A Carotenoid Health Index Based on Plasma Carotenoids and Health Outcomes
Abstract
:1. Background
2. Search Methodology and Analysis
| Reference | Partition Measure | % Diff Cut 1-2 | % Diff Cut 2-3 | % Diff Cut 3-4 |
|---|---|---|---|---|
| Akbaraly 2007 [22] | quartile cutoffs | 23.5% | 8.8% | −2.4% |
| Epplein 2009 [23] | quartile cutoffs | 13.5% | 5.2% | −4.2% |
| Epplein 2009 [24] | median tertiles | 51.5% | 26.6% | 0.5% |
| Goodman 1998 [25] | quartile cutoffs | 5.6% | −1.9% | −11.2% |
| Jenab 2006 [26] | quartile cutoffs | 25.3% | 12.9% | 1.4% |
| Shardell 2011 [27] | quartile cutoffs | 14.8% | 3.1% | −2.8% |
| Yuan 2001 [28] | quartile cutoffs | 17.0% | 5.40% | 1.1% |
| Average Adjustment | +21.6% | +8.6% | −2.5% |
| Min | cut 1-2 | cut 2-3 | cut 3-4 | cut 4-5 | Max | |
|---|---|---|---|---|---|---|
| α-carotene | 0.01 | 0.07 | 0.11 | 0.16 | 0.27 | 1.06 |
| β-carotene | 0.02 | 0.24 | 0.42 | 0.64 | 1.05 | 8.00 |
| β-cryptoxanthin | 0.01 | 0.05 | 0.06 | 0.08 | 0.12 | 0.60 |
| lutein | 0.02 | 0.18 | 0.23 | 0.29 | 0.36 | 0.81 |
| zeaxanthin | 0.01 | 0.04 | 0.05 | 0.06 | 0.09 | 0.28 |
| lycopene | 0.03 | 0.31 | 0.47 | 0.67 | 1.03 | 6.47 |
| TOTAL, SUM | 0.10 | 0.89 | 1.34 | 1.90 | 2.92 | 17.22 |
| Adjustment | +23% | +14% | +6% | −2% | ||
| Adjusted SUM | 1.095 | 1.53 | 2.01 | 2.86 |
| First Author, Year | Partition of Carotenoid Concentrations † | Cutoff 1 | Cutoff 2 | Cutoff 3 | Cutoff 4 | Cutoff 5 | Benefit, Fraction ‡ | Which carotenoids? § | Which sex? | What outcome? |
|---|---|---|---|---|---|---|---|---|---|---|
| Akbaraly 2009 [29] | qnt cutoffs, men | 1.61 | 2.3 | 2.9 | yes, Qn2-5 | total | men | all cause mortality | ||
| qnt cutoffs, women | 2.3 | 3.25 | 4.04 | no | total | women | all cause mortality | |||
| Bates 2011 [30] | 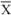 ± SD, men, calc. SD ± SD, men, calc. SD | 0.636 | 1.15 | 1.664 | yes | α-car, lut/zea | men | all cause mortality | ||
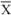 ± SD, women, calc. SD ± SD, women, calc. SD | 0.687 | 1.299 | 1.911 | no | all | women | all cause mortality | |||
| deWaart 2001 [31] | 90% range, men | 0.99 | yes | β-cryp, lut/zea | both | all cause mortality | ||||
| 90% range, women | 1.16 | yes | β-cryp, lut/zea | both | all cause mortality | |||||
| Lauretani 2008 [32] | trt cutoffs, 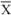 ± SD ± SD | 1.11 | 1.466 | 1.8 | 1.971 | 2.49 | yes, Qn2-5 | total | all cause mortality | |
| qrt cutoffs, indiv, adj | ||||||||||
| Li 2010 [33] | 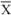 ± SD ± SD | 0.883 | 1.297 | 1.649 | yes, Qr2-4 | α-car | both | all cause mortality | ||
| Mayne 2004 [34] | qrt median, min, max | 0.58 | 1.29 | 2 | yes | lyc, α-car, total | all cause, CVD | |||
| Ray 2006 [35] | 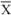 ± SD, men ± SD, men | 1.038 | 1.452 | 1.995 | yes, Qr2-4 | total | women | all cause mortality | ||
| Sahyoun 1996 [36] | 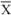 ± SD, women ± SD, women | 1.48 | 1.7 | 2.39 | 3.08 | 3.3 | yes, T2-3 | total | both | all cause mortality |
| qrt cutoffs, indiv carot, total | 1.56 | 1.7 | 2.53 | 3.08 | 3.5 | |||||
| Shardell 2011 [27] | qrt cutoffs | 1.01 | 1.33 | 1.75 | yes, Qr2-4 | total, α-car, lyc | both | all cause mortality | ||
| Akbaraly 2007 [22] | 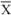 ± SD ± SD | 1.16 | 1.65 | 2.35 | yes | lyc, lut/zea | both | cognition | ||
| Alipanah 2009 [37] | 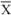 ± SD, women, 5-95% ± SD, women, 5-95% | 1.13 | 2.09 | 3.03 | yes | total | women | walking speed | ||
| Yang 2008 [38] | 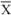 ± SD, men, indiv carot, adj ± SD, men, indiv carot, adj | 1.87 | 4.22 | 6.57 | no | women | osteoporosis | |||
| D’Odorico 2000 [39] | 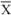 ± SD, women, indiv carot, adj ± SD, women, indiv carot, adj | 1.17 | 1.98 | 2.79 | yes, Qn5 | α-car, β-car | both | atheroscl. lesions | ||
| qnt cutoffs, indiv carot, adj | 1.46 | 2.78 | 4.1 | |||||||
| Dwyer 2004 [21] | qnt medians, indiv carot, adj | 1.313 | 2.014 | 2.86 | yes | lut, zea, β-crypt, α-car | both | intima-media thick. | ||
| Hak 2003 [40] | qrt cutoffs, total-lyc | 1.117 | 1.44 | 1.694 | 2.051 | 2.856 | no | men | 2nd myo. infarction | |
| Hozawa 2009 [41] | qrt medians, cutoffs | 1.08 | 1.29 | 1.57 | yes, Qr4 | total w/o lyc | both | hypertension | ||
| Hozawa 2007 [42] | qrt cutoffs, ranges, total | 0.829 | 1.056 | 1.357 | 1.59 | 1.99 | yes | total w/o lyc | both | inflamm. measures |
| Beydoun 2011 [43] | trt cutoffs, ranges, indiv, adj | 0.863 | 1.183 | 1.622 | yes, Qr3-4 | total | both | metabolic syndrome | ||
| Sugiura 2008 [44] | g.mean, 25, 75% indiv, adj men | 2.99 | 4.85 | yes, T3 | β-car, β-crypt | both | metabolic syndrome | |||
| Suzuki 2011 [45] | g.mean, 25, 75% indiv, adj women | 2.054 | 2.728 | 3.42 | yes, T3 | β-crypt, β-car | men | metabolic syndrome | ||
| qrt cutoffs, indiv, adj | 3.078 | 4.081 | 5.16 | yes | β-crypt, β-car, α-car | women | metabolic syndrome | |||
