [CrossRef]
- Vaughan, V.C.; Hassing, M.-R.; Lewandowski, P.A. Marine polyunsaturated fatty acids and cancer therapy. Br. J. Cancer 2013, 108, 486–492. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, O.Y.; Cho, Y.; Chung, J.H.; Jung, Y.-S.; Hwang, G.-S.; Shin, M.-J. Plasma phospholipid fatty acid composition in ischemic stroke: Importance of docosahexaenoic acid in the risk for intracranial atherosclerotic stenosis. Atherosclerosis 2012, 225, 418–424. [Google Scholar] [CrossRef]
- Quinn, J.F.; Raman, R.; Thomas, R.G.; Yurko-Mauro, K.; Nelson, E.B.; Dyck, C.V.; Galvin, J.E.; Emond, J.; Jack, C.R.; Weiner, M.; et al. Docosahexaenoic acid supplementation and cognitive decline in alzheimer disease: A randomized trial. JAMA 2010, 304, 1903–1911. [Google Scholar] [CrossRef]
- Park, Y.; Lee, A.; Shim, S.C.; Lee, J.H.; Choe, J.Y.; Ahn, H.; Choi, C.B.; Sung, Y.K.; Bae, S.C. Effect of n-3 polyunsaturated fatty acid supplementation in patients with rheumatoid arthritis: A 16-week randomized, double-blind, placebo-controlled, parallel-design multicenter study in Korea. J. Nutr. Biochem. 2013, 24, 1367–1372. [Google Scholar] [CrossRef]
- Rizos, E.C.; Evangelia, E.; Ntzani, E.E.; Eftychia Bika, E.; Kostapanos, M.S.; Elisaf, M.S. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA 2012, 308, 1204–1033. [Google Scholar]
- Mori, T.A.; Bao, D.Q.; Burke, V.; Puddey, I.B.; Beilin, L.J. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension 1999, 34, 253–260. [Google Scholar] [CrossRef]
- Rousseau-Ralliard, D.; Moreau, D.; Guilland, J.C.; Raederstorff, D.; Grynberg, A. Docosahexaenoic acid, but not eicosapentaenoic acid, lowers ambulatory blood pressure and shortens interval qt in spontaneously hypertensive rats in vivo. Prostaglandins Leukot. Essent. Fatty Acids 2009, 80, 269–277. [Google Scholar] [CrossRef]
- Yuan, J.; Akiyama, M.; Nakahama, K.; Sato, T.; Uematsu, H.; Morita, I. The effects of polyunsaturated fatty acids and their metabolites on osteoclastogenesis in vitro. Prostaglandins Other Lipid Mediat. 2010, 92, 85–90. [Google Scholar] [CrossRef]
- Zhu, M.; Van Dyke, T.E.; Gyurko, R. Resolvin E1 regulates osteoclast fusion via DC-STAMP and NFATc1. FASEB J. 2013, 29. [Google Scholar] [CrossRef]
- Wardhana; Surachmanto, E.S.; Datau, E.A. The role of omega-3 fatty acids contained in olive oil on chronic inflammation. Acta Med. Indones. 2011, 43, 138–143. [Google Scholar]
- Kobayashi, Y.; Take, I.; Yamashita, T.; Mizoguchi, T.; Ninomiya, T.; Hattori, T.; Kurihara, S.; Ozawa, H.; Udagawa, N.; Takahashi, N. Prostaglandin e2 receptors ep2 and ep4 are down-regulated during differentiation of mouse osteoclasts from their precursors. J. Biol. Chem. 2005, 280, 24035–24042. [Google Scholar] [CrossRef]
- Jiang, J.; Lv, H.S.; Lin, J.H.; Jiang, D.F.; Chen, Z.K. Ltb4 can directly stimulate human osteoclast formation from pbmc independent of rankl. Artif. Cells Blood Substit. Immobil. Biotechnol. 2005, 33, 391–403. [Google Scholar] [CrossRef]
- Chen, Z.K.; Lv, H.S.; Jiang, J. Ltb4 can stimulate human osteoclast differentiation dependent of rankl. Artif. Cells Blood Substit. Immobil. Biotechnol. 2010, 38, 52–56. [Google Scholar] [CrossRef]
- Krönke, G.; Uderhardt, S.; Katzenbeisser, J.; Schett, G. The 12/15-lipoxygenase pathway promotes osteoclast development and differentiation. Autoimmunity 2009, 42, 383–385. [Google Scholar] [CrossRef]
- Fong, L.; Muhlhausler, B.S.; Gibson, R.A.; Xian, C.J. Perinatal maternal dietary supplementation of ω3-fatty acids transiently affects bone marrow microenvironment, osteoblast and osteoclast formation, and bone mass in male offspring. Endocrinology 2012, 153, 2455–2465. [Google Scholar] [CrossRef]
- Kukita, T.; Wada, N.; Kukita, A.; Kakimoto, T.; Sandra, F.; Toh, K.; Nagata, K.; Iijima, T.; Horiuchi, M.; Matsusaki, H.; et al. Rankl-induced dc-stamp is essential for osteoclastogenesis. J. Exp. Med. 2004, 200, 941–946. [Google Scholar] [CrossRef]
