Selenium and Prostate Cancer Prevention: Insights from the Selenium and Vitamin E Cancer Prevention Trial (SELECT)
Abstract
:1. Introduction
2. Selenium
2.1. Dietary Sources and Supplements
2.2. Selenium Metabolism and Biological Activities
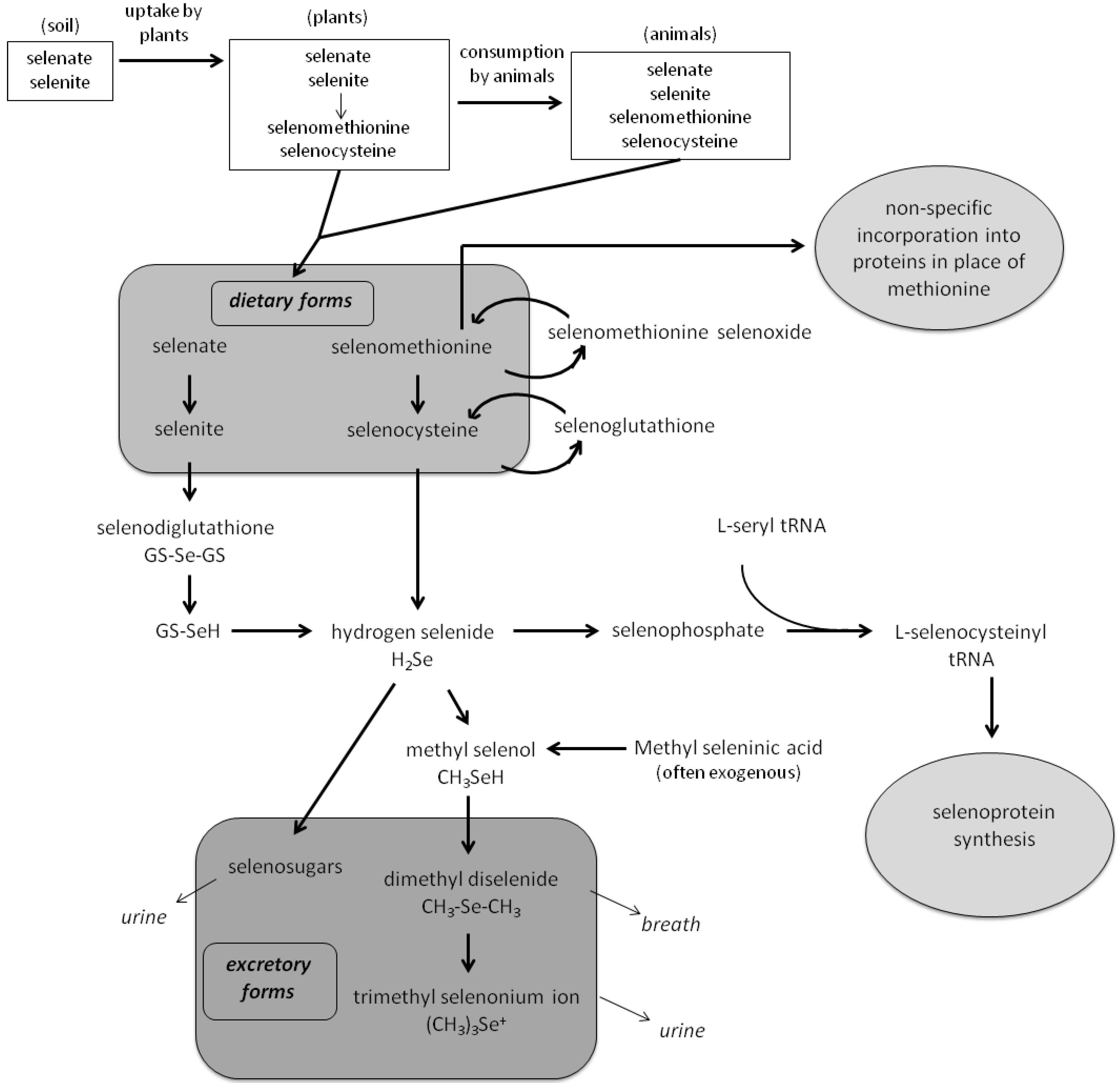
2.3. Nutritional Requirements
2.4. Prostate Cancer Prevention by Selenium—Rationale for SELECT
2.4.1. Epidemiological Studies
| Animal Model [Ref.] | Agent and Dose | Results |
|---|---|---|
| Studies relevant to SELECT trial design | ||
| N-Nitroso-N-methylurea (MNU) + testosterone-treated Wistar-Unilever rats [42] | l-selenomethionine (1.5 or 3 mg/kg diet) dl-α-tocopherol (4000 or 2000 mg/kg diet) l-selenomethionine ( 3 mg/kg diet ) + dl-α-tocopherol (2000 or 5000 mg/kg diet) Selenized yeast (target Se levels of 9 or 3 mg/kg diet) Control | No effect on prostate cancer incidence in any group |
| Testosterone + estradiol-treated NBL rat [43] | l-selenomethionine (1.5 or 3.0 mg/kg diet) dl-α-tocopherol (4000 or 2000 mg/kg diet) Control | No effect on prostate tumor incidence, multiplicity, or death in any group |
| Studies on selenium in combination with other agents only | ||
| Lady transgenic mice [44] | α-tocopherol succinate (800 IU) +
l-selenomethionine (200 μg) + lycopene (50 mg) α-tocopherol succinate (800 IU) + l-selenomethionine (200 μg) Control | Increased survival ( p < 0.0001) in both treatment groups compared to control, no effect on prostate tumor incidence in any group |
| Lady transgenic mice [45] | α-tocopherol succinate (800 IU) +
l-selenomethionine (200 μg) + lycopene (50 mg) Control | Four-fold decrease in prostate cancer incidence in animals treated with 3 agents combined compared to control animals ( p < 0.0001) |
| Studies on other forms of selenium | ||
| Transgenic adenocarcinoma mouse prostate (TRAMP) model [46] | Methylseleninic acid (3 mg selenium/kg body weight) for 10 weeks Methylseleninic acid (3 mg selenium/kg body weight) for 16 weeks Control | Decreased cancer-specific mortality in methylseleninic acid groups compared to control group ( p10 weeks = 0.0078, p16 weeks = 0.0385) |
| MNU + testosterone-treated Wistar rats [47] | Sodium selenite (4 mg/L in drinking water/day) Control | No effect on prostate intraepithelial neoplasia, decreased prostate cancer multiplicity by 44.6% in sodium selenite group compared to control group |
2.4.2. Clinical Trials
3. SELECT
3.1. Rationale and Objectives
3.2. Agent Formulation and Dose
3.3. Trial Design and Outcome Ascertainment
3.4. Recruitment, Enrollment, Cohort, and Baseline Characteristics
3.5. Primary Endpoint Results
| First Report, October 2008 | Second Report, July 2011 | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 8696) | Vitamin E (n = 8737) | Selenium (n = 8752) | Selenium + Vitamin E (n = 8703) | Placebo (n = 8696) | Vitamin E (n = 8737) | Selenium (n = 8752) | Selenium + Vitamin E (n = 8702) | |
