The Clinical Response to Gluten Challenge: A Review of the Literature
Abstract
:1. Introduction
2. Method
3. Results
3.1. CD-Specific Symptoms in Pediatric Patients with Diagnosed or Suspected CD
| Author (year) | Age Group and Age | Diagnosed/Suspected CD | Time on Gluten-Free Diet | Gluten Type and Dose | Duration of Challenge | CD-Symptoms | CD-Antibodies | Mucosal Immunohistology | Sugar Absorption Test |
|---|---|---|---|---|---|---|---|---|---|
| Mayer et al. (1989) [31] | Children 3.5Mdn (1.8–9.6) years | Diagnosed by biopsy (n = 37) | ≥1 year, 17Mdn month | 10 g/day gluten either as biscuit or as powder | 60Mdn (14–205) days | Acute symptoms in 13% (4/32) within 12 h and symptoms in 0% (0/31) within ~7 months | Increased AGA-IgA and IgG in 65% (20/31) within 15 days | Worsening histology score by Whitehead in 68% (21/31) at 2 month, 84% at 3 month, and 97% within 2 years | Decreased blood xylose within 15 days, remained low up to 150 day |
| Packer et al. (1978) [33] | Children 9.9M (3.0–15.3) years | Diagnosed by biopsy (n =32) | 6.5M years (0.25–11.0) | ≥10 g/day as 4 slices white bread | Up to 3 month | Symptoms in 60% (19/32) within 3 months | Increase in villous atrophy in 78% (25/32) within 3 months | ||
| Hamilton et al. (1972) [18] | Children 7.2M ± 1.5SD years | Diagnosed by biopsy (n = 23) | 3.8M years | 2.25 g/day as wheat gluten followed by1 slice/day of bread or equivalent flour (~2–3 g of gluten 1) | 6 days 6 days–15 months | Symptoms in 4% (1/23) at 4 day, in 8% (1/12) at 1 month, in 25% (3/12) at 6 months | Mucosal lesions in 7% (1/13) within 6 days, 92% (11/12) within 1 year, and 100% within 15 months | ||
| Mavromichalis et al. (1976) [43] | Children 6.5M years | Diagnosed by biopsy (n = 23) | 6.5M year (1.5–10) (n = 11 on gluten-free diet) | 20 g/day as gluten-containing diet | 4–9 weeks | Worsening histology score (III or IV on scale I–IV) in 100% (11/11) within 4–9 weeks Increased IEL in 100% (11/11) within 4–9 weeks | |||
| Hansson et al. (1997) [44] | Children 4Mdn (1–18) years | Diagnosed by biopsy (ESPGAN) (n = 57) | (n = 20 on gluten-free diet) | gluten-containing diet (dose not mentioned) | 2 weeks | Positive -AGA-IgA in 30% (6/20) within 2 weeks and 75% (21/28) within 12 weeks -AGA-IgG 15% (3/20) within 2 weeks and 71% (20/28) within 12 weeks -EMA-IgA 35% (7/20) within 2 weeks and 71% (20/28) within 12 weeks -AGA-IgA cells in peripheral blood in 75% (15/20) within 2 weeks and 86% (24/28) within 12 weeks | Increased IEL density in 25% (5/20) within 12 weeks | ||
| Hansson et al. (2002) [20] | Children 4Mdn (1–16) years | Diagnosed by biopsy (ESPGAN) (n = 57) | (n = 21 on gluten-free diet and n = 38 on gluten-containing diet | 2–3 slices/day of white bread (~4–9 g/day 1 of gluten) | 12 weeks | Positive -AGA-IgA in 78% (31/40) within 2 weeks, 89% (41/46) within 12 weeks -EMA-IgA in 45% (18/40) within 2 weeks, 91% (42/46) within 12 weeks -tTG-IgA in 45% (18/40) within 2 weeks, 89% (41/46) within 12 weeks | Increased IEL density in 16% (6/38) within 12 weeks | ||
| Schaad et al. (1981) [45] | Children 8.1M (3.1–13.1) years | Diagnosed | 1.5–10 years | 1 g raw cooked gluten/kg/day (~25 g gluten/day 1,2) | 30 days | Increased IEL in 100% (22/22) at 30 day | |||
| Scott et al. (1980) [19] | Children 5.8Mdn (2.9–8.8) years | Diagnosed by biopsy (n = 10) | - | One 20 g-slice/day of bread (~2 g/day 1 gluten) followed by gluten-containing diet | 1 month of bread followed by gluten-containing diet up to 11 month | Mucosal relapse in 100% (10/10) within 2–11 months (7 monthsMdn) Increased IEL within 2–11 months | |||
