Modulation of Immune Function by Polyphenols: Possible Contribution of Epigenetic Factors
Abstract
:1. Introduction
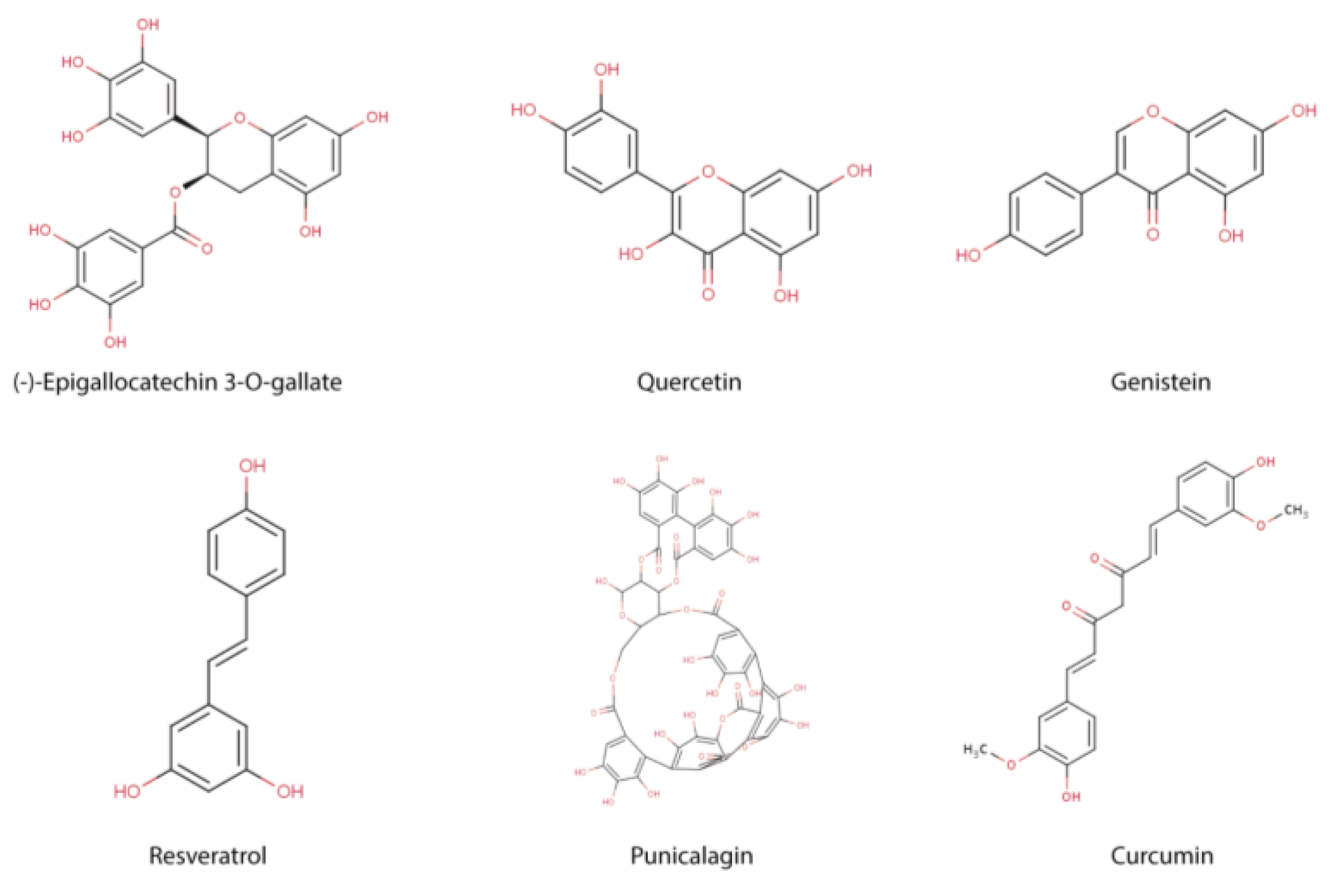
2. Immune Function and Polyphenols
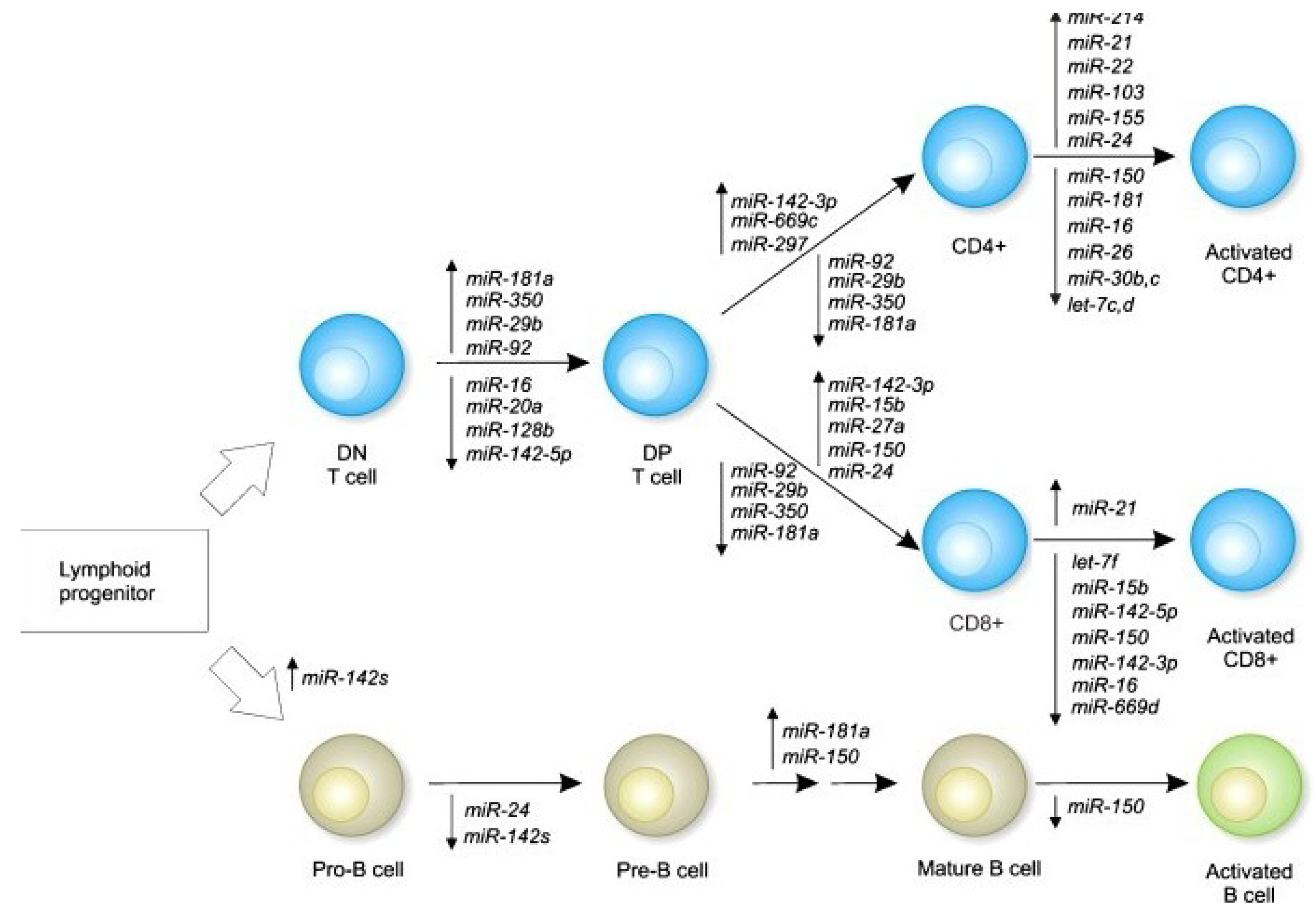
3. Immune Function Modulation by Epigenetic Mechanisms
4. Regulation of Epigenetic Mechanisms by Polyphenols
| Polyphenols | Associated epigenetic mechanism | Transcriptional effect | References |
|---|---|---|---|
| Epigallocathechin-3-gallate | DNMT1 inhibition | Expression | [87] |
| HDAC inhibition | Expression | [90] | |
| miRNAs repression | Expression | [95] | |
| Curcumin | HAT inhibition | Repression | [91] |
| Quercetin | HAT activation and HDAC inhibition | Expression | [93] |
| Genistein | Histone demethylation, HAT activation and SIRT inhibition | Expression | [94] |
5. Modulation of Immune Function by Polyphenols through Epigenetic Mechanisms
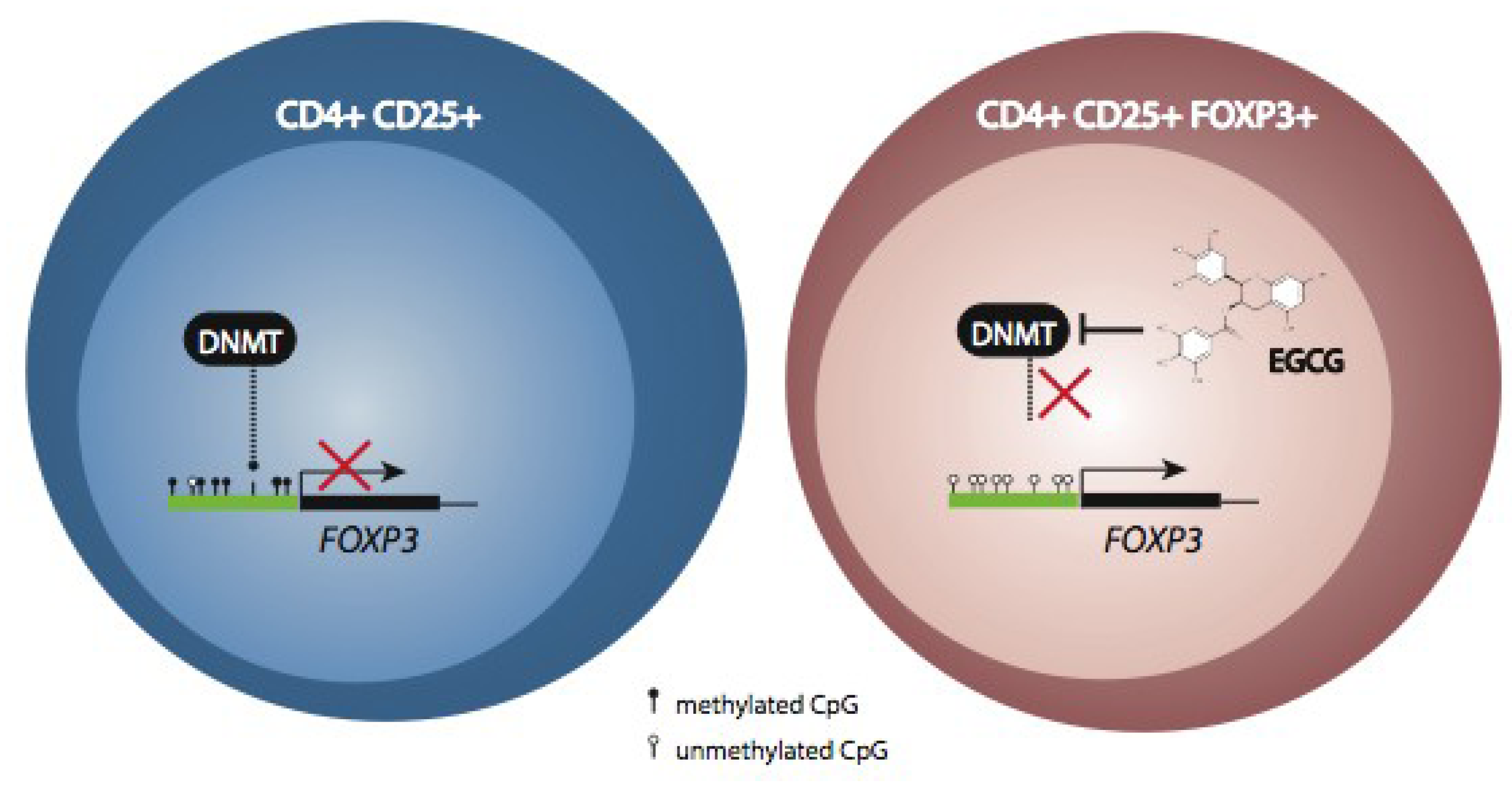
6. Conclusions
Acknowledgments
Conflict of Interest
References
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Recio, M.C.; Andujar, I.; Rios, J.L. Anti-inflammatory agents from plants: Progress and potential. Curr. Med. Chem. 2012, 19, 2088–2103. [Google Scholar]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Antioxidant activity of fresh apples. Nature 2000, 405, 903–904. [Google Scholar]
- Andriantsitohaina, R.; Auger, C.; Chataigneau, T.; Etienne-Selloum, N.; Li, H.; Martinez, M.C.; Schini-Kerth, V.B.; Laher, I. Molecular mechanisms of the cardiovascular protective effects of polyphenols. Br. J. Nutr. 2012, 108, 1532–1549. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, M.; Bilotto, S.; Tedesco, I.; Laratta, B.; Russo, G.L. Dietary polyphenols in cancer prevention: The example of the flavonoid quercetin in leukemia. Ann. N. Y. Acad. Sci. 2012, 1259, 95–103. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar]
- Malireddy, S.; Kotha, S.R.; Secor, J.D.; Gurney, T.O.; Abbott, J.L.; Maulik, G.; Maddipati, K.R.; Parinandi, N.L. Phytochemical antioxidants modulate mammalian cellular epigenome: Implications in health and disease. Antioxid. Redox Signal. 2012, 17, 327–339. [Google Scholar] [CrossRef]
- Fritz, K.S.; Galligan, J.J.; Smathers, R.L.; Roede, J.R.; Shearn, C.T.; Reigan, P.; Petersen, D.R. 4-Hydroxynonenal inhibits sirt3 via thiol-specific modification. Chem. Res. Toxicol. 2011, 24, 651–662. [Google Scholar]
- Jagdeo, J.; Brody, N. Complementary antioxidant function of caffeine and green tea polyphenols in normal human skin fibroblasts. J. Drugs Dermatol. 2011, 10, 753–761. [Google Scholar]
- Zhu, Q.; Zheng, Z.P.; Cheng, K.W.; Wu, J.J.; Zhang, S.; Tang, Y.S.; Sze, K.H.; Chen, J.; Chen, F.; Wang, M. Natural polyphenols as direct trapping agents of lipid peroxidation-derived acrolein and 4-hydroxy-trans-2-nonenal. Chem. Res. Toxicol. 2009, 22, 1721–1727. [Google Scholar] [CrossRef]
