Extracts, Anthocyanins and Procyanidins from Aronia melanocarpa as Radical Scavengers and Enzyme Inhibitors
Abstract
:1. Introduction
2. Experimental Section
2.1. Plant Material
2.2. Chemicals
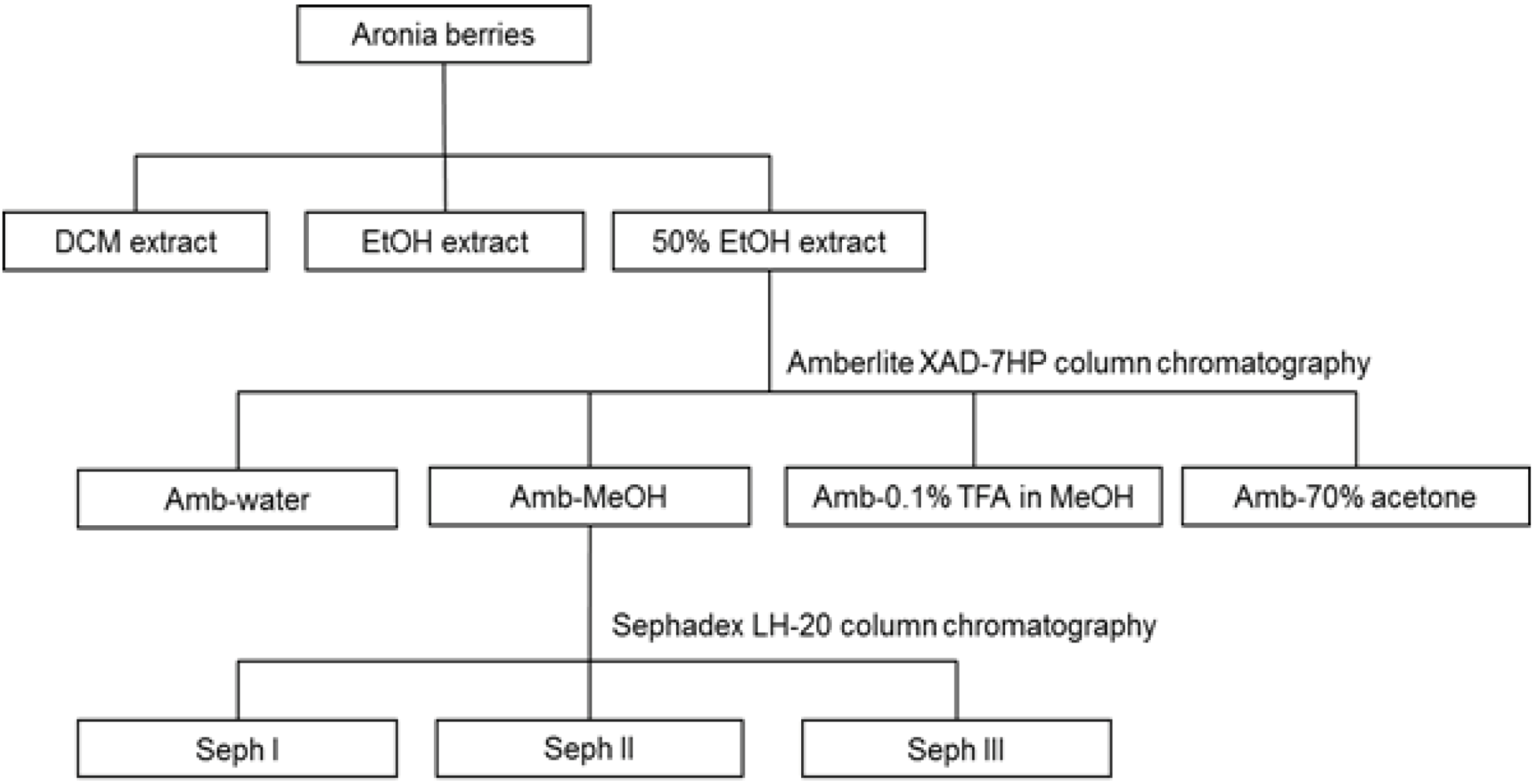
2.3. Extraction and Fractionation
2.4. Isolation of Anthocyanins
2.5. Isolation of Procyanidins
2.6. Analysis
2.6.1. TLC
2.6.2. HPLC
2.6.3. UV Measurements
2.6.4. Thiolysis
2.6.5. Mass Spectrometry
2.6.6. NMR
2.7. DPPH Radical Scavenging
2.8. Inhibition of 15-Lipoxygenase (15-LO)
2.9. Inhibition of Xanthine Oxidase (XO)
2.10. α-Glucosidase Inhibitory Activity
2.11. Statistics
3. Results and Discussion
3.1. Extraction and Chemical Characterization
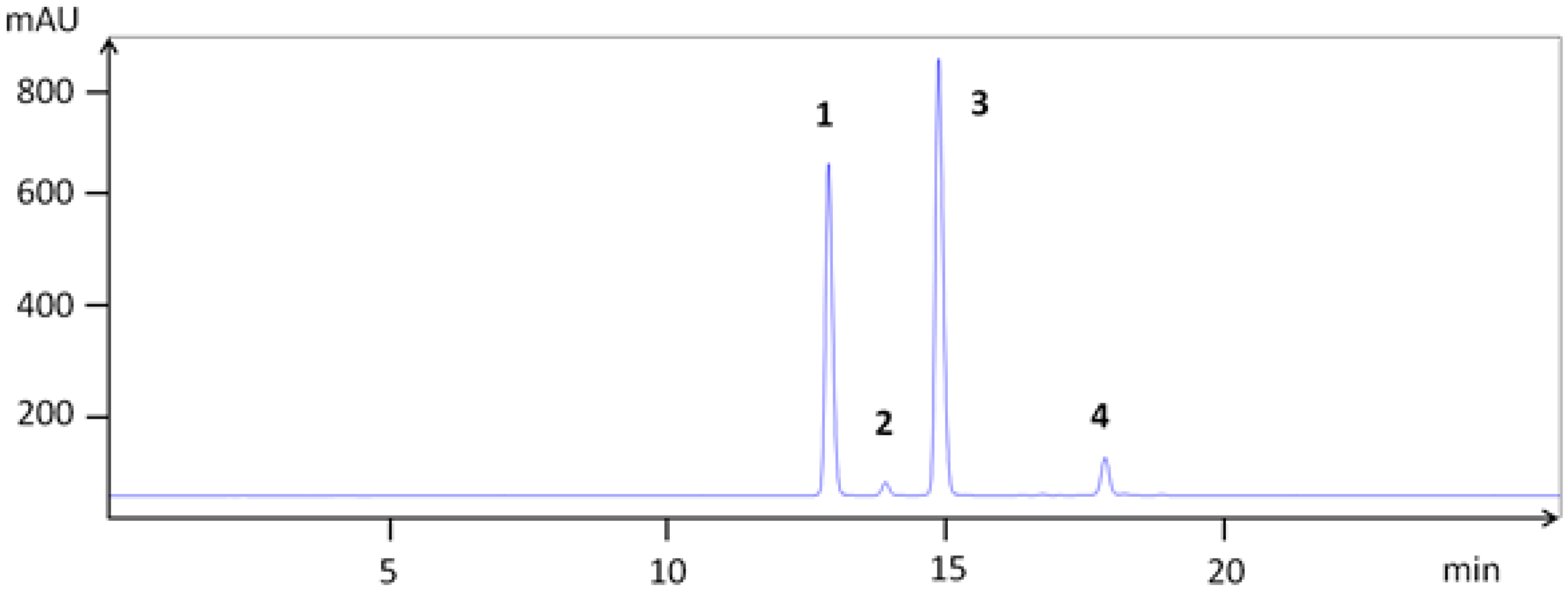
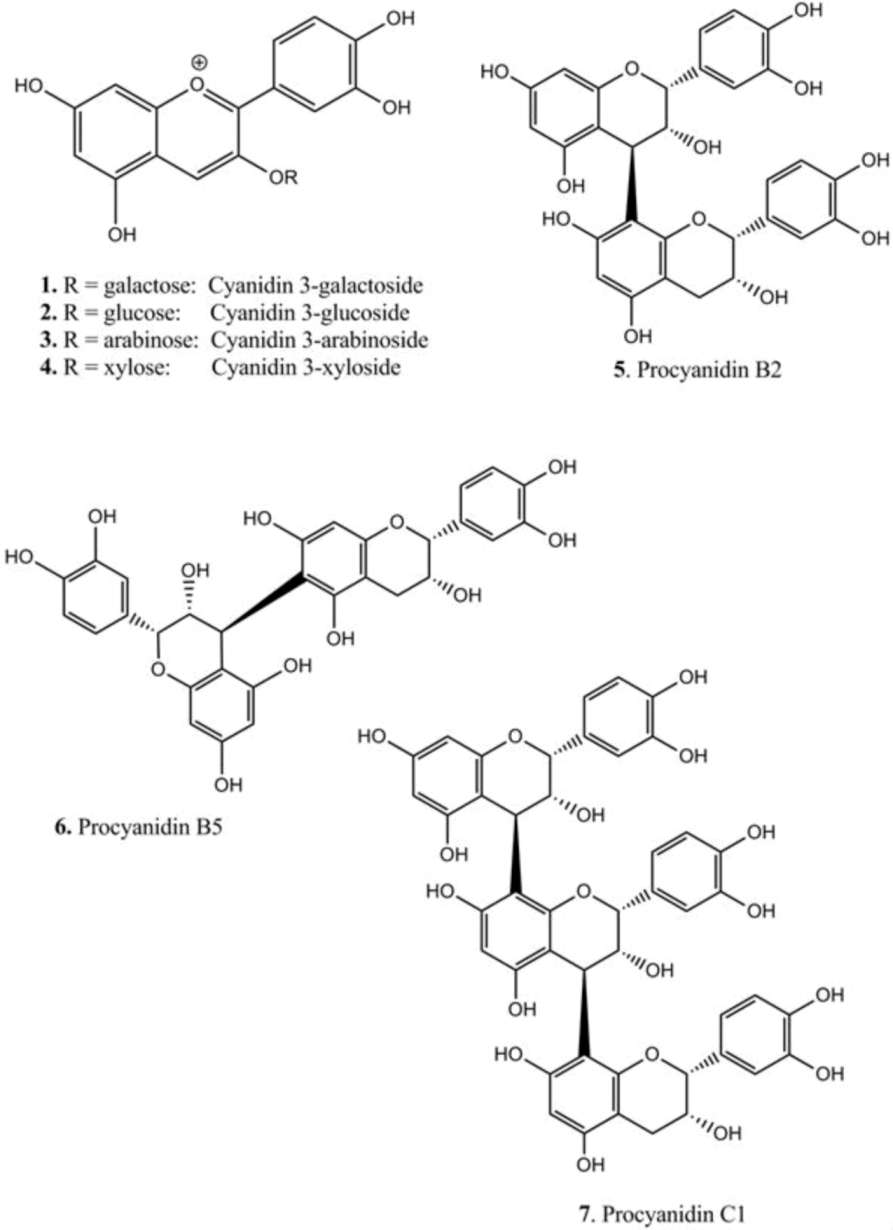
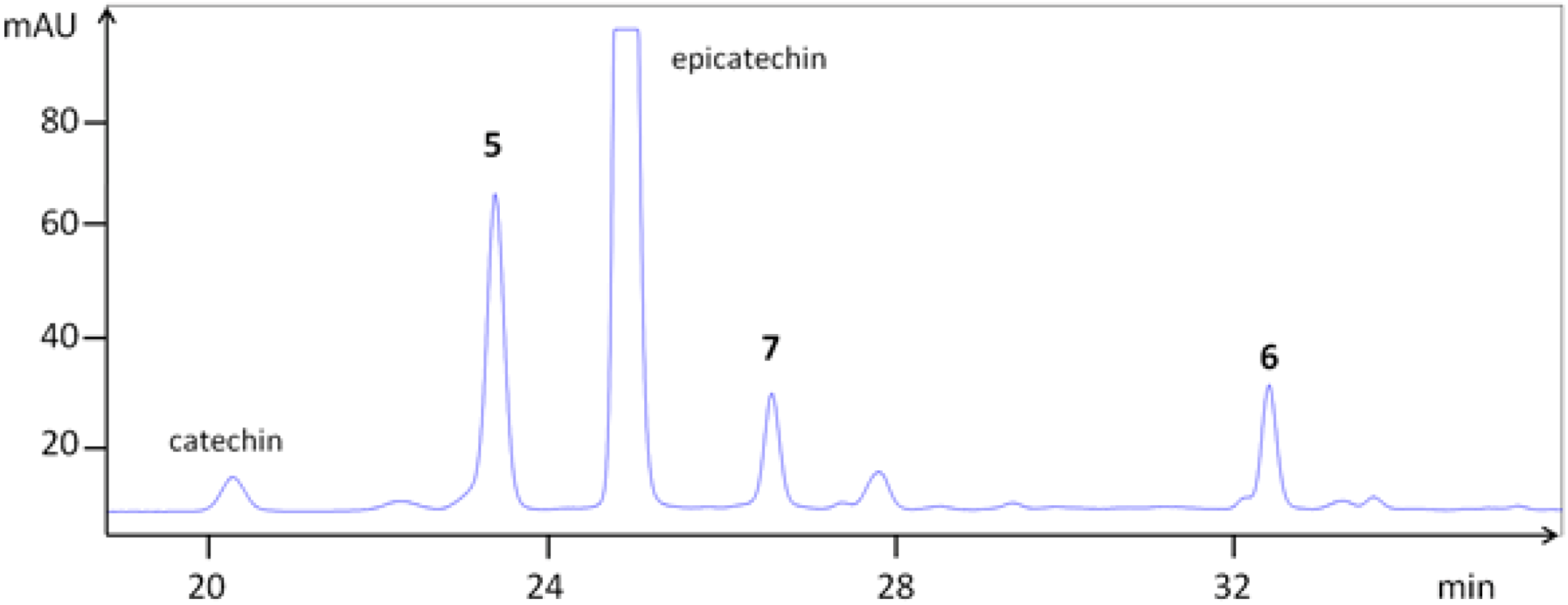
| Compounds | TLC | HPLC | LCMS | [M + H]+ | Fragments (am→u) | |
|---|---|---|---|---|---|---|
| rRF | tR (min) | tR (min) | ||||
| 1 | cyanidin 3-galactoside | 12.9 | 22.0 | 449.104 | 287.044 | |
| 2 | cyanidin 3-glucoside | 13.9 | 22.2 | 449.102 | 287.044 | |
| 3 | cyanidin 3-arabinoside | 15.0 | 22.7 | 419.084 | 287.039 | |
| 4 | cyanidin 3-xyloside | 17.8 | 24.3 | 419.085 | 287.040 | |
| epicatechin | 79 | 25.1 | 28.5 | 291.078 | ||
| 5 | epi-(4β→8)-epi (B2) | 58 | 23.4 | 29.9 | 579.157 | 291.078 |
| 6 | epi-(4β→6)-epi (B5) | 67 | 32.5 | 35.6 | 579.168 | 291.084 |
| 7 | epi-(4β→8)-epi-(4β→8)-epi (C1) | 51 | 26.7 | 32.7 | 867.216 | 579.143, 291.080 |
3.2. Biochemical Activities
| Material | DPPH | α-Glucosidase | 15-Lipoxygenase |
|---|---|---|---|
| IC50a (µg/mL) #/ | |||
| IC50 a (µg/mL) | IC50a (µg/mL) | ||
| % scavenging at 10.4 µg/mL ^ | |||