| Ford 2003 [46] | median, 25, 75%, indiv & total | 0.883 | 1.297 | 1.649 | yes, Qr2-4 | all 5 indiv. | both | high CRP | ||
| Hughes 2009 [47] | qrt cutoffs, total | 0.63 | 0.92 | 1.51 | no | both | isoprostanes | |||
| Akbaraly 2008 [48] | qnt medians, indiv, adjusted | 1.82 | 2.55 | 3.43 | yes, Q4 | total | both | dysglycemia | ||
| Coyne 2005 [49] | qrt cutoffs, ranges | 0.8 | 1.39 | 1.93 | 2.53 | 4.09 | yes | all 5 indiv. | both | fast glucose, OGTT |
| Hozawa 2006 [50] | trt cutoffs, indiv, adj | 0.98 | 1.29 | 1.66 | yes | total | both | diabetes | ||
| Suzuki 2002 [51] | qrt medians, indiv, adj | 3.14 | 5.03 | yes | all 5 indiv. | both | high Hb1Ac | |||
| Wang 2006 [52] | 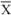 ± SD, total, β-car, cases ± SD, total, β-car, cases | 0.619 | 1.32 | 1.77 | 2.602 | no | all 5 indiv. | women | diabetes | |
| Connett 1989 [53] | 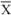 ± SD, total, β-car, controls ± SD, total, β-car, controls | 0.925 | 1.59 | 2.25 | yes, Qn4-5 | total, β-car | both | lung cancer | ||
| trt medians, total & indiv | 1.05 | 1.81 | 2.57 | |||||||
| Epplein 2009 [23] | g.mean, 5-95th%, cases | 1.67 | 2.53 | 3.72 | yes, T2-3 | total, all 5 indiv. | men | lung cancer | ||
| Ito 2003 [54] | g.mean, 5-95th%, controls | 1.74 | yes, Qr2-4 | total, α-car, β-car, lyc, crypt | both | lung cancer | ||||
| qrt cutoffs, men | 1.87 | |||||||||
| Ito 2005 [55] | qrt cutoffs, women | 1.22 | 1.69 | 2.53 | yes, Qr4 | α-car, β-car, lyc, crypt | men | lung cancer | ||
| qrt cutoffs, total & indiv. | 1.87 | 2.76 | 3.93 | no | women | lung cancer | ||||
| Yuan 2001 [28] | qrt cutoffs, cases | 0.743 | 0.941 | 1.222 | yes, Qr3-4 | β-crypt, total (smokers) | men | lung cancer | ||
| Dorjgochoo [56] | qrt cutoffs, controls | 1.89 | 2.43 | 2.99 | no | none | women | breast cancer | ||
| qrt cutoffs, total & indiv. | 1.86 | 2.21 | 2.86 | women | ||||||
| Epplein 2009 [23] | 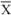 ± SD, cases ± SD, cases | 2.072 | 2.771 | 3.583 | no | none | women | breast cancer | ||
| Ito 1999 [57] | 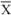 ± SD, controls ± SD, controls | 0.513 | 0.847 | 1.181 | yes | all 5 indiv.& total | women | breast cancer | ||
| trt cutoffs, indiv, adj | 0.655 | 1.181 | 1.707 | women | ||||||
| Kabat 2009 [58] | 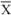 ± SD, controls ± SD, controls | 1.65 | 2.4 | yes, T3 | α-car | women | breast cancer | |||
| Maillard 2010 [59] | qrt cutoffs, medians, total | 1.39 | 2.19 | 2.99 | no | none | women | breast cancer | ||
| Rock 2005 [60] | trt cutoffs, total | 1.038 | 1.537 | 2.182 | 2.867 | 4.189 | yes, Qr4 | total | women | breast cancer |
| Rock 2009 [61] | qnt cutoffs, total & indiv | 1.656 | 2.452 | yes, T2-3 | total | women | breast cancer | |||
| Sato 2002 [62] | qnt medians, total & indiv | 1.131 | 1.683 | 2.231 | yes, Qn5 | β-car, lyc | women | breast cancer | ||
| Tamimi 2005 [63] | 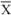 ± SD, cases ± SD, cases | 1.01 | 1.48 | 1.85 | 2.27 | 3.05 | yes, Qn5 | α-car, β-car, lut/zea, total | women | breast cancer |
| Toniolo 2001 [64] | 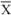 ± SD, controls ± SD, controls | 1.438 | 2.306 | 3.174 | yes, Qr2-4 | β-car, lut, cryp, total | women | breast cancer | ||
| qrt cutoffs, total, cases | 1.527 | 2.593 | 3.659 | women | ||||||
| Chang 2005 [65] | qrt cutoffs, total, control | 0.82 | 1.18 | 1.45 | yes | α-car, β-car, β-cryp, lut/zea, | men | prostate cancer | ||
| qrt medians, total & indiv | 0.88 | 1.14 | 1.63 | men | ||||||
| Gill 2009 [66] | qrt cutoffs, indiv, adj | 1.842 | 2.552 | 3.31 | 4.712 | no | none | men | prostate cancer | |
| Goodman 2003 [67] | qnt cutoffs, indiv, adj | 1.076 | 1.466 | 1.856 | yes, Qr3-4 | lut, zea, β-crypt | both | lung/prostate cancer | ||
| Huang 2003 [68] | qnt cutoffs, lyc & total | 1.03 | 1.476 | 1.91 | no | none | men | prostate cancer | ||
| Key 2007 [69] | qrt cutoffs, indiv, adj | 1.27 | 1.82 | 2.32 | yes, Qr4 | lyc, total | men | prostate cancer, esp. advanced | ||
| Lu 2001 [70] | qnt medians, indiv, adj | 0.602 | 0.832 | 1.222 | yes, Q3-4 | lyc, zea, lut, β-crypt | men | prostate cancer | ||
| Peters 2007 [71] | qrt cutoffs, min, max, indiv, adj | 1.233 | 1.777 | 2.225 | 2.708 | 3.843 | no | lyc, β-car | men | prostate cancer |
| Vogt 2002 [72] | qrt medians, indiv, adj | 0.879 | 1.285 | 1.745 | yes, Q4 | lyc | men | prostate cancer, esp. advanced | ||
| Zhang 2007 [73] | qrt cutoffs, men | 0.673 | 1.064 | 1.444 | 2.218 | yes, Q4 | lyc | men | prostate cancer | |
| Jiang 2005 [74] | qrt cutoffs, women | 1.36 | 1.92 | 2.5 | yes, Q4 | α-car, β-car, total | men | colorectal cancer | ||
| qrt medians, total & indiv | 1.91 | 2.64 | 3.06 | no | all | women | colorectal cancer | |||
| Steck-Scott 2004 [75] | trt medians, men, total & indiv | 0.972 | 1.376 | 1.78 | 2.495 | yes, Q4 | α-car, β-car, total | both | polyps in colon | |