| Bürginn-Wolff et al. (1991) [46] | Children 4 months–18 years | Diagnosed by biopsy (n = 135) | - | Gluten-containing diet (dose not mentioned) | Up to 15 year | Positive AGA-IgA in 97% (28/29) within 3 months, 85% (73/86) within 1 year, and 49% within ≥3 years EMA-IgA 65% (13/20) within 3 months, 84% (49/58) within 1 year, and 93% within ≥3 years | Abnormal mucosa in 72% (31/43) within 1 month, 94% (31/33) within 7–10 months, 95% (18/19) within 20 months | ||
| Ascher et al. (1990) [30] | Children 1.4 Mdn (0.5–16.5) years | Diagnosed by biopsy (ESPGAN) (n = 45) | 1 year | Gluten-containing diet (dose not mentioned) | 3–31 months | Strong symptoms in 4% (2/45) within 1–2 weeks | Positive AGA-IgA in 90% (38/42) of not-IgA deficient patients within 10 months | ||
| Bodé et al. (1983) [47] | Children 2.8Mdn (0.3–15.5) years | Diagnosed by biopsy (ESPGAN) (n = 14) | ≥1 year | ≥10 g/day (type not mentioned) | 3 months–2 years | Positive AGA-IgG in 79% (11/14) within 3 months–2 years AGA-IgA in 57% (8/14) within 3 months–2 years | |||
| Danielsson et al. (1990) [48] | Children ~2M (1–5.6) years | Diagnosed by biopsy (n = 67) | 0.9–1.4 years | 10 g/day as gluten-containing diet | 0.5–4.4 years | Abnormal histology score (II–IV on scale I–IV) in 96% (64/67) within 2 years | |||
| Berg et al (1997) [34] | Children ~1 year | Diagnosed by biopsy (n = 34) | 1–1.5 years | Gluten-containing diet or 3–15 g/day of gluten | Symptoms in 32% (11/34) within 4–5 months | Abnormal histology in 100% (23/23) in patients without symptoms within 4–5 months | |||
| Troncone et al. (1994) [37] | Children 7.3M (4.9–9.8) years | Suspected (n = 6) and diagnosed by biopsy (n = 9) | 6 yearsMdn (3–8) | 10 g/day as biscuits or pasta | 30Mdn days (14 days–6 months) | Symptoms in 42% (5/12) within 6 months | Positive AGA-IgA in ~AGA-IgA in ~50% at 2 weeks, ~25% at 1 month, ~50% at 2 month, ~70% at 3 month, 57% within 6 months AGA-IgG in ~10% at 1 month, ~25% at 2 month, ~50% at 3 month, 29% within 6 months EMA-IgA in ~77% (10/13) at 1 month, 93% within 6 months | Increased urinary cellulose/mannitol ratio in 86% (12/14) within 3 months | |
| Korponay-Szabó et al. (1997) [38] | Children 5.1Mdn (1.9–15.3) years | Suspected (n = 67) and diagnosed by biopsy (ESPGAN) (n = 90) | Not reported | 5–10 g/day as purified gluten | 6 weeks–2 years | -Mild symptoms in 34.3% (46/134) -Severe symptoms in 2.9% (4/134) of patients with histological relapse within 6 weeks–2 years | Positive EMA-IgA or -IgG in 66% at 3 month, 90% at 6 month, and 88% (134/153) within 21 months | Abnormal histology score (scale I-III by Fontaine and Navarro) in 88% (134/153) within 2 years. Relapse time (A + B) 5Mdn month (1.8–26.5) and (C) 6Mdn month (1.4–25.3) | |
| Rolles et al. (1976) [32] | Children 5.7M (1.5–15) years | Suspected (n = 35) | 4.2M (1–10) years | 20 g/day as gluten powder | 4–13 weeks | Mild to severe symptoms in 29% (10/35) within 4–13 weeks | Abnormal histology score (scale 3 or 4 on 0–4) in 51% (18/35) within 4–13 weeks | ||
| Lancaster et al. (1976) [49] | Children 11.5M (5–16.5) years | Suspected (n = 16) | 6.9M (1.5–13) years | 10 g/day as wheat protein | Up to 24 month | Decrease in Vh in 62% (10/16) within 3 months, in 81% (13/16) within 3–24 months Increased in IEL density in 100% (13/13) within 3 months | |||
| Laurin et al. (2002) [42] | Children 3.8Mdn (2.7–8.8) years | Suspected (n = 25) | ≥1 year | 1.4Mdn g/day (0.2–4.3) as gluten-containing diet | 13Mdn week (5 week–1 year) | Symptoms in 79% within 4 weeks, 96% (23/24) within 15 weeks | Positive -AGA-IgA in 25% (5/20) within 4 weeks, 75% within 8 weeks -AGA-IgG in 0% (0/19) within 4 weeks, 5% (5/20) within 8 weeks -EMA-IgA in 65% (13/20) within 4 weeks, 75% within 8 weeks -EMA-IgG in 16% (3/19) within 4 weeks, 25% (5/20) within 8 weeks | -Abnormal histology score (3 or 4 on scale 0–4 by Marsh) in 91% (21/23) within 1 year -Increased IEL count in 96% (22/23) within 1 year | |
