Celiac Disease, Inflammation and Oxidative Damage: A Nutrigenetic Approach
Abstract
:Abbreviations
| AA | arachidonic acid |
| COX-2 | cycloxygenase-2 |
| cPLA2 | cytosolic phospholipase A2 |
| CD | celiac disease |
| DHA | docosahexaenoic acid |
| EPA | eicosapentaenoic acid |
| GDF | gluten free diet |
| EGFR | epidermal growth factor receptor |
| HLA | human leukocytes antigen |
| IFN-γ | interferon-gamma |
| GSSG | oxidised glutathione |
| GSH | reduced glutathione |
| NO | nitric oxide |
| iNOS | inducible-nitric oxide synthase |
| PUFAs | polyunsaturated fatty acids |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| TJ | tight junctions |
| tTG | tissue transglutaminase |
1. Introduction
2. Molecular Mechanisms on the Toxic Effects of Gluten
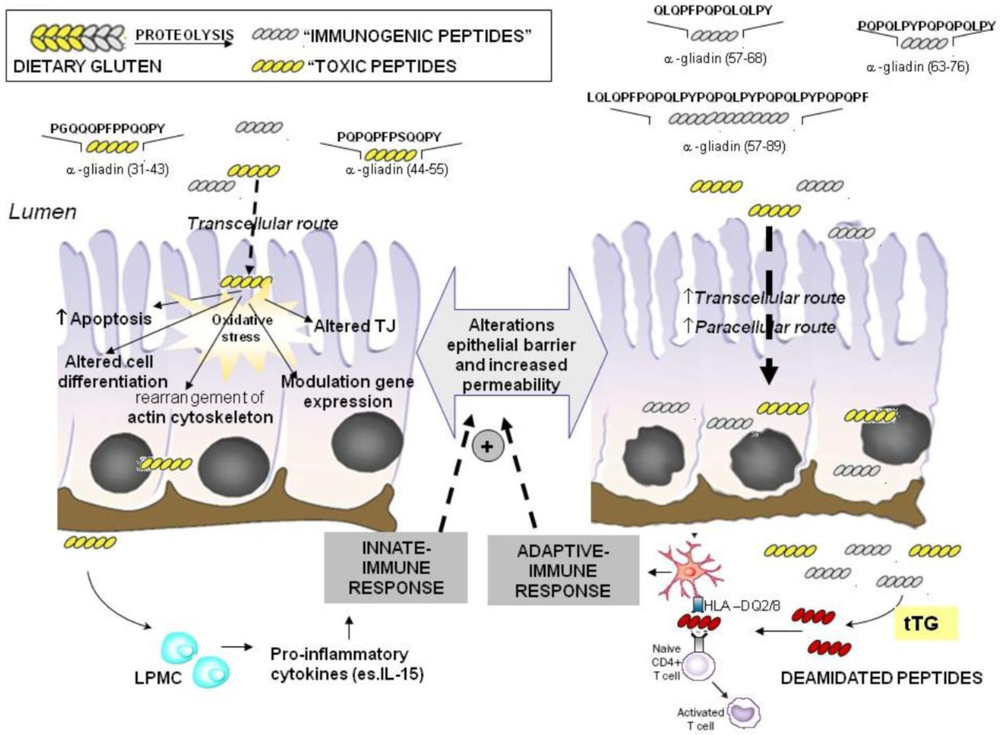
2.1. Imunomodulatory Effects of Gluten Peptides
2.2. No-Immune Mediated Cytotoxicity of Gluten: Effect on Oxidative Stress and Gene Expression
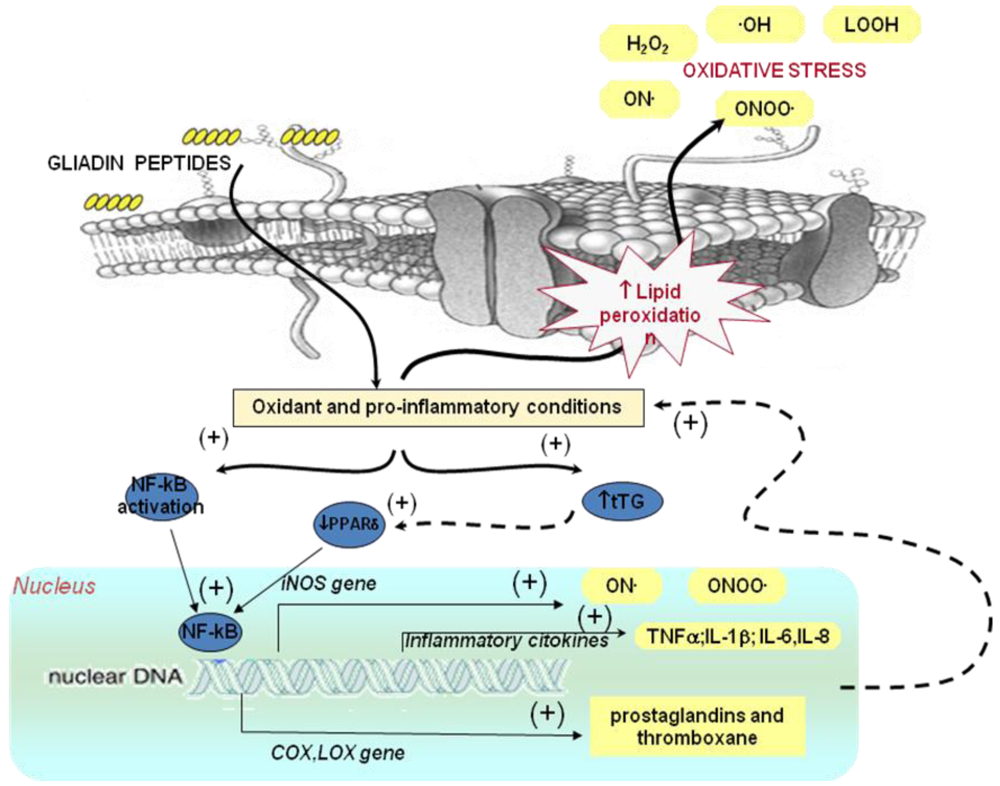
2.2.1. Effect of Gluten on Oxidative Stress
| Intestine (intestinal biopsies) | ↑ level of lipid hydroperoxides (LOOH) |
| ↓ reduced glutathione (GSH) levels | |
| ↓ glutathione peroxidase (GPx) activity | |
| ↓ glutathione reductase (GR) activity | |
| ↑ superoxide dismutase (SOD) activity | |
| ↓ paraoxonase-1 (PON1) and paraoxonase-3 (PON3) expression | |
| ↑ inducible-nitric oxide synthase (iNOS) expression | |
| Blood (plasma and blood cells) | ↑ lipid hydroperoxides (LOOH) level in plasma |
| ↑ thiobarbituric acid-reactive substances levels in plasma and lipoproteins | |
| ↑ carbonyl groups levels in plasma | |
| ↑ 8-hydroxyguanosine (8-oxodG) in DNA in leukocytes | |
| ↑ NO metabolite levels plasma | |
| ↑ nitrotyrosine levels in plasma | |
| ↓ reduced glutathione levels in plasma | |
| ↑ blood superoxide dismutase (SOD) activity | |
| ↓ blood glutathione peroxidase (GPx) activity | |
| ↓ blood glutathione reductase (GR) activity | |
| ↓ alpha-tocopherol levels in plasma and in erythrocytes | |
| ↓ plasma ascorbic acid levels | |
| ↓ plasma retinol levels | |
| Urine | ↑ 8-hydroxyguanosine (8-oxodG) in DNA metabolite levels |
2.2.2. Effect of Gluten on Gene Expression: Relationship with Oxidative Stress and Inflammation
3. Nutritional Genomics in Celiac Disease
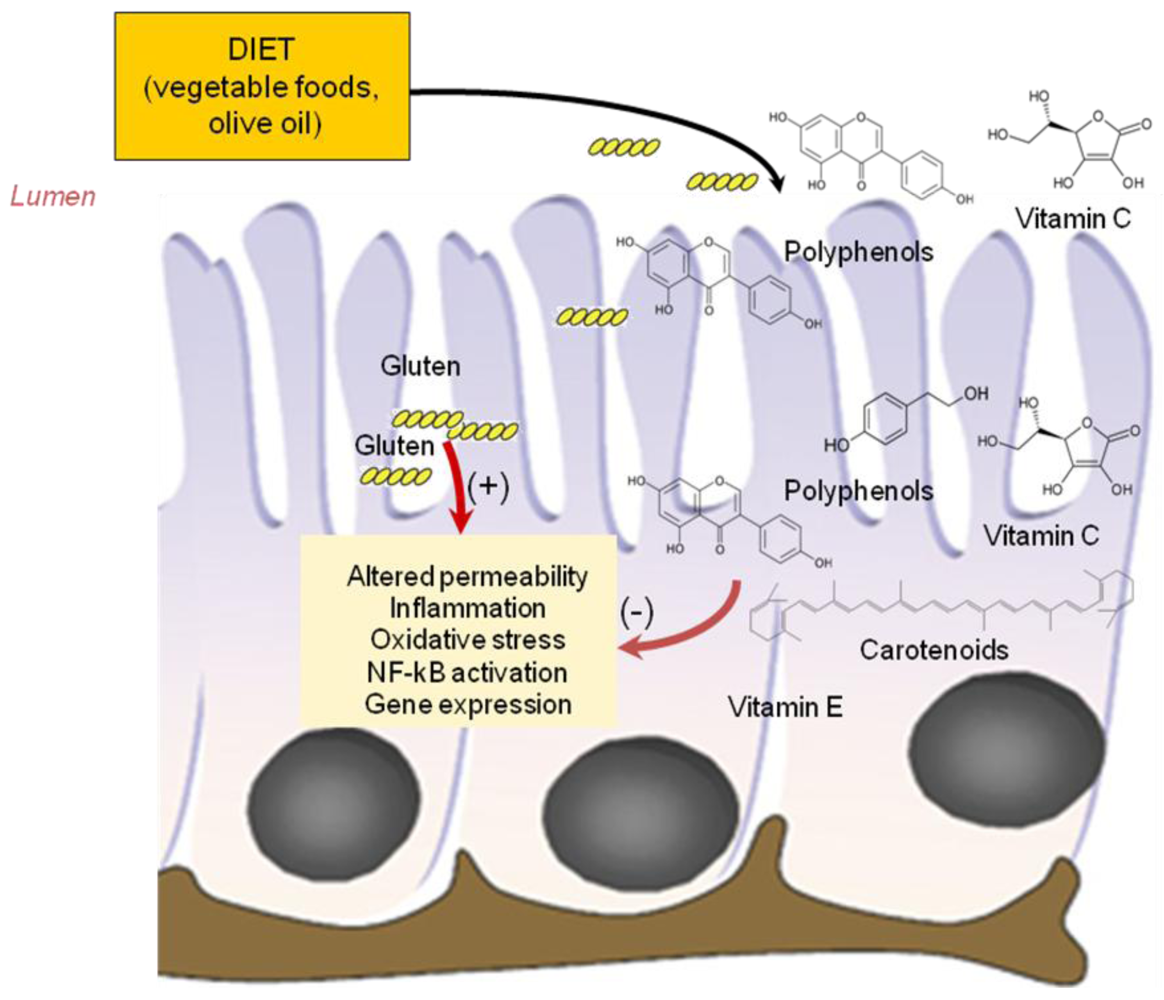
3.1. Antioxidant Vitamins
3.2. Phyochemicals: Polyphenols and Carotenoids
3.3. Fatty Acids
4. Conclusions
Conflict of Interest
References
- Sollid, L.M.; Jabri, B. Is celiac disease an autoimmune disorder? Curr. Opin. Immunol. 2005, 17, 595–600. [Google Scholar] [CrossRef]
- Van Heel, D.A.; West, J. Recent advances in coeliac disease. Gut 2006, 36, 864–874. [Google Scholar]
