Can Scientific Evidence Support Using Bangladeshi Traditional Medicinal Plants in the Treatment of Diarrhoea? A Review on Seven Plants
Abstract
:1. Background
| Scientific name | Synonym | English name | Family |
|---|---|---|---|
| Diospyros peregrina Gürke | D. biflora Blanco | Gaub Persimmon Riber Ebony | Ebenaceae |
| D. citrifolia Wall. ex A.DC. | |||
| D. embryopteris Pers. | |||
| D. glutinifera (Roxb.) Wall. | |||
| D. glutinosa J.König ex Roxb. | |||
| D. malabarica (Desr.) Kostel. | |||
| D. siamensis Hochr. | |||
| Embryopteris gelatinifera G.Don | |||
| E. glutinifera Roxb. | |||
| E. glutinifolia Link | |||
| E. peregrina Gaertn. | |||
| Heritiera littoralis Dryand. | Amygdalus littoralis (Dryand.) Kuntze | Looking-glass mangrove | Sterculiaceae |
| Balanopteris tothila Gaertn. | |||
| H. minor Bojer | |||
| Ixora coccinea L. | Pavetta coccinea (L.) Blume | Jungleflame ixora | Rubiaceae |
| Scarlet jungleflame | |||
| Pongamia pinnata (L.) Pierre | Cytisus pinnatus L. | Indian beech tree Pongam tree | Fabaceae |
| Dalbergia arborea Willd. | |||
| Derris indica (Lam.) Bennet | |||
| Galedupa indica Lam. | |||
| G. pinnata (L.) Taub. | |||
| G. pungum J.G.Gmel. | |||
| Millettia pinnata L. | |||
| M. novo-guineensis Kaneh. & Hatus. | |||
| P. glabra Vent. | |||
| P. mitis (L.) Kurz | |||
| P. xerocarpa Hassk. | |||
| Pterocarpus flavus Lour. | |||
| Robinia mitis L. | |||
| Rhizophora mucronata Lam. | Mangium candelarium Rumphius | True mangrove | Rhizophoraceae |
| R. candelaria Wight & Am. | |||
| R. longissima Blanco | |||
| R. macrorrhiza Griff. | |||
| Xylocarpus granatum König | X. obovatus A. Juss. | Puzzle nut tree | Meliaceae |
| Carapa granatum (Koen.) Alston | Cannon ball tree | ||
| Xylocarpus moluccensis M. Roem. | Carapa moluccensis Lam. | Meliaceae |
2. Traditional Antidiarrhoeal Plants from Bangladesh
2.1. Diospyros peregrina Gürke (Ebenaceae)

2.1.1. Traditional Use and Plant Parts Used
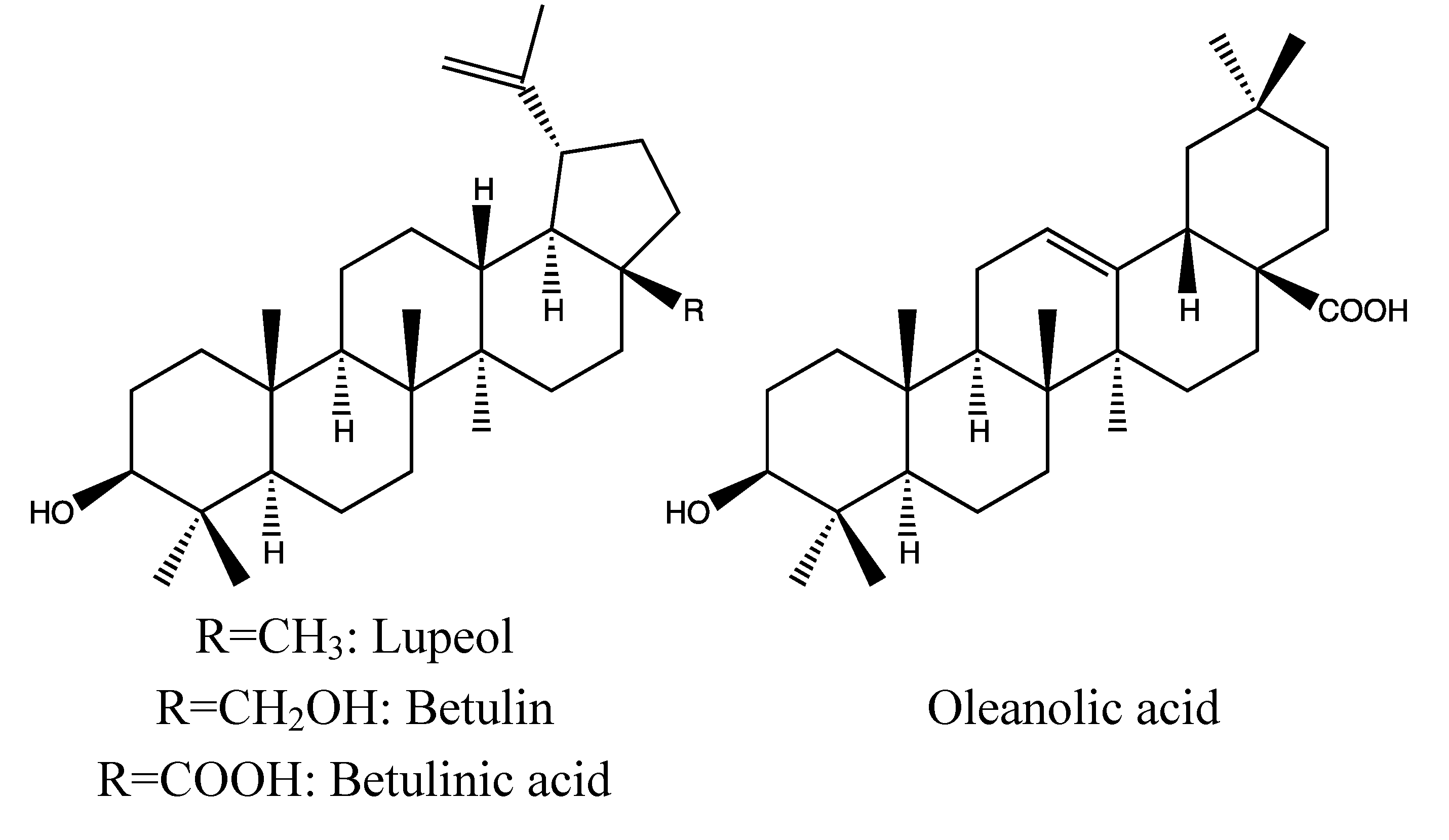
2.1.2. Chemical Composition (Figure 2)
- Triterpenoid: Lupeol [37].
- Triterpenoid: betulinic acid [32].
2.1.3. Bioactivity
2.1.4. Toxicity
2.1.5. Comments
2.2. Heritiera littoralis Dryand (Sterculiaceae)

2.2.1. Traditional Use and Plant Parts Used
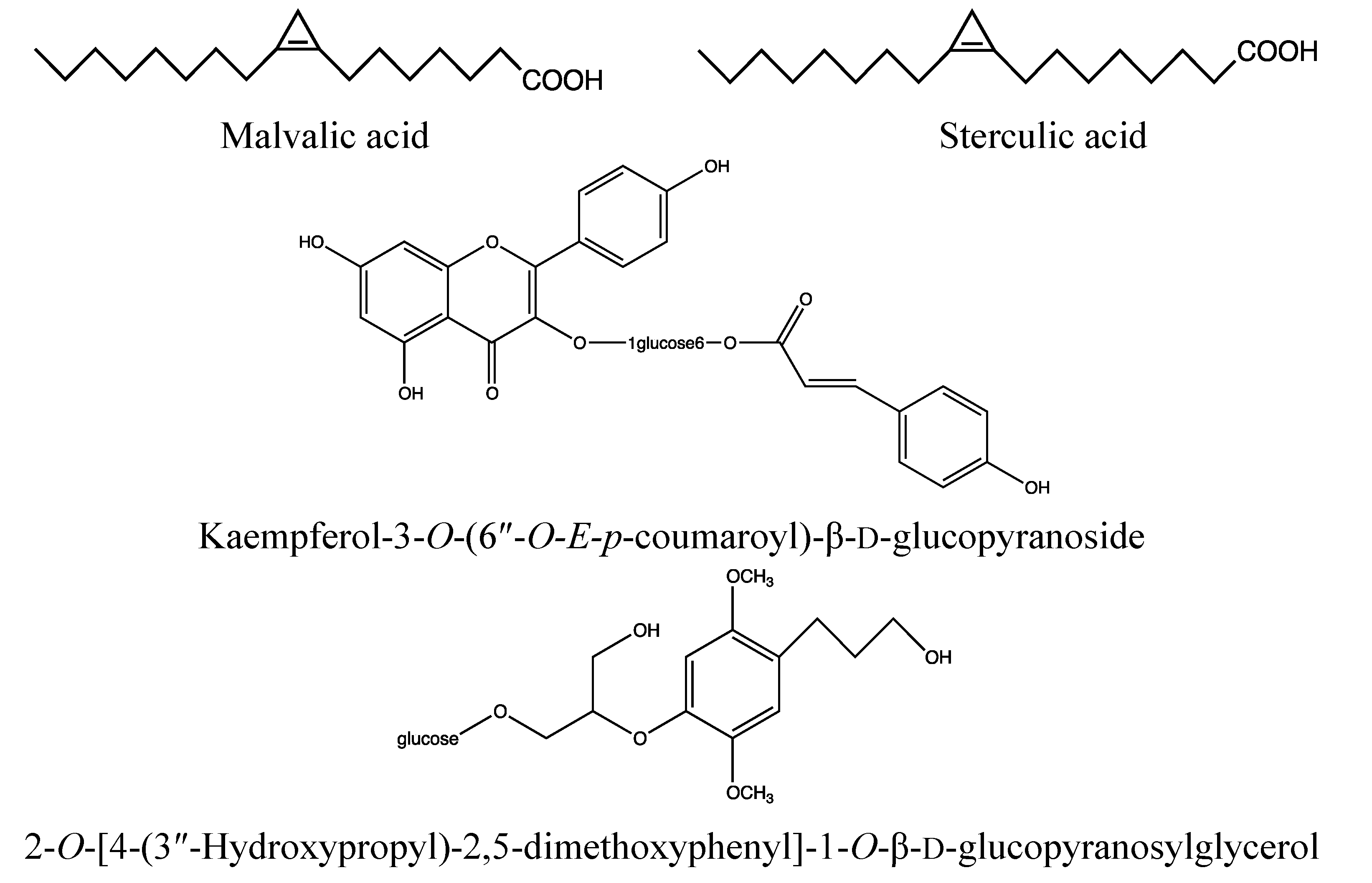
2.2.2. Chemical Composition (Figure 4)
- Flavonoids: quercitrin, quercetin, kaempferol-3-O-(6″-O-E-p-coumaroyl)-β-d-glucopyranoside, kaempferol, kaempferitrin, myricetin, eriodictyol, afzelin, astragalin, tribuloside, catechin [73];
- Fatty acids: malvalic, sterculic, palmitic, oleic and linoleic acid [75].
- Triterpenoid: friedelin [77].