| Prostate cancer | ||||||||
| No. events | 416 | 473 | 432 | 437 | 529 | 620 | 575 | 555 |
| HR (99% CI) | 1 (reference) | 1.13 (0.95–1.35) | 1.04 (0.87–1.24) | 1.05 (0.88–1.25) | 1 (reference) | 1.17 (1.004–1.36) a | 1.09 (0.93–1.27) | 1.05 (0.89–1.22) |
| Method of diagnosis, n (%) | ||||||||
| Prostate biopsy | 404 (97) | 458 (97) | 419 (97) | 420 (96) | n.r. b | n.r. | n.r. | n.r. |
| Other/unknown | 12 (3) | 15 (3) | 13 (3) | 17 (4) | n.r. | n.r. | n.r. | n.r. |
| Gleason score, n (%) | ||||||||
| 2–6 | 240 (66) | 249 (63) | 217 (60) | 220 (60) | ||||
| 4–6 | 286 (69) | 310 (67) | 281 (64) | 281 (63) | ||||
| 7 | 101 (28) | 124 (31) | 124 (34) | 115 (32) | 102 (24) | 118 (25) | 135 (31) | 124 (28) |
| 8–10 | 24 (7) | 23 (6) | 20 (6) | 30 (8) | 31 (7) | 37 (8) | 26 (6) | 40 (9) |
| Not graded | 51 | 77 | 71 | 72 | 110 | 155 | 133 | 110 |
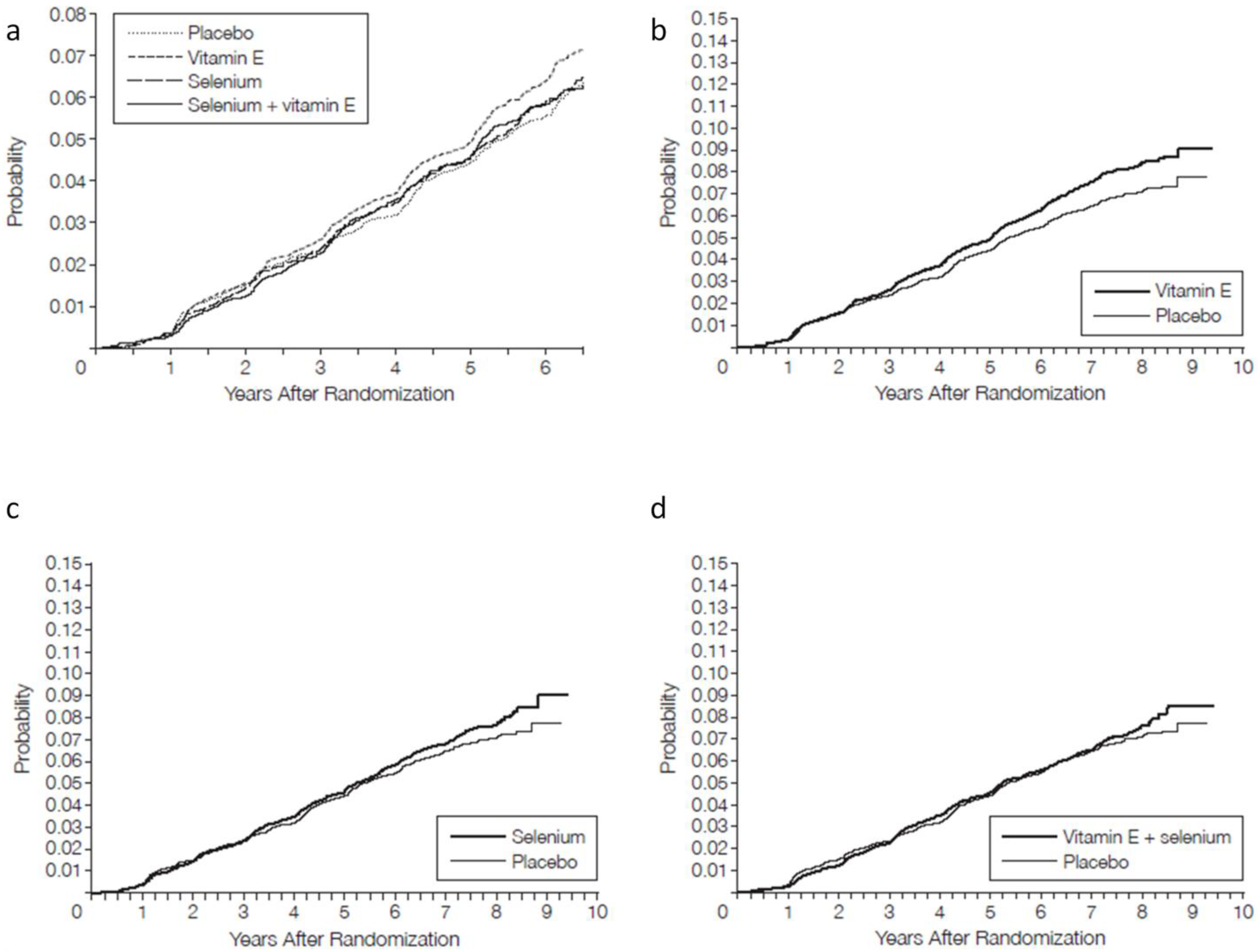
3.6. Secondary Endpoints and Adverse Outcomes
3.7. Adherence to Study Supplements
3.8. Follow-Up
| First Report, October 2008 | Second Report, July 2011 | |||||||
|---|---|---|---|---|---|---|---|---|
| Trial Arm | Placebo (n = 8696) | Vitamin E (n = 8737) | Selenium (n = 8752) | Selenium + Vitamin E (n = 8703) | Placebo (n = 8696) | Vitamin E (n = 8737) | Selenium (n = 8752) | Selenium + Vitamin E (n = 8702) |
| Any cancer, including prostate a | ||||||||
| No. events | 824 | 856 | 837 | 846 | 1108 | 1190 | 1132 | 1149 |
| HR (99% CI) | 1 (reference) | 1.03 (0.91–1.17) | 1.01 (0.89–1.15) | 1.02 (0.90–1.16) | 1 (reference) | 1.07 (0.96–1.19) | 1.02 (0.92–1.14) | 1.02 (0.92–1.12) |
| Lung cancer | ||||||||
| No. events | 67 | 67 | 75 | 78 | 92 | 104 | 94 | 104 |
| HR (99% CI) | 1 (reference) | 1.00 (0.64–1.55) | 1.12 (0.73–1.72) | 1.16 (0.76–1.78) | 1 (reference) | 1.11 (0.76–1.61) | 1.02 (0.70–1.50) | 1.11 (0.76–1.62) |
| Colorectal cancer | ||||||||
| No. events | 60 | 66 | 63 | 77 | 75 | 85 | 74 | 93 |
| HR (99% CI) | 1 (reference) | 1.09 (0.69–1.73) | 1.05 (0.66–1.67) | 1.28 (0.82–2.00) | 1 (reference) | 1.09 (0.72–1.64) | 0.96 (0.63–1.46) | 1.21 (0.81–1.81) |
| Other primary cancer | ||||||||
| No. events | 306 | 274 | 292 | 290 | 579 | 570 | 557 | 594 |
| HR (99% CI) | 1 (reference) | 0.89 (0.72–1.10) | 0.95 (0.77–1.17) | 0.94 (0.76–1.16) | 1 (reference) | 0.97 (0.83–1.14) | 0.96 (0.83–1.13) | 1.02 (0.92–1.14) |
| Diabetes | ||||||||
| No. events | 669 | 700 | 724 | 660 | 869 | 918 | 913 | 875 |
| HR (99% CI) | 1 (reference) | 1.04 (0.91–1.18) | 1.07 (0.94–1.22) | 0.97 (0.85–1.11) | 1 (reference) | 1.05 (0.93–1.17) | 1.04 (0.93–1.17) | 0.99 (0.89–1.12) |
| Any cardiovascular event | ||||||||
| No. events | 1050 | 1034 | 1080 | 1041 | n.r. b | n.r. | n.r. | n.r. |
| HR (99% CI) | 1 (reference) | 0.98 (0.88–1.09) | 1.02 (0.92–1.13) | 0.99 (0.89–1.10) | ||||