- Wieser, H.; Koehler, P. The biochemical basis of celiac disease. Cereal Chem. 2008, 85, 1–13. [Google Scholar]
- Diosdado, B.; van Oort, E.; Wijmenga, C. “Coelionomics”: Towards understanding the molecular pathology of coeliac disease. Clin. Chem. Lab. Med. 2005, 43, 685–695. [Google Scholar]
- Koning, F.; Schuppan, D.; Cerf-Bensussan, N.; Sollid, L.M. Pathomechanisms in celiac disease. Best Pract. Res. Clin. Gastroenterol. 2005, 19, 373–387. [Google Scholar]
- Jabri, B.; Kasarda, D.D.; Green, P.H.R. Innate and adaptive immunity: The Yin and Yang of celiac disease. Immunol. Rev. 2005, 206, 219–231. [Google Scholar]
- Mamone, G.; Picarello, G.; Addeo, F.; Ferranti, P. Proteomic analysis in allergy and intolerance to wheat products. Expert Rev. Proteomics 2011, 8, 95–115. [Google Scholar]
- Ciccocioppo, R.; di Sabatino, A.; Corazza, G.R. The immune recognition of gluten in coeliac disease. Clin. Exp. Immunol. 2005, 140, 408–416. [Google Scholar]
- Shan, L.; Molberg, Ø.; Parrot, I.; Hausch, F.; Filiz, F.; Gray, G.M.; Sollid, L.M.; Khosla, C. Structural basis for gluten intolerance in celiac sprue. Science 2002, 297, 2275–2279. [Google Scholar]
- Trynka, G.; Zhernakova, A.; Romanos, J.; Franke, L.; Hunt, K.A.; Turner, G.; Bruinenberg, M.; Heap, G.A.; Platteel, M.; Ryan, A.W.; et al. Coeliac disease-associated risk variants in TNFAIP3 and REL implicate altered NF-kappaB signalling. Gut 2009, 58, 1078–1083. [Google Scholar] [CrossRef]
- Vader, W.; Stepniak, D.; Kooy, Y.; Mearin, L.; Thompson, A.; van Rood, J.J.; Spaenij, L.; Koning, F. The HLA-DQ2 gene dose effect in celiac disease is directly related to the magnitude and breadth of gluten-specific T cell responses. Proc. Natl. Acad. Sci. USA 2003, 100, 12390–12395. [Google Scholar]
- Harmon, G.S.; Lebeck, L.K.; Weidner, N. Gluten-dependent enteropathy and atypical human leukocyte antigen alleles. Hum. Pathol. 2011, 42, 1112–1126. [Google Scholar]
- Karell, K.; Louka, A.S.; Moodie, S.J.; Ascher, H.; Clot, F.; Greco, L.; Ciclitira, P.J.; Sollid, L.M.; Partanen, J. European genetics cluster on celiac disease. Hum. Immunol. 2003, 64, 469–477. [Google Scholar]
- Calder, P.C.; Albers, R.; Antoine, J.M.; Blum, S.; Bourdet-Sicard, R.; Ferns, G.A.; Folkerts, G.; Friedmann, P.S.; Frost, G.S.; Guarner, F.; et al. Inflammatory disease processes and interactions with nutrition. Br. J. Nutr. 2009, 101, 1–45. [Google Scholar]
- Matysiak-Budnik, T.; Candalh, C.; Dugave, C.; Namane, A.; Cellier, C.; Cerf-Bensussan, N.; Heyman, M. Alterations of the intestinal tran sport and processing of gliadin peptides in celiac disease. Gastroenterology 2003, 125, 696–707. [Google Scholar]
- Trynka, G.; Wijmenga, C.; van Heel, D.A. A genetic perspective on coeliac disease. Trends Mol. Med. 2010, 16, 537–550. [Google Scholar]
