Salty Taste Acuity Is Affected by the Joint Action of αENaC A663T Gene Polymorphism and Available Zinc Intake in Young Women
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Study Design and Subjects
2.2. Genotyping
2.3. Sensory Evaluation
2.4. Dietary Assessment
2.5. Anthropometric and Biochemical Assessment
2.6. Statistical Analysis
3. Results
| Men | Women | P e | |
|---|---|---|---|
| (n = 104) | (n = 103) | ||
| Age (years) | 23.6 ± 2.2 | 23.6 ± 2.3 | 0.87 |
| BMI (kg/m2) | 23.0 ± 2.1 | 20.9 ± 1.8 | <0.001 |
| Albumin (g/dL) | 4.9 ± 0.3 | 4.8 ± 0.2 | 0.02 |
| Hemoglobin (g/dL) | 15.8 ± 1.0 | 13.4 ± 0.9 | <0.001 |
| Energy intake (kcal/day) | 2116.2 ± 449.3 | 1630.0 ± 355.8 | <0.001 |
| Sodium intake (mg) | 4114.2 ± 113.8 | 3071.5 ± 78.8 | <0.001 |
| Sodium intake (mg/1000 kcal) | 1960.6 ± 45.2 | 1907.7 ± 41.8 | 0.39 |
| Zinc intake (mg/1000 kcal) | |||
| Total zinc | 4.2 ± 0.9 | 4.4 ± 1.1 | 0.22 |
| Available zinc b | 1.8 ± 0.5 | 1.9 ± 0.6 | 0.19 |
| <EAR c | 40 (38.5) | 58 (56.3) | 0.01 |
| Serum zinc (μM) | 14.4 ± 2.8 | 13.6 ± 2.5 | 0.04 |
| Serum deficiency d | 13 (12.5) | 11 (10.7) | 0.68 |
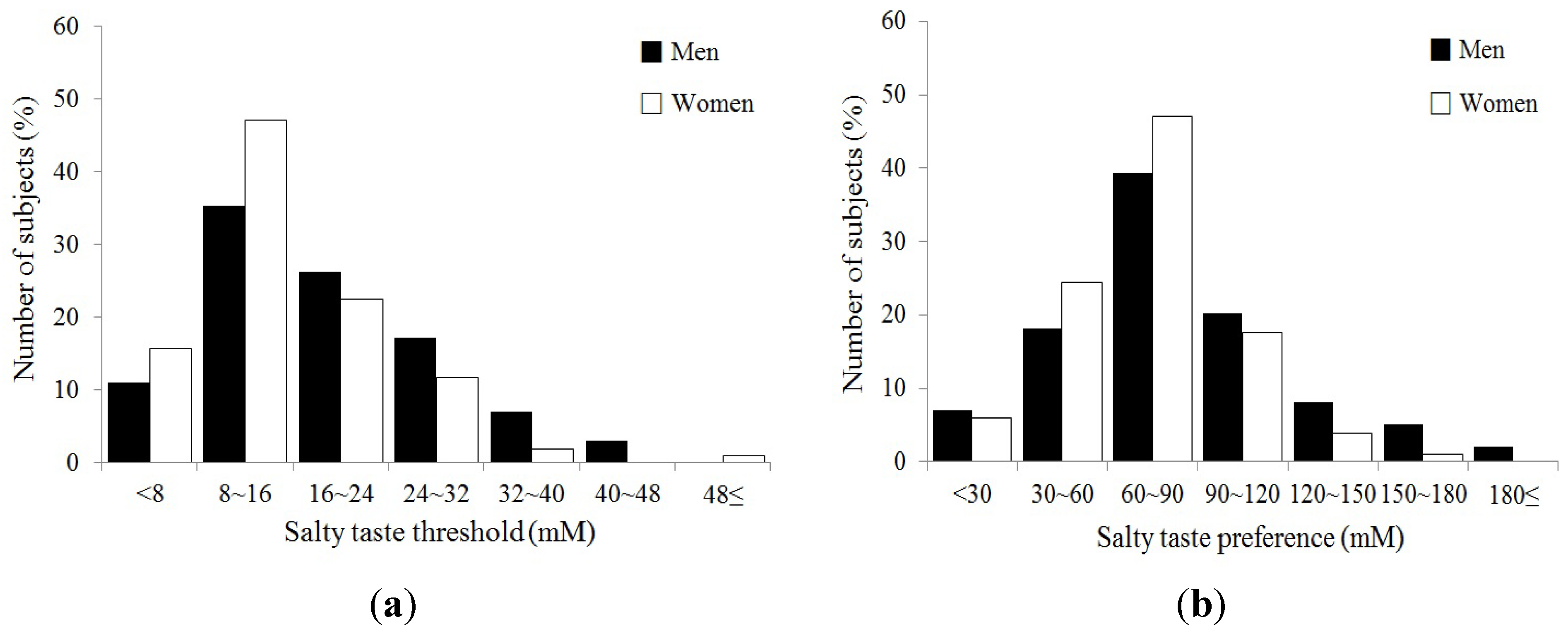
| Salty taste threshold (mM) | 19.3 ± 1.2 | 15.8 ± 0.9 | 0.02 |
| Salty taste preference (mM) | 86.0 ± 4.0 | 75.4 ± 3.0 | 0.03 |
| αENaC A663T Genotypes | AA | AT | TT | P c |
|---|---|---|---|---|
| Men | ||||
| n (%) b | 36 (34.6) | 50 (48.1) | 18 (17.3) | |
| Frequency of A allele | 0.59 | |||
| Salty taste threshold (mM) | 20.3 ± 12.0 | 18.6 ± 12.6 | 19.0 ± 11.0 | 0.58 |
| Salty taste preference (mM) | 81.9 ± 40.0 | 89.3 ± 40.0 | 85.0 ± 45.1 | 0.83 |
| Zinc intake (mg/1000 kcal) | ||||
| Total zinc | 4.3 ± 0.2 | 4.2 ± 0.1 | 4.0 ± 0.2 | 0.25 |
| Available zinc | 1.9 ± 0.1 | 1.8 ± 0.0 | 1.8 ± 0.1 | 0.32 |
| Women | ||||
| n (%) b | 37 (35.9) | 53 (51.5) | 13 (12.6) | |
| Frequency of A allele | 0.62 | |||
| Salty taste threshold (mM) | 18.0 ± 11.5 | 14.7 ± 7.5 | 14.0 ± 6.1 | 0.28 |
| Salty taste preference (mM) | 71.7 ± 31.7 | 79.8 ± 28.3 | 67.6 ± 31.2 | 0.69 |
| Zinc intake (mg/1000 kcal) | ||||
| Total zinc | 4.5 ± 0.2 | 4.2 ± 0.1 | 4.7 ± 0.4 | 0.94 |