| Laurin et al. (2003) [50] | Children 3.8Mdn (2.7–8.8) years | Suspected (n = 25) | ≥1 year | 1.4Mdn g/day (0.2–4.3) as gluten-containing diet | Up to 3 month | Positive -AGA-IgA in 90% (16/18) within 8 weeks -EMA-IgA in 90% within 8 weeks | |||
| Valletta et al. (1990) [40] | Children 3.8M (2.7–8.8) years | Suspected (n = 17) | 0.4–8 years | Gluten-containing diet with 5, 10, 15 g/day gliadin at age 1–3, 3–5, and 5–10 years, respectively | 20–45 days | -Symptoms in 59% (10/17) -Food refusal in 100% (17/17) within 45 days | Positive -AGA-IgA in 94% (16/17) within 15–35 days -EMA-IgA in 90% within 2 months | -Worsening histology in 94% (16/17) within 25–45 days -Increased IEL score in 100% (17/17) within 25–45 days | |
| Jansson et al. (2001) [51] | Children 2.7M ± 1SD years | Suspected (n = 54) | ≥1 year | Gluten powder 0–4 weeks: 0.2 g/kg/day (~2.6 g/day 1,3) (A) 0.5 g/kg/day (~6.5 g/day 1,3) (B) 4–8 weeks: 0.5 g/kg/day (~6.5 g/day) (A and B) | 4–8 weeks | A and B: Positive -AGA-IgA in 76% (38/50) at 2 week, 88% at 4 week, 94% at 8 week no difference between A and B -EMA-IgA in 59% (32/54) at 2 week, 65% at 4 week, 67% at 8 week no difference between A and B | A and B: Worsening histology score (≥3 increase on scale 4–16) in 94% (51/54) at 4 week and 100% at 8 week | ||
| Wauters et al. (1991) [39] | Children 5.6M (2–16) years | Suspected (n = 17) | 46M months (10–168) | Gluten powder: 750 mg/kg bw/day (~14 g/day 4) with max 20 g/day | 3 months | Symptoms in 24% (4/17) within 3 months | Positive -AGA-IgA in 90% (9/10) within 6 weeks, and 100% (7/7) within 12 weeks -AGA-IgG in 90% (9/10) within 6 weeks, and 100% (7/7) within 12 weeks | Villous atrophy in 59% (10/17) within 12 weeks | |
| Savilahti et al. (1983) [35] | Children 1.6M years | Suspected (n = 19) | 0.7–2.3 years | Gluten-containing diet (dose not mentioned) | 0.1–1.1 year | Symptoms in 26% (5/19) within 0.1–1.1 year | Positive AGA-IgA in 73% (11/15) within 0.1–1.1 year | Abnormal mucosa in 95% (18/19) within 0.1–1.1 year | |
| Rolles et al. (1975) [52] | Children 0.5–5.7 years | Suspected (n = 16) | 0.1–5 years | 20 g/day as gluten powder | Up to 1.5 year | Symptoms in 33% (5/15) within 28 days | Histology score (3 or 4 on scale 0–4) in 80% (4/5) within 1.5 years | Decreased blood xylose in 40% (6/15) within 1 day, 67% (10/15) within 2–7 days, and 100% (15/15) within 14–28 days | |
| Bonamico et al. (2005) [36] | Children and adolescents 9.2M (5.4–19) years | Suspected (n = 24) | Three gluten-containing meals/day (n = 24) | up to 2 month | Symptoms after in vivo challenge in 33% (8/24) within few days–2 months | Positive EMA-IgA in 63% (15/24) within 2 months | Abnormal histology score (3 on scale 0–3 by Marsh) in 87% (13/15) within 2 months | ||
| Mäki et al. (1989) [41] | Adolescents 16.6M (14.3–22.1) years | Suspected (n = 9) and diagnosed by biopsy (ESPGAN) (n = 20) | ~8M years (3.0–16.0) | ≥10 g/day as gluten-containing diet | Up to 2 year | Symptoms in 32% (7/22) anti-reticulin positives within 2.4–24 months | Positive -AGA-IgA in 79% (23/29) within 2.4–24 months -AGA-IgG in 62% (18/29) within 2.4–24 months | Lower Vh in 85% (23/27) within 2.4–24 months. 15% (4/27) did not relapse in 2 years | |
| Lancaster-Smith et al. (1975) [53] | Adults | Diagnosed by biopsy (n = 11) | 4.3 (1–15) years | 25 g as single gluten dose (n = 8) or 10 or 20 g/day as gluten-containing diet (n = 4) | Single-dose (A) 7 days (B) | A: Increased IEL in 24–48 h B: Increased IEL in 100% within 1 week | |||