- Cornell, H.J.; Wills-Johnson, G. Structure-activity relationships in coeliac-toxic gliadin peptides. Amino Acid 2001, 21, 243–253. [Google Scholar]
- Auricchio, S.; Barone, M.V.; Troncone, R. Dietary proteins and mechanismsof gastrointestinal diseases: Gliadin as a model. J. Pediatr. Gastroenterol. Nutr. 2004, 39, S738–S739. [Google Scholar]
- Hausch, F.; Shan, L.; Santiago, N.A.; Gray, G.M.; Khosla, C. Intestinal digestive resistance of immunodominant gliadin peptides. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, 996–1003. [Google Scholar]
- Folk, J.E. Mechanism and basis for specificity of transglutaminasecatalyzede-(g-glutamyl) lysine bond formation. Adv. Enzymol. Relat. Areas Mol. Biol. 1983, 54, 1–56. [Google Scholar]
- Kagnoff, M.F. Overview and pathogenesis of celiac disease. Gastroenterology 2005, 128, 10–18. [Google Scholar]
- Maiuri, L.; Ciacci, C.; Ricciardelli, I.; Vacca, L.; Raia, V.; Auricchio, S.; Picard, J.; Osman, M.; Quarantino, S.; Londei, M. Association between innate response to gliadin and activation of pathogenic T cells in celiac disease. Lancet 2003, 362, 30–37. [Google Scholar]
- Pender, S.L.F.; Lionetti, P.; Murch, S.H.; Wathan, N.; MacDonald, T.T. Proteolytic degradation of intestinal mucosa extracellular matrix after lamina propria T cell activation. Gut 1996, 39, 284–290. [Google Scholar]
- Elli, L.; Dolfini, E.; Bardella, M.T. Gliadin cytotoxicity and in vitro cell cultures. Toxicol. Lett. 2003, 46, 1–8. [Google Scholar]
- Maiuri, L.; Troncone, R.; Mayer, M.; Coletta, S.; Picarelli, A.; de Vincenzi, M.; Pavone, V.; Auricchio, S. In vitro activities of A-gliadin related synthetic peptides: Damaging affect on the atrophic coeliac mucosa and activation of mucosal immune response in the treated coeliac mucosa. Scand. J. Gastroenterol. 1996, 31, 247–253. [Google Scholar]
- Schumann, M.; Richter, J.F.; Wedell, I.; Moos, V.; Zimmermann-Kordmann, M.; Schneider, T.; Daum, S.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Mechanisms of epithelial translocation of the a2-gliadin-33mer in coeliac sprue. Gut 2008, 57, 747–754. [Google Scholar]
- Heyman, M.; Menard, S. Pathways of gliadin transport in celiac disease. Ann. N. Y. Acad. Sci. 2009, 1165, 274–278. [Google Scholar]
- Zimmer, K.P.; Fischer, I.; Mothes, T.; Weissen-Plenz, G.; Schmitz, M.; Wieser, H.; Büning, J.; Lerch, M.M.; Ciclitira, P.C.; Weber, P.; et al. Endocytotic segregation of gliadin peptide 31–49 in enterocytes. Gut 2010, 59, 300–310. [Google Scholar]
- Luciani, A.; Villella, V.R.; Vasaturo, A.; Giardino, I.; Pettoello-Mantovani, M.; Guido, S.; Cexus, O.N.; Peake, N.; Londei, M.; Quaratino, S.; et al. Lysosomal accumulation of gliadin p31–43 peptide induces oxidative stress and tissue transglutaminase-mediated PPARgamma downregulation in intestinal epithelial cells and coeliac mucosa. Gut 2010, 59, 311–319. [Google Scholar]