| Available zinc | 2.0 ± 0.1 | 1.9 ± 0.0 | 2.0 ± 0.2 | 0.93 |
| Zinc Intake | P b | |||||
|---|---|---|---|---|---|---|
| Tertile 1 (lowest) | Tertile 2 | Tertile 3 (highest) | ||||
| Total zinc | ||||||
| Men | ||||||
| Threshold (mM) | 20.8 ± 2.4 | 19.7 ± 1.8 | 17.2 ± 2.0 | 0.44 | ||
| Preference (mM) | 85.08 ± 7.9 | 93.7 ± 6.1 | 79.1 ± 6.5 | 0.36 | ||
| Women | ||||||
| Threshold (mM) | 17.1 ± 1.9 | 17.6 ± 1.5 | 12.8 ± 1.0 | 0.04 | ||
| Preference (mM) | 77.2 ± 5.7 | 76.6 ± 4.6 | 72.5 ± 5.1 | 0.63 | ||
| Available zinc | ||||||
| Men | ||||||
| Threshold (mM) | 20.6 ± 2.2 | 18.7 ± 2.0 | 18.5 ± 1.9 | 0.73 | ||
| Preference (mM) | 82.1 ± 8.2 | 89.3 ± 6.4 | 86.6 ± 6.1 | 0.49 | ||
| Women | ||||||
| Threshold (mM) | 17.6 ± 1.9 | 17.5 ± 1.5 | 12.2 ± 1.0 | 0.02 | ||
| Preference (mM) | 79.8 ± 5.4 | 72.6 ± 4.9 | 73.6 ± 5.2 | 0.60 | ||
| Zinc Intake | αENaC A663T Genotype | ||||||
|---|---|---|---|---|---|---|---|
| AA | AT or TT | ||||||
| β | SE | p | β | SE | p | ||
| Men | |||||||
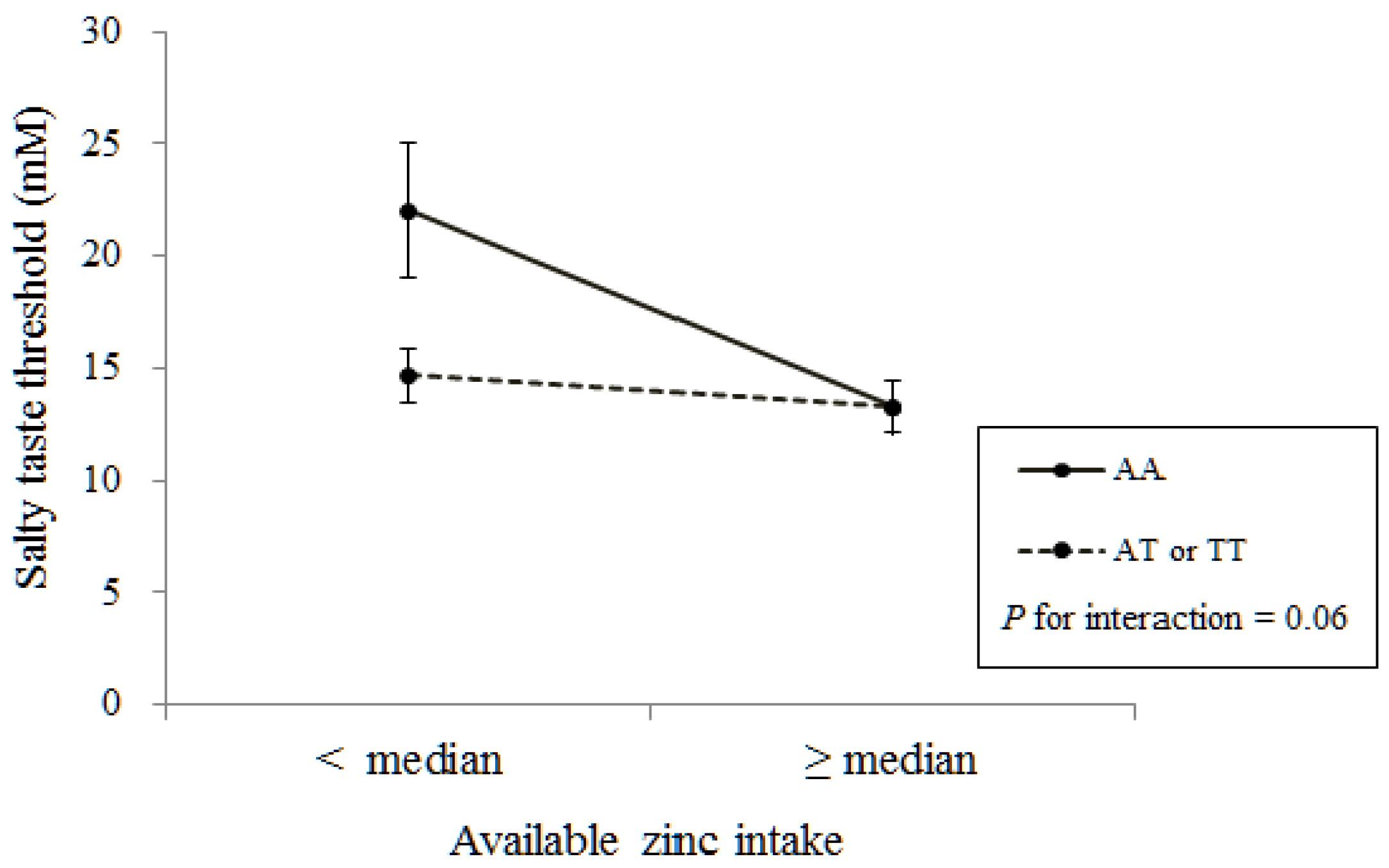
| Total Zinc | 0.040 | 0.50 | 0.94 | −0.947 | 0.51 | 0.07 | |
| Available Zinc | 0.038 | 0.40 | 0.93 | −0.731 | 0.47 | 0.13 | |
| Women | |||||||
| Total zinc | −0.765 | 0.41 | 0.07 | −0.190 | 0.32 | 0.56 | |
| Available zinc | −0.833 | 0.35 | 0.02 | −0.261 | 0.28 | 0.36 | |
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Meneton, P.; Jeunemaitre, X.; de Wardener, H.E.; MacGregor, G.A. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol. Rev. 2005, 85, 679–715. [Google Scholar] [CrossRef]
- Whelton, P.K.; Appel, L.J.; Sacco, R.L.; Anderson, C.A.; Antman, E.M.; Campbell, N.; Dunbar, S.B.; Frohlich, E.D.; Hall, J.E.; Jessup, M.; et al. Sodium, blood pressure, and cardiovascular disease: Further evidence supporting the american heart association sodium reduction recommendations. Circulation 2012, 126, 2880–2889. [Google Scholar] [CrossRef]
- Bibbins-Domingo, K.; Chertow, G.M.; Coxson, P.G.; Moran, A.; Lightwood, J.M.; Pletcher, M.J.; Goldman, L. Projected effect of dietary salt reductions on future cardiovascular disease. N. Engl. J. Med. 2010, 362, 590–599. [Google Scholar] [CrossRef]