| Cardiovascular events, grade ≥4 | ||||||||
| No. events | n.r. | n.r. | n.r. | n.r. | 969 | 909 | 939 | 943 |
| HR (99% CI) | 1 (reference) | 0.93 (0.83–1.05) | 0.97 (0.86–1.09) | 0.97 (0.86–1.09) | ||||
| Deaths, all cause | ||||||||
| No. events | 382 | 358 | 378 | 359 | 564 | 571 | 551 | 542 |
| HR (99% CI) | 1 (reference) | 0.93 (0.77–1.13) | 0.99 (0.82–1.19) | 0.94 (0.77–1.13) | 1 (reference) | 1.01 (0.86–1.17) | 0.98 (0.84–1.14) | 0.96 (0.82–1.12) |
| Bioadherence Trial arm | Placebo (n = 285) | Vitamin E (n = 290) | Selenium (n = 277) | Selenium + Vitamin E (n = 257) | ||||
| Serum selenium, μg/L | ||||||||
| Baseline, mean | 137.6 | 135.9 | 135.0 | 136.4 | n.r. | n.r. | n.r. | n.r. |
| IQR | 124.7–151.8 | 122.4–148.4 | 123.4–145.9 | 122.9–150.0 | ||||
| 6-months visit, mean | 137.4 | 138.4 | 223.4 | 227.0 | ||||
| IQR | 123.3–152.0 | 124.1–154.0 | 198.6–251.8 | 199.4–251.2 | ||||
| 1st annual visit, mean | 138.1 | 137.7 | 232.4 | 228.5 | ||||
| IQR | 125.2–152.2 | 120.1–139.9 | 204.2–261.4 | 205.5–258.1 | ||||
| 2nd annual visit, mean | 132.0 | 129.8 | 228.0 | 220.7 | ||||
| IQR | 120.8–143.1 | 126.2–158.6 | 206.3–256.9 | 194.0–249.5 | ||||
| 4th annual visit, mean | 140.1 | 143.8 | 251.6 | 253.1 | ||||
| IQR | 124.3–150.8 | 126.2–158.6 | 218.7–275.0 | 210.5–283.0 | ||||
4. Discussion
4.1. Agent: Selenium Formulation and Dose
4.2. Cohort: Baseline Selenium Status, Age, Genetics
4.2.1. Baseline Selenium Status
4.2.2. Genetics
4.2.3. Age
4.3. Design
4.4. Does Selenium Really Prevent Prostate Cancer?
5. Future Directions
| Agent | Optimal dose range for selenium has not been established. |
| l-selenomethionine may not be most active formulation of selenium. | |
| A better understanding of selenium biology is necessary. | |
| Cohort | Baseline selenium status of participants was too high for the men to receive additional benefit from selenium supplementation. |
| Genotype of various selenoproteins of the cohort should be taken into consideration. | |
| Intervening in older men may miss a critical window for preventive activities of selenium. | |
| Design | Possible lag to effect may have occurred. |
| Subgroup analyses were not addressed. |
Acknowledgments
Conflict of Interest
References
- SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations); Howlader, N.; Noone, A.M.; Krapcho, M.; Neyman, N.; Aminou, R.; Altekruse, S.F.; Kosary, C.L.; Ruhl, J.; Tatalovich, Z.; Cho, H.; et al. (Eds.) National Cancer Institute: Bethesda, MD, USA, 2012; Available online: http://seer.cancer.gov/csr/1975_2009_pops09/ (accessed on 13 December 2012).
- American Cancer Society, Cancer Facts and Figures 2012; American Cancer Society: Atlanta, GA, USA, 2012.
- Potosky, A.L.; Legler, J.; Albertsen, P.C.; Stanford, J.L.; Gilliland, F.D.; Hamilton, A.S.; Eley, J.W.; Stephenson, R.A.; Harlan, L.C. Health outcomes after prostatectomy or radiotherapy for prostate cancer: Results from the Prostate Cancer Outcomes Study. J. Natl. Cancer Inst. 2000, 92, 1582–1592. [Google Scholar] [CrossRef]