| Wakai 2005 [76] | trt medians, women, total & indiv, | 1.48 | 1.59 | 2.21 | yes, T2-3 | total | men | colorectal cancer | ||
| qrt cutoffs, total & indiv | 2.06 | 2.55 | 2.96 | no | α-car, total | women | colorectal cancer | |||
| Jenab 2006 [26] | qrt cutoffs, medians, indiv, adj | 1.294 | 1.811 | 2.494 | yes, Qr4 | β-cryp, zea, total | both | gastric cancer | ||
| Persson 2008 [77] | qrt cutoffs, indiv, adj | 0.668 | 1.109 | 1.723 | 2.274 | 2.932 | yes, Qr3-4 | α-car, β-car | men | gastric cancer |
| Yuan 2004 [78] | qrt cutoffs, total & indiv. | 0.571 | 0.818 | 1.138 | yes, Qr4 | α-car, β-car, lyc | men | gastric cancer | ||
| Goodman 1998 [25] | trt medians, indiv, adj | 1.71 | 2.183 | 2.826 | yes, Qr3-4 | crypt, total | women | cervical dysplasia | ||
| Nagata 1999 [79] | trt cutoffs, indiv, adj | 2.357 | 3.518 | 5.707 | yes, T2-3 | α-car, lyc | women | cervical dysplasia | ||
| Schiff 2001 [80] | 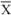 ± SD, cases ± SD, cases | 2.141 | 2.728 | yes, T3 | α-car, β-crypt, lut/zea | women | cervical dysplasia | |||
| Nomura 1997 [81] | 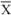 ± SD, controls ± SD, controls | 0.713 | 1.521 | 2.329 | yes, T2-3 | α-car, β-car, β-crypt, total | men | upper aerodigestive tract cancer | ||
| trt cutoffs, indiv, adj | 0.955 | 1.774 | 2.593 | men | ||||||
| Delcourt 2006 [82] | trt cutoffs, indiv, adj | 1.119 | 3.085 | yes, T2-3 | zea, lut, α-car | both | age-related maculopathy, cataract |
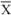 = arithmetic mean, SD = standard deviation, adj = individual carotenoids summed and adjusted to better fit total carotenoid approximations; ‡ Qn = quintile, Qr = quartile, T = tertile. The quartiles with statistically different outcomes compared to the lowest partition are listed; § α-car = α-carotene, β-car = β-carotene, lut = lutein, zea = zeaxanthin, lyc = lycopene.
= arithmetic mean, SD = standard deviation, adj = individual carotenoids summed and adjusted to better fit total carotenoid approximations; ‡ Qn = quintile, Qr = quartile, T = tertile. The quartiles with statistically different outcomes compared to the lowest partition are listed; § α-car = α-carotene, β-car = β-carotene, lut = lutein, zea = zeaxanthin, lyc = lycopene.3. Summary of Studies
| Averages across Percentiles, µM | Cutoff 1 | Cutoff 2 | Cutoff 3 | Cutoff 4 | Cutoff 5 |
|---|---|---|---|---|---|
| 10-18% | 20-40% | 50-62.5% | 66-80% | 84-90% | |
| All Studies, N = 62 | 1.114 | 1.468 | 1.893 | 2.522 | 3.069 |
| SEM ‡ | 0.078 | 0.079 | 0.085 | 0.129 | 0.204 |
| Men Only, N = 21 | 1.091 | 1.359 | 1.735 | 2.263 | 2.923 |
| Women Only, N = 28 | 1.237 | 1.800 | 2.336 | 3.025 | 3.411 |
| No Benefit studies, N = 10 | 1.251 | 1.747 | 2.357 | 2.873 | 3.641 |
| Benefit Studies, N = 52 | 1.123 | 1.463 | 1.874 | 3.012 | 3.679 |
| Carotenoid Health Index, µM | <1 | 1 to <1.5 | 1.5 to <2.5 | 2.5 to <4 | ≥4 |
| Very High Risk | High Risk | Moderate Risk | Low Risk | Very Low Risk | |
 | |||||
4. Threshold, Dose-Response, or Triage Mechanism
5. Protective Concentration of Total Carotenoids
6. Implications of the Carotenoid Health Index
7. Conclusions
Supplementary Files
Conflict of Interest
References
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit, and cancer. I. Epidemiology. Cancer Causes Control 1991, 2, 325–357. [Google Scholar] [CrossRef] [PubMed]
- Block, G.; Patterson, B.; Subar, A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr. Cancer 1992, 18, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Doll, R.; Peto, R. The causes of cancer: Quantitative estimates of avoidable risks of cancer in the United States today. J. Natl. Cancer Inst. 1981, 66, 1191–1308. [Google Scholar]
- Willett, W.C. Diet and cancer: One view at the start of the millennium. Cancer Epidemiol. Biomark. Prev. 2001, 10, 3–8. [Google Scholar]
- Bingham, S.; Luben, R.; Welch, A.; Low, Y.L.; Khaw, K.T.; Wareham, N.; Day, N. Associations between dietary methods and biomarkers, and between fruits and vegetables and risk of ischaemic heart disease, in the EPIC Norfolk Cohort Study. Int. J. Epidemiol. 2008, 37, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.A.; Luben, R.; Welch, A.; Wareham, N.; Khaw, K.-T.; Day, N. Are imprecise methods obscuring a relation between fat and breast cancer? Lancet 2003, 362, 212–214. [Google Scholar] [PubMed]
- Freedman, L.S.; Potischman, N.; Kipnis, V.; Midthune, D.; Schatzkin, A.; Thompson, F.E.; Troiano, R.P.; Prentice, R.; Patterson, R.; Carroll, R.; Subar, A.F. A comparison of two dietary instruments for evaluating the fat-breast cancer relationship. Int. J. Epidemiol. 2006, 35, 1011–1021. [Google Scholar]
- Dahm, C.C.; Keogh, R.H.; Spencer, E.A.; Greenwood, D.C.; Key, T.J.; Fentiman, I.S.; Shipley, M.J.; Brunner, E.J.; Cade, J.E.; Burley, V.J.; et al. Dietary fiber and colorectal cancer risk: A nested case-control study using food diaries. J. Natl. Cancer Inst. 2010, 102, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Liu, S.; Solomon, C.G.; Willett, W.C. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 2001, 345, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Stampfer, M.J.; Hu, F.B.; Manson, J.E.; Rimm, E.B.; Willett, W.C. Primary prevention of coronary heart disease in women through diet and lifestyle. N. Engl. J. Med. 2000, 343, 16–22. [Google Scholar]
