Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Uncoupling Protein 3 Gene Expression in C2C12 Muscle Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Cell Culture
2.3. Preparation of EPA and DHA
2.4. Cytotoxicity Assay
2.5. Real-Time qRT-PCR
2.6. Construction of Human Uncoupling Protein 3 (UCP3)/Luc Reporter Gene
2.7. Transfection and Luciferase Activity
2.8. Statistical Analysis
3. Results
3.1. Effects of EPA and DHA on Cell Viability of Muscle Cells

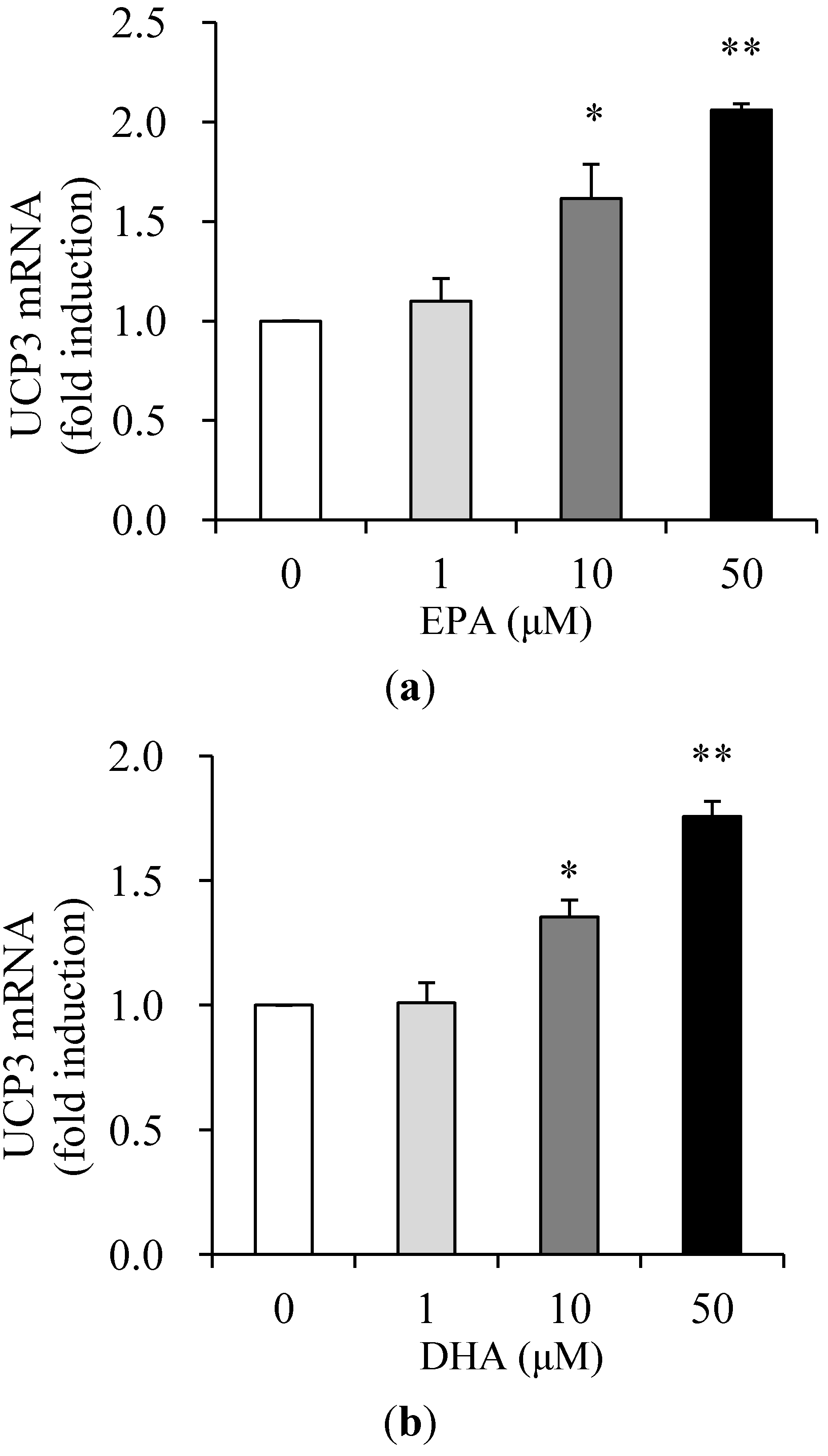
3.2. Effects of EPA and DHA on UCP3 Expression
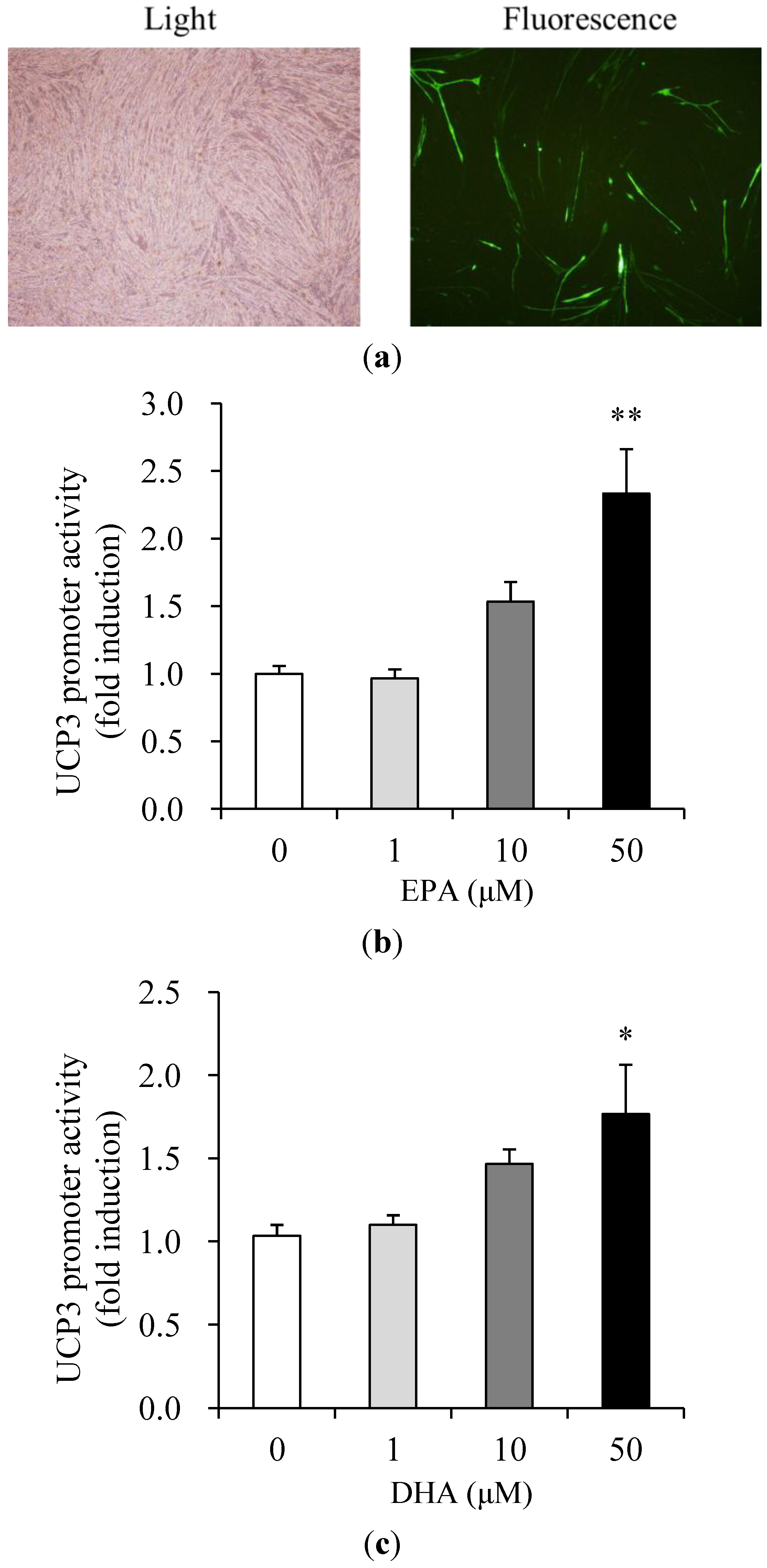
3.3. Effects of EPA and DHA on UCP3 Promoter Activity
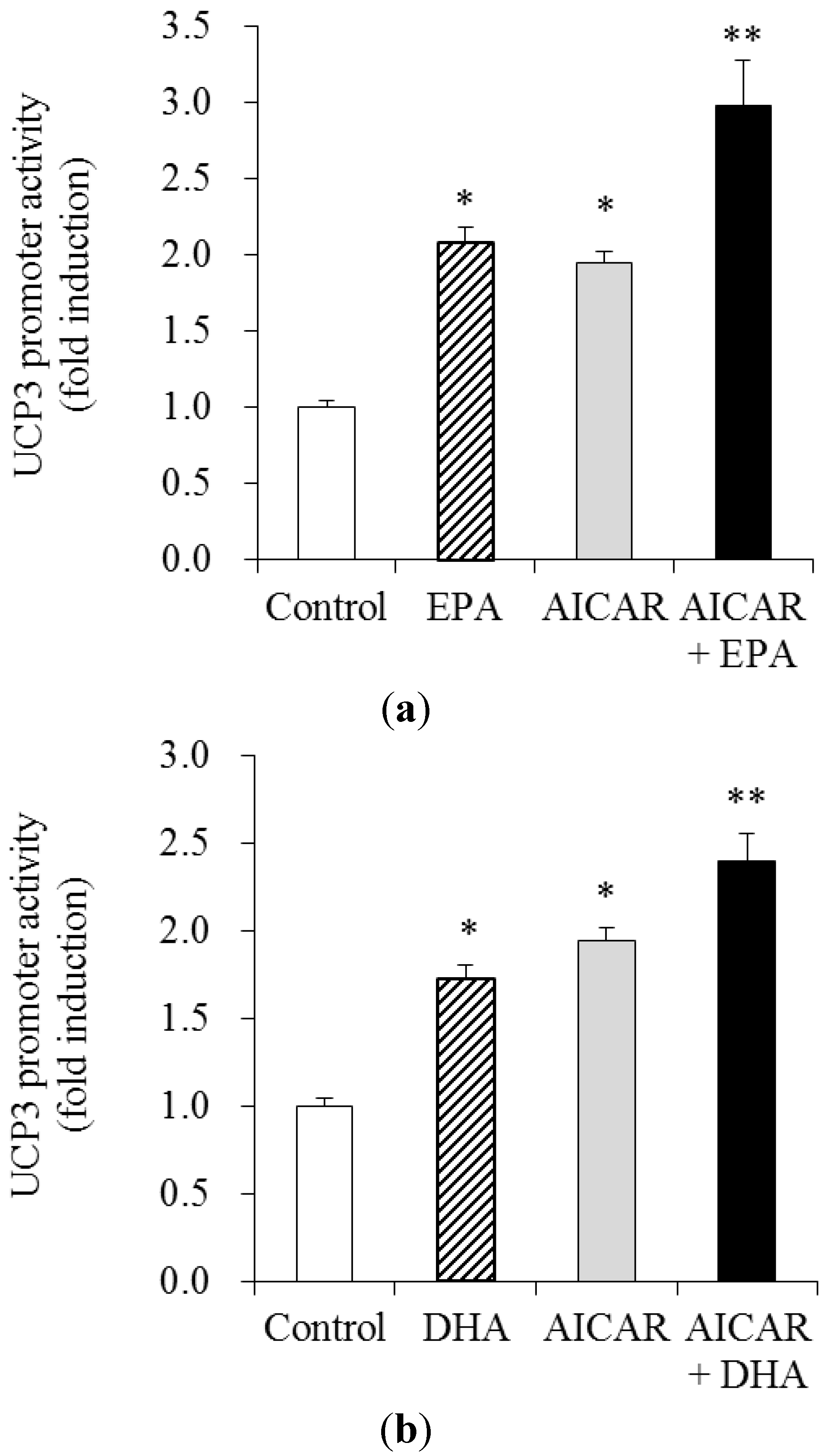
3.4. Effects of AICAR on EPA- and DHA—Stimulated UCP3 Promoter Activity
4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Ricquier, D.; Casteilla, L.; Bouillaud, F. Molecular studies of the uncoupling protein. FASEB J. 1991, 5, 2237–2242. [Google Scholar]
- Gimeno, R.E.; Dembski, M.; Weng, X.; Deng, N.; Shyjan, A.W.; Gimeno, C.J.; Iris, F.; Ellis, S.J.; Woolf, E.A.; Tartaglia, L.A. Cloning and characterization of an uncoupling protein homolog: A potential molecular mediator of human thermogenesis. Diabetes 1997, 46, 900–906. [Google Scholar]
- Ricquier, D.; Bouillaud, F. Mitochondrial uncoupling proteins: From mitochondria to the regulation of energy balance. J. Physiol. 2000, 529, 3–10. [Google Scholar]