- Rivabene, R.; Mancini, E.; de Vincenzi, M. In vitro cytotoxic effect of wheat gliadin-derived peptides on the Caco-2 intestinal cell line is associated with intracellular oxidative imbalance: Implications for coeliac disease. Biochim. Biophys. Acta 1453, 152–160. [Google Scholar]
- Di Sabatino, A.; Ciccocioppo, R.; D’Alò, S.; Parroni, R.; Millimaggi, M.; Cifone, M.G.; Corazza, G.R. Intraepithelial and lamina propria lymphocytes show distinct patterns of apoptosis whereas both populations are active in Fas based cytotoxicity in coeliac disease. Gut 2001, 49, 380–386. [Google Scholar]
- Giovannini, C.; Sanchez, M.; Straface, E.; Scazzocchio, B.; Silano, M.; de Vincenzi, M. Induction of apoptosis in CaCo-2 cells by wheat gliadin peptides. Toxicology 2000, 145, 63–71. [Google Scholar]
- Giovannini, C.; Mancini, E.; de Vincenzi, M. Inhibition of the cellular metabolism of Caco-2 cells by prolamin peptides from cereals toxic for coeliacs. Toxicol. In Vitro 1996, 10, 533–538. [Google Scholar]
- Giovannini, C.; Maiuri, L.; de Vincenzi, M. Mytotoxic effect of prolamin-derived peptides on in vitro cultures of cell line Caco-2: Implications for coeliac disease. Toxicol. In Vitro 1994, 9, 251–255. [Google Scholar]
- Dolfini, E.; Elli, L.; Dasdia, T.; Bufardeci, B.; Colleoni, M.P.; Costa, B.; Floriani, I.; Falini, M.L.; Guerrieri, N.; Forlani, F.; et al. In vitro cytotoxic effect of bread wheat gliadin on the LoVo human adenocarcinoma cell line. Toxicol. In Vitro 2002, 16, 331–337. [Google Scholar] [CrossRef]
- Lavö, B.; Knutson, L.; Lööf, L.; Hällgren, R. Gliadin challenge-induced jejunal prostaglandin E2 secretion in celiac disease. Gastroenterologist 1990, 99, 703–709. [Google Scholar]
- Stojiljković, V.; Todorović, A.; Pejić, S.; Kasapović, J.; Saicić, Z.S.; Radlović, N.; Pajović, S.B. Antioxidant status and lipid peroxidation in small intestinal mucosa of children with celiac disease. Clin. Biochem. 2009, 42, 1431–1437. [Google Scholar]
- Stahlberg, M.R.; Hietanen, E.; Maki, M. Mucosal biotransformation rates in the small intestine of children. Gut 1988, 29, 1058–1063. [Google Scholar]
- Sido, B.; Hack, V.; Hochlehnert, A.; Lipps, H.; Herfarth, C.; Droge, W. Impairment of intestinal glutathione synthesis in patients with inflammatory bowel disease. Gut 1998, 42, 485–492. [Google Scholar]
- Daniels, I.; Cavill, D.; Murray, I.A.; Long, R.G. Elevated expression of iNOS mRNA and protein in coeliac disease. Clin. Chim. Acta 2005, 356, 134–142. [Google Scholar]