2.2.3. Bioactivity
2.2.4. Toxicity
2.2.5. Comments
2.3. Ixora coccinea L. (Rubiaceae)

2.3.1. Traditional Use and Plant Parts Used
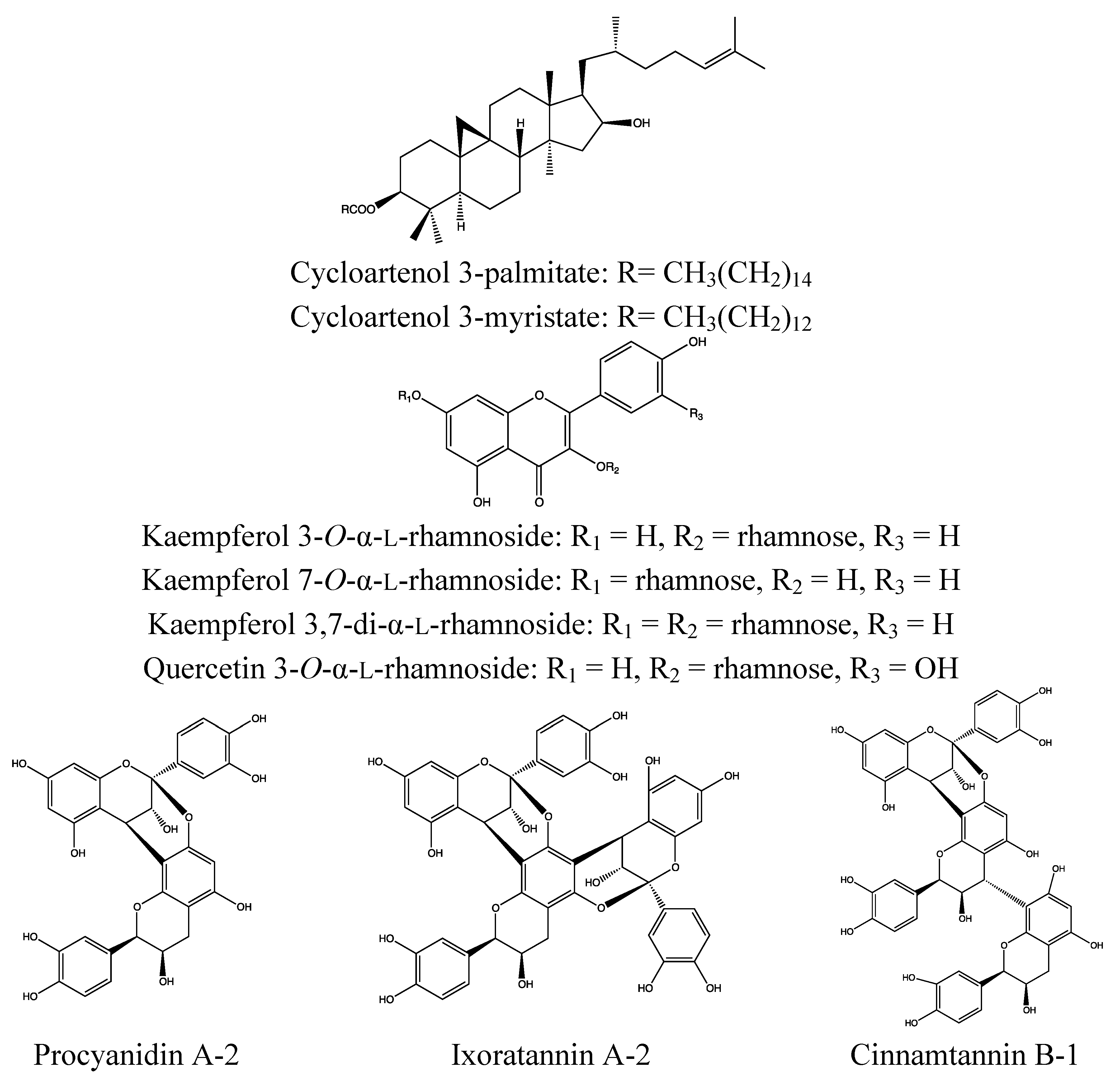
2.3.2. Chemical Composition (Figure 6)
- Triterpenoids: lupeol, 3-acetylbetulic acid, betunolic acid, α-amyrin, β-amyrin, ursolic acid, 3-acetylursolic acid, oleanonic acid [92];
- Sterols: 6β-hydroxystigmast-4-en-3-one, sitosteryl-3-O-β-d-glucoside, β-sitosterol, stigmasterol [92];
- Flavonoids: kaempferol, kaempferol-7-O-α-rhamnoside, kaempferitrin, luteolin, (−)-epicatechin, (+)-catechin [92];
- Diterpenoids: 16a-hydro-19-acetoxy-(−)kauran-17-oic acid, 16a-hydro-19-ol-(−)-kauran-17-oic acid [92];
- Quinones: 1,4-dihydroxy-3-methylanthraquinone, tocopherylquinone [92];
- Peptides: ixorapeptides I and II [92].
- Fatty acids: palmitic, stearic, oleic and linoleic acid [93];
2.3.3. Bioactivity
2.3.4. Toxicity
2.3.5. Comments
2.4. Pongamia pinnata (L.) Pierre (Fabaceae)

2.4.1. Traditional Use and Plant Parts Used
2.4.2. Chemical Composition (Figure 8)

2.4.3. Bioactivity
2.4.4. Toxicity
2.4.5. Comments
2.5. Rhizophora mucronata Lamk. (Rhizophoraceae)

2.5.1. Traditional Use and Plant Parts Used
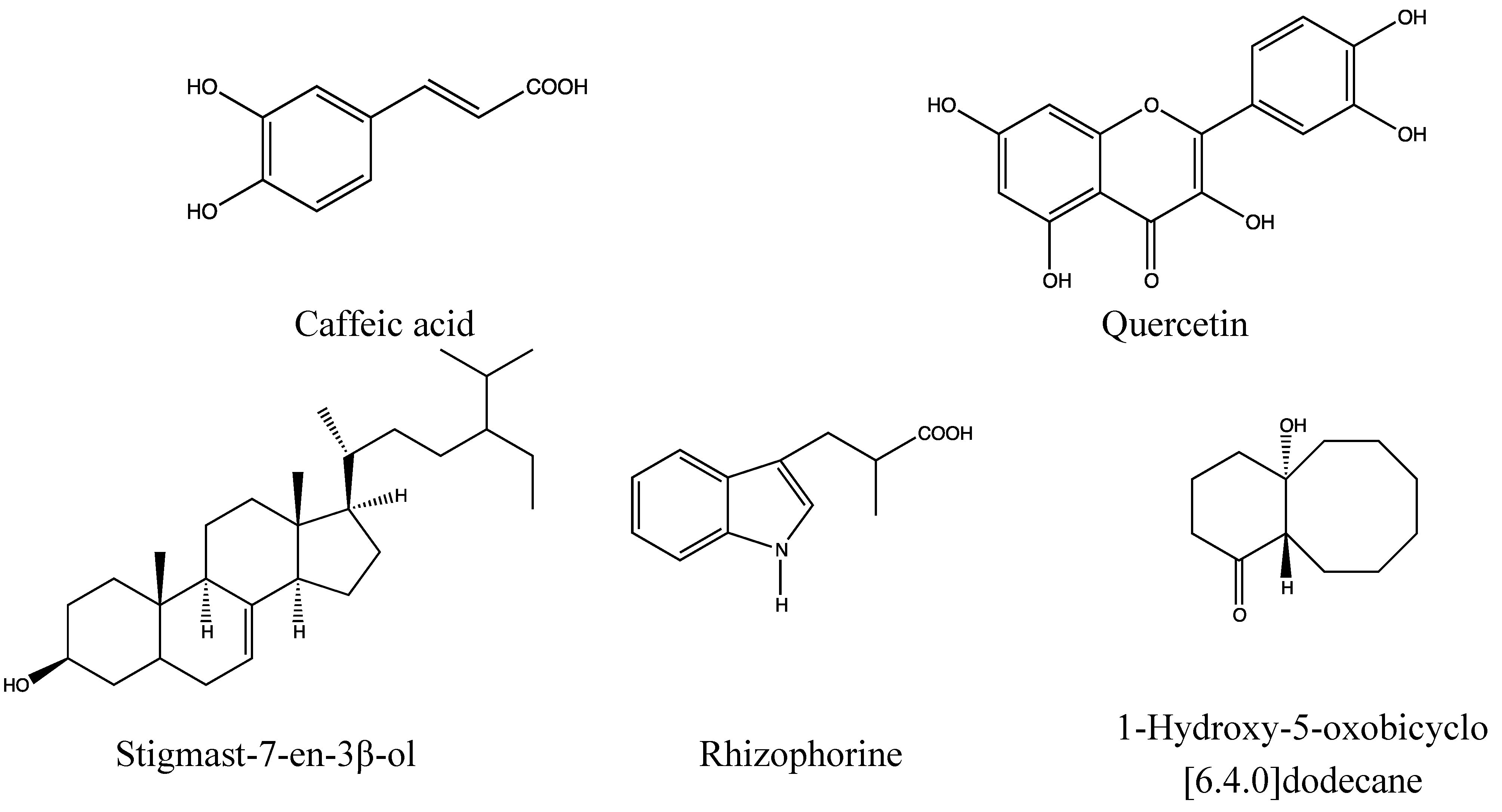
2.5.2. Chemical Composition (Figure 10)
- Phenolic acid: caffeic acid [161].
2.5.3. Bioactivity
2.5.4. Toxicity
2.5.5. Comments
2.6. Xylocarpus granatum König (Meliaceae)

2.6.1. Traditional Use and Plant Parts Used
2.6.2. Chemical Composition (Figure 12)
- Flavonoids: catechin, epicatechin [197];
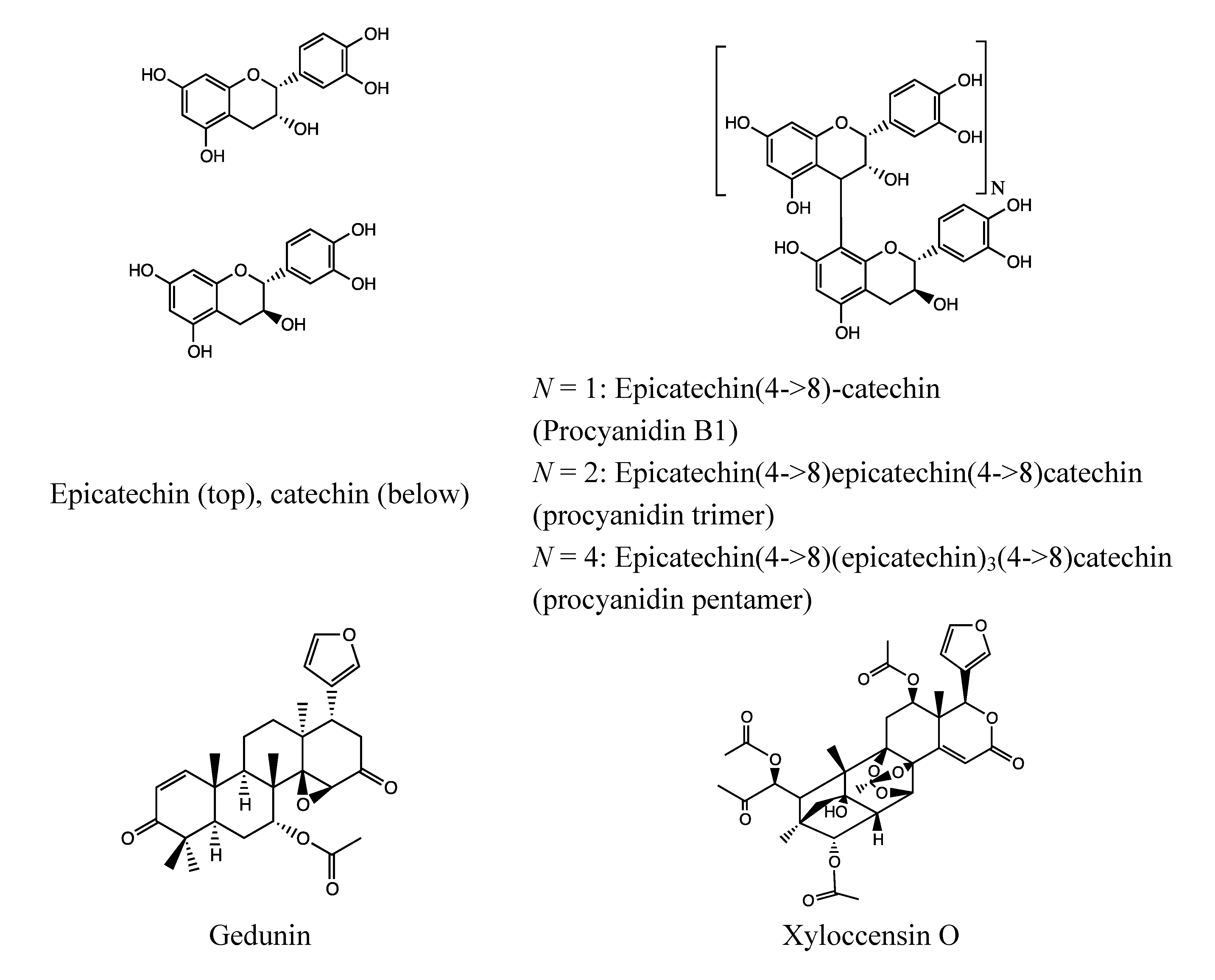
2.6.3. Bioactivity
2.6.4. Toxicity
2.6.5. Comments
2.7. Xylocarpus moluccensis M. Roem (Meliaceae)

2.7.1. Traditional Use and Plant Parts Used
2.7.2. Chemical Composition (Figure 14)
2.7.3. Bioactivity Studies
2.7.4. Toxicity
2.7.5. Comments
3. General Discussion
4. Conclusions
Acknowledgements
Conflict of Interest
References
- Kosek, M.; Bern, C.; Guerrant, R.L. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull. World Health Organ. 2003, 81, 197–204. [Google Scholar]
- World Health Organization (WHO), The World Health Report 2005—Make Every Mother and Child Count; WHO: Geneva, Switzerlands, 2005.
- World Health Organization (WHO), The World Health Report 2007—A Safer Future: Global Public Health in the 21st Century; WHO: Geneva, Switzerlands, 2007.