- (KCDC) Korean Centers for Disease Control and Prevetion. 2011 Korean National Health and Nutrition Examinaiton Survey; Jeon, B.Y., Ed.; KCDC: Osong, Korea, 2012. [Google Scholar]
- (WHO) World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases; Report of a Joint Who/Fao Expert Consultation, WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Hayes, J.E.; Sullivan, B.S.; Duffy, V.B. Explaining variability in sodium intake through oral sensory phenotype, salt sensation and liking. Physiol. Behav. 2010, 100, 369–380. [Google Scholar] [CrossRef]
- Kim, G.H.; Lee, H.M. Frequent consumption of certain fast foods may be associated with an enhanced preference for salt taste. J. Hum. Nutr. Diet. 2009, 22, 475–480. [Google Scholar] [CrossRef]
- Pangborn, R.M.; Pecore, S.D. Taste perception of sodium chloride in relation to dietary intake of salt. Am. J. Clin. Nutr. 1982, 35, 510–520. [Google Scholar]
- DeSimone, J.A.; Lyall, V. Taste receptors in the gastrointestinal tract iii. Salty and sour taste: Sensing of sodium and protons by the tongue. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G1005–G1010. [Google Scholar] [CrossRef]
- Hellekant, G.; Ninomiya, Y. On the taste of umami in chimpanzee. Physiol. Behav. 1991, 49, 927–934. [Google Scholar] [CrossRef]
- Hellekant, G.; DuBois, G.E.; Roberts, T.W.; Van der Wel, H. On the gustatory effect of amiloride in the monkey (macaca mulatto). Chem. Senses 1988, 13, 89–93. [Google Scholar] [CrossRef]
- Lin, W.; Finger, T.E.; Rossier, B.C.; Kinnamon, S.C. Epithelial Na+ channel subunits in rat taste cells: Localization and regulation by aldosterone. J. Comp. Neurol. 1999, 405, 406–420. [Google Scholar] [CrossRef]
- Chandrashekar, J.; Kuhn, C.; Oka, Y.; Yarmolinsky, D.A.; Hummler, E.; Ryba, N.J.; Zuker, C.S. The cells and peripheral representation of sodium taste in mice. Nature 2010, 464, 297–301. [Google Scholar] [CrossRef]
- Ambrosius, W.T.; Bloem, L.J.; Zhou, L.; Rebhun, J.F.; Snyder, P.M.; Wagner, M.A.; Guo, C.; Pratt, J.H. Genetic variants in the epithelial sodium channel in relation to aldosterone and potassium excretion and risk for hypertension. Hypertension 1999, 34, 631–637. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kato, N.; Ishinaga, Y.; Yamori, Y.; Yazaki, Y. Evaluation of selected polymorphisms of the mendelian hypertensive disease genes in the Japanese population. Hypertens. Res. 2001, 24, 515–521. [Google Scholar] [CrossRef]