- Platz, E.A.; Willett, W.C.; Colditz, G.A.; Rimm, E.B.; Spiegelman, D.; Giovannucci, E. Proportion of colon cancer risk that might be preventable in a cohort of middle-aged US men. Cancer Causes Control 2000, 11, 579–588. [Google Scholar]
- Mozaffarian, D.; Kamineni, A.; Carnethon, M.; Djousse, L.; Mukamal, K.J.; Siscovick, D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: The cardiovascular health study. Arch. Intern. Med. 2009, 169, 798–807. [Google Scholar]
- Bazzano, L.A.; Li, T.Y.; Joshipura, K.J.; Hu, F.B. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care 2008, 31, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Rimm, E.B.; Stampfer, M.J.; Colditz, G.A.; Ascherio, A.; Willett, W.C. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 1994, 54, 2390–2397. [Google Scholar] [PubMed]
- Williams, R. Biochemical Individuality, 1st ed; McGraw-Hill: New York, NY, USA, 1998. [Google Scholar]
- Campbell, D.R.; Gross, M.D.; Martini, M.C.; Grandits, G.A.; Slavin, J.L.; Potter, J.D. Plasma carotenoids as biomarkers of vegetable and fruit intake. Cancer Epidemiol. Biomark. Prev. 1994, 3, 493–500. [Google Scholar]
- Forman, M.; Lanza, E.; Yong, L.; Holden, J.; Graubard, B.; Beecher, G.; Meltiz, M.; Brown, E.; Smith, J. The correlation between two dietary assessments of carotenoid intake and plasma carotenoid concentrations: Application of a carotenoid food-composition database. Am. J. Clin. Nutr. 1993, 58, 519–524. [Google Scholar]
- Jansen, M.C.J.F.; van Kappel, A.L.; Ocké, M.C.; van’t Veer, P.; Boshuizen, H.C.; Riboli, E.; Bueno-de-Mesquita, H.B. Plasma carotenoid levels in Dutch men and women, and the relation with vegetable and fruit consumption. Eur. J. Clin. Nutr. 2004, 58, 1386–1395. [Google Scholar]
- Al-Delaimy, W.K.; Ferrari, P.; Slimani, N.; Pala, V.; Johansson, I.; Nilsson, S.; Mattisson, I.; Wirfalt, E.; Galasso, R.; Palli, D.; et al. Plasma carotenoids as biomarkers of intake of fruits and vegetables: Individual-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur. J. Clin. Nutr. 2005, 59, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Valtuena, S.; Pellegrini, N.; Bianchi, M.A.; Ardigo, D.; Franzini, L.; Scazzina, F.; Monti, L.; Zavaroni, I.; Brighenti, F. Intervention study with a high or low antioxidant capacity diet: Effects on circulating β-carotene. Eur. J. Clin. Nutr. 2009, 63, 1220–1225. [Google Scholar]
- Dwyer, J.H.; Paul-Labrador, M.J.; Fan, J.; Shircore, A.M.; Merz, C.N.B.; Dwyer, K.M. Progression of carotid intima-media thickness and plasma antioxidants: The los angeles atherosclerosis study. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 313–319. [Google Scholar]
- Akbaraly, N.T.; Faure, H.; Gourlet, V.; Favier, A.; Berr, C. Plasma carotenoid levels and cognitive performance in an elderly population: Results of the EVA study. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 308–316. [Google Scholar]
- Epplein, M.; Shvetsov, Y.; Wilkens, L.; Franke, A.; Cooney, R.; Le Marchand, L.; Henderson, B.; Kolonel, L.; Goodman, M. Plasma carotenoids, retinol, and tocopherols and postmenopausal breast cancer risk in the Multiethnic Cohort Study: A nested case-control study. Breast Cancer Res. 2009, 11, R49. [Google Scholar] [CrossRef] [PubMed]
- Epplein, M.; Franke, A.A.; Cooney, R.V.; Morris, J.S.; Wilkens, L.R.; Goodman, M.T.; Murphy, S.P.; Henderson, B.E.; Kolonel, L.N.; Le Marchand, L. Association of plasma micronutrient levels and urinary isoprostane with risk of lung cancer: The multiethnic cohort study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1962–1970. [Google Scholar]
- Goodman, M.T.; Kiviat, N.; McDuffie, K.; Hankin, J.H.; Hernandez, B.; Wilkens, L.R.; Franke, A.; Kuypers, J.; Kolonel, L.N.; Nakamura, J.; et al. The association of plasma micronutrients with the risk of cervical dysplasia in Hawaii. Cancer Epidemiol. Biomark. Prev. 1998, 7, 537–544. [Google Scholar]
- Jenab, M.; Riboli, E.; Ferrari, P.; Friesen, M.; Sabate, J.; Norat, T.; Slimani, N.; Tjonneland, A.; Olsen, A.; Overvad, K.; et al. Plasma and dietary carotenoid, retinol and tocopherol levels and the risk of gastric adenocarcinomas in the European prospective investigation into cancer and nutrition. Br. J. Cancer 2006, 95, 406–415. [Google Scholar]
- Shardell, M.D.; Alley, D.E.; Hicks, G.E.; El-Kamary, S.S.; Miller, R.R.; Semba, R.D.; Ferrucci, L. Low-serum carotenoid concentrations and carotenoid interactions predict mortality in US adults: The third national health and nutrition examination survey. Nutr. Res. 2011, 31, 178–189. [Google Scholar]