- Karasawa, K.; Uzuhashi, Y.; Hirota, M.; Otani, H. A matured fruit extract of date palm tree (Phoenix dactylifera L.) stimulates the cellular immune system in mice. J. Agric. Food Chem. 2011, 59, 11287–11293. [Google Scholar] [CrossRef]
- John, C.M.; Sandrasaigaran, P.; Tong, C.K.; Adam, A.; Ramasamy, R. Immunomodulatory activity of polyphenols derived from cassia auriculata flowers in aged rats. Cell Immunol. 2011, 271, 474–479. [Google Scholar] [CrossRef]
- Robinson, D.S.; Larche, M.; Durham, S.R. Tregs and allergic disease. J. Clin. Investig. 2004, 114, 1389–1397. [Google Scholar]
- Boden, E.K.; Snapper, S.B. Regulatory T cells in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2008, 24, 733–741. [Google Scholar] [CrossRef]
- Costantino, C.M.; Baecher-Allan, C.; Hafler, D.A. Multiple sclerosis and regulatory T cells. J. Clin. Immunol. 2008, 28, 697–706. [Google Scholar] [CrossRef]
- Boissier, M.C.; Assier, E.; Biton, J.; Denys, A.; Falgarone, G.; Bessis, N. Regulatory T cells (treg) in rheumatoid arthritis. Joint Bone Spine 2009, 76, 10–14. [Google Scholar] [CrossRef]
- Brusko, T.; Atkinson, M. Treg in type 1 diabetes. Cell Biochem. Biophys. 2007, 48, 165–175. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Miyara, M.; Costantino, C.M.; Hafler, D.A. Foxp3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010, 10, 490–500. [Google Scholar] [CrossRef]
- Wong, C.P.; Nguyen, L.P.; Noh, S.K.; Bray, T.M.; Bruno, R.S.; Ho, E. Induction of regulatory T cells by green tea polyphenol egcg. Immunol. Lett. 2011, 139, 7–13. [Google Scholar] [CrossRef]
- Yang, J.; Yang, X.; Li, M. Baicalin, a natural compound, promotes regulatory T cell differentiation. BMC Complement. Altern. Med. 2012, 12, 64. [Google Scholar] [CrossRef]
- Wang, H.K.; Yeh, C.H.; Iwamoto, T.; Satsu, H.; Shimizu, M.; Totsuka, M. Dietary flavonoid naringenin induces regulatory T cells via an aryl hydrocarbon receptor mediated pathway. J. Agric. Food Chem. 2012, 60, 2171–2178. [Google Scholar]
- Zhu, J.; Paul, W.E. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol. Rev. 2010, 238, 247–262. [Google Scholar] [CrossRef]
- Wang, J.; Pae, M.; Meydani, S.N.; Wu, D. Green tea epigallocatechin-3-gallate modulates differentiation of naive CD4+ T cells into specific lineage effector cells. J. Mol. Med. (Berl.) 2013, 91, 485–495. [Google Scholar] [CrossRef]
- Yang, J.; Yang, X.; Chu, Y.; Li, M. Identification of baicalin as an immunoregulatory compound by controlling t(h)17 cell differentiation. PLoS One 2011, 6, e17164. [Google Scholar] [CrossRef]
- LeBien, T.W.; Tedder, T.F. B lymphocytes: How they develop and function. Blood 2008, 112, 1570–1580. [Google Scholar] [CrossRef]
- Zunino, S.J.; Storms, D.H. Resveratrol alters proliferative responses and apoptosis in human activated b lymphocytes in vitro. J. Nutr. 2009, 139, 1603–1608. [Google Scholar]