- Shigemura, N.; Ohkuri, T.; Sadamitsu, C.; Yasumatsu, K.; Yoshida, R.; Beauchamp, G.K.; Bachmanov, A.A.; Ninomiya, Y. Amiloride-sensitive nacl taste responses are associated with genetic variation of enac alpha-subunit in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R66–R75. [Google Scholar]
- Aliani, M.; Udenigwe, C.C.; Girgih, A.T.; Pownall, T.L.; Bugera, J.L.; Eskin, M.N. Zinc deficiency and taste perception in the elderly. Crit. Rev. Food Sci. Nutr. 2013, 53, 245–250. [Google Scholar] [CrossRef]
- Henkin, R.I.; Martin, B.M.; Agarwal, R.P. Decreased parotid saliva gustin/carbonic anhydrase VI secretion: An enzyme disorder manifested by gustatory and olfactory dysfunction. Am. J. Med. Sci. 1999, 318, 380–391. [Google Scholar] [CrossRef]
- Wright, A.L.; King, J.C.; Baer, M.T.; Citron, L.J. Experimental zinc depletion and altered taste perception for nacl in young adult males. Am. J. Clin. Nutr. 1981, 34, 848–852. [Google Scholar]
- Stewart-Knox, B.J.; Simpson, E.E.; Parr, H.; Rae, G.; Polito, A.; Intorre, F.; Andriollo Sanchez, M.; Meunier, N.; O’Connor, J.M.; Maiani, G.; et al. Taste acuity in response to zinc supplementation in older europeans. Br. J. Nutr. 2008, 99, 129–136. [Google Scholar]
- Takeda, N.; Takaoka, T.; Ueda, C.; Toda, N.; Kalubi, B.; Yamamoto, S. Zinc deficiency in patients with idiopathic taste impairment with regard to angiotensin converting enzyme activity. Auris Nasus Larynx 2004, 31, 425–428. [Google Scholar]
- Yamagata, T.; Nakamura, Y.; Yamagata, Y.; Nakanishi, M.; Matsunaga, K.; Nakanishi, H.; Nishimoto, T.; Minakata, Y.; Mune, M.; Yukawa, S. The pilot trial of the prevention of the increase in electrical taste thresholds by zinc containing fluid infusion during chemotherapy to treat primary lung cancer. J. Exp. Clin. Cancer Res. 2003, 22, 557–563. [Google Scholar]