- Lutterodt, G.D. Inhibition of microlax-induced experimental diarrhoea with narcotic-like extracts of Psidium guajava leaf in rats. J. Ethnopharmacol. 1992, 37, 151–157. [Google Scholar] [CrossRef]
- Albert, M.J.; Faruque, A.S.G.; Faruque, S.M.; Sack, R.B.; Mahalanabis, D. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. J. Clin. Microbiol. 1999, 37, 3458–3464. [Google Scholar]
- Sack, R.B.; Siddique, A.K.; Longini, I.M.; Nizam, A.; Yunus, M.; Islam, M.S.; Morris, J.G.; Ali, A.; Huq, A.; Nair, G.B.; et al. A 4-year study of the epidemiology of Vibrio cholerae in four rural areas of Bangladesh. J. Infect. Dis. 2003, 187, 96–101. [Google Scholar] [CrossRef]
- Qadri, F.; Khan, A.I.; Faruque, A.S.G.; Begum, Y.A.; Chowdhury, F.; Nair, G.B.; Salam, M.A.; Sack, D.A.; Svennerholm, A.-M. Enterotoxigenic Escherichia coli and Vibrio cholerae diarrhea, Bangladesh, 2004. Emerg. Infect. Dis. 2005, 11, 1104–1107. [Google Scholar] [CrossRef]
- Palombo, E.A. Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: modes of action and effects on intestinal function. Phytother. Res. 2006, 20, 717–724. [Google Scholar] [CrossRef]
- Barrett, M. The Handbook of Clinically Tested Herbal Remedies; Haworth Herbal Press: Binghamton, NY, USA, 2004. [Google Scholar]
- Dillinger, T.L.; Barriga, P.; Escarcega, S.; Jimenez, M.; Lowe, D.S.; Grivetti, L.E. Food of the gods: cure for humanity? A cultural history of the medicinal and ritual use of chocolate. J. Nutr. 2000, 130, 2057S–2072S. [Google Scholar]
- Alam, M.A.; Akter, R.; Subhan, N.; Rahman, M.M.; Majumder, M.M.; Nahar, L.; Sarker, S.D. Antidiarrhoeal property of the hydroethanolic extract of the flowering tops of Anthocephalus cadamba. Braz. J. Pharmacogn. 2008, 18, 155–159. [Google Scholar]
- Bruins, M.J.; Cermak, R.; Kiers, J.L.; van der Meulen, J.; van Amelsvoort, J.M.M.; van Klinken, B.J.W. In vivo and in vitro effects of tea extracts on enterotoxigenic Escherichia coli-induced intestinal fluid loss in animal models. J. Pediat. Gastroenterol. Nutr. 2006, 43, 459–469. [Google Scholar] [CrossRef]
- Chen, J.-C.; Ho, T.-Y.; Chang, Y.-S.; Wu, S.-L.; Hsiang, C.-Y. Anti-diarrheal effect of Galla Chinensis on the Escherichia coli heat-labile enterotoxin and ganglioside interaction. J. Ethnopharmacol. 2006, 103, 385–391. [Google Scholar] [CrossRef]
- Chen, J.-C.; Huang, L.-J.; Wu, S.-L.; Kuo, S.-C.; Ho, T.-Y.; Hsiang, C.-Y. Ginger and its bioactive component inhibit enterotoxigenic Escherichia coli heat-labile enterotoxin-induced diarrhea in mice. J. Agric. Food Chem. 2007, 55, 8390–8397. [Google Scholar] [CrossRef]
- Lakhsminarayana, M.; Shivkumar, H.; Rimaben, P.; Bhargava, V.K. Antidiarrhoeal activity of leaf extract of Moringa oleifera in experimentally induced diarrhoea in rats. Int. J. Phytomed. 2011, 3, 68–74. [Google Scholar]
- Oi, H.; Matsuura, D.; Miyake, M.; Ueno, M.; Takai, I.; Yamamoto, T.; Kubo, M.; Moss, J.; Noda, M. Identification in traditional herbal medications and confirmation by synthesis of factors that inhibit cholera toxin-induced fluid accumulation. Proc. Natl. Acad. Sci. USA 2002, 99, 3042–3046. [Google Scholar]
- Rahman, T.; Rahman, K.A.; Rajia, S.; Alamgir, M.; Khan, M.T.H.; Choudhuri, M.S.K. Evaluation of antidiarrhoeal activity of cardamom (Elettaria cardamomum) on mice models. Orient. Pharm. Exp. Med. 2008, 8, 130–134. [Google Scholar] [CrossRef]
- Asif, H.M.; Usmanghni, K.; Akram, M.; Akhtar, N.; Jabeen, Q.; Shah, S.M.A.; Rehman, R.; Ahmed, K.; Saeed, T. Herbal treatment of secretory diarrhea. Int. J. Phytomed. 2010, 2, 425–429. [Google Scholar]
- Cai, Y.; Evans, F.J.; Roberts, M.F.; Phillipson, J.D.; Zenk, M.H.; Gleba, Y.Y. Biological and chemical investigation of dragon’s blood from Croton species of South America. Part 1. Polyphenolic compounds from Croton lechleri. Phytochemistry 1991, 30, 2033–2040. [Google Scholar]
- Lewis, W.H.; Elvin-Lewis, M.P.F. Medical Botany: Plants Affecting Human Health; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Fischer, H.; Machen, T.E.; Widdicombe, J.H.; Carlson, T.J.S.; King, S.R.; Chow, J.W.S.; Illek, B. A novel extract SB-300 from the stem bark latex of Croton lechleri inhibits CFTR-mediated chloride secretion in human colonic epithelial cells. J. Ethnopharmacol. 2004, 93, 351–357. [Google Scholar] [CrossRef]
- Patz, J.A.; Campbell-Lendrum, D.; Holloway, T.; Foley, J.A. Impact of regional climate change on human health. Nature 2005, 438, 310–317. [Google Scholar]
- Iftekhar, M.S.; Islam, M.R. Degeneration of Bangladesh’s Sundarbans mangroves: A management issue. Int. Forestry Rev. 2004, 6, 123–135. [Google Scholar] [CrossRef]
- Mandal, R.N.; Das, C.S.; Naskar, K.R. Dwindling Indian sundarban mangrove: The way out. Sci. Cult. 2010, 76, 275–282. [Google Scholar]
- Upadhyay, V.P.; Ranjan, R.; Singh, J.S. Human-mangrove conflicts: The way out. Curr. Sci. 2002, 83, 1328–1336. [Google Scholar]
- Li, M.-Y.; Xiao, Q.; Pan, J.-Y.; Wu, J. Natural products from semi-mangrove flora: Source, chemistry and bioactivities. Nat. Prod. Rep. 2009, 26, 281–298. [Google Scholar] [CrossRef]
- Datta, D.; Chattopadhyay, R.N.; Deb, S. Prospective livelihood opportunities from mangroves of the Sunderbans, India. Res. J. Environ. Sci. 2011, 5, 536–543. [Google Scholar] [CrossRef]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants; Lalit Mohan Basu: Allahabad, India, 1935. [Google Scholar]
- Male Flowers of Diospyros malabarica from Ebenaceae. Rarely Seen in Cultivation. Available online: http://en.wikipedia.org/wiki/File:Malabar_Ebony.jpg (accessed on 14 April 2013).
- Chopra, R.N.; Nayar, S.L.; Chopra, I.C. Glossary of Indian Medicinal Plants; Council of Scientific & Industrial Research: New Dehli, India, 1956. [Google Scholar]
- Sinha, B.N.; Bansal, S.K. A review of phytochemical and biological studies of Diospyros species used in folklore medicine of Jharkand. J. Nat. Remedies 2008, 8, 11–17. [Google Scholar]
- Misra, P.S.; Misra, G.; Nigam, S.K.; Mitra, C.R. Constituents of Diospyros peregrina fruit and seed. Phytochemistry 1971, 10, 904–905. [Google Scholar]
- Chinsembu, K.C.; Hedimbi, M. An ethnobotanical survey of plants used to manage HIV/AIDS opportunistic infections in Katima Mulilo, Caprivi region, Namibia. J. Ethnobiol. Etnomed. 2010, 6, 25. [Google Scholar] [CrossRef]
- Kantamreddi, V.S.S.; Wright, C.V. Investigation of Diospyros species for antiplasmodial properties. Evid. Based Complement. Altern. Med. 2008, 5, 187–190. [Google Scholar] [CrossRef]
- Wagh, V.V.; Jain, A.K.; Kadel, C. Ethnomedicinal plants used for curing dysentery and diarrhea by tribals of Jhabua district (Madhya Pradesh). Indian J. Nat. Prod. Resour. 2011, 2, 256–260. [Google Scholar]
- Gupta, R.K.; Tiwari, R.D. Chemical examination of the bark of Diospyros peregrina. Proc. Indian Nat. Sci. Acad. Sect. A 1964, 34, 180–181. [Google Scholar]
- Sundararamaiah, T.; Ramraj, S.K.; Rao, K.L.; Vimalabai, V. Isolation of the lupeol group of triterpenes from Dillenia indica Linn. and Diospyros peregrina. J. Indian Chem. Soc. 1976, 53, 638. [Google Scholar]
- Chauhan, J.S.; Kumari, G. A new leucoanthocyanin from the stem of Diospyros peregrina. J. Indian Chem. Soc. 1978, 55, 1068–1070. [Google Scholar]
- Chauhan, J.S.; Kumari, G. Nonadecan-7-ol-2-one, an aliphatic ketol from Diospyros peregrina. Phytochemistry 1980, 19, 2637–2638. [Google Scholar]
- Gupta, R.K.; Tiwari, R.D. Chemical examination of the leaves of Diospyros peregrina Gurke. Indian J. Chem. 1964, 2, 129–130. [Google Scholar]
- Jain, N.; Alam, M.S.; Kamil, M.; Ilyas, M.; Ali, M. Peregrinol: A new lupene type triterpene from Diospyros peregrina. Pharmazie 1992, 47, 559–560. [Google Scholar]
- Bhaumik, T.; Dey, A.K.; Das, P.C.; Mukhopadhyay, A.K.; Chatterjee, M.A. Triterpenes of Diospyros peregrina Gurke: Partial syntheses of olean-9(11),12-dien-3-one and ursan-9(11),12-dien-3-one (marsformosanone). Indian J. Chem. Sect. B Org. Med. Chem. 1981, 20B, 664–668. [Google Scholar]
- Pareek, R.B.; Vidyapati, T.J.; Bhutani, K.K. Chemical examination of the fruits of Diospyros peregrina. J. Teaching Res. Chem. 2008, 15, 6–11. [Google Scholar]
- Jain, N.; Yadava, R. A novel chromenoflavone from the fruits of Diospyros peregrina. Fitoterapia 1996, 67, 348–350. [Google Scholar]
- Sahu, R.; Dewanjee, S.; Dua, T.K.; Gangopadhyay, M.; Das, A.K.; Dey, S.P. Dereplication coupled with in vitro antioxidant assay of two flavonoid glycosides from Diospyros peregrina fruit. Nat. Prod. Res. 2012, 26, 454–459. [Google Scholar] [CrossRef]
- Sampurna, T.; Rao, T.S.S. Chemical examination of the fruits of Diospyros peregrina Gurke. Asian J. Chem. 2004, 16, 540–542. [Google Scholar]
- Begum, S.A.; Hosain, M.M.; Ahmed, M.F.; Muttakin, M.A.; Rahman, M.M. Vitamin C content in tropical fruits and vegetables available in different districts of Bangladesh. J. Bangladesh Acad. Sci. 2009, 33, 231–234. [Google Scholar]
- Chauhan, J.S.; Saraswat, M.; Kumari, G. Structure of a new dihydroflavonol glycoside from Diospyros peregrina roots. Planta Med. 1979, 35, 373–375. [Google Scholar] [CrossRef]
- Chauhan, J.S.; Saraswat, M.; Kumari, G. Structure of a new flavanone glycoside from Diospyros peregrina roots. Indian J. Chem. Sect. B Org. Med. Chem. 1982, 21B, 169–170. [Google Scholar]
- Rouf, R.; Uddin, S.J.; Shilpi, J.A.; Toufiq-ur-rahman, M.; Ferdous, M.M.; Sarker, S.D. Anti-diarrhoeal properties of Diospyros peregrina in the castor oil-induced diarrhoea model in mice. Ars. Pharm. 2006, 47, 81–89. [Google Scholar]
- Dewanjee, S.; Maiti, A.; Kundu, M.; Mandal, S.C. Evaluation of anthelmintic activity of extracts of Diospyros peregrina, Coccinia grandis and Schima wallichii. Dhaka Univ. J. Pharm. Sci. 2007, 6, 121–123. [Google Scholar]
- Dewanjee, S.; Kundu, M.; Maiti, A.; Majumdar, R.; Majumdar, A.; Mandal, S.C. In vitro evaluation of antimicrobial activity of crude extract from plants Diospyros peregrina, Coccinia grandis and Swietenia macrophylla. Trop. J. Pharm. Res. 2007, 6, 773–778. [Google Scholar]
- Dewanjee, S.; Maiti, A.; Mandal, S.C. Antioxidant activity of the methanol extract of Diospyros peregrina fruit in alloxan induced diabetic rats. Pharmacologyonline 2007, 3, 80–86. [Google Scholar]
- Dewanjee, S.; Bose, S.K.; Sahu, R.; Mandal, S.C. Antidiabetic effect of matured fruits of Diospyros peregrina in alloxan-induced diabetic rats. Int. J. Green. Pharm. 2008, 2, 95–99. [Google Scholar] [CrossRef]
- Dewanjee, S.; Sahu, R.; Mandal, V.; Maiti, A.; Mandal, S.C. Antidiabetic and antioxidant activity of the methanol extract of Diospyros peregrina fruit on type I diabetic rats. Pharm. Biol. 2009, 47, 1149–1153. [Google Scholar] [CrossRef]
- Dewanjee, S.; Gangopadhyay, M.; Das, A.K. Multimodal approaches of the hydroalcoholic extract of fruits in diabetic therapy. Nat. Prod. Res. 2011, 25, 827–833. [Google Scholar] [CrossRef]
- Dewanjee, S.; Maiti, A.; Sahu, R.; Dua, T.K.; Mandal, V. Effective control of type 2 diabetes through antioxidant defense by edible fruits of Diospyros peregrina. Evid. Based Complement. Altern. Med. 2011, 2011, 675397. [Google Scholar] [CrossRef]
- Kumar, K.E.; Mastan, S.K.; Sreekanth, N.; Chaitanya, G.; Sumalatha, G.; Krishna, P.V. Hypoglycemic and antihyperglycemic activity of aqueous extract of Diospyros peregrina fruits in normal and alloxan-induced diabetic rabbits. Pharmacologyonline 2008, 3, 250–256. [Google Scholar]
- Venu Gopal, Y.; Ravindranath, A.; Kalpana, G.; Raju, A.B.; Reddy, P. Antitumor activity of Diospyros peregrina on Ehrlich ascites carcinoma in mice. J. Sci. Res. 2011, 3, 413–419. [Google Scholar]
- Hussan Reza, K.; Jeevanandham, S.; Kumervelrajen, R. Evaluation of Diospyros peregrina gum as a novel binder in tablet formulation. Int. Res. J. Pharm. Appl. Sci. 2012, 2, 53–60. [Google Scholar]
- Singh, N.; Nath, R.; Gupta, M.L. A pharmacological evaluation of anti-stress activity of Diospyros peregrina Gurke. Indian J. Pharm. 1988, 20, 102–108. [Google Scholar]
- Tomlinson, P.B. The Botany of Mangroves; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]
- Heritiera littoralis (Green Fruit). Location: Oahu, Keehi Lagoon. Available online: http://en.wikipedia.org/wiki/File:Starr_080530-4634_Heritiera_littoralis.jpg (accessed on 14 April 2013).