- Ricquier, D.; Bouillaud, F. The uncoupling protein homologues: UCP1, UCP2, UCP3, StUCP and AtUCP. Biochem. J. 2000, 345, 161–179. [Google Scholar] [CrossRef]
- Brand, M.D.; Esteves, T.C. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005, 2, 85–93. [Google Scholar] [CrossRef]
- Costford, S.R.; Chaudhry, S.N.; Crawford, S.A.; Salkhordeh, M.; Harper, M.E. Long-term high-fat feeding induces greater fat storage in mice lacking UCP3. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1018–E1024. [Google Scholar]
- Baillie, R.A.; Takada, R.; Nakamura, M.; Clarke, S.D. Coordinate induction of peroxisomal acyl-CoA oxidase and UCP-3 by dietary fish oil: A mechanism for decreased body fat deposition. Prostaglandins Leukot. Essent. Fatty Acids 1999, 60, 351–356. [Google Scholar] [CrossRef]
- Cha, S.H.; Fukushima, A.; Sakuma, K.; Kagawa, Y. Chronic docosahexaenoic acid intake enhances expression of the gene for uncoupling protein 3 and affects pleiotropic mRNA levels in skeletal muscle of aged C57BL/6NJcl mice. J. Nutr. 2001, 131, 2636–2642. [Google Scholar]
- Hwang, C.S.; Lane, M.D. Up-regulation of uncoupling protein-3 by fatty acid in C2C12 myotubes. Biochem. Biophys. Res. Commun. 1999, 258, 464–469. [Google Scholar]
- Kola, B.; Grossman, A.B.; Korbonits, M. The role of AMP-activated protein kinase in obesity. Front. Horm. Res. 2008, 36, 198–211. [Google Scholar]
- Ahmadian, M.; Abbott, M.J.; Tang, T.; Hudak, C.S.; Kim, Y.; Bruss, M.; Hellerstein, M.K.; Lee, H.Y.; Samuel, V.T.; Shulman, G.I.; et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 2011, 13, 739–748. [Google Scholar] [CrossRef]