- DeWimone, J.; Lyall, V. Amiloride-Sensitive Ion Channels. In The Senses: A Comprehensive Reference, 1st ed.; Smith, D., Firestein, S., Beauchamp, G.K., Eds.; Elsevier: Amsterdam, The Netherland, Boston, MA, USA, 2008. [Google Scholar]
- Eny, K.M.; Wolever, T.M.; Corey, P.N.; El-Sohemy, A. Genetic variation in tas1r2 (ile191val) is associated with consumption of sugars in overweight and obese individuals in 2 distinct populations. Am. J. Clin. Nutr. 2010, 92, 1501–1510. [Google Scholar] [CrossRef]
- Kim, U.K.; Jorgenson, E.; Coon, H.; Leppert, M.; Risch, N.; Drayna, D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science 2003, 299, 1221–1225. [Google Scholar] [CrossRef]
- Fushan, A.A.; Simons, C.T.; Slack, J.P.; Drayna, D. Association between common variation in genes encoding sweet taste signaling components and human sucrose perception. Chem. Senses 2010, 35, 579–592. [Google Scholar] [CrossRef]
- Stewart-Knox, B.J.; Simpson, E.E.; Parr, H.; Rae, G.; Polito, A.; Intorre, F.; Meunier, N.; Andriollo-Sanchez, M.; O’Connor, J.M.; Coudray, C.; et al. Zinc status and taste acuity in older europeans: The zenith study. Eur. J. Clin. Nutr. 2005, 59, S31–S36. [Google Scholar]
- Meligaard, M.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Watanabe, M.; Kudo, H.; Fukuoka, Y.; Hatakeyama, A.; Kudo, H.; Kodama, H.; Izumo, Y.; Sasaki, H. Salt taste perception and salt intake in older people. Geriatr. Gerontol. Int. 2008, 8, 62–64. [Google Scholar] [CrossRef]
- Paik, H.; Kim, K. DS24; Seoul Nation University, Human Nutrition Lab. & Sook Myung University, AI/DB Lab.: Seoul, Korea, 1997. [Google Scholar]
- Lonnerdal, B. Dietary factors influencing zinc absorption. J. Nutr. 2000, 130, 1378–1383. [Google Scholar]
- Brown, K.H.; Rivera, J.A.; Bhutta, Z.; Gibson, R.S.; King, J.C.; Lonnerdal, B.; Ruel, M.T.; Sandtrom, B.; Wasantwisut, E.; Hotz, C. International zinc nutrition consultative group (izincg) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004, 25, S99–S203. [Google Scholar]
- Murphy, S.P.; Beaton, G.H.; Calloway, D.H. Estimated mineral intakes of toddlers: Predicted prevalence of inadequacy in village populations in Egypt, Kenya, and Mexico. Am. J. Clin. Nutr. 1992, 56, 565–572. [Google Scholar]