- Daengrot, C.; Ponglimanont, C.; Karalai, C. Chemical Constituents from the Barks of Heritiera Littoralis. In Proceedings of the 31st Annual Congress on Science and Technology of Thailand, Suranaree, Thailand, 18–20 October 2005.
- Bandaranayake, W.M. Traditional and medicinal uses of mangroves. Mangroves Salt Marshes 1998, 2, 133–148. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. Bioactivities, bioactive compounds and chemical constitutions of mangrove plants. Wetlands Ecol. Manag. 2002, 10, 451–452. [Google Scholar] [CrossRef]
- Pattanaik, C.; Reddy, C.S.; Dhal, N.K.; Das, R. Utilisation of mangrove forests in Bhitarkanika wildlife sanctuary, Orissa. Indian J. Tradit. Knowl. 2008, 7, 598–603. [Google Scholar]
- Miles, D.H.; Lho, D.S.; de la Cruz, A.A.; Gomez, E.D.; Weeks, J.A.; Atwood, J.L. Toxicants from mangrove plants. 3. Heritol, a novel ichthyotoxin from the mangrove plant Heritiera littoralis. J. Org. Chem. 1987, 52, 2930–2932. [Google Scholar] [CrossRef]
- Bhargava, N. Ethnobotanical studies of the tribes of Andaman and Nicobar Islands, India. I. Onge. Econ. Bot. 1983, 37, 110–119. [Google Scholar] [CrossRef]
- Dahdouh-Guebas, F.; Collin, S.; lo Seen, D.; Rönnbäck, P.; Depommier, D.; Ravishankar, T.; Koedam, N. Analysing ethnobotanical and fishery-related importance of mangroves of the East-Godavari Delta (Andhra Pradesh, India) for conservation and management purposes. J. Ethnobiol. Ethnomed. 2006, 2, 24. [Google Scholar] [CrossRef]
- Tewtrakul, S.; Tansakul, P.; Daengrot, C.; Ponglimanont, C.; Karalai, C. Anti-inflammatory principles from Heritiera littoralis bark. Phytomedicine 2010, 17, 851–855. [Google Scholar] [CrossRef]
- Balasooriya, S.J.; Sotheeswaran, S. Economically useful plants of Sri Lanka. Part IV. Screening of Sri Lanka plants for tannins. J. Natl. Sci. Counc. Sri Lanka 1982, 10, 213–219. [Google Scholar]
- Tian, Y.; Wu, J.; Zhang, S. Flavonoids from leaves of Heritiera littoralis D. J. Chin. Pharm. Sci. 2004, 13, 214–216. [Google Scholar]
- Takeda, Y.; Miyazaki, K.; Shimizu, H.; Masuda, T.; Hirata, E.; Takushi, A.; Shinzato, T.; Otsuka, H. A new phenylpropanoid-glycerol conjugate from Heritiera littoralis Dryand. Nat. Med. 2000, 54, 22–25. [Google Scholar]
- Gaydou, E.M.; Ramanoelina, A.R.P.; Rasoarahona, J.R.E.; Combres, A. Fatty acid composition of Sterculia seeds and oils from Madagascar. J. Agric. Food Chem. 1993, 41, 64–66. [Google Scholar]
- Miles, D.H.; Chittawong, V.; Lho, D.-S.; Payne, A.M.; de la Cruz, A.A.; Gomez, E.D.; Weeks, J.A.; Atwood, J.L. Toxicants from mangrove plants, VII. Vallapin and vallapianin, novel sesquiterpene lactones from the mangrove plant Heritiera littoralis. J. Nat. Prod. 1991, 54, 286–289. [Google Scholar] [CrossRef]
- Miles, D.H.; de la Cruz, A.A.; Ly, A.-M.; Lho, D.S.; Gomez, E.D.; Weeks, J.A.; Atwood, J.L. Toxicants from mangrove plants. Ichthyotoxins from the Philippine plant Heritiera littoralis. Am. Chem. Soc. Symp. Ser. 1987, 330, 491–501. [Google Scholar]
- Miles, D.H.; Ly, A.-M.; Chittawong, V.; de la Cruz, A.A.; Gomez, E.D. Toxicants from mangrove plants, VI. Heritonin, a new piscicide from the mangrove plant Heritiera littoralis. J. Nat. Prod. 1989, 52, 896–898. [Google Scholar] [CrossRef]
- Dang, H.C.T. Antioxidants in Heritiera fomes, a Medicinal Plant from the Mangrove Forest of Bangladesh (in Norwegian). Master Thesis, School of Pharmacy, University of Oslo, Oslo, Norway, 2007. [Google Scholar]
- Tao, W.Q.; Xu, M.B.; Huang, L.Y.; Miao, S.Y. Antibacterial activity of extracts from four mangrove species in vitro. Med. Plant 2012, 3, 38–41. [Google Scholar]
- Latha, P.G.; Panikkar, K.R.; Suja, S.R.; Abraham, A.; Rajasekharan, S. Chemistry, pharmacognosy, pharmacology and botany of Ixora coccinea—A review. J. Med. Arom. Plant Sci. 2001, 23, 670–676. [Google Scholar]
- A Specimen of Ixora coccinea, Also Known as the Jungle Flame, Shot in Miami, Florida. Available online: http://en.wikipedia.org/wiki/File:IxoraCoccineaMiami.JPG (accessed on 14 April 2013).
- Nayak, S.; Udupa, L.; Udupa, S. Altered antioxidant enzyme profile in wound healing. Indian J. Clin. Biochem. 2003, 18, 75–79. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Tiu, F.; Rideout, J.A. New cycloartenol esters from Ixora coccinea. Nat. Prod. Res. 2004, 18, 319–323. [Google Scholar] [CrossRef]
- Ratnasooriya, W.D.; Deraniyagala, S.A.; Bathige, S.D.N.K.; Goonasekara, C.L.; Jayakody, J.R.A.C. Antinociceptive action of aqueous extract of the leaves of Ixora coccinea. Acta Biol. Hung. 2005, 56, 21–34. [Google Scholar] [CrossRef]
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer: New York, NY, USA, 2007. [Google Scholar]
- Alam, M.K. Medical ethnobotany of the Marma tribe of Bangladesh. Econ. Bot. 1992, 46, 330–335. [Google Scholar] [CrossRef]
- Baliga, M.S.; Kurian, P.J. Ixora coccinea Linn.: Traditional uses, phytochemistry and pharmacology. Chin. J. Integr. Med. 2012, 18, 72–79. [Google Scholar]
- Zachariah, R.; Nair, C.R.S.; Panicker, P.V. Anti-inflammatory and anti-mitotic activities of lupeol isolated from the leaves of Ixora coccinea Linn. Indian J. Pharm. Sci. 1994, 56, 129–131. [Google Scholar]
- Idowu, T.O.; Ogundaini, A.O.; Salau, A.O.; Obuotor, E.M.; Bezabih, M.; Abegaz, B.M. Doubly linked, A-type proanthocyanidins trimer and other constituents of Ixora coccinea leaves and their antioxidant and antibacterial properties. Phytochemistry 2010, 71, 2092–2098. [Google Scholar] [CrossRef]
- Sumathy, H.; Sangeetha, J.; Vijayalakhsmi, K. Chromatographic fingerprint analysis of Ixora coccinea methanolic flower extract. Int. J. Pharm. Sci. Drug Res. 2011, 3, 327–330. [Google Scholar]
- Lee, C.-L.; Liao, Y.-C.; Hwang, T.-L.; Wu, C.-C.; Chang, F.-R.; Wu, Y.-C. Ixorapeptide I and ixorapeptide II, bioactive peptides isolated from Ixora coccinea. Bioorg. Med. Chem. Lett. 2010, 20, 7354–7357. [Google Scholar]
- Yadava, R.N. Analysis of the fixed oil from the roots of Ixora coccinea Linn. Asian J. Chem. 1989, 1, 307–308. [Google Scholar]
- Srinivasan, G.V.; Sharanappa, P.; Udayan, P.S.; Leela, N.K.; Vijayan, K.K. Chemical composition and antimicrobial activity of the essential oil of Ixora coccinea L. root. J. Med. Arom. Plant Sci. 2010, 32, 27–30. [Google Scholar]
- Maniyar, Y.; Bhixavatimath, P.; Agashikar, N.V. Antidiarrheal activity of Ixora coccinea Linn. in rats. J. Ayurveda Integr. Med. 2010, 1, 287–291. [Google Scholar] [CrossRef]
- Annapurna, J.; Amarnath, P.V.S.; Amar Kumar, D.; Ramakrishna, S.V.; Raghavan, K.V. Antimicrobial activity of Ixora coccinea leaves. Fitoterapia 2003, 74, 291–293. [Google Scholar] [CrossRef]
- Sasidharan, V.K. Search for antibacterial and antifungal activity of some plants of Kerala. Acta Pharm. 1997, 47, 47–51. [Google Scholar]
- Selvaraj, N.; Lakshmanan, B.; Mazumder, P.M.; Karuppusamy, M.; Jena, S.S.; Pattnaik, A.K. Evaluation of wound healing and antimicrobial potentials of Ixora coccinea root extract. Asia Pac. J. Trop. Med. 2011, 959–963. [Google Scholar]
- Shyamal, S.; Latha, P.G.; Suja, S.R.; Shine, V.J.; Anuja, G.I.; Sini, S.; Pradeep, S.; Shikha, P.; Rajasekharan, S. Hepatoprotective effect of three herbal extracts on aflatoxin B1-intoxicated rat liver. Singap. Med. J. 2010, 51, 326–331. [Google Scholar]
- Torey, A.; Sasidharan, S.; Latha, L.Y.; Sudhakaran, S.; Ramanathan, S. Antioxidant activity and total phenolic content of methanol extracts of Ixora coccinea. Pharm. Biol. 2010, 48, 1119–1123. [Google Scholar] [CrossRef]
- Momin, F.N.; Kalai, B.R.; Shikalgar, T.S.; Naikwade, N.S. Cardioprotective effect of methanolic extract of Ixora coccinea Linn. leaves on doxorubicin-induced cardiac toxicity in rats. Indian J. Pharmacol. 2012, 44, 178–183. [Google Scholar] [CrossRef]
- Haridass, S.; Sekar, S.; Vijayan, R.; Jayakumar, S.; Thtmizharasu, S.; Krishnamurthy, V.; Chidambaram, S.B. Relative antioxidant and cytotoxic activities of Ixora coccinea flower extracts. J. Pharm. Res. 2012, 5, 1403–1408. [Google Scholar]
- Wongwattanasathien, O.; Kangsadalampai, K.; Tongyonk, L. Antimutagenicity of some flowers grown in Thailand. Food Chem. Toxicol. 2010, 48, 1045–1051. [Google Scholar]
- Maniyar, Y.; Bhixavatimath, P. Evaluation of the hypoglycaemic and hypolipidaemic activities of the aqueous extract of the leaves of Ixora coccinea Linn in diabetic rats. J. Clin. Diagnost. Res. 2011, 5, 1381–1384. [Google Scholar]
- Bhattacharya, A.; Kar, D.R.; Sengupta, A.; Ghosh, G.; Mishra, S.K. Evaluation of anti-inflammatory and analgesic activity of Ixora coccinea flower extract. Asian J. Chem. 2011, 23, 4369–4372. [Google Scholar]
- Kensa, V.M. Larvicidal effects of selected plant leaves extract against Anopheles mosquitoes. Ecol. Environ. Conserv. Paper 2011, 17, 375–380. [Google Scholar]
- Seetha, D.B.; Sudhakaran-Nair, C.R.; Velayudha-Panicker, P. Pharmacognostical and pharmacological studies on the root of Ixora coccinea Linn. (Rubicaceae). Indian J. Pharm. Sci. 1991, 53, 92–93. [Google Scholar]
- Latha, P.G.; Panikkar, K.R. Inhibition of chemical carcinogenesis in mice by Ixora coccinea flowers. Pharm. Biol. 2000, 38, 152–156. [Google Scholar]
- Atiq-Ur-Rahman; Taqvi, S.I.H.; Versiani, M.A.; Ikram, A.; Ahmed, S.K. Effects of whole flower and fractions from Ixora coccinea Linn. on cardiovascular system: A preliminary report. J. Chem. Soc. Pak. 2012, 34, 758–766. [Google Scholar]
- Howell, A.B.; Reed, J.D.; Krueger, C.G.; Winterbottom, R.; Cunningham, D.G.; Leahy, M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry 2005, 66, 2281–2291. [Google Scholar] [CrossRef]
- Chopade, V.V.; Tankar, A.N.; Pande, V.V.; Tekade, A.R.; Gowekar, N.M.; Bhandari, S.R.; Khandake, S.N. Pongamia pinnata: Phytochemical constituents, traditional uses and pharmacological properties: A review. Int. J. Green Pharm. 2008, 2, 72–75. [Google Scholar] [CrossRef]
- Pandey, A.; Khatri, P.; Patel, P.; Jakhetia, V.; Sharma, S. Phytopharmacognostic and pharmacological review of Pongamia pinnata family Fabaceae. J. Global Pharm. Technol. 2009, 3, 1–6. [Google Scholar]