- Lorente-Cebrián, S.; Bustos, M.; Marti, A.; Martinez, J.A.; Moreno-Aliaga, M.J. Eicosapentaenoic acid stimulates AMP-activated protein kinase and increases visfatin secretion in cultured murine adipocytes. Clin. Sci. (Lond.) 2009, 117, 243–249. [Google Scholar]
- Putman, C.T.; Kiricsi, M.; Pearcey, J.; MacLean, I.M.; Bamford, J.A.; Murdoch, G.K.; Dixon, W.T.; Pette, D. AMPK activation increases uncoupling protein-3 expression and mitochondrial enzyme activities in rat muscle without fibre type transitions. J. Physiol. 2003, 551, 169–178. [Google Scholar]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for General Users and for Biologist Programmers. In Bioinformatics Methods and Protocols: Methods in Molecular Biology; Misener, S., Krawetz, S.A., Eds.; Humana Press: Totowa, NJ, USA, 2000; pp. 365–386. [Google Scholar]
- Nabben, M.; Hoeks, J. Mitochondrial uncoupling protein 3 and its role in cardiac- and skeletal muscle metabolism. Physiol. Behav. 2008, 94, 259–269. [Google Scholar] [CrossRef]
- Flachs, P.; Rossmeisl, M.; Bryhn, M.; Kopecky, J. Cellular and molecular effects of n-3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin. Sci. (Lond.) 2009, 116, 1–16. [Google Scholar]
- Li, J.J.; Huang, C.J.; Xie, D. Anti-obesity effects of conjugated linoleic acid, docosahexaenoic acid, and eicosapentaenoic acid. Mol. Nutr. Food Res. 2008, 52, 631–645. [Google Scholar] [CrossRef]