- Stanford, J.L.; Feng, Z.; Hamilton, A.S.; Gilliland, F.D.; Stephenson, R.A.; Eley, J.W.; Albertsen, P.C.; Harlan, L.C.; Potosky, A.L. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: The Prostate cancer outcomes study. JAMA 2000, 283, 354–360. [Google Scholar]
- Wilson, K.M.; Giovannucci, E.L.; Mucci, L.A. Lifestyle and dietary factors in the prevention of lethal prostate cancer. Asian J. Androl. 2012, 14, 365–374. [Google Scholar] [CrossRef]
- Moyer, V.A. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2012, 157, 120–134. [Google Scholar]
- Parnes, H.L. Prostate cancer prevention: Do the 5-ARIs make the grade? Am. J. Bioeth. 2011, 11, 30–31. [Google Scholar]
- Thompson, I.M.; Goodman, P.J.; Tangen, C.M.; Lucia, M.S.; Miller, G.J.; Ford, L.G.; Lieber, M.M.; Cespedes, R.D.; Atkins, J.N.; Lippman, S.M.; et al. The influence of finasteride on the development of prostate cancer. N. Engl. J. Med. 2003, 349, 215–224. [Google Scholar] [CrossRef]
- Cohen, Y.C.; Liu, K.S.; Heyden, N.L.; Carides, A.D.; Anderson, K.M.; Daifotis, A.G.; Gann, P.H. Detection bias due to the effect of finasteride on prostate volume: A modeling approach for analysis of the Prostate Cancer Prevention Trial. J. Natl. Cancer Inst. 2007, 99, 1366–1374. [Google Scholar] [CrossRef]
- Andriole, G.L.; Bostwick, D.G.; Brawley, O.W.; Gomella, L.G.; Marberger, M.; Montorsi, F.; Pettaway, C.A.; Tammela, T.L.; Teloken, C.; Tindall, D.J.; et al. Effect of dutasteride on the risk of prostate cancer. N. Engl. J. Med. 2010, 362, 1192–1202. [Google Scholar] [CrossRef]
- Theoret, M.R.; Ning, Y.-M.; Zhang, J.J.; Justice, R.; Keegan, P.; Pazdur, R. The risks and benefits of 5α-reductase inhibitors for prostate-cancer prevention. N. Engl. J. Med. 2011, 365, 97–99. [Google Scholar] [CrossRef]
- Heinonen, O.P.; Albanes, D.; Virtamo, J.; Taylor, P.R.; Huttunen, J.K.; Hartman, A.M.; Haapakoski, J.; Malila, N.; Rautalahti, M.; Ripatti, S.; et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: Incidence and mortality in a controlled trial. J. Natl. Cancer Inst. 1998, 90, 440–446. [Google Scholar] [CrossRef]
- Clark, L.C.; Combs, G.F.; Turnbull, B.W.; Slate, E.H.; Chalker, D.K.; Chow, J.; Davis, L.S.; Glover, R.A.; Graham, G.F.; Gross, E.G.; et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 1996, 276, 1957–1963. [Google Scholar] [CrossRef]
- Terry, N.; Zayed, A.M.; De Souza, M.P.; Tarun, A.S. Selenium in Higher Plants. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef]
- Li, H.F.; McGrath, S.P.; Zhao, F.J. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol. 2008, 178, 92–102. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Beck, M.A.; Levander, O.A.; Handy, J. Selenium deficiency and viral infection. J. Nutr. 2003, 133, 1463–1467. [Google Scholar]