- Satish Kumar, B.N. Phytochemistry and pharmacological studies of Pongamia pinnata (Linn.) Pierre. Int. J. Pharm. Sci. Rev. Res. 2011, 9, 12–19. [Google Scholar]
- Khatri, P.; Patel, R. A phytochemical overview of various parts of Pongamia pinnata (Karanj). World J. Pharm. Res. 2013, 2, 146–165. [Google Scholar]
- Pongamia pinnata—Karanj, Indian Beech Tree, Honge Tree, Pongam Tree or Panigrahi at Deer Park in Shamirpet, Rangareddy District, Andhra Pradesh, India. Available online: http://en.wikipedia.org/wiki/File:Pongamia_pinnata_%28Karanj%29_near_Hyderabad_W_IMG_7633.jpg (accessed on 14 April 2013).
- Muthu, C.; Ayyanar, M.; Raja, N.; Ignacimuthu, S. Medicinal plants used by traditional healers in Kancheepuram district of Tamil Nadu, India. J. Ethnobiol. Ethnomed. 2006, 2, 43. [Google Scholar] [CrossRef]
- Pandikumar, P.; Chellappandian, M.; Mutheeswaran, S.; Ignacimuthu, S. Consensus of local knowledge on medicinal plants amongst traditional healers in Mayialadumparai block of Theni district, Tamil Nadu, India. J. Ethnopharmacol. 2011, 134, 354–362. [Google Scholar] [CrossRef]
- Ragupathy, S.; Newmaster, S.G. Valorizing the “Irulas” traditional knowledge of medicinal plants in the Kodiakkal Reserve Forest, India. J. Ethnobiol. Ethnomed. 2009, 5, 10. [Google Scholar] [CrossRef]
- Awashti, A.K. Ethnobotanical studies of the Negrito islanders of Andaman islands, India—The great Andamanese. Econ. Bot. 1991, 45, 274–280. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, V.C.; Tewari, D.D. Documentation and determination of consensus about phytotherapeutic veterinary practices among the Tharu tribal community of Uttar Pradesh, India. Trop. Animal Health Prod. 2012, 44, 863–872. [Google Scholar] [CrossRef]
- Jain, S.K. Medicinal plant lore of the tribals of Bastar. Econ. Bot. 1965, 19, 236–250. [Google Scholar] [CrossRef]
- Garg, G.P. A new component from leaves of Pongamia glabra. Planta Med. 1979, 37, 73–74. [Google Scholar] [CrossRef]
- Mahey, S.; Sharma, P.; Seshadri, T.R.; Mukerjee, T.R. Structure & synthesis of glabrachromene, a new constituent of Pongamia glabra. Indian J. Chem. 1972, 10, 585–588. [Google Scholar]
- Malik, S.B.; Seshadri, T.R.; Sharma, P. Minor components of the leaves of Pongamia glabra. Indian J. Chem. 1976, 14, 229–230. [Google Scholar]
- Malik, S.B.; Sharma, P.; Seshadri, T.R. Furanoflavonoids from the leaves of Pongamia glabra. Indian J. Chem. 1977, 15, 536–538. [Google Scholar]
- Sagwan, S.; Rao, D.V.; Sharma, R.A. Studies on GC/MS spectroscopic analysis of some different in vivo plant extracts of (Karanj) Pongamia pinnata (L.) Pierre. J. Global Pharma Technol. 2012, 8, 1–10. [Google Scholar]
- Talapatra, B.; Mallik, A.K.; Talapatra, S.K. Triterpenoids and flavonoids from the leaves of Pongamia glabra Vent. Demethylation studies on 5-methoxy-furanoflavones. J. Indian Chem. Soc. 1985, 62, 408–409. [Google Scholar]
- Shoba, F.G.; Thomas, M. Study of antidiarrhoeal activity of four medicinal plants in castor-oil induced diarrhoea. J. Ethnopharmacol. 2001, 76, 73–76. [Google Scholar] [CrossRef]
- Brijesh, S.; Daswani, P.G.; Tetali, P.; Rojatkar, S.R.; Antia, N.H.; Birdi, T.J. Studies on Pongamia pinnata (L.) Pierre leaves: Understanding the mechanism(s) of action in infectious diarrhea. J. Zhejiang Univ. Sci. B 2006, 7, 665–674. [Google Scholar] [CrossRef]
- Dahikar, S.B.; Arote, S.R.; Tambekar, D.H.; Yeole, P.G.; Bhutada, S.A. Antibacterial properties of Pongamia pinnata Linn. (Fabaceae) against enteric bacterial pathogens. Biotechnol. Indian J. 2009, 3, 35–38. [Google Scholar]
- Johnson, D.B.; Shringi, B.N.; Patidar, D.K.; Chalichem, N.S.S.; Javvadi, A.K. Screening of antimicrobial activity of alcoholic & aqueous extracts of some indigenous plants. Indo-Global J. Pharm. Sci. 2011, 1, 186–193. [Google Scholar]
- Ramesh, K.; Gifson Gnanadhas, Y.; Karthick, N.; Umamaheswari, S. Plasmid mediated drug resistance and anti-MRSA activity of Pongamia glabra and Ocimum basilicum. J. Pharm. Res. 2011, 4, 2466–2467. [Google Scholar]
- Sumathi, R.; Pavni, S.; Sivakumar, T. Antimicrobial evaluation of lipid extract of Pongamia pinnata leaves. Res. J. Pharm. Technol. 2009, 2, 714–718. [Google Scholar]
- Sajid, Z.I.; Anwar, F.; Shabir, G.; Rasul, G.; Alkharfy, K.M.; Gilani, A.-H. Antioxidant, antimicrobial properties and phenolics of different solvent extracts from bark, leaves and seeds of Pongamia pinnata (L.) Pierre. Molecules 2012, 17, 3917–3932. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, S.; Nag, R.; Chaturvedi, A.; Nag, T.N. Antimicrobial activity and cellular toxicity of flavonoid extracts from Pongamia pinnata and Vitex negundo. Rom. Biotechnol. Lett. 2011, 16, 6396–6400. [Google Scholar]
- Umera Begam, A.K.; Manoharan, N.; Sirajudeen, J.; Abdul Jameel, A. Effect of some Indian traditional plants on few common pathogens. Adv. Appl. Sci. Res. 2010, 1, 205–211. [Google Scholar]
- Gupta, V.; Sharma, M. In vitro antioxidant studies on methanolic extracts of Pongamia pinnata (L.) Pierre: A great versatile leguminous plant. J. Pharm. Res. 2011, 4, 2241–2244. [Google Scholar]
- Badole, S.L.; Bodhankar, S.L. Antihyperglycemic activity of cycloart-23-ene-3β, 25-diol isolated from stem bark of Pongamia pinnata in alloxan induced diabetic mice. Res. J. Phytochem. 2009, 3, 18–24. [Google Scholar] [CrossRef]
- Badole, S.L.; Bodhankar, S.L. vestigation of antihyperglycaemic activity of aqueous and petroleum ether extract of stem bark of Pongamia pinnata on serum glucose level in diabetic mice. J. Ethnopharmacol. 2009, 123, 115–120. [Google Scholar] [CrossRef]
- Badole, S.L.; Bodhankar, S.L. Antidiabetic activity of cycloart-23-ene-3β, 25-diol isolated from Pongamia pinnata (L. Pierre) in streptozocine-nicotinamide induced diabetic mice. Eur. J. Pharmacol. 2010, 632, 103–109. [Google Scholar] [CrossRef]
- Sikarwar, M.S.; Patil, M.B. Antidiabetic activity of Pongamia pinnata leaf extracts in alloxan-induced diabetic rats. Int. J. Ayurveda Res. 2010, 1, 199–204. [Google Scholar] [CrossRef]
- Badole, S.L.; Zanwar, A.A.; Khopade, A.N.; Bodhankar, S.L. In vitro antioxidant and antimicrobial activity cycloart-23-ene-3β, 25-diol (B2) isolated from Pongamia pinnata (L. Pierre). Asia Pac. J. Trop. Med. 2011, 4, 910–916. [Google Scholar]
- Jaiswal, N.; Yadav, P.P.; Maurya, R.; Srivastava, A.K.; Tamrakar, A.K. Karanjin from Pongamia pinnata induces GLUT4 translocation in skeletal muscle cells in a phosphatidylinositol-3-kinase-independent manner. Eur. J. Pharmacol. 2011, 670, 22–28. [Google Scholar] [CrossRef]
- Tamrakar, A.K.; Jaiswal, N.; Yadav, P.P.; Maurya, R.; Srivastava, A.K. Pongamol from Pongamia pinnata stimulates glucose uptake by increasing surface GLUT4 level in skeletal muscle. Mol. Cell. Endocrinol. 2011, 339, 98–104. [Google Scholar] [CrossRef]
- Tamrakar, A.K.; Yadav, P.P.; Tiwari, P.; Maurya, R.; Srivastava, A.K. Identification of pongamol and karanjin as lead compounds with antihyperglycemic activity from Pongamia pinnata fruits. J. Ethnopharmacol. 2008, 118, 435–439. [Google Scholar] [CrossRef]
- Prabha, T.; Dorababu, M.; Goel, S.; Agarwal, P.K.; Singh, A.; Joshi, V.K.; Goel, R.K. Effect of methanolic extract of Pongamia pinnata Linn seed on gastro-duodenal ulceration and mucosal offensive and defensive factors in rats. Indian J. Exp. Biol. 2009, 47, 649–659. [Google Scholar]
- Vismaya; Belagihally, S.M.; Rajashekhar, S.; Jayaram, V.B.; Dharmesh, S.M.; Thirumakudalu, S.K.C. Gastroprotective properties of karanjin from Karanja (Pongamia pinnata) seeds; Role as antioxidant and H+, K+-ATPase inhibitor. Evid. Based Complement. Altern. Med. 2011. [Google Scholar] [CrossRef]
- Bhatia, G.; Puri, A.; Maurya, R.; Yadav, P.P.; Khan, M.M.; Khanna, A.K.; Saxena, J.K. Anti-dyslipidemic and antioxidant activities of different fractions of Pongamia pinnata (Lin.) fruits. Med. Chem. Res. 2008, 17, 281–289. [Google Scholar] [CrossRef]
- Sagar, S.K.; Sehgal, S.S. Effects of aqueous extract of deoiled neem (Azadirachta indica A. Juss) seed kernel and karanja (Pongamia glabra Vent) seed kernel against Culex quinquefasciatus. J. Commun. Dis. 1996, 28, 260–269. [Google Scholar]
- Sagar, S.K.; Sehgal, S.S.; Agarwala, S.P. Bioactivity of ethanol extract of Karanja (Pongamia glabra Vent) seed coat against mosquitoes. J. Commun. Dis. 1999, 31, 107–111. [Google Scholar]
- Abdul Rahuman, A.; Bagavan, A.; Kamaraj, C.; Vadivelu, M.; Zahir, A.A.; Elango, G.; Pandiyan, G. Evaluation of indigenous plant extracts against larvae of Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol. Res. 2009, 104, 637–643. [Google Scholar] [CrossRef]
- Swathi, S.; Murugananthan, G.; Ghosh, S.K. Oviposition deterrent activity from the ethanolic extract of Pongamia pinnata, Coleus forskohlii, and Datura stramonium leaves against Aedes aegypti and Culex quinquefasciatus. Pharmacogn. Mag. 2010, 6, 320–322. [Google Scholar] [CrossRef]
- Samuel, A.J.S.J.; Radhamani, S.; Gopinath, R.; Kalusalingam, A.; Vimala, A.G.K.A.; Husain, H.A. In vitro screening of anti-lice activity of Pongamia pinnata leaves. Korean J. Parasitol. 2009, 47, 377–380. [Google Scholar] [CrossRef]
- Shanthi, S.; Elamathy, S.; Panneerselvam, A.; Radha, N. Antixanthomonas activity of Pongamia pinnata Linn. leaves—Cow urine extract—Natural cost effective ecofriendly remedy to bacterial leaf blight of paddy (BLB). J. Pharm. Res. 2011, 4, 650–652. [Google Scholar]
- Srinivasan, K.; Muruganandan, S.; Lal, J.; Chandra, S.; Tandan, S.K.; Prakash, V.R. Evaluation of anti-inflammatory activity of Pongamia pinnata leaves in rats. J. Ethnopharmacol. 2001, 78, 151–157. [Google Scholar] [CrossRef]
- Baki, M.A.; Khan, A.; Al-Bari, M.A.A.; Mosaddik, A.; Sadik, G.; Mondal, K.A.M.S.H. Sub-acute toxicological studies of pongamol isolated from Pongamia pinnata. Res. J. Med. Med. Sci. 2007, 2, 53–57. [Google Scholar]
- Rhizophora mucronata Propagules at Iriomote is. Okinawa, Japan. Available online: http://en.wikipedia.org/wiki/File:Rhizophora_mucronata_Propagules.jpg (accessed on 14 April 2013).