- Ter Steege, J.; Buurman, W.; Arends, J.W.; Forget, P. Presence of inducible nitric oxide synthase, nitrotyrosine, CD68, and CD14 in the small intestine in celiac disease. Lab. Invest. 1997, 77, 29–36. [Google Scholar]
- Beckett, C.G.; Dell’Olio, D.; Ellis, H.J.; Rosen-Bronson, S.; Ciclitira, P.J. The detection and localization of inducible nitric oxide synthase production in the small intestine of patients with coeliac disease. Eur. J. Gastroenterol. Hepatol. 1998, 10, 641–647. [Google Scholar]
- Holmgren Peterson, K.; Falth-Magnusson, K.; Magnusson, K.E.; Stenhammar, L.; Sundqvist, T. Children with celiac disease express inducible nitric oxide synthase in the small intestine during gluten challenge. Scand. J. Gastroenterol. 1998, 33, 939–943. [Google Scholar]
- Murray, I.A.; Daniels, I.; Coupland, K; Smith, J.A.; Long, R.G. Increased activity and expression of iNOS in human duodenal enterocytes from patients with celiac disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, 319–326. [Google Scholar]
- Murray, I.A.; Bullimore, D.W.; Long, R.G. Fasting plasma nitric oxide products in coeliac disease. Eur. J. Gastroenterol. Hepatol. 2003, 15, 1091–1095. [Google Scholar]
- Ter Steege, J.C.; Koster-Kamphuis, L.; van Straaten, E.A.; Forget, P.P.; Buurman, W.A. Nitrotyrosine in plasma of celiac disease patients as detected by a new sandwich ELISA. Free Radic. Biol. Med. 1998, 25, 953–963. [Google Scholar]
- Ertekin, V.; Selimoğlu, M.A.; Türkan, Y.; Akçay, F. Serum nitric oxide levels in children with celiac disease. J. Clin. Gastroenterol. 2005, 39, 782–785. [Google Scholar]
- Van Straaten, E.A.; Koster-Kamphuis, L.; Bovee-Oudenhoven, I.M.; van der Meer, R.; Forget, P.P. Increased urinary nitric oxide oxidation products in children with active coeliac disease. Acta Paediatr. 1999, 88, 528–531. [Google Scholar]
- Högberg, L.; Webb, C.; Fälth-Magnusson, K.; Forslund, T.; Magnusson, K.E.; Danielsson, L.; Ivarsson, A.; Sandström, O.; Sundqvist, T. Children with screening-detected coeliac disease show increased levels of nitric oxide products in urine. Acta Paediatr. 2011, 100, 1023–1027. [Google Scholar]
- Sundqvist, T.; Laurin, P.; Fälth-Magnusson, K.; Magnusson, K.E.; Stenhammar, L. Significantly increased levels of nitric oxide products in urine of children with celiac disease. J. Pediatr. Gastroenterol. Nutr. 1998, 27, 196–198. [Google Scholar]
- Stojiljković, V.; Todorović, A.; Radlović, N.; Pejić, S.; Mladenović, M.; Kasapović, J.; Pajović, S.B. Antioxidant enzymes, glutathione and lipid peroxidation in peripheral blood of children affected by coeliac disease. Ann. Clin. Biochem. 2007, 44, 537–543. [Google Scholar]
- Odetti, P.; Valentini, S.; Aragno, I.; Garibaldi, S.; Pronzato, M.A.; Rolandi, E.; Barreca, T. Oxidative stress in subjects affected by celiac disease. Free Radic. Res. 1998, 29, 7–24. [Google Scholar]