- Dumont, E.; Vanhaecke, F.; Cornelis, R. Selenium speciation from food source to metabolites: A critical review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar] [CrossRef]
- Thomson, C.D.; Chisholm, A.; McLachlan, S.K.; Campbell, J.M. Brazil nuts: An effective way to improve selenium status. Am. J. Clin. Nutr. 2008, 87, 379–384. [Google Scholar]
- Schrauzer, G.N. Nutritional selenium supplements: Product types, quality, and safety. J. Am. Coll. Nutr. 2001, 20, 1–4. [Google Scholar]
- Medina, D.; Thompson, H.; Ganther, H.; Ip, C. Se-methylselenocysteine: A new compound for chemoprevention of breast cancer. Nutr. Cancer 2001, 40, 12–17. [Google Scholar] [CrossRef]
- Combs, G.F., Jr.; Gray, W.P. Chemopreventive agents: Selenium. Pharmacol. Ther. 1998, 79, 179–192. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Gladyshev, V.N. The outcome of selenium and vitamin E cancer prevention trial (SELECT) reveals the need for better understanding of selenium biology. Mol. Interv. 2009, 9, 18–21. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Gladyshev, V.N. How selenium has altered our understanding of the genetic code. Mol. Cell. Biol. 2002, 22, 3565–3576. [Google Scholar] [CrossRef]
- Davis, C.D.; Tsuji, P.A.; Milner, J.A. Selenoproteins and cancer prevention. Annu. Rev. Nutr. 2012, 32, 73–95. [Google Scholar] [CrossRef]
- Lee, K.H.; Jeong, D. Bimodal actions of selenium essential for antioxidant and toxic pro-oxidant activities: The selenium paradox (Review). Mol. Med. Rep. 2012, 5, 299–304. [Google Scholar]
- Letavayova, L.; Vlckova, V.; Brozmanova, J. Selenium: From cancer prevention to DNA damage. Toxicology 2006, 227, 1–14. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ogra, Y.; Ishiwata, K.; Takayama, H.; Aimi, N.; Suzuki, K.T. Selenosugars are key and urinary metabolites for selenium excretion within the required to low-toxic range. Proc. Natl. Acad. Sci. USA 2002, 99, 15932–15936. [Google Scholar]
- Kim, Y.S.; Milner, J. Molecular targets for selenium in cancer prevention. Nutr. Cancer 2001, 40, 50–54. [Google Scholar] [CrossRef]
- El-Bayoumy, K.; Sinha, R. Molecular chemoprevention by selenium: A genomic approach. Mutat. Res. 2005, 591, 224–236. [Google Scholar] [CrossRef]
- El-Bayoumy, K.; Sinha, R. Mechanisms of mammary cancer chemoprevention by organoselenium compounds. Mutat. Res. 2004, 551, 181–197. [Google Scholar] [CrossRef]
- Panel on Dietary Antioxidants and Related Compounds; Subcommittee on Upper Reference Levels of Nutrients; Subcommittee on Interpretation and Use of Dietary Reference Intakes; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; Food and Nutrition Board; Institute of Medicine. In Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academy Press: Washington, DC, USA, 2000; p. 506.