- Sanbongi, C.; Suzuki, N.; Sakane, T. Polyphenols in chocolate, which have antioxidant activity, modulate immune functions in humans in vitro. Cell Immunol. 1997, 177, 129–136. [Google Scholar] [CrossRef]
- Hassanain, E.; Silverberg, J.I.; Norowitz, K.B.; Chice, S.; Bluth, M.H.; Brody, N.; Joks, R.; Durkin, H.G.; Smith-Norowitz, T.A. Green tea (Camelia sinensis) suppresses b cell production of ige without inducing apoptosis. Ann. Clin. Lab. Sci. 2010, 40, 135–143. [Google Scholar]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Gonzalez, R.; Ballester, I.; Lopez-Posadas, R.; Suarez, M.D.; Zarzuelo, A.; Martinez-Augustin, O.; Sanchez de Medina, F. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef]
- Wang, K.; Ping, S.; Huang, S.; Hu, L.; Xuan, H.; Zhang, C.; Hu, F. Molecular mechanisms underlying the in vitro anti-inflammatory effects of a flavonoid-rich ethanol extract from Chinese propolis (poplar type). Evid. Based Complement. Altern. Med. 2013, 2013, 127672. [Google Scholar]
- Park, K.I.; Kang, S.R.; Park, H.S.; Lee, D.H.; Nagappan, A.; Kim, J.A.; Shin, S.C.; Kim, E.H.; Lee, W.S.; Chung, H.J.; et al. Regulation of proinflammatory mediators via NF-kappab and p38 mapk-dependent mechanisms in raw 264.7 macrophages by polyphenol components isolated from korea lonicera japonica thunb. Evid. Based Complement. Altern. Med. 2012, 2012, 828521. [Google Scholar]
- Lai, Z.R.; Ho, Y.L.; Huang, S.C.; Huang, T.H.; Lai, S.C.; Tsai, J.C.; Wang, C.Y.; Huang, G.J.; Chang, Y.S. Antioxidant, anti-inflammatory and antiproliferative activities of kalanchoe gracilis (L.) dc stem. Am. J. Chin. Med. 2011, 39, 1275–1290. [Google Scholar]
- Soromou, L.W.; Zhang, Z.; Li, R.; Chen, N.; Guo, W.; Huo, M.; Guan, S.; Lu, J.; Deng, X. Regulation of inflammatory cytokines in lipopolysaccharide-stimulated raw 264.7 murine macrophage by 7-O-methyl-naringenin. Molecules 2012, 17, 3574–3585. [Google Scholar] [CrossRef]
- Olivera, A.; Moore, T.W.; Hu, F.; Brown, A.P.; Sun, A.; Liotta, D.C.; Snyder, J.P.; Yoon, Y.; Shim, H.; Marcus, A.I.; et al. Inhibition of the NF-kappaB signaling pathway by the curcumin analog, 3,5-bis(2-pyridinylmethylidene)-4-piperidone (ef31): Anti-inflammatory and anti-cancer properties. Int. Immunopharmacol. 2012, 12, 368–377. [Google Scholar]
- Capiralla, H.; Vingtdeux, V.; Venkatesh, J.; Dreses-Werringloer, U.; Zhao, H.; Davies, P.; Marambaud, P. Identification of potent small-molecule inhibitors of stat3 with anti-inflammatory properties in raw 264.7 macrophages. FEBS J. 2012, 279, 3791–3799. [Google Scholar] [CrossRef]
- Drummond, E.M.; Harbourne, N.; Marete, E.; Martyn, D.; Jacquier, J.; O’Riordan, D.; Gibney, E.R. Inhibition of proinflammatory biomarkers in thp1 macrophages by polyphenols derived from chamomile, meadowsweet and willow bark. Phytother. Res. 2012, 27, 588–594. [Google Scholar]
- Schindler, R.; Mancilla, J.; Endres, S.; Ghorbani, R.; Clark, S.C.; Dinarello, C.A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 1990, 75, 40–47. [Google Scholar]