| Lähdeaho et al. (2011) [54] | Adults: 49Mdn (21–68) years | Diagnosed by biopsy (n = 21) | 11Mdn (2–34) years | 1–3 g/day (biscuits) (A) 3–5 g/day (biscuits) (B) | 12 weeks | A: Symptoms in 64% (7/11) within 3 months B: Symptoms in 80% (8/10) within 3 months | A: Positive tTG-IgA in 36% (4/11) within 12 weeks B: Positive tTG-IgA in 50% (5/10) within 12 weeks | A: decreased Vh/Cd in 64% (7/11) within 12 weeks A: Increased IEL in 55% (6/11) patients within 12 weeks B: decreased Vh/Cd ratio in 70% (7/10) within 12 weeks B: Increased IEL in 80% (8/10) patients within 12 weeks | |
| Leffler et al. (2012) [24] | Adults 43M ± 14SD years | Diagnosed by biopsy (n = 20) | 5 years | 3 or 7.6 g/day (bread) | 2 weeks | Increased symptom severity at 3 day, 1 and 2 week | Positive -tTG-IgA in 25% (5/20) within 2 weeks (increase to 50% 2 weeks post-challenge) -DGP-IgA/IgG in 30% (6/20) within 2 weeks | Abnormal histology score (3 or 4 on scale 0–4 by Marsh) in 68% (13/19) within 2 weeks Increased IEL within 2 weeks | Increase in urinary lactulose:mannitol ratio within 2 weeks |
| Montgomory (1988) [55] | Adults 40M (17–74) years | Diagnosed by biopsy (n = 13 on GFD) | 13Mdm (6–27) months | 2.5–5 g/day | 14 months | Positive AGA-IgA in 17% (11/13) within 3–14 months | Increased Vh within 14 months: no effect Increased IEL within 14 months | ||
| Brottveit (2011) [56] | Adults41M (16–65) years | Diagnosed by biopsy (n = 13 on GFD) | 13.9 (0.8–31.6) years | 40 g/day (four slices bread) | 3 days | Abnormal histology score (3 or 4 on scale 0–4 by Marsh) in 23% (4/13) within 3 days | |||
| Daveson (2011) [57] | Adults 44M (25–58) years | Diagnosed by biopsy (n = 10 on GFD in control group) | ≥6 months | 16 g/day (two slices bread) | 5 days | Abnormal histology score (3 or 4 on scale 0–4 by Marsh) in 70% (7/10) within 1 week Increased IEL at 1 week | |||
| Cornell et al. (2005) [25] | Adults 18–70 year | Diagnosed by biopsy (n = 21 on placebo) | Not reported | 3 Cracker biscuits (~1.3 g/day gluten) | 2 weeks | >5 Episodes of moderate-to-severe symptoms in 33% (7/21) on placebo within 2 weeks challenge and the following 10 week | Positive tTGA >5 U/mL in 19% (4/21) within 2 weeks and 3–15 weeks post-challenge | -Increased lymphocyte score in 83% (5/6) at 2 week -Increased epithelial stunting in 50% (3/6) at 2 week | |
| Tye-Din et al. (2010) [26] | Adults 41Mdn (21–67) years | Diagnosed by biopsy (n = 10 on placebo) | ≥8 weeks | 16 g/day Wheat flour slurry | 3 days | Symptoms increased within 1 week, 75% of symptoms were mild | No positive tTGA and DGP-IgA/IgG at 6 day | ||
| Kelly et al. (2013) [27] | Adults 18–65 years | Diagnosed by biopsy (n = 44 on placebo) | ≥6 weeks | 2.7 g/day Gluten powder (3 × daily 0.9 g) | 6 weeks | Symptoms increased in 80% within 6 weeks. Plateau at 3 week | Positive tTG-IgA > 10 U/mL in 30% (13/44) at 6 week | Increase in urinary lactulose:mannitol ratio. Plateau at 4 week | |
| Leffler et al. (2012b) [28] | Adults 18–72 years | Diagnosed by biopsy (n = 14 on placebo) | ≥6 weeks | 2.4 g/day Gluten powder (3 × daily 0.8 g) | 2 weeks | Symptoms increased in 50% within 2 weeks | No positive tTG-IgA at 2 week | Increase in urinary lactulose:mannitol ratio at 2 week | |
| Tack et al. 2010 [58] | Adults 55Mdn (20–68) years | Diagnosed by biopsy (n = 7 on placebo) | 7.5 (2–40) years | 7 g/day (5 toasts) | 2 weeks | Symptoms increased in 43% (3/7) within 2 weeks | Positive -tTG-IgA in 14% (1/7) -AGA-IgA in 14% (1/7) -AGA-IgG in 14% (1/7) No positive EMA-IgA within 2 weeks | -Increased tTG-IgA deposits in 71% (5/7) -Abnormal histology score (3 or 4 on scale 0–4 by Marsh) in 23% (2/7) Within 2 weeks | |