- Lavy, A.; Ben Amotz, A.; Aviram, M. Increased susceptibility to undergo lipid peroxidation of chylomicrons and low-density lipoprotein in celiac disease. Ann. Nutr. Metab. 1993, 37, 68–74. [Google Scholar]
- Szaflarska-Poplawska, A.; Siomek, A.; Czerwionka-Szaflarska, M.; Gackowski, D.; Rózalski, R.; Guz, J.; Szpila, A.; Zarakowska, E.; Olinski, R. Oxidatively damaged DNA/oxidative stress in children with celiac disease. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1960–1965. [Google Scholar]
- Hozyasz, K.K.; Chelchowska, M.; Laskowska-Klita, T. Vitamin E levels in patients with celiac disease. Med. Wieku Rozwoj. 2003, 7, 593–604. [Google Scholar]
- Rothem, L.; Hartman, C.; Dahan, A.; Lachter, J.; Eliakim, R.; Shamir, R. Paraoxonases are associated with intestinal inflammatory diseases and intracellularly localized to the endoplasmic reticulum. Free Radic. Biol. Med. 2007, 43, 730–739. [Google Scholar]
- Maiuri, M.C.; de Stefano, D.; Mele, G.; Iovine, B.; Bevilacqua, M.A.; Greco, L.; Auricchio, S.; Carnuccio, R. Gliadin increases iNOS gene expression in interferon—Stimulated RAW 264.7 cells through a mechanism involving NF-κB. Naunyn Schmiedebergs Arch. Pharmacol. 2003, 368, 63–71. [Google Scholar] [CrossRef]
- de Stefano, D.; Maiuri, M.C.; Iovine, B.; Ialenti, A.; Bevilacqua, M.A.; Carnuccio, R. The role of NF-κB, IRF-1, and STAT-1α transcription factors in the iNOS gene induction by gliadin and IFN-γin RAW 264.7 macrophages. J. Mol. Med. 2006, 84, 65–74. [Google Scholar] [CrossRef]
- Thomas, K.E.; Sapone, A.; Fasano, A.; Vogel, S.N. Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88-dependent: Role of the innate immune response in celiac disease. J. Immunol. 2006, 176, 2512–2521. [Google Scholar]
- Maiuri, L.; Ciacci, C.; Ricciardelli, I.; Vacca, L.; Raia, V.; Rispo, A.; Griffin, M.; Issekutz, T.; Quaratino, S.; Londei, M. Unexpected role of surface transglutaminase type II in celiac disease. Gastroenterology 2005, 129, 1400–1413. [Google Scholar]
- Luciani, A.; Villella, V.R.; Vasaturo, A.; Giardino, I.; Pettoello-Mantovani, M.; Guido, S.; Cexus, O.N.; Peake, N.; Londei, M.; Quaratino, S.; et al. Lysosomal accumulation of gliadin p31e43 peptide induces oxidative stress and tissue transglutaminase-mediated PPARg downregulation in intestinal epithelial cells and coeliac mucosa. Gut 2010, 59, 311–319. [Google Scholar]
- de Re, V.; Simula, M.P.; Notarpietro, A.; Canzonieri, V.; Cannizzaro, R.; Toffoli, G. Do gliadin and tissue transglutaminase mediate PPAR downregulation in intestinal cells of patients with coeliac disease? Gut 2010, 59, 1730–1731. [Google Scholar]