- Essafi-Benkhadir, K.; Refai, A.; Riahi, I.; Fattouch, S.; Karoui, H.; Essafi, M. Quince (Regulation of proinflammatory mediators via nf-kappab and p38 mapk-dependent mechanisms in raw 264.7 macrophages by polyphenol components isolated from korea lonicera japonica thunb) peel polyphenols modulate lps-induced inflammation in human thp-1-derived macrophages through NF-kappab, p38mapk and akt inhibition. Biochem. Biophys. Res. Commun. 2012, 418, 180–185. [Google Scholar]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radi. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Fernandes, E.S.; de Oliveira Machado, M.; Becker, A.M.; de Andrade, F.; Maraschin, M.; de Silva, E.L. Yerba mate (Ilex paraguariensis) enhances the gene modulation and activity of paraoxonase-2: In vitro and in vivo studies. Nutrition 2012, 28, 1157–1164. [Google Scholar]
- Rosenblat, M.; Elias, A.; Volkova, N.; Aviram, M. Monocyte-macrophage membrane possesses free radicals scavenging activity: Stimulation by polyphenols or by paraoxonase 1 (pon1). Free Radic. Res. 2013, 47, 257–267. [Google Scholar] [CrossRef]
- Byun, E.B.; Sung, N.Y.; Byun, E.H.; Song, D.S.; Kim, J.K.; Park, J.H.; Song, B.S.; Park, S.H.; Lee, J.W.; Kim, J.H. The procyanidin trimer c1 inhibits lps-induced mapk and NF-kappab signaling through tlr4 in macrophages. Int. Immunopharmacol. 2013, 15, 450–456. [Google Scholar] [CrossRef]
- Kim, S.; Joo, Y.E. Theaflavin inhibits lps-induced il-6, mcp-1, and icam-1 expression in bone marrow-derived macrophages through the blockade of NF-kappab and mapk signaling pathways. Chonnam Med. J. 2011, 47, 104–110. [Google Scholar] [CrossRef]
- Guha, M.; Mackman, N. Lps induction of gene expression in human monocytes. Cell Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Hong, J.W.; Yang, G.E.; Kim, Y.B.; Eom, S.H.; Lew, J.H.; Kang, H. Anti-inflammatory activity of cinnamon water extract in vivo and in vitro LPS-induced models. BMC Complement. Altern. Med. 2012, 12, 237. [Google Scholar] [CrossRef]
- Xie, C.; Kang, J.; Ferguson, M.E.; Nagarajan, S.; Badger, T.M.; Wu, X. Blueberries reduce pro-inflammatory cytokine TNF-alpha and IL-6 production in mouse macrophages by inhibiting NF-kappab activation and the mapk pathway. Mol. Nutr. Food Res. 2011, 55, 1587–1591. [Google Scholar] [CrossRef]
- Kolehmainen, M.; Mykkanen, O.; Kirjavainen, P.V.; Leppanen, T.; Moilanen, E.; Adriaens, M.; Laaksonen, D.E.; Hallikainen, M.; Puupponen-Pimia, R.; Pulkkinen, L.; et al. Bilberries reduce low-grade inflammation in individuals with features of metabolic syndrome. Mol. Nutr. Food Res. 2012, 56, 1501–1510. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Llorach, R.; Rotches-Ribalta, M.; Guillen, M.; Casas, R.; Arranz, S.; Valderas-Martinez, P.; Portoles, O.; Corella, D.; et al. Differential effects of polyphenols and alcohol of red wine on the expression of adhesion molecules and inflammatory cytokines related to atherosclerosis: A randomized clinical trial. Am. J. Cli.l Nutr. 2012, 95, 326–334. [Google Scholar] [CrossRef]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33 (Suppl.), 245–254. [Google Scholar]