- (WHO) World Health Organization. Trace Elements in Human Nutrition and Health: 5. Zinc; World Health Organization: Geneva, Switzerland, 1996; pp. 72–104.
- (KNS) Korean Nutrition Society. Dietary Reference Intakes for Koreans; 1st revision; KNS: Seoul, Korea, 2010.
- Watanabe, M.; Asatsuma, M.; Ikui, A.; Ikeda, M.; Yamada, Y.; Nomura, S.; Igarashi, A. Measurements of several metallic elements and matrix metalloproteinases (mmps) in saliva from patients with taste disorder. Chem. Senses 2005, 30, 121–125. [Google Scholar] [CrossRef]
- McDaid, O.; Stewart-Knox, B.; Parr, H.; Simpson, E. Dietary zinc intake and sex differences in taste acuity in healthy young adults. J. Hum. Nutr. Diet. 2007, 20, 103–110. [Google Scholar] [CrossRef]
- Stähler, F.; Riedel, K.; Demgensky, S.; Neumann, K.; Dunkel, A.; Täubert, A.; Raab, B.; Behrens, M.; Raguse, J.-D.; Hofmann, T.; et al. A role of the epithelial sodium channel in human salt taste transduction? Chemosens. Percept. 2008, 1, 78–90. [Google Scholar]
- Pratt, J.H. Central role for enac in development of hypertension. J. Am. Soc. Nephrol. 2005, 16, 3154–3159. [Google Scholar] [CrossRef]
- Samaha, F.F.; Rubenstein, R.C.; Yan, W.; Ramkumar, M.; Levy, D.I.; Ahn, Y.J.; Sheng, S.; Kleyman, T.R. Functional polymorphism in the carboxyl terminus of the alpha-subunit of the human epithelial sodium channel. J. Biol. Chem. 2004, 279, 23900–23907. [Google Scholar] [CrossRef]
- Tong, Q.; Menon, A.G.; Stockand, J.D. Functional polymorphisms in the alpha-subunit of the human epithelial Na+ channel increase activity. Am. J. Physiol. Renal Physiol. 2006, 290, F821–F827. [Google Scholar]
- Yan, W.; Suaud, L.; Kleyman, T.R.; Rubenstein, R.C. Differential modulation of a polymorphism in the cooh terminus of the alpha-subunit of the human epithelial sodium channel by protein kinase cdelta. Am. J. Physiol. Renal Physiol. 2006, 290, F279–F288. [Google Scholar] [CrossRef]
- Nilsson, B. Taste acuity of the human palate: III. Studies with taste solutions on subjects in different age groups. Acta Odontol. Scand. 1979, 37, 235–252. [Google Scholar] [CrossRef]