- Simula, M.P.; Cannizzaro, R.; Canzonieri, V.; Pavan, A.; Maiero, S.; Toffoli, G.; de Re, V. PPAR signaling pathway and cancer-related proteins are involved in celiac disease-associated tissue damage. Mol. Med. 2010, 16, 199–209. [Google Scholar]
- Maiuri, M.C.; de Stefano, D.; Mele, G.; Fecarotta, S.; Greco, L.; Troncone, R.; Carnuccio, R. Nuclear factor kappa B is activated in small intestinal mucosa of celiac patients. J. Mol. Med. 2003, 81, 373–379. [Google Scholar]
- Flohè, L.; Brigelius-Flohe, R.; Saliou, C.; Traber, M.G.; Packer, L. Redox regulation of NF-κB activation. Free Radic. Biol. Med. 1997, 22, 1115–1126. [Google Scholar]
- Ohmori, Y.; Hamilton, T.A. Cooperative interaction between interferon (IFN) stimulus response element and kappa B sequence motifs controls IFN gamma- and lipopolysaccharide-stimulated transcription from the murine IP 10 promoter. J. Biol. Chem. 1993, 268, 6677–6688. [Google Scholar]
- Vincentini, O.; Quaranta, M.G.; Viora, M.; Agostoni, C.; Silano, M. Docosahexaenoic acid modulates in vitro the inflammation of celiac disease in intestinal epithelial cells via the inhibition of cPLA2. Clin. Nutr. 2011, 30, 541–546. [Google Scholar]
- Friis, S.; Anthonsen, D.; Norén, O.; Sjöström, H. Gamma-type gliadins cause secretion of prostaglandin E2 in patients with coeliac disease. Clin. Chim. Acta 1994, 231, 173–183. [Google Scholar]
- Juuti-Uusitalo, K.; Mäki, M.; Kaukinen, K.; Collin, P.; Visakorpi, T.; Vihinen, M.; Kainulainen, H. cDNA microarray analysis of gene expression in coeliac disease jejunal biopsy samples. J. Autoimmun. 2004, 22, 249–265. [Google Scholar]
- Juuti-Uusitalo, K.; Mäki, M.; Kainulainen, H.; Isola, J.; Kaukinen, K. Gluten affects epithelial differentiation-associated genes in small intestinal mucosa of coeliac patients. Clin. Exp. Immunol. 2007, 150, 294–305. [Google Scholar]
- Cataldo, F.; Lio, D.; Marino, V.; Scola, L.; Crivello, A.; Corazza, G.R. Working Group of the SIGEP; Working Group of “Club del Tenue”. Plasma cytokine profiles in patients with celiac disease and selective IgA deficiency. Pediatr. Allergy Immunol. 2003, 14, 320–324. [Google Scholar] [CrossRef]
- Calder, P.C. Fatty acids and inflammation: The cutting edge between food and pharma. Eur. J. Pharmacol. 2011, 668, 50–58. [Google Scholar]
- Goetzl, E.J.; Wasserman, S.I.; Gigli, I.; Austen, K.F. Enhancement of random migration and chemotactic response of human leukocytes by ascorbic acid. J. Clin. Invest. 1974, 53, 813–818. [Google Scholar]
- Cook-Mills, J.M.; McCary, C.A. Isoforms of vitamin E differentially regulate inflammation. Endocr. Metab. Immune Disord. Drug Targets 2010, 10, 348–366. [Google Scholar]