- He, L.; Hannon, G.J. Micrornas: Small rnas with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef]
- Portela, A.; Esteller, M. Epigenetic modifications and human disease. Nat. Biotechnol. 2010, 28, 1057–1068. [Google Scholar] [CrossRef]
- Cheung, P.; Lau, P. Epigenetic regulation by histone methylation and histone variants. Mol. Endocrinol. 2005, 19, 563–573. [Google Scholar] [CrossRef]
- Sonkoly, E.; Stahle, M.; Pivarcsi, A. Micrornas and immunity: Novel players in the regulation of normal immune function and inflammation. Semin. Cancer Biol. 2008, 18, 131–140. [Google Scholar] [CrossRef]
- Chen, C.Z.; Li, L.; Lodish, H.F.; Bartel, D.P. Micrornas modulate hematopoietic lineage differentiation. Science 2004, 303, 83–86. [Google Scholar] [CrossRef]
- Li, Q.J.; Chau, J.; Ebert, P.J.; Sylvester, G.; Min, H.; Liu, G.; Braich, R.; Manoharan, M.; Soutschek, J.; Skare, P.; et al. Mir-181a is an intrinsic modulator of t cell sensitivity and selection. Cell 2007, 129, 147–161. [Google Scholar]
- Neilson, J.R.; Zheng, G.X.; Burge, C.B.; Sharp, P.A. Dynamic regulation of mirna expression in ordered stages of cellular development. Genes Dev. 2007, 21, 578–589. [Google Scholar] [CrossRef]
- Monticelli, S.; Ansel, K.M.; Xiao, C.; Socci, N.D.; Krichevsky, A.M.; Thai, T.H.; Rajewsky, N.; Marks, D.S.; Sander, C.; Rajewsky, K.; et al. Microrna profiling of the murine hematopoietic system. Genome Biol. 2005, 6, R71. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, S.; Mayr, C.; Bartel, D.P.; Lodish, H.F. Mir-150, a microrna expressed in mature B and T cells, blocks early b cell development when expressed prematurely. Proc. Natl. Acad. Sci. USA 2007, 104, 7080–7085. [Google Scholar]
- Floess, S.; Freyer, J.; Siewert, C.; Baron, U.; Olek, S.; Polansky, J.; Schlawe, K.; Chang, H.D.; Bopp, T.; Schmitt, E.; et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007, 5, e38. [Google Scholar]
- Lal, G.; Zhang, N.; van der Touw, W.; Ding, Y.; Ju, W.; Bottinger, E.P.; Reid, S.P.; Levy, D.E.; Bromberg, J.S. Epigenetic regulation of foxp3 expression in regulatory T cells by DNA methylation. J. Immunol. 2009, 182, 259–273. [Google Scholar]
- Liu, J.; Lluis, A.; Illi, S.; Layland, L.; Olek, S.; von Mutius, E.; Schaub, B. T regulatory cells in cord blood-foxp3 demethylation as reliable quantitative marker. PLoS One 2010, 5, e13267. [Google Scholar]
- Jeker, L.T.; Zhou, X.; Gershberg, K.; de Kouchkovsky, D.; Morar, M.M.; Stadthagen, G.; Lund, A.H.; Bluestone, J.A. Microrna 10a marks regulatory t cells. PLoS One 2012, 7, e36684. [Google Scholar]