| DCM | >167 # | Inactive | >83 |
| EtOH | >167 # | Inactive | >83 |
| 50% EtOH | 25.0 ± 5.0 # | 3.5 ± 0.1 | >83 |
| Amb-MeOH | 3.8 ± 0.2 # | 0.55 ± 0.01 | 56.7 ± 0.7 |
| Seph I | 3.1 ± 0.5 # | nt b | 30.3 ± 0.7 |
| Seph II | 12.0 ± 2.8 # | nt b | 91.0 ± 4.8 |
| Seph III | 4.0 ± 0.5 # | nt b | 33.0 ± 2.0 |
| Compound 1 | 39.0 ± 2.9 ^ | 1.54 ± 0.1 | 71.5 ± 1.8 |
| Compound 2 | 37.0 ± 0.9 ^ | 0.87 ± 0.2 | 73.3 ± 2.1 |
| Compound 3 | 40.0 ± 0.4 ^ | 0.37 ± 0.08 | 58.7 ± 2.5 |
| Compound 4 | 25.0 ± 5.0 ^ | 5.5 ± 1.6 | >83 |
| Compound 5 | 4.7 ± 0.3 # | 4.7 ± 0.2 | 65.1 ± 2.6 |
| Compound 6 | 5.2 ± 0.1 # | 5.5 ± 0.1 | 72.3 ± 5.7 |
| Compound 7 | 3.2 ± 0.1 # | 3.8 ± 0.2 | 57.6 ± 2.0 |
| Quercetin (control) | 3.0 ± 0.2 # | nt b | 26.0 ± 2.0 |
| Acarbose (control) | nt b | 130.0 ± 20.0 | nt b |
| Material | % inhibition at a concentration of 42 µg/mL |
|---|---|
| DCM | Inactive |
| EtOH | Inactive |
| 50% EtOH | Inactive |
| Amb-MeOH | 26.3 ± 3.4 |
| Seph I | 32.2 ± 7.8 |
| Seph II | 19.9 ± 7.6 |
| Seph III | 46.5 ± 5.9 |
| Compound 5 | 12.7 ± 2.8 |
| Compound 6 | 6.6 ± 0.7 |
| Compound 7 | 15.5 ± 1.5 |
| % inhibition at a concentration of 17 µg/mL | |
| Compound 1 | 11.9 ± 4.4 |
| Compound 2 | 20.9 ± 3.4 |
| Compound 3 | 39.1 ± 2.1 |
| Compound 4 | 11.4 ± 2.8 |
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. J. Med. Food 2010, 13, 255–269. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdylo, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef]
- Roobha, J.J.; Saravanakumar, M.; Aravinthan, K.M.; Devi, S.P. Antioxidant analysis of anthocyanin extracted from Musa acuminata bract. J. Pharm. Res. 2011, 4, 1488–1492. [Google Scholar]