- Bernardo, D.; Martínez-Abad, B.; Vallejo-Diez, S.; Montalvillo, E.; Benito, V.; Anta, B.; Fernández-Salazar, L.; Blanco-Quirós, A.; Garrote, J.A.; Arranz, E. Ascorbate-dependent decrease of the mucosal immune inflammatory response to gliadin in coeliac disease patients. Allergol. Immunopathol. 2012, 40, 3–8. [Google Scholar] [Green Version]
- Hecker, M.; Preiss, C.; Klemm, V.; Busse, R. Inhibition by antioxidants of nitric oxide synthase expression in murine macrophages: Role of nuclear factor kappa B and interferon regulatory factor 1. Br. J. Pharmacol. 1996, 118, 2178–2184. [Google Scholar]
- Heber, D.; Lu, Q.Y. Overview of mechanisms of action of lycopene. Exp. Biol. Med. 2002, 227, 920–923. [Google Scholar]
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999, 65, 337–353. [Google Scholar]
- de Stefano, D.; Maiuri, M.C.; Simeon, V.; Grassia, G.; Soscia, A.; Cinelli, M.P.; Carnuccio, R. Lycopene, quercetin and tyrosol prevent macrophage activation induced by gliadin and IFN-gamma. Eur. J. Pharmacol. 2007, 566, 192–199. [Google Scholar]
- Suzuki, T.; Hara, H. Role of flavonoids in intestinal tight junction regulation. J. Nutr. Biochem. 2011, 22, 401–408. [Google Scholar]
- Lee, J.Y.; Sohn, K.H.; Rhee, S.H.; Hwang, D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 2001, 276, 16683–16689. [Google Scholar]
- Zapata-Gonzalez, F.; Rueda, F.; Petriz, J.; Domingo, P.; Villarroya, F.; Diaz-Delfin, J.; de Madariaga, M.A.; Domingo, J.C. Human dendritic cell activities are modulated by the omega-3 fatty acid, docosahexaenoic acid, mainly through PPAR(gamma):RXR heterodimers: Comparison with other polyunsaturated fatty acids. J. Leukoc. Biol. 2008, 84, 1172–1182. [Google Scholar]
- Vincentini, O.; Quaranta, M.G.; Viora, M.; Agostoni, C.; Silano, M. Docosahexaenoic acid modulates in vitro the inflammation of celiac disease in intestinal epithelial cells via the inhibition of cPLA2. Clin. Nutr. 2011, 30, 541–546. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ferretti, G.; Bacchetti, T.; Masciangelo, S.; Saturni, L. Celiac Disease, Inflammation and Oxidative Damage: A Nutrigenetic Approach. Nutrients 2012, 4, 243-257. https://doi.org/10.3390/nu4040243
Ferretti G, Bacchetti T, Masciangelo S, Saturni L. Celiac Disease, Inflammation and Oxidative Damage: A Nutrigenetic Approach. Nutrients. 2012; 4(4):243-257. https://doi.org/10.3390/nu4040243
Chicago/Turabian StyleFerretti, Gianna, Tiziana Bacchetti, Simona Masciangelo, and Letizia Saturni. 2012. "Celiac Disease, Inflammation and Oxidative Damage: A Nutrigenetic Approach" Nutrients 4, no. 4: 243-257. https://doi.org/10.3390/nu4040243
APA StyleFerretti, G., Bacchetti, T., Masciangelo, S., & Saturni, L. (2012). Celiac Disease, Inflammation and Oxidative Damage: A Nutrigenetic Approach. Nutrients, 4(4), 243-257. https://doi.org/10.3390/nu4040243