- Steinfelder, S.; Floess, S.; Engelbert, D.; Haeringer, B.; Baron, U.; Rivino, L.; Steckel, B.; Gruetzkau, A.; Olek, S.; Geginat, J.; et al. Epigenetic modification of the human ccr6 gene is associated with stable ccr6 expression in T cells. Blood 2011, 117, 2839–2846. [Google Scholar] [CrossRef]
- Allan, R.S.; Zueva, E.; Cammas, F.; Schreiber, H.A.; Masson, V.; Belz, G.T.; Roche, D.; Maison, C.; Quivy, J.P.; Almouzni, G.; et al. An epigenetic silencing pathway controlling t helper 2 cell lineage commitment. Nature 2012, 487, 249–253. [Google Scholar] [CrossRef]
- Syrbe, U.; Jennrich, S.; Schottelius, A.; Richter, A.; Radbruch, A.; Hamann, A. Differential regulation of P-selectin ligand expression in naive versus memory CD4+ T cells: Evidence for epigenetic regulation of involved glycosyltransferase genes. Blood 2004, 104, 3243–3248. [Google Scholar] [CrossRef]
- Ji, H.; Ehrlich, L.I.; Seita, J.; Murakami, P.; Doi, A.; Lindau, P.; Lee, H.; Aryee, M.J.; Irizarry, R.A.; Kim, K.; et al. Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature 2010, 467, 338–342. [Google Scholar] [CrossRef]
- Bullwinkel, J.; Ludemann, A.; Debarry, J.; Singh, P.B. Epigenotype switching at the cd14 and cd209 genes during differentiation of human monocytes to dendritic cells. Epigenetics Off. J. DNA Methylation Soc. 2011, 6, 45–51. [Google Scholar] [CrossRef]
- Medzhitov, R.; Horng, T. Transcriptional control of the inflammatory response. Nat. Rev. Immunol. 2009, 9, 692–703. [Google Scholar] [CrossRef]
- Nicodeme, E.; Jeffrey, K.L.; Schaefer, U.; Beinke, S.; Dewell, S.; Chung, C.W.; Chandwani, R.; Marazzi, I.; Wilson, P.; Coste, H.; et al. Suppression of inflammation by a synthetic histone mimic. Nature 2010, 468, 1119–1123. [Google Scholar]
- Escoubet-Lozach, L.; Benner, C.; Kaikkonen, M.U.; Lozach, J.; Heinz, S.; Spann, N.J.; Crotti, A.; Stender, J.; Ghisletti, S.; Reichart, D.; et al. Mechanisms establishing TLR4-responsive activation states of inflammatory response genes. PLoS Genet. 2011, 7, e1002401. [Google Scholar] [CrossRef]
- Hargreaves, D.C.; Horng, T.; Medzhitov, R. Control of inducible gene expression by signal-dependent transcriptional elongation. Cell 2009, 138, 129–145. [Google Scholar] [Green Version]
- Ramirez-Carrozzi, V.R.; Braas, D.; Bhatt, D.M.; Cheng, C.S.; Hong, C.; Doty, K.R.; Black, J.C.; Hoffmann, A.; Carey, M.; Smale, S.T. A unifying model for the selective regulation of inducible transcription by cpg islands and nucleosome remodeling. Cell 2009, 138, 114–128. [Google Scholar] [CrossRef]
- Garber, M.; Yosef, N.; Goren, A.; Raychowdhury, R.; Thielke, A.; Guttman, M.; Robinson, J.; Minie, B.; Chevrier, N.; Itzhaki, Z.; et al. A high-throughput chromatin immunoprecipitation approach reveals principles of dynamic gene regulation in mammals. Mol. Cell 2012, 47, 810–822. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat. Rev. Immunol. 2010, 10, 365–376. [Google Scholar] [CrossRef]
- Ivashkiv, L.B. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2012, 34, 216–223. [Google Scholar] [CrossRef]