- Yuan, J.-M.; Ross, R.K.; Chu, X.-D.; Gao, Y.-T.; Yu, M.C. Prediagnostic Levels of serum β-cryptoxanthin and retinol predict smoking-related lung cancer risk in Shanghai, China. Cancer Epidemiol. Biomark. Prev. 2001, 10, 767–773. [Google Scholar]
- Akbaraly, T.N.; Favier, A.; Berr, C. Total plasma carotenoids and mortality in the elderly: Results of the Epidemiology of Vascular Ageing (EVA) study. Br. J. Nutr. 2009, 101, 86–92. [Google Scholar]
- Bates, C.J.; Hamer, M.; Mishra, G.D. Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: The national diet and nutrition survey of people aged 65 years and over. Br. J. Nutr. 2011, 105, 123–132. [Google Scholar]
- De Waart, F.; Schouten, E.; Stalenhoef, A.; Kok, F. Serum carotenoids, α-tocopherol and mortality risk in a prospective study among Dutch elderly. Int. J. Epidemiol. 2001, 30, 136–143. [Google Scholar]
- Lauretani, F.; Semba, R.D.; Dayhoff-Brannigan, M.; Corsi, A.M.; Di Iorio, A.; Buiatti, E.; Bandinelli, S.; Guralnik, J.M.; Ferrucci, L. Low total plasma carotenoids are independent predictors of mortality among older persons: The InCHIANTI study. Eur. J. Nutr. 2008, 47, 335–340. [Google Scholar]
- Li, C.; Ford, E.S.; Zhao, G.; Balluz, L.S.; Giles, W.H.; Liu, S. Serum α-carotene concentrations and risk of death among US Adults: The third national health and nutrition examination survey follow-up study. Arch. Intern. Med. 2011, 171, 507–515. [Google Scholar]
- Mayne, S.; Cartmel, B.; Lin, H.; Zheng, T.; Goodwin, W.J. Low plasma lycopene concentration is associated with increased mortality in a cohort of patients with prior oral, pharynx or larynx cancers. J. Am. Coll. Nutr. 2004, 23, 34–42. [Google Scholar]
- Ray, A.L.; Semba, R.D.; Walston, J.; Ferrucci, L.; Cappola, A.R.; Ricks, M.O.; Xue, Q.-L.; Fried, L.P. Low serum selenium and total carotenoids predict mortality among older women living in the community: The women’s health and aging studies. J. Nutr. 2006, 136, 172–176. [Google Scholar]
- Sahyoun, N.R.; Jacques, P.F.; Russell, R.M. Carotenoids, vitamins C and E, and mortality in an elderly population. Am. J. Epidemiol. 1996, 144, 501–511. [Google Scholar] [PubMed]
- Alipanah, N.; Varadhan, R.; Sun, K.; Ferrucci, L.; Fried, L.P.; Semba, R.D. Low serum carotenoids are associated with a decline in walking speed in older women. J. Nutr. Health Aging 2009, 13, 170–175. [Google Scholar]
- Yang, Z.; Zhang, Z.; Penniston, K.L.; Binkley, N.; Tanumihardjo, S.A. Serum carotenoid concentrations in postmenopausal women from the United States with and without osteoporosis. Int. J. Vitam. Nutr. Res. 2008, 78, 105–111. [Google Scholar]
- D’Odorico, A.; Martines, D.; Kiechl, S.; Egger, G.; Oberhollenzer, F.; Bonvicini, P.; Sturniolo, G.C.; Naccarato, R.; Willeit, J. High plasma levels of α- and β-carotene are associated with a lower risk of atherosclerosis: Results from the Bruneck study. Atherosclerosis 2000, 153, 231–239. [Google Scholar]
- Hak, A.E.; Stampfer, M.J.; Campos, H.; Sesso, H.D.; Gaziano, J.M.; Willett, W.; Ma, J. Plasma carotenoids and tocopherols and risk of myocardial infarction in a low-risk population of US male physicians. Circulation 2003, 108, 802–807. [Google Scholar]
- Hozawa, A.; Jacobs, D.R.; Steffes, M.W.; Gross, M.D.; Steffen, L.M.; Lee, D.-H. Circulating carotenoid concentrations and incident hypertension: The Coronary Artery Risk Development in Young Adults (CARDIA) study. J. Hypertens. 2009, 27, 237–242. [Google Scholar]
- Hozawa, A.; Jacobs, D.R.; Steffes, M.W.; Gross, M.D.; Steffen, L.M.; Lee, D.-H. Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: The Coronary Artery Risk Development in Young Adults (CARDIA)/Young Adult Longitudinal Trends in Antioxidants (YALTA) Study. Clin. Chem. 2007, 53, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Shroff, M.R.; Chen, X.; Beydoun, H.A.; Wang, Y.; Zonderman, A.B. Serum antioxidant status is associated with metabolic syndrome among US adults in recent national surveys. J. Nutr. 2011, 141, 903–913. [Google Scholar]
- Sugiura, M.; Nakamura, M.; Ogawa, K.; Ikoma, Y.; Matsumoto, H.; Ando, F.; Shimokata, H.; Yano, M. Associations of serum carotenoid concentrations with the metabolic syndrome: Interaction with smoking. Br. J. Nutr. 2008, 100, 1297–1306. [Google Scholar]
- Suzuki, K.; Ito, Y.; Inoue, T.; Hamajima, N. Inverse association of serum carotenoids with prevalence of metabolic syndrome among Japanese. Clin. Nutr. 2011, 30, 369–375. [Google Scholar]
- Ford, E.S.; Liu, S.; Mannino, D.M.; Giles, W.H.; Smith, S.J. C-reactive protein concentration and concentrations of blood vitamins, carotenoids, and selenium among United States adults. Eur. J. Clin. Nutr. 2003, 57, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.J.; Mayne, S.T.; Blumberg, J.B.; Ribaya-Mercado, J.D.; Johnson, E.J.; Cartmel, B. Plasma carotenoids and biomarkers of oxidative stress in patients with prior head and neck cancer. Biomark. Insights 2009, 4, 17–26. [Google Scholar]
- Akbaraly, T.N.; Fontbonne, A.; Favier, A.; Berr, C. Plasma carotenoids and onset of dysglycemia in an elderly population. Diabetes Care 2008, 31, 1355–1359. [Google Scholar]