- Ruzickova, J.; Rossmeisl, M.; Prazak, T.; Flachs, P.; Sponarova, J.; Veck, M.; Tvrzicka, E.; Bryhn, M.; Kopecky, J. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids 2004, 39, 1177–1185. [Google Scholar] [CrossRef]
- Flachs, P.; Horakova, O.; Brauner, P.; Rossmeisl, M.; Pecina, P.; Franssen-van Hal, N.; Ruzickova, J.; Sponarova, J.; Drahota, Z.; Vlcek, C.; et al. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce beta-oxidation in white fat. Diabetologia 2005, 48, 2365–2375. [Google Scholar] [CrossRef]
- Echtay, K.S.; Roussel, D.; St-Pierre, J.; Jekabsons, M.B.; Cadenas, S.; Stuart, J.A.; Harper, J.A.; Roebuck, S.J.; Morrison, A.; Pickering, S.; et al. Superoxide activates mitochondrial uncoupling proteins. Nature 2002, 415, 96–99. [Google Scholar] [CrossRef]
- Kuriki, K.; Nagaya, T.; Tokudome, Y.; Imaeda, N.; Fujiwara, N.; Sato, J.; Goto, C.; Ikeda, M.; Maki, S.; Tajima, K.; Tokudome, S. Plasma concentrations of (n-3) highly unsaturated fatty acids are good biomarkers of relative dietary fatty acid intakes: A cross-sectional study. J. Nutr. 2003, 133, 3643–3650. [Google Scholar]
- Desvergne, B.; Wahli, W. Peroxisome proliferator-activated receptors: Nuclear control of metabolism. Endocr. Rev. 1999, 20, 649–688. [Google Scholar] [CrossRef]
- Joubert, R.; Métayer, C.S.; Swennen, Q.; Sibut, V.; Crochet, S.; Cailleau-Audouin, E.; Buyse, J.; Decuypere, E.; Wrutniak-Cabello, C.; Cabello, G.; et al. The β-adrenergic system is involved in the regulation of the expression of avian uncoupling protein in the chicken. Domest. Anim. Endocrinol. 2010, 38, 115–125. [Google Scholar] [CrossRef]