- Satoh, T.; Takeuchi, O.; Vandenbon, A.; Yasuda, K.; Tanaka, Y.; Kumagai, Y.; Miyake, T.; Matsushita, K.; Okazaki, T.; Saitoh, T.; et al. The jmjd3-irf4 axis regulates m2 macrophage polarization and host responses against helminth infection. Nat. Immunol. 2010, 11, 936–944. [Google Scholar] [CrossRef]
- Ishii, M.; Wen, H.; Corsa, C.A.; Liu, T.; Coelho, A.L.; Allen, R.M.; Carson, W.F.T.; Cavassani, K.A.; Li, X.; Lukacs, N.W.; et al. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood 2009, 114, 3244–3254. [Google Scholar] [CrossRef]
- Wu, C.; Li, A.; Leng, Y.; Li, Y.; Kang, J. Histone deacetylase inhibition by sodium valproate regulates polarization of macrophage subsets. DNA Cell Biol. 2012, 31, 592–599. [Google Scholar]
- Graff, J.W.; Dickson, A.M.; Clay, G.; McCaffrey, A.P.; Wilson, M.E. Identifying functional micrornas in macrophages with polarized phenotypes. J. Biol. Chem. 2012, 287, 21816–21825. [Google Scholar]
- Zhang, Y.; Zhang, M.; Zhong, M.; Suo, Q.; Lv, K. Expression profiles of mirnas in polarized macrophages. Int. J. Mol. Med. 2013, 31, 797–802. [Google Scholar]
- Cai, X.; Yin, Y.; Li, N.; Zhu, D.; Zhang, J.; Zhang, C.Y.; Zen, K. Re-polarization of tumor-associated macrophages to pro-inflammatory m1 macrophages by microrna-155. J. Mol. Cell Biol. 2012, 4, 341–343. [Google Scholar] [CrossRef]
- Banerjee, S.; Xie, N.; Cui, H.; Tan, Z.; Yang, S.; Icyuz, M.; Abraham, E.; Liu, G. Microrna let-7c regulates macrophage polarization. J. Immunol. 2013, 190, 6542–6549. [Google Scholar]
- Arranz, A.; Doxaki, C.; Vergadi, E.; Martinez de la Torre, Y.; Vaporidi, K.; Lagoudaki, E.D.; Ieronymaki, E.; Androulidaki, A.; Venihaki, M.; Margioris, A.N.; et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc. Natl. Acad. Sci. USA 2012, 109, 9517–9522. [Google Scholar] [CrossRef]
- Rajendran, P.; Ho, E.; Williams, D.E.; Dashwood, R.H. Dietary phytochemicals, hdac inhibition, and DNA damage/repair defects in cancer cells. Clin. Epigenetics 2011, 3, 4. [Google Scholar]
- Fang, M.Z.; Wang, Y.; Ai, N.; Hou, Z.; Sun, Y.; Lu, H.; Welsh, W.; Yang, C.S. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003, 63, 7563–7570. [Google Scholar]
- Lee, W.J.; Shim, J.Y.; Zhu, B.T. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol. Pharmacol. 2005, 68, 1018–1030. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, Z.; Jones, W.; Pavlovicz, R.E.; Liu, S.; Yu, J.; Li, P.K.; Lin, J.; Fuchs, J.R.; Marcucci, G.; et al. Curcumin is a potent DNA hypomethylation agent. Bioorg. Med. Chem. Lett. 2009, 19, 706–709. [Google Scholar]
- Nandakumar, V.; Vaid, M.; Katiyar, S.K. (-)-Epigallocatechin-3-gallate reactivates silenced tumor suppressor genes, cip1/p21 and p16ink4a, by reducing DNA methylation and increasing histones acetylation in human skin cancer cells. Carcinogenesis 2011, 32, 537–544. [Google Scholar]