- Levander, O.A.; Morris, V.C. Dietary selenium levels needed to maintain balance in North American adults consuming self-selected diets. Am. J. Clin. Nutr. 1984, 39, 809–815. [Google Scholar]
- Whanger, P.D. Selenium and its relationship to cancer: An update. Br. J. Nutr. 2004, 91, 11–28. [Google Scholar] [CrossRef]
- Yang, G.; Zhou, R.; Yin, S.; Gu, L.; Yan, B.; Liu, Y.; Li, X. Studies of safe maximal daily dietary selenium intake in a seleniferous area in China. I. Selenium intake and tissue selenium levels of the inhabitants. J. Trace Elem. Electrolytes Health Dis. 1989, 3, 77–87. [Google Scholar]
- Fulgoni, V.L., III; Keast, D.R.; Bailey, R.L.; Dwyer, J. Foods, fortificants, and supplements: Where do Americans get their nutrients? J. Nutr. 2011, 141, 1847–1854. [Google Scholar] [CrossRef]
- Bailey, R.L.; Gahche, J.J.; Lentino, C.V.; Dwyer, J.T.; Engel, J.S.; Thomas, P.R.; Betz, J.M.; Sempos, C.T.; Picciano, M.F. Dietary supplement use in the United States, 2003–2006. J. Nutr. 2011, 141, 261–266. [Google Scholar] [CrossRef]
- Bailey, R.L.; Fulgoni, V.L., III; Keast, D.R.; Dwyer, J.T. Dietary supplement use is associated with higher intakes of minerals from food sources. Am. J. Clin. Nutr. 2011, 94, 1376–1381. [Google Scholar] [CrossRef]
- Webber, M.M.; Perez-Ripoll, E.A.; James, G.T. Inhibitory effects of selenium on the growth of DU-145 human prostate carcinoma cells in vitro. Biochem. Biophys. Res. Commun. 1985, 130, 603–609. [Google Scholar] [CrossRef]
- Etminan, M.; FitzGerald, J.M.; Gleave, M.; Chambers, K. Intake of selenium in the prevention of prostate cancer: A systematic review and meta-analysis. Cancer Causes Control. 2005, 16, 1125–1131. [Google Scholar] [CrossRef]
- Nelson, M.A.; Reid, M.; Duffield-Lillico, A.J.; Marshall, J.R. Prostate cancer and selenium. Urol. Clin. North. Am. 2002, 29, 67–70. [Google Scholar] [CrossRef]
- McCormick, D.L.; Rao, K.V.; Johnson, W.D.; Bosland, M.C.; Lubet, R.A.; Steele, V.E. Null activity of selenium and vitamin E as cancer chemopreventive agents in the rat prostate. Cancer Prev. Res. (Phila.) 2010, 3, 381–392. [Google Scholar] [CrossRef]
- Ozten, N.; Horton, L.; Lasano, S.; Bosland, M.C. Selenomethionine and α-tocopherol do not inhibit prostate carcinogenesis in the testosterone plus estradiol-treated NBL rat model. Cancer Prev. Res. (Phila.) 2010, 3, 371–380. [Google Scholar] [CrossRef]
- Venkateswaran, V.; Klotz, L.H.; Ramani, M.; Sugar, L.M.; Jacob, L.E.; Nam, R.K.; Fleshner, N.E. A combination of micronutrients is beneficial in reducing the incidence of prostate cancer and increasing survival in the Lady transgenic model. Cancer Prev. Res. (Phila.) 2009, 2, 473–483. [Google Scholar] [CrossRef]
- Venkateswaran, V.; Fleshner, N.E.; Sugar, L.M.; Klotz, L.H. Antioxidants block prostate cancer in lady transgenic mice. Cancer Res. 2004, 64, 5891–5896. [Google Scholar] [CrossRef]
- Wang, L.; Bonorden, M.J.; Li, G.X.; Lee, H.J.; Hu, H.; Zhang, Y.; Liao, J.D.; Cleary, M.P.; Lu, J. Methyl-selenium compounds inhibit prostate carcinogenesis in the transgenic adenocarcinoma of mouse prostate model with survival benefit. Cancer Prev. Res. (Phila) 2009, 2, 484–495. [Google Scholar] [CrossRef]
- Bespalov, V.G.; Panchenko, A.V.; Murazov Ia, G.; Chepik, O.F. Influence of sodium selenite on carcinogenesis of the prostate and other organs induced by methylnitrosourea and testosterone in rats. Vopr. Onkol. 2011, 57, 486–492. [Google Scholar]
- Shamberger, R.J.; Frost, D.V. Possible protective effect of selenium against human cancer. Can. Med. Assoc. J. 1969, 100, 682–682. [Google Scholar]
- Shamberger, R.J.; Willis, C.E. Selenium distribution and human cancer mortality. CRC Crit. Rev. Clin. Lab. Sci. 1971, 2, 211–221. [Google Scholar] [CrossRef]
- Schrauzer, G.N. Anticarcinogenic effects of selenium. Cell. Mol. Life Sci. 2000, 57, 1864–1873. [Google Scholar] [CrossRef]
- Brooks, J.D.; Metter, E.J.; Chan, D.W.; Sokoll, L.J.; Landis, P.; Nelson, W.G.; Muller, D.; Andres, R.; Carter, H.B. Plasma selenium level before diagnosis and the risk of prostate cancer development. J. Urol. 2001, 166, 2034–2038. [Google Scholar] [CrossRef]
- Yoshizawa, K.; Willett, W.C.; Morris, S.J.; Stampfer, M.J.; Spiegelman, D.; Rimm, E.B.; Giovannucci, E. Study of prediagnostic selenium level in toenails and the risk of advanced prostate cancer. J. Natl. Cancer Inst. 1998, 90, 1219–1224. [Google Scholar] [CrossRef]
- World Cancer Research Fund, American Institute for Cancer Research; Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; AICR: Washington DC, USA, 2007. Available online: http://www.dietandcancerreport.org/expert_report/index.php (accessed on 26 November 2012).