- Yao, Y.; Sang, W.; Zhou, M.; Ren, G. Antioxidant and α-glucosidase inhibitory activity of colored grains in China. J. Agric. Food Chem. 2010, 58, 770–774. [Google Scholar] [CrossRef]
- Mansouri, E.; Panahi, M.; Ghaffari, M.A.; Ghorbani, A. Effects of grape seed proanthocyanidin extract on oxidative stress and antioxidant enzymes in kidney of rats with diabetic nephropathy. Rom. J. Biophys. 2011, 21, 243–252. [Google Scholar]
- Kumar, B.; Gupta, S.K.; Nag, T.C.; Srivastava, S.; Saxena, R. Green tea prevents hyperglycemia-induced retinal oxidative stress and inflammation in streptozotocin-induced diabetic rats. Ophthalmic Res. 2012, 47, 103–108. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Leso, V.; Trovato-Salinaro, A.; Ventimiglia, B.; Cavallaro, M.; Scuto, M.; Rizza, S.; Zanoli, L.; Neri, S.; Castellino, P. Oxidative stress, glutathione status, sirtuin and cellular stress response in type 2 diabetes. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 729–736. [Google Scholar] [CrossRef]
- Naudi, A.; Jove, M.; Ayala, V.; Cassanye, A.; Serrano, J.; Gonzalo, H.; Boada, J.; Prat, J.; Portero-Otin, M.; Pamplona, R. Cellular dysfunction in diabetes as maladaptive response to mitochondrial oxidative stress. Exp. Diabetes Res. 2012. [Google Scholar] [CrossRef]
- Li, F.; Tang, H.; Xiao, F.; Gong, J.; Peng, Y.; Meng, X. Protective effect of Salidroside from Rhodiolae radix on diabetes-induced oxidative stress in mice. Molecules 2011, 16, 9912–9924. [Google Scholar]
- Dobrian, A.D.; Lieb, D.C.; Cole, B.K.; Fishwick, D.A.T.; Chakrabarti, S.K.; Nadler, J.L. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog. Lipid Res. 2011, 50, 115–131. [Google Scholar]