| Kumar et al. (1979) [59] | Adolescents 16.1M (14–21) years Adults: 37.7M (17–59) years | Suspected (n = 28) | Adolescents: 6.4M (1–14) years Adults: 2.8M (0.75–7) years | ≥4 Slices bread (~10 g/day gluten) | Adolescents 4–17.5 weeks (23Mdn) Adults: median 4–25.5 weeks (11.5Mdn) | Adolescents: Symptoms in 67% (6/9) within 1 h–2 weeks, no symptoms in 33% within 1 year Adults: Symptoms in 84% (16/19) within 4 days–3 months, no symptoms in 16% within 1 year | Adolescents: Decreased Vh in 56% (5/9) within 8 weeks, 100% within 10 momths Increased IEL in 100% within 10 months Adults: Decreased Vh in 95% (18/19) within 7 weeks, still 95% within 1 year Increased IEL in 95% within 7 weeks | ||
| Wahab (2001) [60] | Adults 40M (16–74) years | Suspected (n = 37) | 30 g/day on top of GCD | 2 months | Symptoms in 55% (17/38) within 3 months | Positive AGA-IgA in 22% (8/37) within 2 months Positive EMA-IgA in 17% (4/23)within 2 months | Abnormal histology score (2, 3 or 4 on scale 0–4 by Marsh) in 32% (12/38) at 2 month | ||
| Kaukinen et al. (2005) [61] | Adults 45M (19–70) years | Suspected (n = 21) | Not reported | ≥15 g/day (5 Slices of bread) | 6 months | -Increased tTG-IgA deposits in 24% (5/21) |
3.2. CD-Specific Symptoms in Adults with Diagnosed or Suspected CD
3.3. Antibodies in Pediatric Patients with Diagnosed or Suspected CD
3.3.1. AGA-IgA and AGA-IgG Antibodies
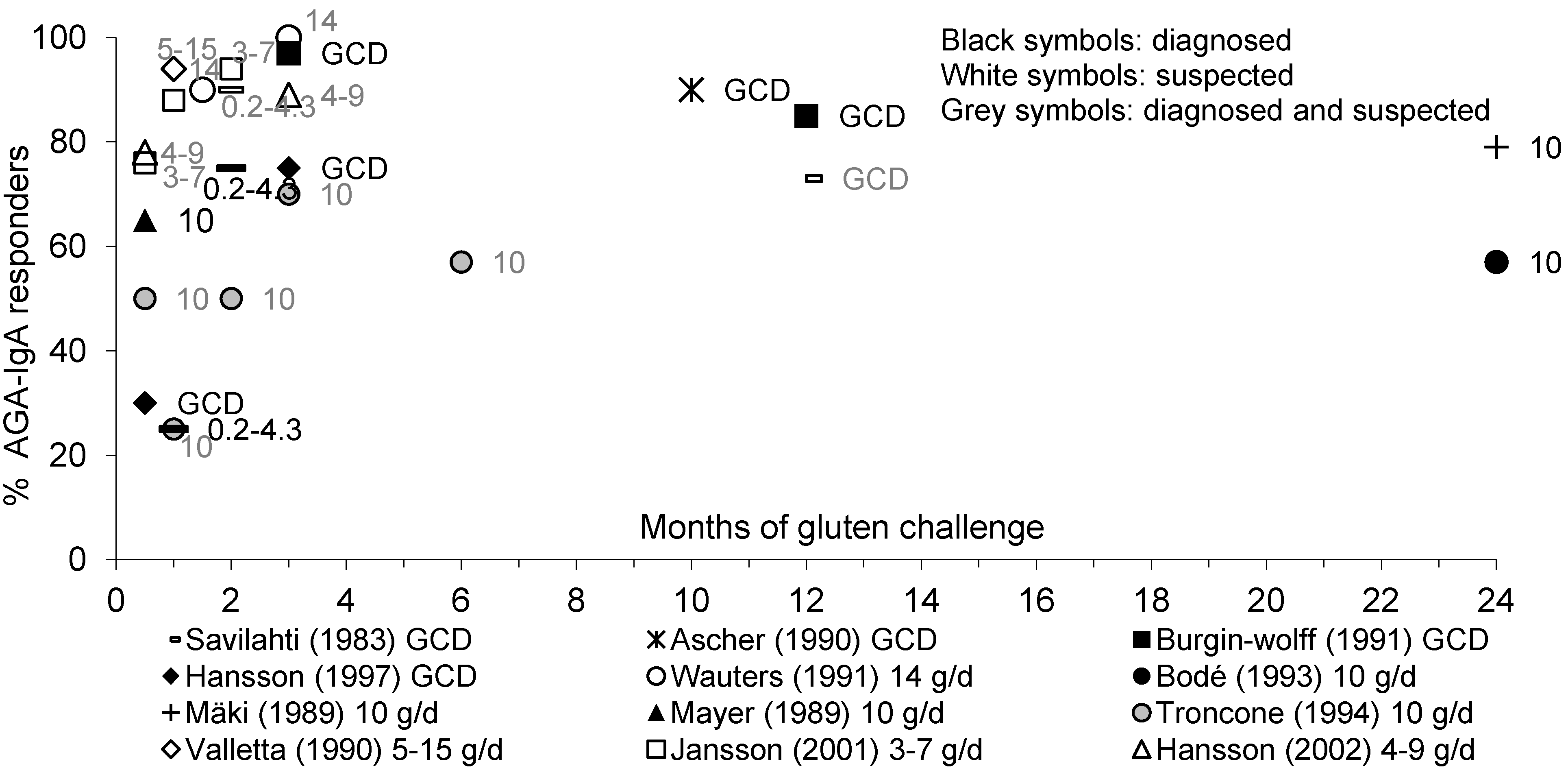
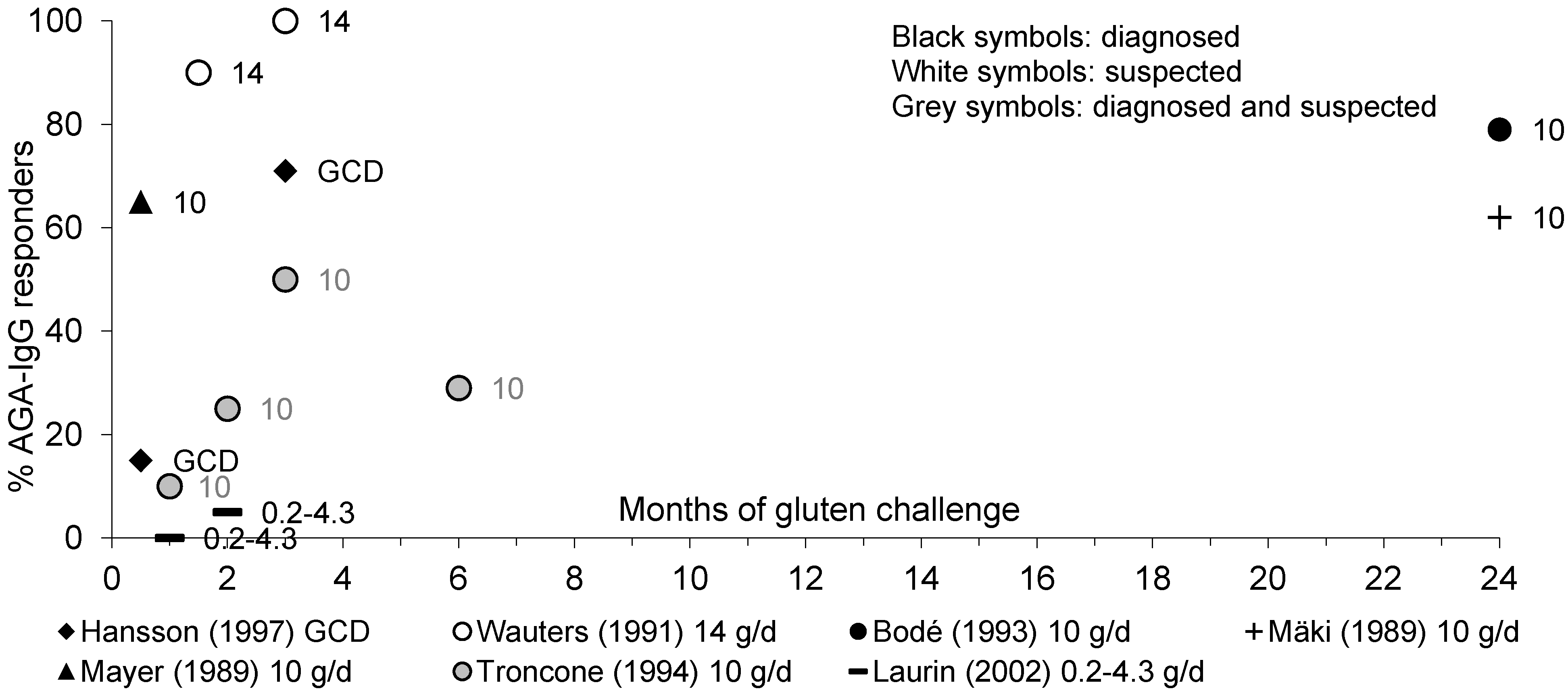
3.3.2. EMA-IgA Antibodies
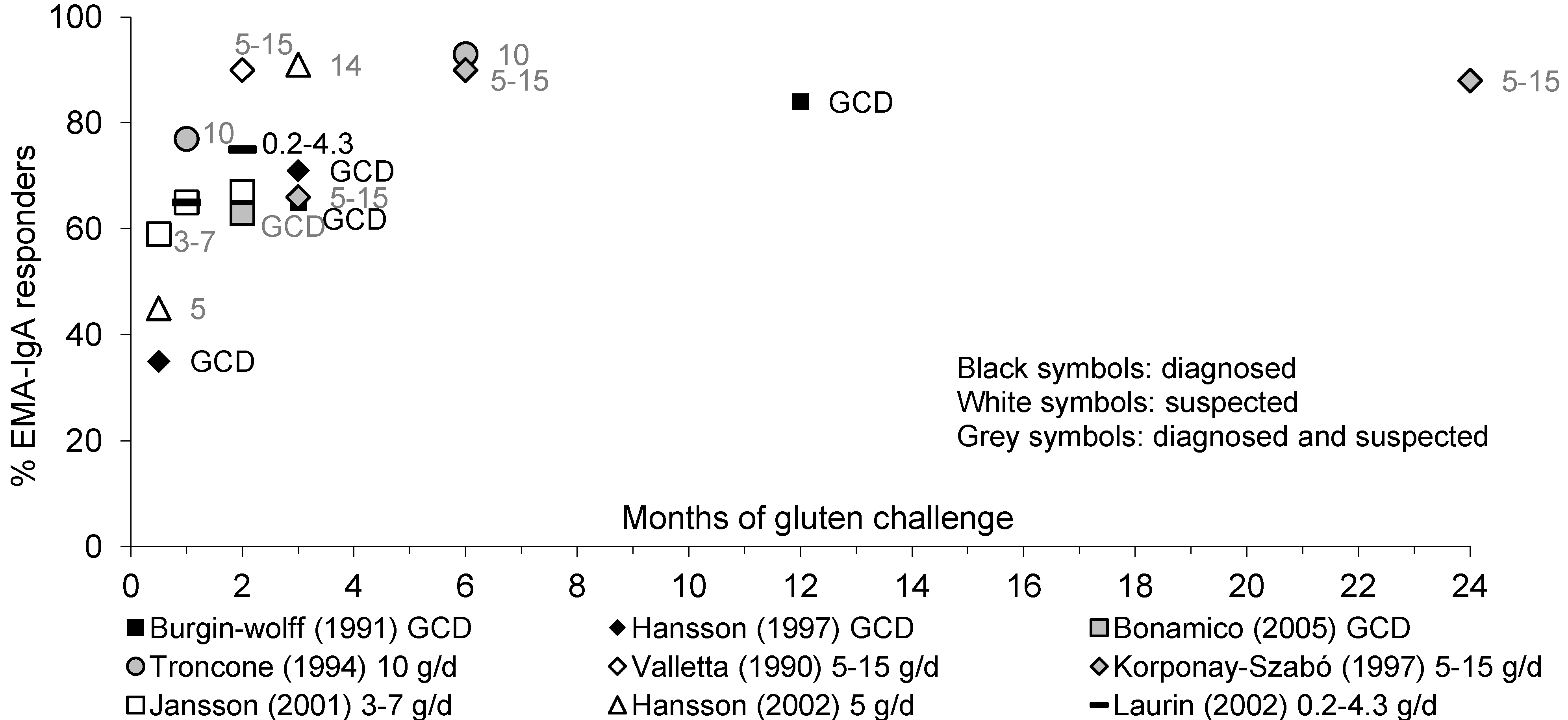
3.3.3. tTG-IgA Antibodies
3.3.4. Antibodies in Pediatric Patients: Summary
3.4. Antibodies in Adult Patients with Diagnosed or Suspected CD
3.4.1. AGA-IgA and EMA-IgA Antibodies
3.4.2. tTG-IgA and DGP-IgA/IgG Antibodies
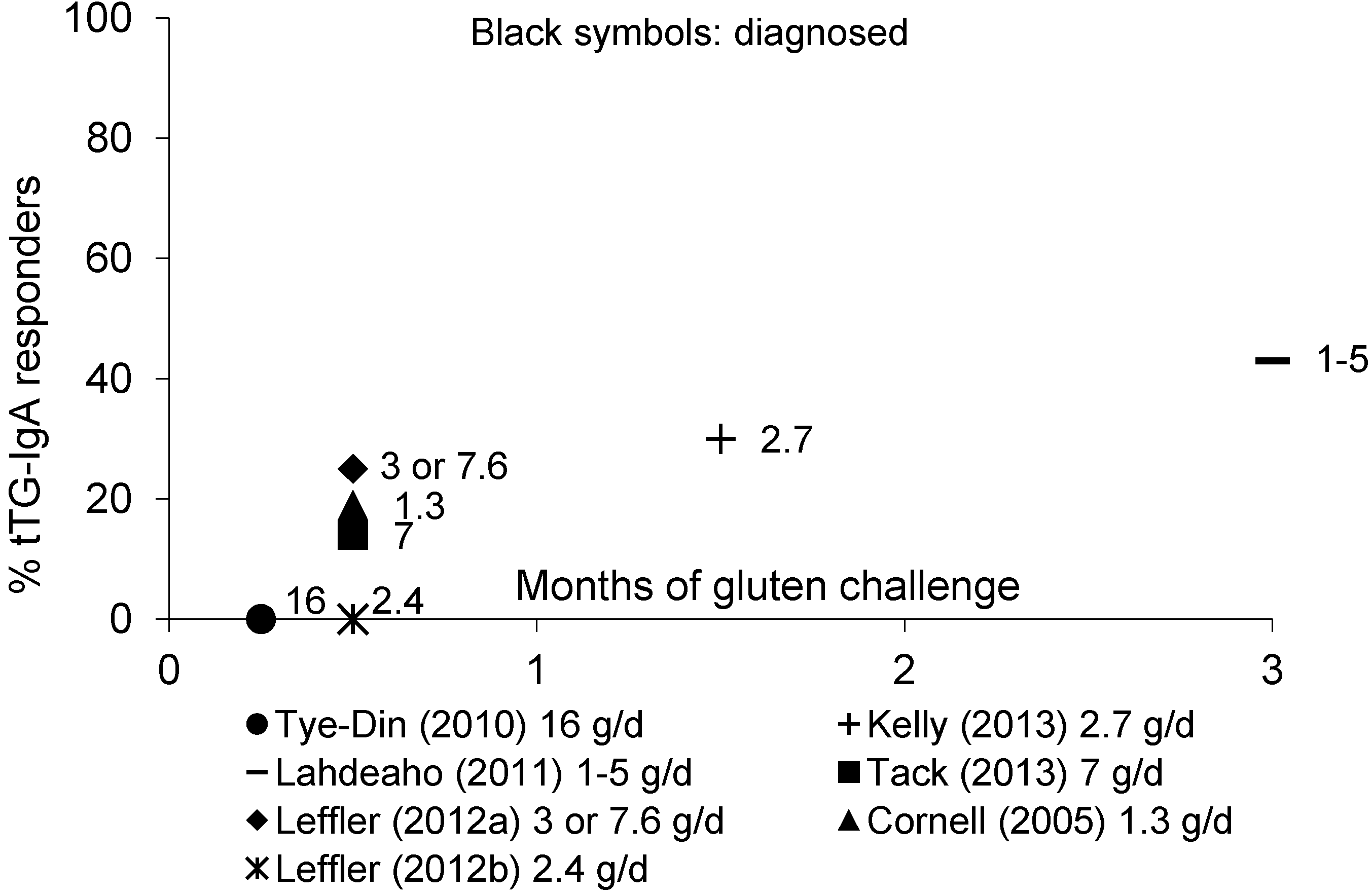
3.4.3. Antibodies in Adult Patients: Summary
3.5. Mucosal Immunohistology in Pediatric Patients with Diagnosed or Suspected CD
3.5.1. Mucosal IEL
3.5.2. Mucosal Histology
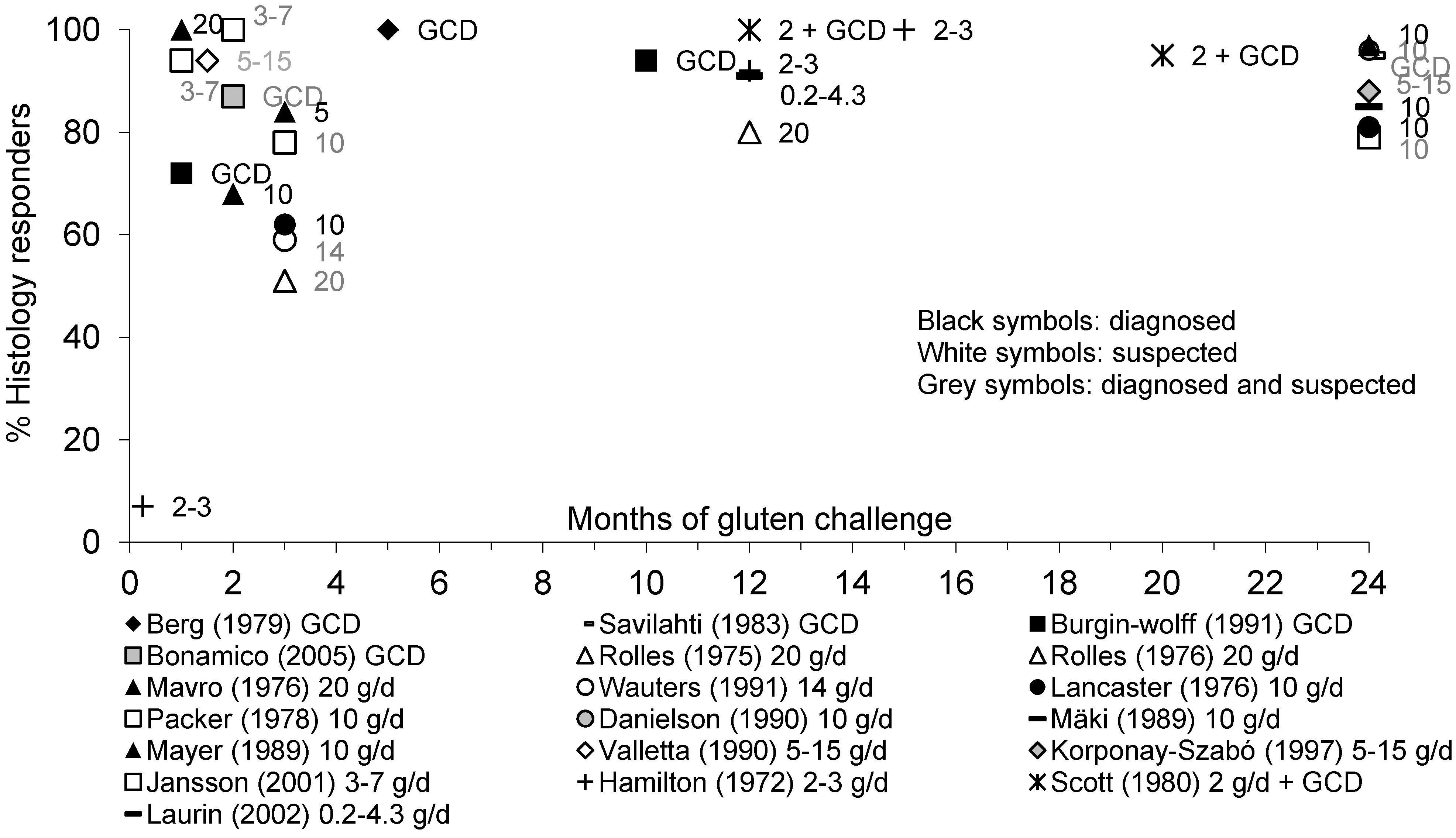
3.5.3. Mucosal Immunohistology: Summary
3.6. Mucosal Immunohistology in Adult Patients with Diagnosed or Suspected CD
3.6.1. Mucosal IEL
3.6.2. Mucosal Histology
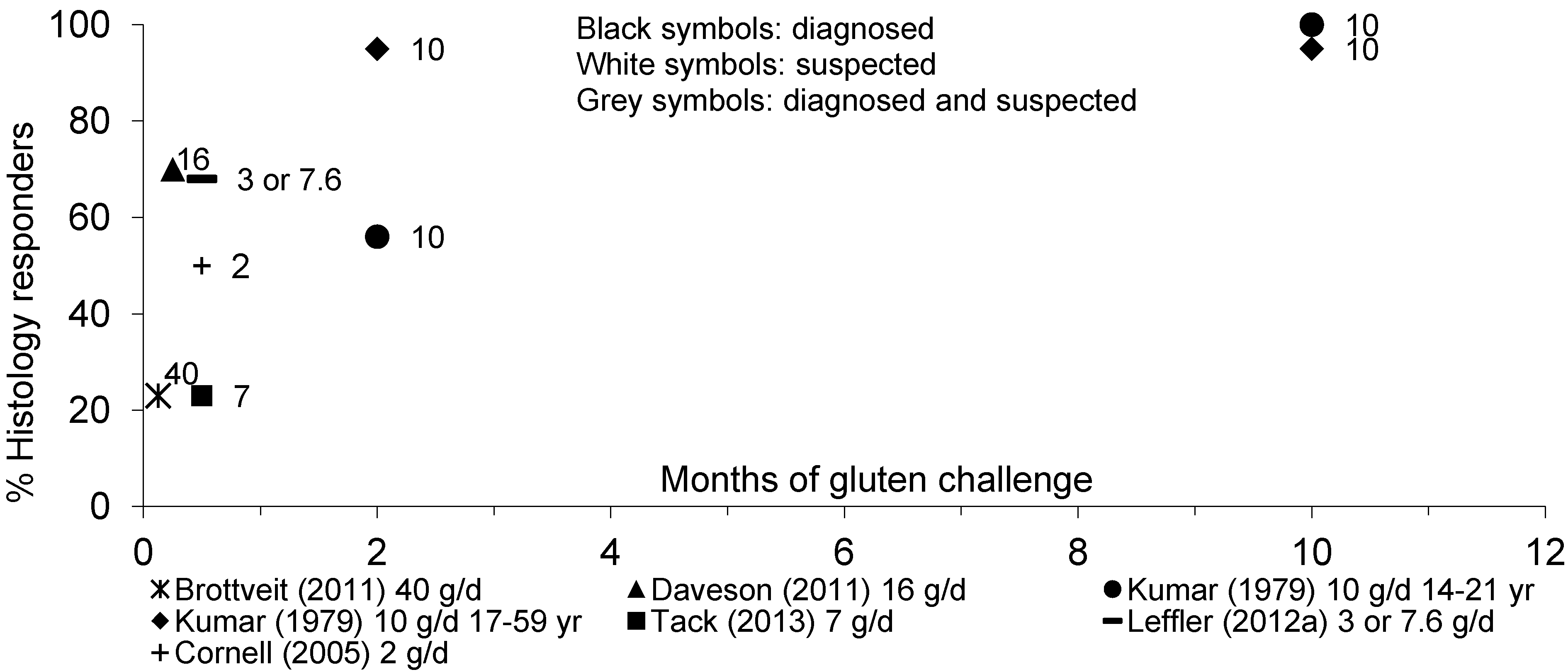
3.6.3. Mucosal tTGA-IgA Deposits
3.6.4. Mucosal Immunohistology: Summary
4. Discussion
4.1. Strength and Weaknesses
4.2. Occurrence of Symptoms in Response to Gluten
4.3. Occurrence of Antibodies in Response to Gluten
4.4. Occurrence of Histological Changes in Response to Gluten
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Coeliac Disease: Recognition and Assessment of Coeliac Disease, 2009. Available online: http://www.nice.org.uk/nicemedia/pdf/cg86fullguideline.pdf (accessed on 7 August 2013).
- Richey, R.; Howdle, P.; Shaw, E.; Stokes, T.; Guideline Development Group. Recognition and assessment of coeliac disease in children and adults: Summary of NICE guidance. BMJ 2009, 338, b1684. [Google Scholar] [CrossRef]
- Walker-Smith, J.A.; Guandalini, S.; Schmitz, J.; Shmerling, D.H.; Visakopi, J.L. Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch. Dis. Child 1990, 65, 909–911. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabo, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A.; American College of Gastroenterology. ACG clinical guidelines: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013, 108, 656–676. [Google Scholar] [CrossRef]
- Hill, I.D.; Dirks, M.H.; Liptak, G.S.; Colletti, R.B.; Fasano, A.; Guandalini, S.; Hoffenberg, E.J.; Horvath, K.; Murray, J.A.; Pivor, M.; et al. Guideline for the diagnosis and treatment of celiac disease in children: Recommendations of the North American society for pediatric gastroenterology, hepatology and nutrition. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 1–19. [Google Scholar] [CrossRef]