- Yu, S.Y.; Zhu, Y.J.; Li, W.G. Protective role of selenium against hepatitis B virus and primary liver cancer in Qidong. Biol. Trace Elem. Res. 1997, 56, 117–124. [Google Scholar] [CrossRef]
- Blot, W.J.; Li, J.Y.; Taylor, P.R.; Guo, W.; Dawsey, S.; Wang, G.Q.; Yang, C.S.; Zheng, S.F.; Gail, M.; Li, G.Y. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J. Natl. Cancer Inst. 1993, 85, 1483–1492. [Google Scholar] [CrossRef]
- Li, J.Y.; Taylor, P.R.; Li, B.; Dawsey, S.; Wang, G.Q.; Ershow, A.G.; Guo, W.; Liu, S.F.; Yang, C.S.; Shen, Q. Nutrition intervention trials in Linxian, China: multiple vitamin/mineral supplementation, cancer incidence, and disease-specific mortality among adults with esophageal dysplasia. J. Natl. Cancer Inst. 1993, 85, 1492–1498. [Google Scholar] [CrossRef]
- Duffield-Lillico, A.J.; Dalkin, B.L.; Reid, M.E.; Turnbull, B.W.; Slate, E.H.; Jacobs, E.T.; Marshall, J.R.; Clark, L.C. Nutritional Prevention of Cancer Study Group. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: An analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003, 91, 608–612. [Google Scholar] [CrossRef]
- Sabichi, A.L.; Lee, J.J.; Taylor, R.J.; Thompson, I.M.; Miles, B.J.; Tangen, C.M.; Minasian, L.M.; Pisters, L.L.; Caton, J.R.; Basler, J.W.; et al. Selenium accumulation in prostate tissue during a randomized, controlled short-term trial of l-selenomethionine: A Southwest Oncology Group Study. Clin. Cancer Res. 2006, 12, 2178–2184. [Google Scholar] [CrossRef]
- The ATBC Cancer Prevention Study Group. The α-tocopherol, beta-carotene lung cancer prevention study: Design, methods, participant characteristics, and compliance. Ann. Epidemiol. 1994, 4, 1–10.
- Lippman, S.M.; Goodman, P.J.; Klein, E.A.; Parnes, H.L.; Thompson, I.M.; Kristal, A.R.; Santella, R.M.; Probstfield, J.L.; Moinpour, C.M.; Albanes, D.; et al. Designing the selenium and vitamin E cancer prevention trial (SELECT). J. Natl. Cancer Inst. 2005, 97, 94–102. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M.; Lippman, S.M.; Goodman, P.J.; Albanes, D.; Taylor, P.R.; Coltman, C. SELECT: The selenium and vitamin E cancer prevention trial: Rationale and design. Prostate Cancer Prostatic Dis. 2000, 3, 145–151. [Google Scholar]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The selenium and vitamin E cancer prevention trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The selenium and vitamin E cancer prevention trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef]
- Stranges, S.; Marshall, J.R.; Natarajan, R.; Donahue, R.P.; Trevisan, M.; Combs, G.F.; Cappuccio, F.P.; Ceriello, A.; Reid, M.E. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: A randomized trial. Ann. Intern. Med. 2007, 147, 217–223. [Google Scholar]
- Bleys, J.; Navas-Acien, A.; Guallar, E. Selenium and diabetes: More bad news for supplements. Ann. Intern. Med. 2007, 147, 271–272. [Google Scholar]
- Bleys, J.; Navas-Acien, A.; Guallar, E. Serum selenium and diabetes in U.S. adults. Diabetes Care 2007, 30, 829–834. [Google Scholar] [CrossRef]
- Goodman, P.J.; Hartline, J.A.; Tangen, C.M.; Crowley, J.J.; Minasian, L.M.; Klein, E.A.; Cook, E.D.; Darke, A.K.; Arnold, K.B.; Anderson, K.; et al. Moving a randomized clinical trial into an observational cohort. Clin. Trials 2012, 10, 131–142. [Google Scholar]
- Lu, J.; Jiang, C.; Kaeck, M.; Ganther, H.; Vadhanavikit, S.; Ip, C.; Thompson, H. Dissociation of the genotoxic and growth inhibitory effects of selenium. Biochem. Pharmacol. 1995, 50, 213–219. [Google Scholar]
- Ip, C.; Birringer, M.; Block, E.; Kotrebai, M.; Tyson, J.F.; Uden, P.C.; Lisk, D.J. Chemical speciation influences comparative activity of selenium-enriched garlic and yeast in mammary cancer prevention. J. Agric. Food Chem. 2000, 48, 2062–2070. [Google Scholar] [CrossRef]
- Ip, C.; Thompson, H.J.; Zhu, Z.; Ganther, H.E. In vitro and in vivo studies of methylseleninic acid: Evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention. Cancer Res. 2000, 60, 2882–2886. [Google Scholar]
- Waters, D.J.; Shen, S.; Kengeri, S.S.; Chiang, E.C.; Morris, J.S.; Bostwick, D.G. Prostatic response to supranutritional selenium supplementation: Comparison of the target tissue potency of selenomethionine vs. selenium-yeast on markers of prostatic homeostasis. Nutrients 2012, 4, 1650–1663. [Google Scholar] [CrossRef]
- Influence of Selenium on Biomarkers of Prostate Cancer Risk. Verified July 2011 by Penn State University, ClinicalTrials.gov Identifier: NCT01112449. Available online: http://clinicaltrials.gov/ct2/show/NCT01112449 (accessed on 13 December 2012).