- Patra, J.K.; Thatoi, H.N. Metabolic diversity and bioactivity screening of mangrove plants: A review. Acta Physiol. Plant. 2011, 33, 1051–1061. [Google Scholar] [CrossRef]
- Nurulhuda, M.N.; Chew, L.T.; Mohd, N.M.Y.; Abdul, R.M.A. Tannin properties of Rhizophora mucronata barks of different ages. Holz Roh Werkst. 1990, 48, 381–383. [Google Scholar] [CrossRef]
- Shinoda, Y.; Ogisu, M.; Iwata, S.; Tajima, T. The chemical composition of mangroves II. Gifu Daigaku Nogakubu Kenkyu Hokoku 1985, 50, 155–165. [Google Scholar]
- Rohini, R.M.; Das, A.K. Antidiarrheal and anti-inflammatory activities of lupeol, quercetin, β-sitosterol, adene-5-3-ol and caffeic acid isolated from Rhizophora mucronata bark. Pharm. Lett. 2010, 2, 95–101. [Google Scholar]
- Rohini, R.M.; Das, A.K. Triterpenoids from the stem bark of Rhizophora mucronata. Nat. Prod. Res. 2010, 24, 197–202. [Google Scholar] [CrossRef]
- Rohini, R.M.; Das, A.K. Determination of lupeol, β-sitosterol and quercetin from ethyl acetate extract of Rhizophora mucronata bark by HPTLC technique. Asian J. Pharm. Clin. Res. 2011, 4, 103–105. [Google Scholar]
- Saha, P.K.; Ganguly, T.; Ganguly, S.N.; Sircar, S.M. Rhizophorine, a new indole acid plant growth inhibitor from Rhizophora mucronata. Plant Biochem. J. 1978, 5, 65–68. [Google Scholar]
- Ganguly, S.N.; Sircar, S.M. Gibberellins from mangrove plants. Phytochemistry 1974, 13, 1911–1913. [Google Scholar] [CrossRef]
- Joel, E.L.; Bhimba, V. Isolation and characterization of secondary metabolites from the mangrove plant Rhizophora mucronata. Asia Pac. J. Trop. Med. 2010, 602–604. [Google Scholar] [CrossRef]
- Hogg, R.W.; Gillan, F.T. Fatty acids, sterols and hydrocarbons in the leaves from eleven species of mangrove. Phytochemistry 1984, 23, 93–97. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kumar, A.S.; Kannathasan, K.; Venkatesalu, V. Fatty-acid composition of some mangroves. Chem. Nat. Comp. 2010, 46, 92–94. [Google Scholar] [CrossRef]
- Lakshmi, V.; Misra, A. The novel 1-hydroxy-5-oxobicyclo[6.4.0]dodecane from Rhizophora mucronata. Planta Med. 1995, 61, 382–383. [Google Scholar] [CrossRef]
- Puspitasar, Y.E.; Hartiati, A.M.; Suprayitno, E. The potency of Rhizophora mucronata leaf extract as antidiarrhea. J. Appl. Sci. Res. 2012, 8, 1180–1185. [Google Scholar]
- Laphookhieo, S.; Karalai, C.; Ponglimanont, C. New sesquiterpenoid and triterpenoids from the fruits of Rhizophora mucronata. Chem. Pharm. Bull. 2004, 52, 883–885. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Anjaneyulu, V.; Rao, V.L. New beyerane and isopimarane diterpenoids from Rhizophora mucronata. J. Asian Nat. Prod. Res. 2002, 4, 53–61. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Anjaneyulu, V.; Rao, V.L. Rhizophorin B: A novel beyerane diterpenoid from the Indian mangrove plant Rhizophora mucronata. Indian J. Chem. Sect. B 2000, 39B, 803–807. [Google Scholar]
- Anjaneyulu, A.S.R.; Rao, V.L. Rhizophorin A, a novel secolabdane diterpenoid from the Indian mangrove plant Rhizophora mucronata. Nat. Prod. Lett. 2001, 15, 13–19. [Google Scholar] [CrossRef]
- Richter, A.; Thonke, B.; Popp, M. 1d-1-O-Methyl-muco-inositol in Viscum album and members of the Rhizophoraceae. Phytochemistry 1990, 29, 1785–1786. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Hiader, S.I.; Rabbani, M.M. 25-S-Spirost-5-ene-3β, 14α-diol from Rhizophora mucronata. Fitoterapia 1988, 59, 79. [Google Scholar]
- Premanathan, M.; Kathiresan, K.; Chandra, K. Antiviral evaluation of some marine plants against Semliki forest virus. Pharm. Biol. 1995, 33, 75–77. [Google Scholar] [CrossRef]
- Premanathan, M.; Kathiresan, K.; Yamamoto, N.; Nakashima, H. In vitro anti-human immunodeficiency virus activity of polysaccharide from Rhizophora mucronata Poir. Biosci. Biotechnol. Biochem. 1999, 63, 1187–1191. [Google Scholar] [CrossRef]
- Premnathan, M.; Chandra, K.; Bajpai, S.K.; Kathiresan, K. A survey of some Indian marine plants for antiviral activity. Bot. Mar. 1992, 35, 321–324. [Google Scholar]
- Baskaran, R.; Mohan, P.M. In vitro antibacterial activity of leaf extracts of Rhizophora mucronata L. against multi drug resistant Vibrio spp. isolated from marine water lobster’s larvae hatcheries. Indian J. Geo-Mar. Sci. 2012, 41, 218–222. [Google Scholar]
- Chandrasekaran, M.; Venkatesalu, V.; Sivasankari, S.; Kannathasan, K.; Sajit Khan, A.K.; Prabhakar, K.; Rajendran, S.; Lakhsmi Sarayu, Y. Antibacterial activity of certain mangroves against methicillin resistant Staphylococcus aureus. Seaweed Res. Util. 2006, 28, 165–170. [Google Scholar]
- Rajaretinam, R.K.; PrakashVincent, S.G. Isolation of a novel bioactive compound from Rhizophora mucronata for methicillin resistant Staphylococcus aureus (MRSA) and compound toxicity assessment in zebra fish embryos. J. Pharm. Res. 2010, 3, 2000–2003. [Google Scholar]
- Kusuma, S.; Anil Kumar, P.; Boopalan, K. Potent antimicrobial activity of Rhizophora mucronata. J. Ecobiotechnol. 2011, 3, 40–41. [Google Scholar]
- Ravikumar, S.; Inbaneson, S.J.; Suganthi, P.; Guanadesigan, M. In vitro antiplasmodial activity of ethanolic extracts of mangrove plants from South East coast of India against chloroquine-sensitive Plasmodium falciparum. Parasitol. Res. 2011, 108, 873–878. [Google Scholar] [CrossRef]
- Ravikumar, S.; Inbaneson, S.J.; Suganthi, P.; Venkatesan, M.; Ramu, A. Mangrove plants as a source of lead compounds for the development of new antiplasmodial drugs from South East coast of India. Parasitol. Res. 2011, 108, 1405–1410. [Google Scholar] [CrossRef]
- Agoramoorthy, G.; Chen, F.A.; Venkatesalu, V.; Kuo, D.H.; Shea, P.C. Evaluation of antioxidant polyphenols from selected mangrove plants of India. Asian J. Chem. 2008, 20, 1311–1322. [Google Scholar]
- Vadlapudi, V.; Naidu, K.C. Evaluation of antioxidant potential of selected mangrove plants. J. Pharm. Res. 2009, 2, 1742–1745. [Google Scholar]
- Johnson, T. CRC Ethnobotany Desk Reference; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Cannonball Mangrove (Xylocarpus granatum) in a Mangrove Swamp in the Daintree Region of Queensland, Australia. Located on the Mardjja Botanical Walk (Coordinates Imprecise). Available online: http://en.wikipedia.org/wiki/File:Xylocarpus_granatum.jpg (accessed on 14 April 2013).