- Murch, S.; Jenkins, H.; Auth, M.; Bremner, R.; Butt, A.; France, S.; Furman, M.; Gillett, P.; Kiparissi, F.; Lawson, M.; et al. Joint BSPGHAN and Coeliac UK guidelines for the diagnosis and management of coeliac disease in children. Arch. Dis. Child 2013, 98, 806–811. [Google Scholar] [CrossRef]
- Catassi, C.; Fasano, A. Is this really celiac disease? Pitfalls in diagnosis. Curr. Gastroenterol. Rep. 2008, 10, 466–472. [Google Scholar] [CrossRef]
- Ravelli, A.; Bolognini, S.; Gambarotti, M.; Villanacci, V. Variability of histologic lesions in relation to biopsy site in gluten-sensitive enteropathy. Am. J. Gastroenterol. 2005, 100, 177–185. [Google Scholar] [CrossRef]
- Corazza, G.R.; Villanacci, V.; Zambelli, C.; Milione, M.; Luinetti, O.; Vindigni, C.; Chioda, C.; Albarello, L.; Bartolini, D.; Donato, F. Comparison of the interobserver reproducibility with different histologic criteria used in celiac disease. Clin. Gastroenterol. Hepatol. 2007, 5, 838–843. [Google Scholar] [CrossRef]
- Corazza, G.R.; Villanacci, V. Coeliac disease. J. Clin. Pathol. 2005, 58, 573–574. [Google Scholar] [CrossRef]
- Weile, B.; Hansen, B.F.; Hagerstrand, I.; Hansen, J.P.; Krasilnikoff, P.A. Interobserver variation in diagnosing coeliac disease. A joint study by Danish and Swedish pathologists. APMIS 2000, 108, 380–384. [Google Scholar]
- Freeman, H.J. Refractory celiac disease and sprue-like intestinal disease. World J. Gastroenterol. 2008, 14, 828–830. [Google Scholar] [CrossRef]
- Guidelines for the Management of Patients with Coeliac Disease. British Society of Gastroenterology. Available online: www.bsg.org.uk (accessed on 8 April 2013).
- United States Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 26. Available online: http://ndb.nal.usda.gov/ (accessed on 24 October 2013).
- Setty, M.; Hormaza, L.; Guandalini, S. Celiac disease: Risk assessment, diagnosis, and monitoring. Mol. Diagn. Ther. 2008, 12, 289–298. [Google Scholar] [CrossRef]
- Akobeng, A.K.; Thomas, A.G. Systematic review: Tolerable amount of gluten for people with coeliac disease. Aliment. Pharmacol. Ther. 2008, 27, 1044–1052. [Google Scholar] [CrossRef]
- Hamilton, J.R.; McNeill, L.K. Childhood celiac disease: Response of treated patients to a small uniform daily dose of wheat gluten. J. Pediatr. 1972, 81, 885–893. [Google Scholar] [CrossRef]
- Scott, H.; Ek, J.; Baklien, K.; Brandtzaeg, P. Immunoglobulin-producing cells in jejunal mucosa of children with coeliac disease on a gluten-free diet and after gluten challenge. Scand. J. Gastroenterol. 1980, 15, 81–88. [Google Scholar] [CrossRef]
- Hansson, T.; Dahlbom, I.; Rogberg, S.; Dannaeus, A.; Hopfl, P.; Gut, H.; Kraaz, W.; Klareskog, L. Recombinant human tissue transglutaminase for diagnosis and follow-up of childhood coeliac disease. Pediatr. Res. 2002, 51, 700–705. [Google Scholar] [CrossRef]
- Dutch Food Composition Database Online Version 2013/4.0. Available online: http://www.rivm.nl/en/Topics/Topics/D/Dutch_Food_Composition_Database (accessed on 24 October 2013).
- Van Overbeek, F.M.; Uil-Dieterman, I.G.; Mol, I.W.; Köhler-Brands, L.; Heymans, H.S.; Mulder, C.J. The daily gluten intake in relatives of patients with coeliac disease compared with that of the general Dutch population. Eur. J. Gastroenterol. Hepatol. 1997, 9, 1097–1099. [Google Scholar] [CrossRef]
- World Health Organisation. The WHO Child Growth Standards. Available online: http://www.who.int/childgrowth/en/ (accessed on 7 August 2013).