- Dunn, B.K.; Richmond, E.S.; Minasian, L.M.; Ryan, A.M.; Ford, L.G. A nutrient approach to prostate cancer prevention: The selenium and vitamin E cancer prevention trial (SELECT). Nutr. Cancer 2010, 62, 896–918. [Google Scholar] [CrossRef]
- Waters, D.J.; Shen, S.; Glickman, L.T.; Cooley, D.M.; Bostwick, D.G.; Qian, J.; Combs, G.F.; Morris, J.S. Prostate cancer risk and DNA damage: Translational significance of selenium supplementation in a canine model. Carcinogenesis 2005, 26, 1256–1262. [Google Scholar]
- Chiang, E.C.; Shen, S.; Kengeri, S.S.; Xu, H.; Combs, G.F.; Morris, J.S.; Bostwick, D.G.; Waters, D.J. Defining the optimal selenium dose for prostate cancer risk reduction: insights from the u-shaped relationship between selenium status, DNA damage, and apoptosis. Dose Response 2009, 8, 285–300. [Google Scholar]
- Li, H.; Kantoff, P.W.; Giovannucci, E.; Leitzmann, M.F.; Gaziano, J.M.; Stampfer, M.J.; Ma, J. Manganese superoxide dismutase polymorphism, prediagnostic antioxidant status, and risk of clinical significant prostate cancer. Cancer Res. 2005, 65, 2498–2504. [Google Scholar]
- Penney, K.L.; Schumacher, F.R.; Li, H.; Kraft, P.; Morris, J.S.; Kurth, T.; Mucci, L.A.; Hunter, D.J.; Kantoff, P.W.; Stampfer, M.J.; et al. A large prospective study of SEP15 genetic variation, interaction with plasma selenium levels, and prostate cancer risk and survival. Cancer Prev. Res. (Phila.) 2010, 3, 604–610. [Google Scholar] [CrossRef]
- Combs, G.F., Jr.; Jackson, M.I.; Watts, J.C.; Johnson, L.K.; Zeng, H.; Idso, J.; Schomburg, L.; Hoeg, A.; Hoefig, C.S.; Chiang, E.C.; et al. Differential responses to selenomethionine supplementation by sex and genotype in healthy adults. Br. J. Nutr. 2012, 107, 1514–1525. [Google Scholar] [CrossRef]
- Boyapati, S.M.; Shu, X.O.; Ruan, Z.X.; Dai, Q.; Cai, Q.; Gao, Y.T.; Zheng, W. Soyfood intake and breast cancer survival: A followup of the Shanghai Breast Cancer Study. Breast Cancer Res. Treat. 2005, 92, 11–17. [Google Scholar]
- Freemantle, N. Interpreting the results of secondary end points and subgroup analyses in clinical trials: Should we lock the crazy aunt in the attic? BMJ 2001, 322, 989–991. [Google Scholar] [CrossRef]
- Powles, T.J.; Ashley, S.; Tidy, A.; Smith, I.E.; Dowsett, M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J. Natl. Cancer Inst. 2007, 99, 283–290. [Google Scholar]
- Bera, S.; Rosa, V.D.; Rachidi, W.; Diamond, A.M. Does a role for selenium in DNA damage repair explain apparent controversies in its use in chemoprevention? Mutagenesis 2013, 28, 127–134. [Google Scholar] [CrossRef]
- Ledesma, M.C.; Jung-Hynes, B.; Schmit, T.L.; Kumar, R.; Mukhtar, H.; Ahmad, N. Selenium and vitamin E for prostate cancer: Post-SELECT (Selenium and Vitamin E Cancer Prevention Trial) status. Mol. Med. 2011, 17, 134–143. [Google Scholar]
- Nicastro, H.L.; Trujillo, E.B.; Milner, J.A. Nutrigenomics and cancer prevention. Curr. Nutr. Rep. 2012, 1, 37–43. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nicastro, H.L.; Dunn, B.K. Selenium and Prostate Cancer Prevention: Insights from the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Nutrients 2013, 5, 1122-1148. https://doi.org/10.3390/nu5041122
Nicastro HL, Dunn BK. Selenium and Prostate Cancer Prevention: Insights from the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Nutrients. 2013; 5(4):1122-1148. https://doi.org/10.3390/nu5041122
Chicago/Turabian StyleNicastro, Holly L., and Barbara K. Dunn. 2013. "Selenium and Prostate Cancer Prevention: Insights from the Selenium and Vitamin E Cancer Prevention Trial (SELECT)" Nutrients 5, no. 4: 1122-1148. https://doi.org/10.3390/nu5041122
APA StyleNicastro, H. L., & Dunn, B. K. (2013). Selenium and Prostate Cancer Prevention: Insights from the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Nutrients, 5(4), 1122-1148. https://doi.org/10.3390/nu5041122