- Coyne, T.; Ibiebele, T.I.; Baade, P.D.; Dobson, A.; McClintock, C.; Dunn, S.; Leonard, D.; Shaw, J. Diabetes mellitus and serum carotenoids: Findings of a population-based study in Queensland, Australia. Am. J. Clin. Nutr. 2005, 82, 685–693. [Google Scholar]
- Hozawa, A.; Jacobs, D.R.; Steffes, M.W.; Gross, M.D.; Steffen, L.M.; Lee, D.-H. Associations of serum carotenoid concentrations with the development of diabetes and with insulin concentration: Interaction with smoking: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Am. J. Epidemiol. 2006, 163, 929–937. [Google Scholar]
- Suzuki, K.; Ito, Y.; Nakamura, S.; Ochiai, J.; Aoki, K. Relationship between serum carotenoids and hyperglycemia: A population-based cross-sectional study. J. Epidemiol. 2002, 12, 357–366. [Google Scholar]
- Wang, L.; Liu, S.; Pradhan, A.D.; Manson, J.E.; Buring, J.E.; Gaziano, J.M.; Sesso, H.D. Plasma lycopene, other carotenoids, and the risk of type 2 diabetes in women. Am. J. Epidemiol. 2006, 164, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Connett, J.E.; Kuller, L.H.; Kjelsberg, M.O.; Polk, B.F.; Collins, G.; Rider, A.; Hulley, S.B. Relationship between carotenoids and cancer. The Multiple Risk Factor Intervention Trial (MRFIT) study. Cancer 1989, 64, 126–134. [Google Scholar] [PubMed]
- Ito, Y.; Wakai, K.; Suzuki, K.; Tamakoshi, A.; Seki, N.; Ando, M.; Nishino, Y.; Kondo, T.; Watanabe, Y.; Ozasa, K.; Ohno, Y. JACC study group serum carotenoids and mortality from lung cancer: A case-control study nested in the Japan Collaborative Cohort (JACC) study. Cancer Sci. 2003, 94, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Wakai, K.; Suzuki, K.; Ozasa, K.; Watanabe, Y.; Seki, N.; Ando, M.; Nishino, Y.; Kondo, T.; Ohno, Y.; Tamakoshi, A. Lung cancer mortality and serum levels of carotenoids, retinol, tocopherols, and folic acid in men and women: A case-control study nested in the JACC Study. J. Epidemiol. 2005, 15, S140–S149. [Google Scholar] [CrossRef] [PubMed]
- Dorjgochoo, T.; Gao, Y.-T.; Chow, W.-H.; Shu, X.-O.; Li, H.; Yang, G.; Cai, Q.; Rothman, N.; Cai, H.; Franke, A.A.; Zheng, W.; Dai, Q. Plasma carotenoids, tocopherols, retinol and breast cancer risk: Results from the Shanghai Women Health Study (SWHS). Breast Cancer Res. Treat. 2009, 117, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Gajalakshmi, K.C.; Sasaki, R.; Suzuki, K.; Shanta, V. A study on serum carotenoid levels in breast cancer patients of Indian women in Chennai (Madras), India. J. Epidemiol. 1999, 9, 306–314. [Google Scholar]
- Kabat, G.C.; Kim, M.; Adams-Campbell, L.L.; Caan, B.J.; Chlebowski, R.T.; Neuhouser, M.L.; Shikany, J.M.; Rohan, T.E. Longitudinal study of serum carotenoid, retinol, and tocopherol concentrations in relation to breast cancer risk among postmenopausal women. Am. J. Clin. Nutr. 2009, 90, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Maillard, V.; Kuriki, K.; Lefebvre, B.; Boutron‐Ruault, M.; Lenoir, G.M.; Joulin, V.; Clavel‐Chapelon, F.; Chajès, V. Serum carotenoid, tocopherol and retinol concentrations and breast cancer risk in the E3N‐EPIC study. Int. J. Cancer 2010, 127, 1188–1196. [Google Scholar]
- Rock, C.L.; Flatt, S.W.; Natarajan, L.; Thomson, C.A.; Bardwell, W.A.; Newman, V.A.; Hollenbach, K.A.; Jones, L.; Caan, B.J.; Pierce, J.P. Plasma carotenoids and recurrence-free survival in women with a history of breast cancer. J. Clin. Oncol. 2005, 23, 6631–6638. [Google Scholar]
- Rock, C.L.; Natarajan, L.; Pu, M.; Thomson, C.A.; Flatt, S.W.; Caan, B.J.; Gold, E.B.; Al-Delaimy, W.K.; Newman, V.A.; Hajek, R.A.; Stefanick, M.L.; Pierce, J.P. Women’s Healthy Eating and Living Study Group. Longitudinal biological exposure to carotenoids is associated with breast cancer-free survival in the women’s healthy eating and living study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 486–494. [Google Scholar]
- Sato, R.; Helzlsouer, K.J.; Alberg, A.J.; Hoffman, S.C.; Norkus, E.P.; Comstock, G.W. Prospective study of carotenoids, tocopherols, and retinoid concentrations and the risk of breast cance. Cancer Epidemiol. Biomark. Prev. 2002, 11, 451–457. [Google Scholar]
- Tamimi, R.M.; Hankinson, S.E.; Campos, H.; Spiegelman, D.; Zhang, S.; Colditz, G.A.; Willett, W.C.; Hunter, D.J. Plasma carotenoids, retinol, and tocopherols and risk of breast cance. Am. J. Epidemiol. 2005, 161, 153–160. [Google Scholar]
- Toniolo, P.; van Kappel, A.L.; Akhmedkhanov, A.; Ferrari, P.; Kato, I.; Shore, R.E.; Riboli, E. Serum carotenoids and breast cancer. Am. J. Epidemiol. 2001, 153, 1142–1147. [Google Scholar]
- Chang, S.; Erdman, J.W., Jr.; Clinton, S.K.; Vadiveloo, M.; Strom, S.S.; Yamamura, Y.; Duphorne, C.M.; Spitz, M.R.; Amos, C.I.; Contois, J.H.; Gu, X.; Babaian, R.J.; Scardino, P.T.; Hursting, S.D. Relationship between plasma carotenoids and prostate cancer. Nutr. Cancer 2005, 53, 127–134. [Google Scholar]