- Alvi, K.A.; Crews, P.; Aalbersberg, B.; Prasad, R. Limonoids from the Fijian medicinal plant dabi (Xylocarpus). Tetrahedron 1991, 47, 8943–8948. [Google Scholar] [CrossRef]
- Weiner, M.A. Ethnomedicine in Tonga. Econ. Bot. 1971, 25, 423–450. [Google Scholar] [CrossRef]
- Rouf, R.; Uddin, S.J.; Shilpi, J.A.; Alamgir, M. Assessment of antidiarrhoeal activity of the methanol extract of Xylocarpus granatum bark in mice model. J. Ethnopharmacol. 2007, 109, 539–542. [Google Scholar] [CrossRef]
- Uddin, S.J.; Shilpi, J.A.; Delazar, A.; Nahar, L.; Sarker, S.D. Free radical scavenging activity of some Bangladeshi plant extracts. Orient. Pharm. Exp. Med. 2004, 4, 187–195. [Google Scholar] [CrossRef]
- Wiart, C. Medicinal Plants of Asia and the Pacific; Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Lakhsmi, V.; Saxena, A.; Singh, S.; Pal, R.; Srivastava, S.; Srivastava, M.N. A Process for the Isolation of Standardized Antidiarrhoeal Fraction and Its Active Compounds from the Fruit Seed Coat of Xylocarpus granatum Koen and Its Use Thereof. Indian Pat. Appl. No. 1937/DEL/2006 A, 2006. [Google Scholar]
- Zaridah, M.Z.; Idid, S.Z.; Wan Omar, A.; Khozirah, S. In vitro antifilarial effects of three plant species against adult worms of subperiodic Brugia malayi. J. Ethnopharmacol. 2001, 78, 79–84. [Google Scholar] [CrossRef]
- Wangensteen, H.; Alamgir, M.; Duong, G.M.; Grønhaug, T.E.; Samuelsen, A.B.; Malterud, K.E. Chemical and Biological Studies of Medicinal Plants from the Sundarbans Mangrove Forest. In Advances in Phytotherapy Research; Eddouks, M., Ed.; Research Signpost: Trivandrum, India, 2009; pp. 59–78. [Google Scholar]
- Wangensteen, H.; Duong, G.M.; Alamgir, M.; Sarder, M.; Samuelsen, A.B.; Malterud, K.E. Biological activities of limonoids, catechins, procyanidins and extracts from Xylocarpus granatum. Nat. Prod. Commun. 2006, 1, 985–990. [Google Scholar]
- Cui, J.; Deng, Z.; Li, J.; Fu, H.; Proksch, P.; Lin, W. Phragmalin-type limonoids from the mangrove plant Xylocarpus granatum. Phytochemistry 2005, 66, 2334–2339. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, Q.; Huang, J.S.; Xiao, Z.H.; Qi, S.H.; Li, Q.X.; Zhang, S. Xyloccensins O and P, unique 8,9,30-phragmalin ortho esters from Xylocarpus granatum. Org. Lett. 2004, 6, 1841–1844. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, Q.; Zhang, S.; Li, X.; Xiao, Z.H.; Ding, H.X.; Li, Q.X. Xyloccensins Q-V, six new 8,9,30-phragmalin ortho ester antifeedants from the Chinese mangrove Xylocarpus granatum. Tetrahedron 2005, 61, 8382–8389. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, S.; Xiao, Q.; Li, Q.; Huang, J.; Xiao, Z.; Long, L. Xyloccensin M and N, two new B, D-seco limonoids from Xylocarpus granatum. Z. Naturforsch. Sect. B 2003, 58, 1216–1219. [Google Scholar]
- Wu, J.; Zhang, S.; Xiao, Q.; Li, Q.X.; Huang, H.S.; Long, L.J.; Huang, L.M. Xyloccensin L, a novel limonoid from Xylocarpus granatum. Tetrahedron Lett. 2004, 45, 591–593. [Google Scholar]
- Sundarasivarao, B.; Nazma; Madhusudhanarao, J. Antifungal activity of gedunin. Curr. Sci. 1977, 46, 714–716. [Google Scholar]
- Du, S.; Wang, M.; Zhu, W.; Qin, Z. A new fungicidal lactone from Xylocarpus granatum (Meliaceae). Nat. Prod. Res. 2009, 23, 1316–1321. [Google Scholar] [CrossRef]
- Hu, W.-M.; Wu, J. Protoxylogranatin B, a key biosynthetic intermediate from Xylocarpus granatum: Suggesting an oxidative cleavage biogenetic pathway to limonoid. Open Nat. Prod. J. 2010, 3, 1–5. [Google Scholar] [CrossRef]
- Huo, C.; Guo, D.; Shen, L.; Wang, W.; Zhang, Q.; Zhang, M.; Shi, Q. One new limonoid from seeds of Xylocarpus granatum. Zhongcaoyao 2010, 41, 176–178. [Google Scholar]
- Huo, C.-H.; Dong, C.; Shen, L.-R.; Yin, B.-W.; Sauriol, F.; Li, L.-G.; Zhang, M.-L.; Shi, Q.-W.; Kiyota, H. Xylocarpanoids A and B, unique C28 skeleton limonoids from Xylocarpus granatum. Tetrahedron Lett. 2010, 51, 754–757. [Google Scholar] [CrossRef]
- Kokpol, U.; Chavasiri, W.; Tip-pyang, S.; Veerachato, G.; Zhao, F.L.; Simpson, J.; Weavers, R.T. A limonoid from Xylocarpus granatum. Phytochemistry 1996, 41, 903–905. [Google Scholar] [CrossRef]
- Li, M.-Y.; Wu, J.; Zhang, S.; Xiao, Q.; Li, Q.-X. The absolute stereochemistry of protoxylogranatin A—A new protolimonoid from the seeds of Chinese mangrove Xylocarpus granatum. J. Asian Nat. Prod. 2008, 10, 503–508. [Google Scholar] [CrossRef]
- Li, M.-Y.; Yang, X.-B.; Pan, J.-Y.; Feng, G.; Xiao, Q.; Sinkkonen, J.; Satyanandamurty, T.; Wu, J. Granatumins A–G, limonoids from the seeds of a Krishna mangrove, Xylocarpus granatum. J. Nat. Prod. 2009, 72, 2110–2114. [Google Scholar] [CrossRef]
- Ng, A.S.; Fallis, A.G. Comment: 7α-Acetoxydihydronomilin and mexicanolide: Limonoids from Xylocarpus granatum (Koenig). Can. J. Chem. 1979, 57, 3088–3089. [Google Scholar] [CrossRef]
- Okorie, D.A.; Taylor, D.A.H. Limonoids from Xylocarpus granatum Koenig. J. Chem. Soc. C 1970, 211–213. [Google Scholar] [CrossRef]
- Pan, J.-Y.; Chen, S.-L.; Li, M.-Y.; Li, J.; Yang, M.-H.; Wu, J. Limonoids from the seeds of a Hainan mangrove, Xylocarpus granatum. J. Nat. Prod. 2010, 73, 1672–1679. [Google Scholar] [CrossRef]
- Pudhom, K.; Sommit, D.; Nuclear, P.; Ngamrojanavanich, N.; Petsom, A. Protoxylocarpins F-H, protolimonoids from seed kernels of Xylocarpus granatum. J. Nat. Prod. 2009, 72, 2188–2191. [Google Scholar] [CrossRef]
- Shen, L.; Guo, D.; Yin, B.; Zhang, Q.; Zhao, L.; Huo, C.; Zhang, M.; Shi, Q.; Wang, Y. Chemical constituents of Xylocarpus granatum Koenig. Zhongcaoyao 2009, 40, 1201–1204. [Google Scholar]
- Waratchareeyakul, W.; Chantrapromma, S.; Fun, H.-K.; Razak, I.A; Karalai, C.; Ponglimanont, C. 7-Oxogedunin. Acta Crystallogr. Sect. E 2004, E60, o1964–o1966. [Google Scholar]
- Wu, J.; Li, M.Y.; Zhang, S.; Xiao, Q.; Li, Q.X. Two new limonoids with a 3-O-β-tigloyl group from the seeds of the Chinese mangrove Xylocarpus granatum. Z. Naturforsch. Sect. B 2007, B62, 859–862. [Google Scholar]
- Wu, J.; Zhang, Z.; Bruhn, T.; Xiao, Q.; Ding, H.; Bringmann, G. Xylogranatins F–R: Antifeedants from the Chinese mangrove, Xylocarpus granatum, a new biogenetic pathway to triterpenoids. Chem. Eur. J. 2008, 14, 1129–1144. [Google Scholar] [CrossRef]
- Yang, X.; Yang, S.; Li, M.; Pan, J.; Zheng, Y.; Wu, J. Chemical constituents in seeds of Indian mangrove, Xylocarpus granatum. Zhongcaoyao 2010, 41, 846–851. [Google Scholar]
- Yin, S.; Fan, C.-Q.; Wang, X.-N.; Lin, L.-P.; Ding, J.; Yue, J.-M. Xylogranatins A–D: Novel tetranortriterpenoids with an unusual 9,10-seco scaffold from marine mangrove Xylocarpus granatum. Org. Lett. 2006, 8, 4935–4938. [Google Scholar] [CrossRef]
- Yin, S.; Wang, X.-N.; Fan, C.-Q.; Lin, L.-P.; Ding, J.; Yue, J.-M. Limonoids from the seeds of the marine mangrove Xylocarpus granatum. J. Nat. Prod. 2007, 70, 682–685. [Google Scholar] [CrossRef]
- Yin, B.; Huo, C.; Shen, L.; Wang, C.; Zhao, L.; Wang, Y.; Shi, Q. Protolimonoids from the seeds of Xylocarpus granatum. Biochem. Syst. Ecol. 2009, 37, 218–220. [Google Scholar] [CrossRef]
- Cheng, F.; Zhou, Y.; Wu, J. Chemical constituents of fruit of Xylocarpus granatum. Zhongyaocai 2009, 32, 1220–1223. [Google Scholar]
- Cui, J.X.; Wu, J.; Deng, Z.W.; Proksch, P.; Lin, W.H. Xylocarpins A–I, limonoids from the Chinese mangrove plant Xylocarpus granatum. J. Nat. Prod. 2007, 70, 772–778. [Google Scholar] [CrossRef]
- Cui, J.; Ouyang, J.; Deng, Z.; Lin, W. Structure elucidation of an unprecedented alkaloid and a new limonoid from Xylocarpus granatum. Magn. Res. Chem. 2008, 46, 894–897. [Google Scholar] [CrossRef]
- Cui, J.; Deng, Z.; Xu, M.; Proksch, P.; Li, Q.; Liu, W. Protolimonoids and limonoids from the Chinese mangrove plant Xylocarpus granatum. Helv. Chim. Acta 2009, 92, 139–150. [Google Scholar] [CrossRef]
- Wu, J.; Ding, H.X.; Li, M.Y.; Zhang, S. Xylogranatin E, a new phragmalin with a rare oxygen bridge between C1 and C29, from the fruit of a Chinese mangrove Xylocarpus granatum. Z. Naturforsch. Sect. B 2007, B 62, 569–572. [Google Scholar]
- Wu, J.; Li, M.Y.; Xiao, Z.H.; Zhou, Y.A. Butyrospermol fatty acid esters from the fruit of a Chinese mangrove Xylocarpus granatum. Z. Naturforsch. Sect. B 2006, B61, 1447–1449. [Google Scholar]
- Wu, J.; Zhang, S.; Li, M.Y.; Zhou, Y.; Xiao, Q. Xylogranatins A–D, new mexicanolides from the fruit of a Chinese mangrove Xylocarpus granatum. Chem. Pharm. Bull. 2006, 54, 1582–1585. [Google Scholar] [CrossRef]
- Wu, J.; Zhihui, X.; Yang, S.; Zhang, S.; Xiao, Q.; Ma, C.; Ding, H.; Li, Q. Complete assignments of 1H and 13C NMR data for two 3β,8β-epoxymexicanolides from the fruit of a Chinese mangrove Xylocarpus granatum. Magn. Res. Chem. 2006, 44, 87–89. [Google Scholar] [CrossRef]
- Wu, J.J. Two new mexicanolides from the fruit of the Chinese mangrove Xylocarpus granatum. Z. Naturforsch. Sect. B 2005, B60, 1291–1294. [Google Scholar]
- Zhou, Y.; Cheng, F.; Wu, J.; Zou, K. Polyhydroxylated phragmalins from the fruit of a Chinese mangrove, Xylocarpus granatum. J. Nat. Prod. 2006, 69, 1083–1085. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, J.; Zou, K. Xylogranatinin, a new pyrido[1,2-a]pyrazine alkaloid from the fruit of a Chinese mangrove Xylocarpus granatum. Chem. Nat. Comp. 2007, 43, 426–428. [Google Scholar] [CrossRef]
- Shen, L.-R.; Guo, D.; Yu, Y.-M.; Yin, B.-W.; Zhao, L.; Shi, Q.-W.; Wang, Y.-L.; Huo, C.-H. Chemical constituents of plants from the genus Xylocarpus. Chem. Biodiv. 2009, 6, 1293–1308. [Google Scholar] [CrossRef]
- Phatthalung, P.N.; Chusri, S.; Voravuthikunchai, S.P. Thai ethnomedicinal plants as resistant modifying agents for combating Acinetobacter baumannii infections. BMC Complement. Altern. Med. 2012, 12, 56. [Google Scholar] [CrossRef]
- Alam, M.Q.; Sarder, M.; Awal, M.A.; Sikder, M.M.H.; Daulla, K.A. Antibacterial activity of the crude ethanolic extract of Xylocarpus granatum stem barks. Bangladesh J. Vet. Med. 2006, 4, 69–72. [Google Scholar]
- Choudhury, S.; Sree, A.; Mukherjee, S.C.; Pattnaik, P.; Bapuji, M. In vitro antibacterial activity of extracts of selected marine algae and mangroves against fish pathogens. Asian Fish. Sci. 2005, 18, 285–294. [Google Scholar]
- Misra, S.; Verma, M.; Mishra, S.; Srivastava, S.; Lakhsmi, V.; Misra-Bhattacharya, S. Gedunin and photogedunin of Xylocarpus granatum possess antifilarial activity against human lymphatic filarial parasite Brugia malayi in experimental rodent host. Parasitol. Res. 2011, 109, 1351–1360. [Google Scholar] [CrossRef]
- Lakhsmi, V.; Srivastava, S.; Mishran, S.K.; Srivastava, M.N.; Srivastava, K.; Puri, S.K. Antimalarial activity in Xylocarpus granatum (Koen). Nat. Prod. Res. 2012, 26, 1012–1015. [Google Scholar] [CrossRef]
- Lakhsmi, V.; Singh, N.; Shrivastva, S.; Mishra, S.K.; Dharmani, P.; Mishra, V.; Palit, G. Gedunin and photogedunin of Xylocarpus granatum show significant anti-secretory effects and protect the gastric mucosa of peptic ulcer in rats. Phytomedicine 2010, 17, 569–574. [Google Scholar] [CrossRef]
- Uddin, S.J.; Nahar, L.; Shilpi, J.A.; Shoeb, M.; Borkowski, T.; Gibbons, S.; Middleton, M.; Byres, M.; Sarker, S.D. Gedunin, a limonoid from Xylocarpus granatum, inhibits the growth of CaCo-2 colon cancer cell line in vitro. Phytother. Res. 2007, 21, 757–761. [Google Scholar] [CrossRef]
- Uddin, S.J.; Grice, I.D.; Tiralongo, E. Cytotoxic effects of Bangladeshi medicinal plant extracts. Evid. Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef]
- Uddin, S.J.; Shilpi, J.A.; Alam, S.M.S.; Alamgir, M.; Rahman, M.T.; Sarker, S.D. Antidiarrhoeal activity of the methanol extract of the barks of Xylocarpus moluccensis in castor oil- and magnesium sulphate-induced diarrhoea models in mice. J. Ethnopharmacol. 2005, 101, 139–143. [Google Scholar] [CrossRef]
- Piyagaw, Xylocarpus moluccensis. Available online: http://stuartxchange.org/Piyagaw.html (accessed on 15 February 2013).