- Pacher, P.; Nivorozhkin, A.; Szabó, C. Therapeutic effects of xanthine oxidase inhibitors: Renaissance half a century after the discovery of allopurinol. Pharmacol. Rev. 2006, 58, 87–114. [Google Scholar]
- Simeonov, S.B.; Botushanov, N.P.; Karahanian, E.B.; Pavlova, M.B.; Husianitis, H.K.; Troev, D.M. Effects of Aronia melanocarpa juice as part of the dietary regimen in patients with diabetes mellitus. Folia Med. (Plovdiv) 2002, 44, 20–23. [Google Scholar]
- Valcheva-Kuzmanova, S.; Kuzmanov, K.; Tancheva, S.; Belcheva, A. Hypoglycemic and hypolipidemic effects of Aronia melanocarpa fruit juice in streptozotocin-induced diabetic rats. Methods Find. Exp. Clin. Pharmacol. 2007, 29, 101–105. [Google Scholar]
- Adisakwattana, S.; Yibchok-Anun, S.; Charoenlertkul, P.; Wongsasiripat, N. Cyanidin-3-rutinoside alleviates postprandial hyperglycemia and its synergism with acarbose by inhibition of intestinal α-glucosidase. J. Clin. Biochem. Nutr. 2011, 49, 36–41. [Google Scholar]
- Slimestad, R.; Torskangerpoll, K.; Nateland, H.S.; Johannessen, T.; Giske, N.H. Flavonoids from black chokeberries, Aronia melanocarpa. J. Food Comp. Anal. 2005, 18, 61–68. [Google Scholar]
- Wangensteen, H.; Samuelsen, A.B.; Malterud, K.E. Antioxidant activity in extracts from coriander. Food Chem. 2004, 88, 293–297. [Google Scholar]
- Noro, T.; Oda, Y.; Miyase, T.; Ueno, A.; Fukushima, S. Inhibitors of xanthine oxidase from the flowers and buds of Daphne genkwa. Chem. Pharm. Bull. 1983, 31, 3984–3987. [Google Scholar]
- Matsui, T.; Yoshimoto, C.; Osajima, K.; Oki, T.; Osajima, Y. In vitro survey of α-glucosidase inhibitory food components. Biosci. Biotechnol. Biochem. 1996, 60, 2019–2022. [Google Scholar]