- Leffler, D.; Schuppan, D.; Pallav, K.; Najarian, R.; Goldsmith, J.D.; Hansen, J.; Kabbani, T.; Dennis, M.; Kelly, C.P. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut 2012, 62, 996–1004. [Google Scholar]
- Cornell, H.J.; Macrae, F.A.; Melny, J.; Pizzey, C.J.; Cook, F.; Mason, S.; Bhathal, P.S.; Stelmasiak, T. Enzyme therapy for management of coeliac disease. Anon. Scand. J. Gastroenterol. 2005, 40, 1304–1312. [Google Scholar] [CrossRef]
- Tye-Din, J.A.; Anderson, R.P.; Ffrench, R.A.; Brown, G.J.; Hodsman, P.; Siegel, M.; Botwick, W.; Shreeniwas, R. The effects of ALV003 pre-digestion of gluten on immune response and symptoms in celiac disease in vivo. Clin. Immunol. 2010, 134, 289–295. [Google Scholar] [CrossRef]
- Kelly, C.P.; Green, P.H.; Murray, J.A.; Dimarino, A.; Colatrella, A.; Leffler, D.A.; Alexander, T.; Arsenescu, R.; Leon, F.; Jiang, J.G.; et al. Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: A randomised placebo-controlled study. Aliment. Pharmacol. Ther. 2013, 37, 252–262. [Google Scholar] [CrossRef]
- Leffler, D.A.; Kelly, C.P.; Abdallah, H.Z.; Colatrella, A.M.; Harris, L.A.; Leon, F.; Arterburn, L.A.; Paterson, B.M.; Lan, Z.H.; Murray, J.A. A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am. J. Gastroenterol. 2012, 107, 1554–1562. [Google Scholar] [CrossRef]
- Tack, G.J.; van de Water, J.M.; Bruins, M.J.; Kooy-Winkelaar, E.M.; van Bergen, J.; Bonnet, P.; Vreugdenhil, A.C.; Korponay-Szabo, I.; Edens, L.; von Blomberg, B.M.; et al. Consumption of gluten with gluten-degrading enzyme by celiac patients: A pilot-study. World J. Gastroenterol. 2013, 19, 5837–5847. [Google Scholar] [CrossRef]
- Ascher, H.; Lanner, A.; Kristiansson, B. A new laboratory kit for anti-gliadin IgA at diagnosis and follow-up of childhood celiac disease. J. Pediatr. Gastroenterol. Nutr. 1990, 10, 443–450. [Google Scholar] [CrossRef]
- Mayer, M.; Greco, L.; Troncone, R.; Grimaldi, M.; Pansa, G. Early prediction of relapse during gluten challenge in childhood celiac disease. J. Pediatr. Gastroenterol. Nutr. 1989, 8, 474–479. [Google Scholar] [CrossRef]
- Rolles, C.J.; McNeish, A.S. Standardised approach to gluten challenge in diagnosing childhood coeliac disease. Br. Med. J. 1976, 1, 1309–1311. [Google Scholar] [CrossRef]
- Packer, S.M.; Charlton, V.; Keeling, J.W. Gluten challenge in treated coeliac disease. Arch. Dis. Child 1978, 53, 449–455. [Google Scholar] [CrossRef]
- Berg, N.O.; Lindberg, T. Incidence of coeliac disease and transient gluten intolerance in children in a Swedish urban community. Acta Paediatr. Scand. 1979, 68, 397–400. [Google Scholar] [CrossRef]
- Savilahti, E.; Viander, M.; Perkkio, M.; Vainio, E.; Kalimo, K.; Reunala, T. IgA antigliadin antibodies: A marker of mucosal damage in childhood coeliac disease. Lancet 1983, 1, 320–322. [Google Scholar]
- Bonamico, M.; Sabbatella, L.; di Tola, M.; Vetrano, S.; Ferri, M.; Nenna, R.; Mariani, P.; Picarelli, A. Antiendomysial antibody detection in biopsy culture allows avoidance of gluten challenge in celiac children. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 165–169. [Google Scholar] [CrossRef]
- Troncone, R.; Auricchio, R.; Granata, V. Issues related to gluten-free diet in coeliac disease. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 329–333. [Google Scholar] [CrossRef]
- Korponay-Szabo, I.R.; Kovacs, J.B.; Lorincz, M.; Gorácz, G.; Szabados, K.; Balogh, M. Prospective significance of antiendomysium antibody positivity in subsequently verified celiac disease. J. Pediatr. Gastroenterol. Nutr. 1997, 25, 56–63. [Google Scholar] [CrossRef]
- Wauters, E.A.K.; Jansen, J.; Houwen, R.H.; Veenstra, J.; Ockhuizen, T. Serum IgG and IgA anti-gliadin antibodies as markers of mucosal damage in children with suspected celiac disease upon gluten challenge. J. Pediatr. Gastroenterol. Nutr. 1991, 13, 192–196. [Google Scholar] [CrossRef]
- Valletta, E.A.; Trevisiol, D.; Mastella, G. IgA anti-gliadin antibodies in the monitoring of gluten challenge in celiac disease. J. Pediatr. Gastroenterol. Nutr. 1990, 10, 169–173. [Google Scholar] [CrossRef]
- Maki, M.; Lahdeaho, M.L.; Hallstrom, O.; Viander, M.; Visakorpi, J.K. Postpubertal gluten challenge in coeliac disease. Arch. Dis. Child 1989, 64, 1604–1607. [Google Scholar] [CrossRef]
- Laurin, P.; Wolving, M.; Fälth-Magnusson, K. Even small amounts of gluten cause relapse in children with celiac disease. J. Pediatr. Gastroenterol. Nutr. 2002, 34, 26–30. [Google Scholar] [CrossRef]
- Mavromichalis, J.; Brueton, M.J.; McNeish, A.S.; Anderson, C.M. Evaluation of the intraepithelial lymphocyte count in the jejunum in childhood enteropathies. Gut 1976, 17, 600–603. [Google Scholar] [CrossRef]
- Hansson, T.; Dannaeus, A.; Kraaz, W.; Sjöberg, O.; Klareskog, L. Production of antibodies to gliadin by peripheral blood lymphocytes in children with celiac disease: The use of an enzyme-linked immunospot technique for screening and follow-up. Pediatr. Res. 1997, 41, 554–559. [Google Scholar] [CrossRef]
- Schaad, U.B.; Gaze, H.; Hadorn, B. Intraepithelial lymphocytes before and after gluten challenge in children with celiac disease. Am. J. Dis. Child 1981, 135, 272–273. [Google Scholar]
- Burgin-Wolff, A.; Gaze, H.; Hadziselimovic, F.; Huber, H.; Lentze, M.J.; Nusslé, D.; Reymond-Berthet, C. Antigliadin and antiendomysium antibody determination for coeliac disease. Arch. Dis. Child 1991, 66, 941–947. [Google Scholar] [CrossRef]
- Bode, S.; Weile, B.; Krasilnikoff, P.A.; Gudmand-Høyer, E. The diagnostic value of the gliadin antibody test in celiac disease in children: A prospective study. J. Pediatr. Gastroenterol. Nutr. 1993, 17, 260–264. [Google Scholar] [CrossRef]
- Danielsson, L.; Stenhammar, L.; Astrom, E. Is gluten challenge necessary for the diagnosis of coeliac disease in young children? Scand. J. Gastroenterol. 1990, 25, 957–960. [Google Scholar] [CrossRef]
- Lancaster Smith, M.; Packer, S.; Kumar, P.J.; Harries, J.T. Cellular infiltrate of the jejunum after re introduction of dietary gluten in children with treated coeliac disease. J. Clin. Pathol. 1976, 29, 587–591. [Google Scholar] [CrossRef]
- Laurin, P.; Fälth-Magnusson, K.; Sundqvist, T. Increase in nitric oxide urinary products during gluten challenge in children with coeliac disease. Scand. J. Gastroenterol. 2003, 38, 55–60. [Google Scholar] [CrossRef]
- Jansson, U.H.; Gudjonsdottir, A.H.; Ryd, W.; Kristiansson, B. Two different doses of gluten show a dose-dependent response of enteropathy but not of serological markers during gluten challenge in children with coeliac disease. Acta Paediatr. 2001, 90, 255–259. [Google Scholar]
- Rolles, C.J.; Anderson, M.; McNeish, A.S. Confirming persistence of gluten intolerance in children diagnosed as having coeliac disease in infancy. Arch. Dis. Child 1975, 50, 259–263. [Google Scholar] [CrossRef]