- Gill, J.K.; Franke, A.A.; Morris, J.S.; Cooney, R.V.; Wilkens, L.R.; Le Marchand, L.; Goodman, M.T.; Henderson, B.E.; Kolonel, L.N. Association of selenium, tocopherols, carotenoids, retinol, and 15-isoprostane F2t in serum or urine with prostate cancer risk: The multiethnic cohort. Cancer Causes Control 2009, 20, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Goodman, G.E.; Schaffer, S.; Omenn, G.S.; Chen, C.; King, I. The association between lung and prostate cancer risk, and serum micronutrients. Cancer Epidemiol. Biomark. Prev. 2003, 12, 518–526. [Google Scholar]
- Huang, H.-Y.; Alberg, A.J.; Norkus, E.P.; Hoffman, S.C.; Comstock, G.W.; Helzlsouer, K.J. Prospective study of antioxidant micronutrients in the blood and the risk of developing prostate cancer. Am. J. Epidemiol. 2003, 157, 335–344. [Google Scholar]
- Key, T.J.; Appleby, P.N.; Allen, N.E.; Travis, R.C.; Roddam, A.W.; Jenab, M.; Egevad, L.; Tjonneland, A.; Johnsen, N.F.; Overvad, K.; et al. Plasma carotenoids, retinol, and tocopherols and the risk of prostate cancer in the European prospective investigation into cancer and nutrition study. Am. J. Clin. Nutr. 2007, 86, 672–681. [Google Scholar] [PubMed]
- Lu, Q.-Y.; Hung, J.-C.; Heber, D.; Go, V.L.W.; Reuter, V.E.; Cordon-Cardo, C.; Scher, H.I.; Marshall, J.R.; Zhang, Z.-F. Inverse associations between plasma lycopene and other carotenoids and prostate cancer. Cancer Epidemiol. Biomark. Prev. 2001, 10, 749–756. [Google Scholar]
- Peters, U.; Leitzmann, M.F.; Chatterjee, N.; Wang, Y.; Albanes, D.; Gelmann, E.P.; Friesen, M.D.; Riboli, E.; Hayes, R.B. Serum lycopene, other carotenoids, and prostate cancer risk: A nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol. Biomark. Prev. 2007, 16, 962–968. [Google Scholar] [CrossRef]
- Vogt, T.M.; Mayne, S.T.; Graubard, B.I.; Swanson, C.A.; Sowell, A.L.; Schoenberg, J.B.; Swanson, G.M.; Greenberg, R.S.; Hoover, R.N.; Hayes, R.B.; Ziegler, R.G. Serum lycopene, other serum carotenoids, and risk of prostate cancer in US blacks and white. Am. J. Epidemiol. 2002, 155, 1023–1032. [Google Scholar]
- Zhang, J.; Dhakal, I.; Stone, A.; Ning, B.; Greene, G.; Lang, N.P.; Kadlubar, F.F. Plasma carotenoids and prostate cancer: A population-based case-control study in arkansas. Nutr. Cancer 2007, 59, 46. [Google Scholar]
- Jiang, J.; Suzuki, S.; Xiang, J.; Kuriki, K.; Hosono, A.; Arakawa, K.; Wang, J.; Nagaya, T.; Kojima, M.; Katsuda, N.; Tokudome, S. Plasma carotenoid, α-tocopherol and retinol concentrations and risk of colorectal adenomas: A case-control study in Japan. Cancer Lett. 2005, 226, 133–141. [Google Scholar]
- Steck-Scott, S.; Forman, M.R.; Sowell, A.; Borkowf, C.B.; Albert, P.S.; Slattery, M.; Brewer, B.; Caan, B.; Paskett, E.; Iber, F.; Kikendall, W.; Marshall, J.; Shike, M.; Weissfeld, J.; Snyder, K.; Schatzkin, A.; Lanza, E. he polyp prevention trial study group carotenoids, vitamin a and risk of adenomatous polyp recurrence in the polyp prevention trial. Int. J. Cancer 2004, 112, 295–305. [Google Scholar]
- Wakai, K.; Suzuki, K.; Ito, Y.; Kojima, M.; Tamakoshi, K.; Watanabe, Y.; Toyoshima, H.; Hayakawa, N.; Hashimoto, S.; Tokudome, S.; Suzuki, S.; Kawado, M.; Ozasa, K.; Tamakoshi, A. Serum carotenoids, retinol, and tocopherols, and colorectal cancer risk in a Japanese cohort: Effect modification by sex for carotenoids. Nutr. Cancer 2005, 51, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Persson, C.; Sasazuki, S.; Inoue, M.; Kurahashi, N.; Iwasaki, M.; Miura, T.; Ye, W.; Tsugane, S. lasma levels of carotenoids, retinol and tocopherol and the risk of gastric cancer in Japan: A nested case-control study. Carcinogenesis 2008, 29, 1042–1048. [Google Scholar]
- Yuan, J.-M.; Ross, R.K.; Gao, Y.-T.; Qu, Y.-H.; Chu, X.-D.; Yu, M.C. Prediagnostic levels of serum micronutrients in relation to risk of gastric cancer in Shanghai, China. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1772–1780. [Google Scholar]
- Nagata, C.; Shimizu, H.; Yoshikawa, H.; Noda, K.; Nozawa, S.; Yajima, A.; Sekiya, S.; Sugimori, H.; Hirai, Y.; Kanazawa, K.; Sugase, M.; Kawana, T. Serum carotenoids and vitamins and risk of cervical dysplasia from a case-control study in Japan. Br. J. Cancer 1999, 81, 1234–1237. [Google Scholar]
- Schiff, M.A.; Patterson, R.E.; Baumgartner, R.N.; Masuk, M.; van Asselt-King, L.; Wheeler, C.M.; Becker, T.M. Serum carotenoids and risk of cervical intraepithelial neoplasia in southwestern American Indian women. Cancer Epidemiol. Biomark. Prev. 2001, 10, 1219–1222. [Google Scholar]
- Nomura, A.M.; Ziegler, R.G.; Stemmermann, G.N.; Chyou, P.H.; Craft, N.E. Serum micronutrients and upper aerodigestive tract cancer. Cancer Epidemiol. Biomark. Prev. 1997, 6, 407–412. [Google Scholar]