- Yagi, M.; Ninomiya, K.; Fujita, N.; Suzuki, T.; Iwasaki, R.; Morita, K.; Hosogane, N.; Matsuo, K.; Toyama, Y.; Suda, T.; et al. Induction of dc-stamp by alternative activation and downstream signaling mechanisms. J. Bone Miner. Res. 2007, 22, 992–1001. [Google Scholar] [CrossRef]
- Courtial, N.; Smink, J.J.; Kuvardina, O.N.; Leutz, A.; Göthert, J.R.; Lausen, J. Tal1 regulates osteoclast differentiation through suppression of the master regulator of cell fusion dc-stamp. FASEB J. 2012, 26, 523–532. [Google Scholar] [CrossRef]
- Ishida-Kitagawa, N.; Tanaka, K.; Bao, X.; Kimura, T.; Miura, T.; Kitaoka, Y.; Hayashi, K.; Sato, M.; Maruoka, M.; Ogawa, T.; et al. Siglec-15 protein regulates formation of functional osteoclasts in concert with dnax-activating protein of 12 kda (dap12). J. Biol. Chem. 2012, 287, 17493–17502. [Google Scholar] [CrossRef]
- Asagiri, M.; Sato, K.; Usami, T.; Ochi, S.; Nishina, H.; Yoshida, H.; Morita, I.; Wagner, E.F.; Mak, T.W.; Serfling, E.; et al. Autoamplification of nfatc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 2005, 202, 1261–1269. [Google Scholar] [CrossRef]
- Iwai, K.; Ishii, M.; Ohshima, S.; Miyatake, K.; Saeki, Y. Expression and function of transmembrane-4 superfamily (tetraspanin) proteins in osteoclasts: Reciprocal roles of tspan-5 and net-6 during osteoclastogenesis. Allergol. Int. 2007, 56, 457–463. [Google Scholar] [CrossRef]
- Bassani, S.; Cingolani, L.A.; Valnegri, P.; Folci, A.; Zapata, J.; Gianfelice, A.; Sala, C.; Goda, Y.; Passafaro, M. The x-linked intellectual disability protein tspan7 regulates excitatory synapse development and ampar trafficking. Neuron 2012, 73, 1143–1158. [Google Scholar] [CrossRef]
- Bolia, A.; Gerek, Z.N.; Keskin, O.; Banu Ozkan, S.; Dev, K.K. The binding affinities of proteins interacting with the pdz domain of pick1. Proteins 2012, 80, 1393–1408. [Google Scholar] [CrossRef]
- Ammendrup-Johnsen, I.; Thorsen, T.S.; Gether, U.; Madsen, K.L. Serine 77 in the pdz domain of pick1 is a protein kinase cα phosphorylation site regulated by lipid membrane binding. Biochemistry 2012, 51, 586–596. [Google Scholar] [CrossRef]
- Rucci, N.; DiGiacinto, C.; Orrù, L.; Millimaggi, D.; Baron, R.; Teti, A. A novel protein kinase c alpha-dependent signal to erk1/2 activated by alphavbeta3 integrin in osteoclasts and in chinese hamster ovary (cho) cells. J. Cell Sci. 2005, 118, 3263–3275. [Google Scholar] [CrossRef]
- Sato, K.; Suematsu, A.; Nakashima, T.; Takemoto-Kimura, S.; Aoki, K.; Morishita, Y.; Asahara, H.; Ohya, K.; Yamaguchi, A.; Takai, T.; et al. Regulation of osteoclast differentiation and function by the camk-creb pathway. Nat. Med. 2006, 12, 1410–1416. [Google Scholar]
- Kurihara, N.; Tatsumi, J.; Arai, F.; Iwama, A.; Suda, T. Macrophage-stimulating protein (msp) and its receptor, ron, stimulate human osteoclast activity but not proliferation: Effect of msp distinct from that of hepatocyte growth factor. Exp. Hematol. 1998, 26, 1080–1085. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Akiyama, M.; Nakahama, K.-i.; Morita, I. Impact of Docosahexaenoic Acid on Gene Expression during Osteoclastogenesis in Vitro—A Comprehensive Analysis. Nutrients 2013, 5, 3151-3162. https://doi.org/10.3390/nu5083151
Akiyama M, Nakahama K-i, Morita I. Impact of Docosahexaenoic Acid on Gene Expression during Osteoclastogenesis in Vitro—A Comprehensive Analysis. Nutrients. 2013; 5(8):3151-3162. https://doi.org/10.3390/nu5083151
Chicago/Turabian StyleAkiyama, Masako, Ken-ichi Nakahama, and Ikuo Morita. 2013. "Impact of Docosahexaenoic Acid on Gene Expression during Osteoclastogenesis in Vitro—A Comprehensive Analysis" Nutrients 5, no. 8: 3151-3162. https://doi.org/10.3390/nu5083151
APA StyleAkiyama, M., Nakahama, K.-i., & Morita, I. (2013). Impact of Docosahexaenoic Acid on Gene Expression during Osteoclastogenesis in Vitro—A Comprehensive Analysis. Nutrients, 5(8), 3151-3162. https://doi.org/10.3390/nu5083151