- Lancaster-Smith, M.; Kumar, P.J.; Dawson, A.M. The cellular infiltrate of the jejunum in adult coeliac disease and dermatitis herpetiformis following the reintroduction of dietary gluten. Gut 1975, 16, 683–688. [Google Scholar] [CrossRef]
- Lahdeaho, M.L.; Maki, M.; Laurila, K.; Huhtala, H.; Kaukinen, K. Small-bowel mucosal changes and antibody responses after low- and moderate-dose gluten challenge in celiac disease. BMC Gastroenterol. 2011, 11, 129. [Google Scholar] [CrossRef]
- Montgomery, A.M.; Goka, A.K.; Kumar, P.J.; Farthing, M.J.; Clark, M.L. Low gluten diet in the treatment of adult coeliac disease: Effect on jejunal morphology and serum anti-gluten antibodies. Gut 1988, 29, 1564–1568. [Google Scholar] [CrossRef]
- Brottveit, M.; Raki, M.; Bergseng, E.; Fallang, L.E.; Simonsen, B.; Løvik, A.; Larsen, S.; Løberg, E.M.; Jahnsen, F.L.; Sollid, L.M.; et al. Assessing possible celiac disease by an HLA-DQ2-gliadin Tetramer Test. Am. J. Gastroenterol. 2011, 106, 1318–1324. [Google Scholar] [CrossRef]
- Daveson, A.J.; Jones, D.M.; Gaze, S.; McSorley, H.; Clouston, A.; Pascoe, A.; Cooke, S.; Speare, R.; Macdonald, G.A.; Anderson, R.; et al. Effect of hookworm infection on wheat challenge in celiac disease—A randomised double-blinded placebo controlled trial. PLoS One 2011, 6, e17366. [Google Scholar]
- Tack, G.J.; van de Water, J.M.; Kooy-Winkelaar, E.M.; van Bergen, J.; Meijer, G.A.; von Blomberg, B.M.; Schreurs, M.W.; Bruins, M.J.; Edens, L.; Mulder, C.J.; et al. 379 Can prolyl endoprotease enzyme treatment mitigate the toxic effect of gluten in coeliac patients? Gastroenterology 2010, 138, S54. [Google Scholar]
- Kumar, P.J.; O’Donoghue, D.P.; Stenson, K.; Dawson, A.M. Reintroduction of gluten in adults and children with treated coeliac disease. Gut 1979, 20, 743–749. [Google Scholar] [CrossRef]
- Wahab, P.J.; Crusius, J.B.A.; Meijer, J.W.; Mulder, C.J. Gluten challenge in borderline gluten-sensitive enteropathy. Am. J. Gastroenterol. 2001, 96, 1464–1469. [Google Scholar] [CrossRef]
- Kaukinen, K.; Peraaho, M.; Collin, P.; Partanen, J.; Woolley, N.; Kaartinen, T.; Nuutinen, T.; Halttunen, T.; Mäki, M.; Korponay-Szabo, I. Small-bowel mucosal transglutaminase 2-specific IgA deposits in coeliac disease without villous atrophy: A prospective and randomized clinical study. Scand. J. Gastroenterol. 2005, 40, 564–572. [Google Scholar] [CrossRef]
- Campanella, J.; Biagi, F.; Bianchi, P.I.; Zanellati, G.; Marchese, A.; Corazza, G.R. Clinical response to gluten withdrawal is not an indicator of coeliac disease. Scand. J. Gastroenterol. 2008, 43, 1311–1314. [Google Scholar] [CrossRef]
- Troncone, R.; Caputo, N.; Micillo, M.; Maiuri, L.; Poggi, V. Immunologic and intestinal permeability tests as predictors of relapse during gluten challenge in childhood coeliac disease. Scand. J. Gastroenterol. 1994, 29, 144–147. [Google Scholar] [CrossRef]
- Jansson, U.H.; Kristiansson, B.; Magnusson, P.; Larsson, L.; Albertsson-Wikland, K.; Bjarnason, R. The decrease of IGF-I, IGF-binding protein-3 and bone alkaline phosphatase isoforms during gluten challenge correlates with small intestinal inflammation in children with coeliac disease. Eur. J. Endocrinol. 2001, 144, 417–423. [Google Scholar] [CrossRef]
- Korponay-Szabo, I.; Kovacs, J.; Lorincz, M.; Körmendy, M.; Sashegyi, J. New cases of celiac disease detected by anti-endomysial antibody test in families of gluten-sensitive patients and among children examined for non-specific gastrointestinal complaints. Orv. Hetil. 1993, 134, 15–20. [Google Scholar]
- Baker, P.G.; Read, A.E. Oats and barley toxicity in coeliac patients. Postgrad. Med. J. 1976, 52, 264–268. [Google Scholar] [CrossRef]
- Anand, B.S.; Piris, J.; Truelove, S.C. The role of various cereals in coeliac disease. Q. J. Med. 1978, 47, 101–110. [Google Scholar]
- Leffler, D.A.; Dennis, M.; Edwards George, J.; Jamma, S.; Cook, E.F.; Schuppan, D.; Kelly, C.P. A validated disease-specific symptom index for adults with celiac disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1328–1334.e3. [Google Scholar] [CrossRef]
- Anderson, R.P.; van Heel, D.A.; Tye-Din, J.A.; Barnardo, M.; Salio, M.; Jewell, D.P.; Hill, A.V.S. T cells in peripheral blood after gluten challenge in coeliac disease. Gut 2005, 54, 1217–1223. [Google Scholar] [CrossRef]
- Catassi, C.; Rossini, M.; Ratsch, I.M.; Bearzi, I.; Santinelli, A.; Castagnani, R.; Pisani, E.; Coppa, G.V.; Giorgi, P.L. Dose dependent effects of protracted ingestion of small amounts of gliadin in coeliac disease children: A clinical and jejunal morphometric study. Gut 1993, 34, 1515–1519. [Google Scholar] [CrossRef]
- Hadziselimovic, F.; Bürgin-Wolff, A. P0429: Indication of gluten tolerance development in patients with coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2004, 39, S218–S219. [Google Scholar] [CrossRef]
- Murray, J.A. The widening spectrum of celiac disease. Am. J. Clin. Nutr. 1999, 69, 354–365. [Google Scholar]
- Chang, F.; Mahadeva, U.; Deere, H. Pathological and clinical significance of increased intraepithelial lymphocytes (IELs) in small bowel mucosa. APMIS 2005, 113, 385–399. [Google Scholar] [CrossRef]
- Collin, P.; Wahab, P.J.; Murray, J.A. Intraepithelial lymphocytes and coeliac disease. Best Pract. Res. Clin. Gastroenterol. 2005, 19, 341–350. [Google Scholar] [CrossRef]
- Ferguson, A.; Arranz, E.; O’Mahony, S. Clinical and pathological spectrum of coeliac disease—Active, silent, latent, potential. Gut 1993, 34, 150–151. [Google Scholar] [CrossRef]
- Koskinen, O.; Collin, P.; Korponay-Szabo, I.; Salmi, T.; Iltanen, S.; Haimila, K.; Partanen, J.; Mäki, M.; Kaukinen, K. Gluten-dependent small bowel mucosal transglutaminase 2-specific IgA deposits in overt and mild enteropathy coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 436–442. [Google Scholar] [CrossRef]
- Raki, M.; Fallang, L.E.; Brottveit, M.; Bergseng, E.; Quarsten, H.; Lundin, K.E.; Sollid, L.M. Tetramer visualization of gut-homing gluten-specific T cells in the peripheral blood of celiac disease patients. Proc. Natl. Acad. Sci. USA 2007, 104, 2831–2836. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bruins, M.J. The Clinical Response to Gluten Challenge: A Review of the Literature. Nutrients 2013, 5, 4614-4641. https://doi.org/10.3390/nu5114614
Bruins MJ. The Clinical Response to Gluten Challenge: A Review of the Literature. Nutrients. 2013; 5(11):4614-4641. https://doi.org/10.3390/nu5114614
Chicago/Turabian StyleBruins, Maaike J. 2013. "The Clinical Response to Gluten Challenge: A Review of the Literature" Nutrients 5, no. 11: 4614-4641. https://doi.org/10.3390/nu5114614
APA StyleBruins, M. J. (2013). The Clinical Response to Gluten Challenge: A Review of the Literature. Nutrients, 5(11), 4614-4641. https://doi.org/10.3390/nu5114614