- Sarker, S.D.; Uddin, S.J.; Shilpi, J.A.; Rouf, R.; Ferdous, M.E.M.; Nahar, L. Neuropharmacological properties of Xylocarpus moluccensis. Fitoterapia 2007, 78, 107–111. [Google Scholar] [CrossRef]
- Connolly, J.D.; MacLellan, M.; Okorie, D.A.; Taylor, D.A.H. Limonoids from Xylocarpus moluccensis (Lam.) M. Roem. J. Chem. Soc. Perkin Trans. 1 1976, 19, 1993–1996. [Google Scholar]
- Mulholland, D.A.; Taylor, D.A.H. Limonoids from Australian members of the Meliaceae. Phytochemistry 1992, 31, 4163–4166. [Google Scholar] [CrossRef]
- Taylor, D.A.H. Limonoid extractives from Xylocarpus moluccensis. Phytochemistry 1983, 22, 1297–1299. [Google Scholar] [CrossRef]
- Bercich, M.G.; Cambie, R.C.; Lal, A.R.; Sidwell, D. Chemistry of Fijian plants. XIV. An unsaturated aryl keto acid from Xylocarpus moluccensis. Austr. J. Chem. 1998, 51, 795–797. [Google Scholar]
- Roy, A.D.; Kumar, R.; Gupta, P.; Khaliq, T.; Narender, T.; Aggarwal, V.; Roy, R. Xyloccensin X and Y, two new limonoids from Xylocarpus moluccensis: NMR investigation in mixture. Magn. Res. Chem. 2006, 44, 1054–1057. [Google Scholar] [CrossRef]
- Kubo, I.; Miura, I.; Nakanishi, K. Structure of xylomollin, a secoiridoid hemiacetal acetal. J. Am. Chem. Soc. 1976, 98, 6704–6705. [Google Scholar] [CrossRef]
- Li, M.-Y.; Yang, S.-X.; Pan, J.-Y.; Xiao, Q.; Satyanandamurty, T.; Wu, J. Moluccensins A–G, phragmalins with a conjugated C-30 carbonyl group from a Krishna mangrove, Xylocarpus moluccensis. J. Nat. Prod. 2009, 72, 1657–1662. [Google Scholar]
- Li, J.; Li, M.-Y.; Feng, G.; Xiao, Q.; Sinkkonen, J.; Satyanandamurty, T.; Wu, J. Limonoids from the seeds of a Godavari mangrove, Xylocarpus moluccensis. Phytochemistry 2010, 71, 1917–1924. [Google Scholar] [CrossRef]
- Li, J.; Li, M.-Y.; Satyanandamurty, T.; Wu, J. Godavarin K: A new limonoid with an oxygen bridge between C(1) and C(29) from the Godavari mangrove Xylocarpus moluccensis. Helv. Chim. Acta 2011, 94, 1651–1656. [Google Scholar] [CrossRef]
- Li, M.-Y.; Zhang, J.; Feng, G.; Satyanandamurty, T.; Wu, J. Khayasin and 2′S-methylbutanoylproceranolide: Promising candidate insecticides from the control of the coconut leaf beetle, Brontispa longissima. J. Pestic. Sci. 2011, 36, 22–26. [Google Scholar] [CrossRef]
- Li, J.; Li, M.-Y.; Feng, G.; Zhang, J.; Karonen, M.; Sinkkonen, J.; Satyanandamurty, T.; Wu, J. Moluccensins R–Y, limonoids from the seeds of a mangrove, Xylocarpus moluccensis. J. Nat. Prod. 2012, 75, 1277–1283. [Google Scholar] [CrossRef]
- Pudhom, K.; Sommit, D.; Nuclear, P.; Ngamrojanavanich, N.; Petsom, A. Moluccensins H–J, 30-ketophragmalin limonoids from Xylocarpus moluccensis. J. Nat. Prod. 2010, 73, 263–266. [Google Scholar] [CrossRef]
- Ravangpai, W.; Sommit, D.; Teerawatananond, T.; Sinpranee, N.; Palaga, T.; Pengpreecha, S.; Muangsin, N.; Pudhom, K. Limonoids from seeds of Thai Xylocarpus moluccensis. Bioorg. Med. Chem. Lett. 2011, 21, 4485–4489. [Google Scholar] [CrossRef]
- Wu, J.; Yang, S.-X.; Li, M.-Y.; Feng, G.; Pan, J.-Y.; Xiao, Q.; Sinkkonen, J.; Satyanandamurty, T. Limonoids and tirucallane derivatives from the seeds of a Krishna mangrove, Xylocarpus moluccensis. J. Nat. Prod. 2010, 73, 644–649. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.-X.; Yang, X.-B.; Li, M.-Y.; Feng, G.; Pan, J.-Y.; Satyanandamurty, T.; Wu, J. Mexicanolides from the seeds of a Krishna mangrove, Xylocarpus moluccensis. Chem. Pharm. Bull. 2010, 58, 552–555. [Google Scholar]
- Gunawan, S.; Darmawan, R.; Nanda, M.; Setiawan, A.D.; Fansuri, H. Proximate composition of Xylocarpus moluccensis seeds and their oils. Ind. Crops Prod. 2013, 41, 107–112. [Google Scholar] [CrossRef]
- Li, M.; Wu, J. Khayasin from Xylocarpus moluccensis for Control of Brontispa longissima larvae. Chinese Patent N 101849541 A, 2010. [Google Scholar]
- Li, M.; Wu, J. Application of Xylolimonoid A from Xylocarpus moluccensis as Insecticide for Brontispa longissima. Chinese Patent CN 101849542 A, 2010. [Google Scholar]
- Wisutsitthiwong, C.; Buranaruk, C.; Pudhom, K.; Palaga, T. The plant limonoid 7-oxo-deacetoxygedunin inhibits RANKL-induced osteoclastogenesis by suppressing activation of the NF-κB and MAPK pathways. Biochem. Biophys. Res. Comm. 2011, 415, 361–366. [Google Scholar] [CrossRef]
- Adzu, B.; Amos, S.; Amizan, M.B.; Gamaniel, K. Evaluation of the antidiarrhoeal effects of Zizyphus spina-christi stem bark in rats. Acta Trop. 2003, 87, 245–250. [Google Scholar] [CrossRef]
- Agbor, G.A.; Léopold, T.; Jeanne, N.Y. The antidiarrhoeal activity of Alchornea cordifolia leaf extract. Phytother. Res. 2004, 18, 873–876. [Google Scholar] [CrossRef]
- Atta, A.H.; Mouneir, S.M. Antidiarrhoeal activity of some Egyptian medicinal plant extracts. J. Ethnopharmacol. 2004, 92, 303–309. [Google Scholar] [CrossRef]
- Gabriel, S.E.; Davenport, S.E.; Steagall, R.J.; Vimal, V.; Carlson, T.; Rozhon, E.J. A novel plant-derived inhibitor of cAMP-mediated fluid and chloride secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 1999, 276, G58–G63. [Google Scholar]
- Schuier, M.; Sies, H.; Illek, B.; Fischer, H. Cocoa-related flavonoids inhibit CFTR-mediated chloride transport across T84 human colon epithelia. J. Nutr. 2005, 135, 2320–2325. [Google Scholar]
- Toda, S.; Shirataki, Y. Inhibitory effects of sophoraflavanones B and H in Sophora moorcroftiana Beth ex Baker on copper-ion-induced protein oxidative modification of mice brain homogenate in vitro. Biol. Trace Element Res. 2000, 78, 157–162. [Google Scholar] [CrossRef]
- Kaur, T.; Singh, S.; Dhawan, V.; Ganguly, N.K. Shigella dysenteriae type 1 toxin induced lipid peroxidation in enterocytes isolated from rabbit ileum. Mol. Cell. Biochem. 1998, 178, 169–179. [Google Scholar] [CrossRef]
- Rawal, S.; Majumdar, S.; Dhawan, V.; Vohra, H. Entamoeba histolytica Gal/GalNAc lectin depletes antioxidant defences of target epithelial cells. Parasitology 2004, 128, 617–624. [Google Scholar] [CrossRef]
- Nieto, N.; López-Pedrosa, J.M.; Mesa, M.D.; Torres, M.I.; Fernández, M.I.; Ríos, A.; Suárez, M.D.; Gil, A. Chronic diarrhea impairs intestinal antioxidant defense system in rats at weaning. Dig. Dis. Sci. 2000, 45, 2044–2050. [Google Scholar] [CrossRef]
- Naito, Y.; Takagi, T.; Yoshikawa, T. Neutrophil-dependent oxidative stress in ulcerative colitis. J. Clin. Biochem. Nutr. 2007, 41, 18–26. [Google Scholar] [CrossRef]
- Tomassoni, A.J.; Simone, K. Herbal medicines for children: An illusion of safety? Curr. Opin. Pediatr. 2001, 13, 162–169. [Google Scholar] [CrossRef]
- Farnsworth, N.R. Ethnopharmacology and Drug Development. In Ethnobotany and the Search for New Drugs; Chadwick, D.J., Marsh, J., Eds.; Wiley: Chichester, UK, 1994; pp. 42–76. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wangensteen, H.; Klarpås, L.; Alamgir, M.; Samuelsen, A.B.C.; Malterud, K.E. Can Scientific Evidence Support Using Bangladeshi Traditional Medicinal Plants in the Treatment of Diarrhoea? A Review on Seven Plants. Nutrients 2013, 5, 1757-1800. https://doi.org/10.3390/nu5051757
Wangensteen H, Klarpås L, Alamgir M, Samuelsen ABC, Malterud KE. Can Scientific Evidence Support Using Bangladeshi Traditional Medicinal Plants in the Treatment of Diarrhoea? A Review on Seven Plants. Nutrients. 2013; 5(5):1757-1800. https://doi.org/10.3390/nu5051757
Chicago/Turabian StyleWangensteen, Helle, Line Klarpås, Mahiuddin Alamgir, Anne B. C. Samuelsen, and Karl E. Malterud. 2013. "Can Scientific Evidence Support Using Bangladeshi Traditional Medicinal Plants in the Treatment of Diarrhoea? A Review on Seven Plants" Nutrients 5, no. 5: 1757-1800. https://doi.org/10.3390/nu5051757
APA StyleWangensteen, H., Klarpås, L., Alamgir, M., Samuelsen, A. B. C., & Malterud, K. E. (2013). Can Scientific Evidence Support Using Bangladeshi Traditional Medicinal Plants in the Treatment of Diarrhoea? A Review on Seven Plants. Nutrients, 5(5), 1757-1800. https://doi.org/10.3390/nu5051757