- Kandil, F.E.; Song, L.; Pezzuto, J.M.; Marley, K.; Seigler, D.S.; Smith, M.A.L. Isolation of oligomeric proanthocyanidins from flavonoid-producing cell cultures. In Vitro Cell. Dev. Biol. Plant 2000, 36, 492–500. [Google Scholar]
- Eberhardt, T.L.; Young, R.A. Conifer seed cone proanthocyanidin polymers: Characterization by 13C NMR spectroscopy and determination of antifungal activities. J. Agric. Food Chem. 1994, 42, 1704–1708. [Google Scholar]
- Wangensteen, H.; Dang, H.C.T.; Uddin, S.J.; Alamgir, M.; Malterud, K.E. Antioxidant and antimicrobial effects of the mangrove tree Heritiera fomes. Nat. Prod. Commun. 2009, 4, 371–376. [Google Scholar]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar]
- Kähkönen, M.; Heinonen, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar]
- Matsui, T.; Ueda, T.; Oki., T.; Sugita, K.; Terahara, N.; Matsumoto, K. α-Glucosidase inhibitory action of natural acylated anthocyanins. 1. Survey of natural pigments with potent inhibitory activity. J. Agric. Food Chem. 2001, 49, 1948–1951. [Google Scholar]
- McDougall, G.; Shpiro, F.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase. J. Agric. Food Chem. 2005, 53, 2760–2766. [Google Scholar]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-Glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar]
- Ma, C.-M.; Sato, N.; Li, X.-Y.; Nakamura, N.; Hattori, M. Flavan-3-ol contents, anti-oxidative and α-glucosidase inhibitory activities of Cynomorium songaricum. Food Chem. 2010, 118, 116–119. [Google Scholar]