- Delcourt, C.; Carriere, I.; Delage, M.; Barberger-Gateau, P.; Schalch, W. POLA Study Group. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: The POLA study. Invest. Ophthalmol. Vis. Sci. 2006, 47, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.C.; Ames, B.N. Vitamin K, an example of triage theory: Is micronutrient inadequacy linked to diseases of aging? Am. J. Clin. Nutr. 2009, 90, 889–907. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Seo, J.H.; Kim, H. β-carotene and lutein inhibit hydrogen peroxide-induced activation of NF-κB and IL-8 expression in gastric epithelial AGS cells. J. Nutr. Sci. Vitaminol. 2011, 57, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.P.; Chan, G.M.; Barrett-Reis, B.M.; Fulton, A.B.; Hansen, R.M.; Ashmeade, T.L.; Oliver, J.S.; Mackey, A.D.; Dimmit, R.A.; Hartmann, E.E.; Adamkin, D.H. Effect of carotenoid supplementation on plasma carotenoids, inflammation and visual development in preterm infants. J. Perinatol. 2011. [Google Scholar] [CrossRef]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Noda, K.; Imamura, Y.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neuroprotective effect of an antioxidant, lutein, during retinal inflammation. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1433–1439. [Google Scholar]
- Hantz, H.L.; Young, L.F.; Martin, K.R. Physiologically attainable concentrations of lycopene induce mitochondrial apoptosis in LNCaP human prostate cancer cells. Exp. Biol. Med. 2005, 230, 171–179. [Google Scholar]
- Hwang, E.-S.; Bowen, P.E. Cell cycle arrest and induction of apoptosis by lycopene in LNCaP human prostate cancer cells. J. Med. Food 2004, 7, 284–289. [Google Scholar]
- Kotake-Nara, E.; Kushiro, M.; Zhang, H.; Sugawara, T.; Miyashita, K.; Nagao, A. Carotenoids affect proliferation of human prostate cancer cells. J. Nutr. 2001, 131, 3303–3306. [Google Scholar]
- Livny, O.; Kaplan, I.; Reifen, R.; Polak-Charcon, S.; Madar, Z.; Schwartz, B. Lycopene inhibits proliferation and enhances gap-junction communication of KB-1 human oral tumor cells. J. Nutr. 2002, 132, 3754–3759. [Google Scholar]
- Zhang, L.-X.; Cooney, R.V.; Bertram, J.S. Carotenoids enhance gap junctional communication and inhibit lipid peroxidation in C3H/10T1/2 cells: Relationship to their cancer chemopreventive action. Carcinogenesis 1991, 12, 2109–2114. [Google Scholar]
- Palozza, P.; Serini, S.; Maggiano, N.; Angelini, M.; Boninsegna, A.; Di Nicuolo, F.; Ranelletti, F.O.; Calviello, G. Induction of cell cycle arrest and apoptosis in human colon adenocarcinoma cell lines by β-carotene through down-regulation of cyclin A and Bcl-2 family proteins. Carcinogenesis 2002, 23, 11–18. [Google Scholar]
- Palozza, P.; Serini, S.; Boninsegna, A.; Bellovino, D.; Lucarini, M.; Monastra, G.; Gaetani, S. The growth-inhibitory effects of tomatoes digested in vitro in colon adenocarcinoma cells occur through down regulation of cyclin D1, Bcl-2 and Bcl-xL. Br. J. Nutr. 2007, 98, 789–795. [Google Scholar] [PubMed]
- Palozza, P.; Bellovino, D.; Simone, R.; Boninsegna, A.; Cellini, F.; Monastra, G.; Gaetani, S. Effect of β-carotene-rich tomato lycopene β-cyclase (tlcy-b) on cell growth inhibition in HT-29 colon adenocarcinoma cells. Br. J. Nutr. 2009, 102, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Sestito, R.; Picci, N.; Lanza, P.; Monego, G.; Ranelletti, F.O. The sensitivity to β-carotene growth-inhibitory and proapoptotic effects is regulated by caveolin-1 expression in human colon and prostate cancer cells. Carcinogenesis 2008, 29, 2153–2161. [Google Scholar]
- Hwang, E.-S.; Lee, H.J. Inhibitory effects of lycopene on the adhesion, invasion, and migration of SK-Hep1 human hepatoma cells. Exp. Biol. Med. 2006, 231, 322–327. [Google Scholar]
- Huang, C.-S.; Liao, J.-W.; Hu, M.-L. Lycopene inhibits experimental metastasis of human hepatoma SK-Hep-1 cells in athymic nude mice. J. Nutr. 2008, 138, 538–543. [Google Scholar]
- Chalabi, N.; Satih, S.; Delort, L.; Bignon, Y.-J.; Bernard-Gallon, D.J. Expression profiling by whole-genome microarray hybridization reveals differential gene expression in breast cancer cell lines after lycopene exposure. Biochim. Biophys. Acta 2007, 1769, 124–130. [Google Scholar]
- Centers for Disease Control and Prevention (CDC), National Report on Biochemical Indicators of Diet and Nutrition-Vitamins A and E and Carotenoids; CDC: Atlanta, GA, USA, 2008.
- Maharshak, N.; Shapiro, J.; Trau, H. Carotenoderma-A review of the current literature. Int. J. Dermatol. 2003, 42, 178–181. [Google Scholar]
- The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [CrossRef] [PubMed]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Donaldson, M.S. A Carotenoid Health Index Based on Plasma Carotenoids and Health Outcomes. Nutrients 2011, 3, 1003-1022. https://doi.org/10.3390/nu3121003
Donaldson MS. A Carotenoid Health Index Based on Plasma Carotenoids and Health Outcomes. Nutrients. 2011; 3(12):1003-1022. https://doi.org/10.3390/nu3121003
Chicago/Turabian StyleDonaldson, Michael S. 2011. "A Carotenoid Health Index Based on Plasma Carotenoids and Health Outcomes" Nutrients 3, no. 12: 1003-1022. https://doi.org/10.3390/nu3121003
APA StyleDonaldson, M. S. (2011). A Carotenoid Health Index Based on Plasma Carotenoids and Health Outcomes. Nutrients, 3(12), 1003-1022. https://doi.org/10.3390/nu3121003