- Long, Y.C.; Zierath, J.R. AMP-activated protein kinase signaling in metabolic regulation. J. Clin. Invest. 2006, 116, 1776–1783. [Google Scholar] [CrossRef]
- Lee, W.J.; Kim, M.; Park, H.S.; Kim, H.S.; Jeon, M.J.; Oh, K.S.; Koh, E.H.; Won, J.C.; Kim, M.S.; Oh, G.T.; et al. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARalpha and PGC-1. Biochem. Biophys. Res. Commun. 2006, 340, 291–295. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, M.-S.; Kim, I.-H.; Kim, Y. Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Uncoupling Protein 3 Gene Expression in C2C12 Muscle Cells. Nutrients 2013, 5, 1660-1671. https://doi.org/10.3390/nu5051660
Lee M-S, Kim I-H, Kim Y. Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Uncoupling Protein 3 Gene Expression in C2C12 Muscle Cells. Nutrients. 2013; 5(5):1660-1671. https://doi.org/10.3390/nu5051660
Chicago/Turabian StyleLee, Mak-Soon, In-Hwan Kim, and Yangha Kim. 2013. "Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Uncoupling Protein 3 Gene Expression in C2C12 Muscle Cells" Nutrients 5, no. 5: 1660-1671. https://doi.org/10.3390/nu5051660
APA StyleLee, M.-S., Kim, I.-H., & Kim, Y. (2013). Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Uncoupling Protein 3 Gene Expression in C2C12 Muscle Cells. Nutrients, 5(5), 1660-1671. https://doi.org/10.3390/nu5051660