- Marcu, M.G.; Jung, Y.J.; Lee, S.; Chung, E.J.; Lee, M.J.; Trepel, J.; Neckers, L. Curcumin is an inhibitor of p300 histone acetylatransferase. Med. Chem. 2006, 2, 169–174. [Google Scholar]
- Chen, Y.; Shu, W.; Chen, W.; Wu, Q.; Liu, H.; Cui, G. Curcumin, both histone deacetylase and p300/cbp-specific inhibitor, represses the activity of nuclear factor kappa b and notch 1 in raji cells. Basic Clin. Pharmacol. Toxicol. 2007, 101, 427–433. [Google Scholar] [CrossRef]
- Lee, W.J.; Chen, Y.R.; Tseng, T.H. Quercetin induces fasl-related apoptosis, in part, through promotion of histone h3 acetylation in human leukemia hl-60 cells. Oncol. Rep. 2011, 25, 583–591. [Google Scholar]
- Kikuno, N.; Shiina, H.; Urakami, S.; Kawamoto, K.; Hirata, H.; Tanaka, Y.; Majid, S.; Igawa, M.; Dahiya, R. Genistein mediated histone acetylation and demethylation activates tumor suppressor genes in prostate cancer cells. Int. J. Cancer. 2008, 123, 552–560. [Google Scholar] [CrossRef]
- Arola-Arnal, A.; Blade, C. Proanthocyanidins modulate microrna expression in human hepg2 cells. PLoS One 2011, 6, e25982. [Google Scholar] [CrossRef]
- Milenkovic, D.; Deval, C.; Gouranton, E.; Landrier, J.F.; Scalbert, A.; Morand, C.; Mazur, A. Modulation of mirna expression by dietary polyphenols in apoe deficient mice: A new mechanism of the action of polyphenols. PLoS One 2012, 7, e29837. [Google Scholar]
- Kim, H.J.; Kim, S.H.; Yun, J.M. Fisetin inhibits hyperglycemia-induced proinflammatory cytokine production by epigenetic mechanisms. Evid. Based Complement. Altern. Med. 2012, 2012, 639469. [Google Scholar]
- Yun, J.M.; Jialal, I.; Devaraj, S. Epigenetic regulation of high glucose-induced proinflammatory cytokine production in monocytes by curcumin. J. Nutr. Biochem. 2011, 22, 450–458. [Google Scholar]
- Boesch-Saadatmandi, C.; Loboda, A.; Wagner, A.E.; Stachurska, A.; Jozkowicz, A.; Dulak, J.; Doring, F.; Wolffram, S.; Rimbach, G. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: Role of mir-155. J. Nutr. Biochem. 2011, 22, 293–299. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cuevas, A.; Saavedra, N.; Salazar, L.A.; Abdalla, D.S.P. Modulation of Immune Function by Polyphenols: Possible Contribution of Epigenetic Factors. Nutrients 2013, 5, 2314-2332. https://doi.org/10.3390/nu5072314
Cuevas A, Saavedra N, Salazar LA, Abdalla DSP. Modulation of Immune Function by Polyphenols: Possible Contribution of Epigenetic Factors. Nutrients. 2013; 5(7):2314-2332. https://doi.org/10.3390/nu5072314
Chicago/Turabian StyleCuevas, Alejandro, Nicolás Saavedra, Luis A. Salazar, and Dulcineia S. P. Abdalla. 2013. "Modulation of Immune Function by Polyphenols: Possible Contribution of Epigenetic Factors" Nutrients 5, no. 7: 2314-2332. https://doi.org/10.3390/nu5072314
APA StyleCuevas, A., Saavedra, N., Salazar, L. A., & Abdalla, D. S. P. (2013). Modulation of Immune Function by Polyphenols: Possible Contribution of Epigenetic Factors. Nutrients, 5(7), 2314-2332. https://doi.org/10.3390/nu5072314