- Knaup, B.; Oehme, A.; Valotis, A.; Schreier, P. Anthocyanins as lipoxygenase inhibitors. Mol. Nutr. Food Res. 2009, 53, 617–624. [Google Scholar]
- Madamanchi, N.R.; Vendrov, A.; Runge, M. Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar]
- Pascual-Teresa, S.; Moreno, D.A.; García-Viguera, C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230–242. [Google Scholar]
- Rasmussen, S.E.; Frederiksen, H.; Krogholm, K.S.; Poulsen, L. Dietary proanthocyanidins: Occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol. Nutr. Food Res. 2005, 49, 159–174. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bräunlich, M.; Slimestad, R.; Wangensteen, H.; Brede, C.; Malterud, K.E.; Barsett, H. Extracts, Anthocyanins and Procyanidins from Aronia melanocarpa as Radical Scavengers and Enzyme Inhibitors. Nutrients 2013, 5, 663-678. https://doi.org/10.3390/nu5030663
Bräunlich M, Slimestad R, Wangensteen H, Brede C, Malterud KE, Barsett H. Extracts, Anthocyanins and Procyanidins from Aronia melanocarpa as Radical Scavengers and Enzyme Inhibitors. Nutrients. 2013; 5(3):663-678. https://doi.org/10.3390/nu5030663
Chicago/Turabian StyleBräunlich, Marie, Rune Slimestad, Helle Wangensteen, Cato Brede, Karl E. Malterud, and Hilde Barsett. 2013. "Extracts, Anthocyanins and Procyanidins from Aronia melanocarpa as Radical Scavengers and Enzyme Inhibitors" Nutrients 5, no. 3: 663-678. https://doi.org/10.3390/nu5030663
APA StyleBräunlich, M., Slimestad, R., Wangensteen, H., Brede, C., Malterud, K. E., & Barsett, H. (2013). Extracts, Anthocyanins and Procyanidins from Aronia melanocarpa as Radical Scavengers and Enzyme Inhibitors. Nutrients, 5(3), 663-678. https://doi.org/10.3390/nu5030663