- Sato, K.; Endo, S.; Tomita, H. Sensitivity of three loci on the tongue and soft palate to four basic tastes in smokers and non-smokers. Acta Oto-Laryngol. Suppl. 2002, 122, 74–82. [Google Scholar] [CrossRef]
- Bartoshuk, L.M.; Duffy, V.B.; Miller, I.J. Ptc/prop tasting: Anatomy, psychophysics, and sex effects. Physiol. Behav. 1994, 56, 1165–1171. [Google Scholar] [CrossRef]
- Lowe, N.M.; Dykes, F.C.; Skinner, A.L.; Patel, S.; Warthon-Medina, M.; Decsi, T.; Fekete, K.; Souverein, O.W.; Dullemeijer, C.; Cavelaars, A.E.; et al. Eurreca-estimating zinc requirements for deriving dietary reference values. Crit. Rev. Food Sci. Nutr. 2013, 53, 1110–1123. [Google Scholar] [CrossRef]
- Lowe, N.M.; Fekete, K.; Decsi, T. Methods of assessment of zinc status in humans: A systematic review. Am. J. Clin. Nutr. 2009, 89, 2040–2051. [Google Scholar] [CrossRef]
- Gibson, R.S.; Hess, S.Y.; Hotz, C.; Brown, K.H. Indicators of zinc status at the population level: A review of the evidence. Br. J. Nutr. 2008, 99, S14–S23. [Google Scholar]
- Drewnowski, A. Taste preferences and food intake. Annu. Rev. Nutr. 1997, 17, 237–253. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Noh, H.; Paik, H.-Y.; Kim, J.; Chung, J. Salty Taste Acuity Is Affected by the Joint Action of αENaC A663T Gene Polymorphism and Available Zinc Intake in Young Women. Nutrients 2013, 5, 4950-4963. https://doi.org/10.3390/nu5124950
Noh H, Paik H-Y, Kim J, Chung J. Salty Taste Acuity Is Affected by the Joint Action of αENaC A663T Gene Polymorphism and Available Zinc Intake in Young Women. Nutrients. 2013; 5(12):4950-4963. https://doi.org/10.3390/nu5124950
Chicago/Turabian StyleNoh, Hwayoung, Hee-Young Paik, Jihye Kim, and Jayong Chung. 2013. "Salty Taste Acuity Is Affected by the Joint Action of αENaC A663T Gene Polymorphism and Available Zinc Intake in Young Women" Nutrients 5, no. 12: 4950-4963. https://doi.org/10.3390/nu5124950
APA StyleNoh, H., Paik, H.-Y., Kim, J., & Chung, J. (2013). Salty Taste Acuity Is Affected by the Joint Action of αENaC A663T Gene Polymorphism and Available Zinc Intake in Young Women. Nutrients, 5(12), 4950-4963. https://doi.org/10.3390/nu5124950




