Vitamin A–Not for Your Eyes Only: Requirement for Heart Formation Begins Early in Embryogenesis
Abstract
:Abbreviations
| RA | all-trans-retinoic acid |
| VAD | vitamin A-deficient |
| HH | Hamburger & Hamilton developmental stages for avian embryo |
| ss | somite stage |
| IFT | inflow tract |
1. Function of Vitamin A, Retinoic Acid, Retinoid Receptors and Gene Regulation
2. Vitamin A in Embryonic Development
3. Vitamin A Deficiency, Heart Development and the Avian Embryo
4. Vitamin A is Required to Build the Link Between the Primordial Heart and its Blood Supply
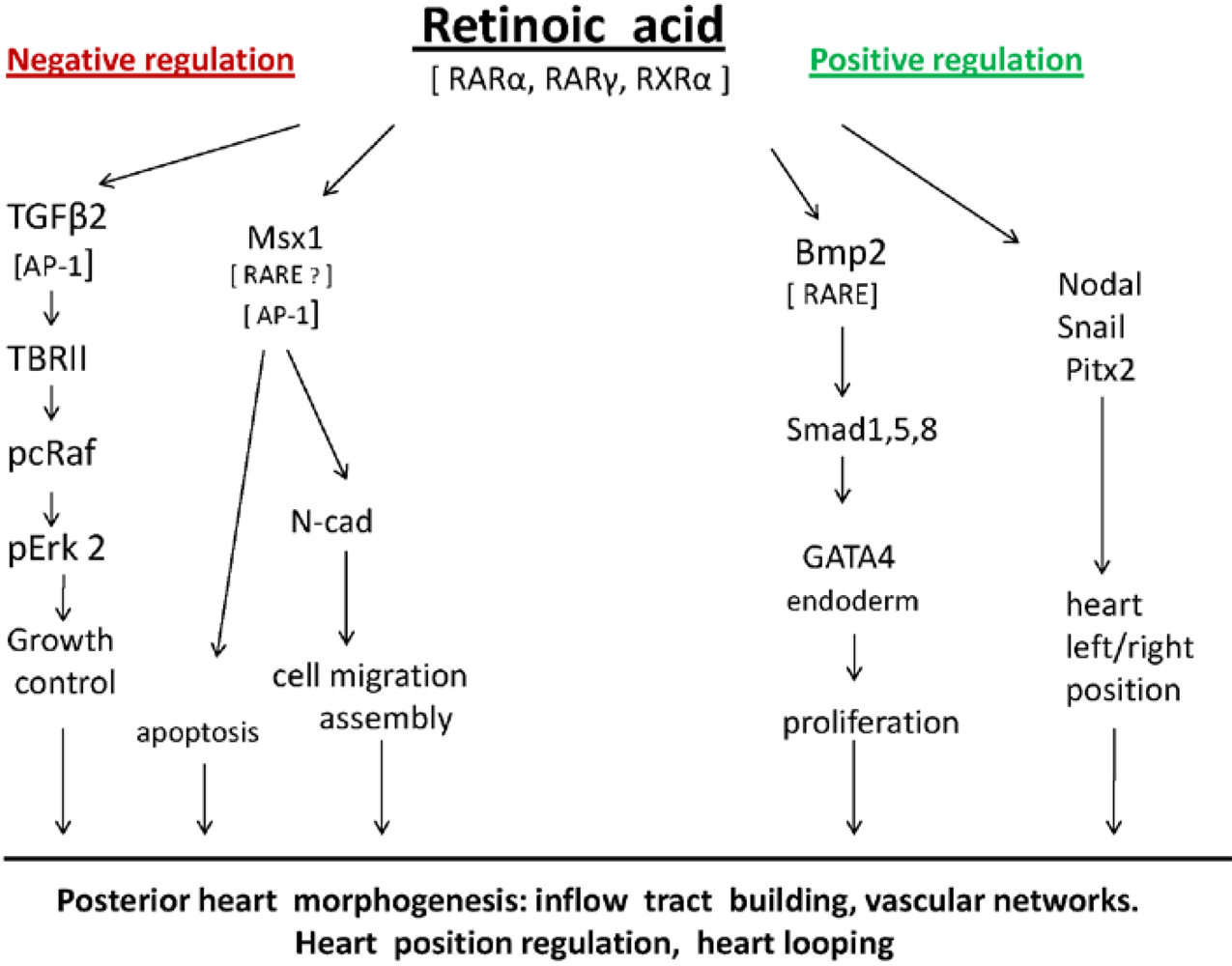
5. Molecules Involved in Retinoic Acid-Regulated Building of the Heart Inflow Tracts
6. Retinoic Acid Regulates N-cadherin During Early Heart Formation [82]
7. TGFβ2 is Negatively Regulated by Endogenous Retinoic Acid during Early Heart Formation
8. Summary and Conclusions
Acknowledgements
References
- Moore, T. Vitamin A; Elsevier Publishing Company: Amsterdam, The Netherlands, 1957. [Google Scholar]
- Blomhoff, R. Overview of vitamin A metabolism and function. In Vitamin A in Health and Disease; Blomhoff, R., Ed.; Marcel Dekker: New York, NY, USA, 1994; pp. 1–35. [Google Scholar]
- Thompson, J.N. The role of vitamin A in reproduction. In The Fat Soluble Vitamins; DeLuca, H.F., Suttie, J.W., Eds.; University of Wisconsin Press: Madison, WI, USA, 1969; pp. 267–281. [Google Scholar]
- Roberts, A.B.; Glick, A.B.; Sporn, M.B. Interrelationships between two families of multifunctional effectors: retinoids and TGF-ß. In Retinoids in Normal Development and Teratogenesis; Morris-Kay, G., Ed.; Oxford Science Public: Oxford, UK, 1992; pp. 137–148. [Google Scholar]
- Gudas, L.J.; Sporn, M.B.; Roberts, A.B. Cellular biology and biochemistry of the retinoids. In The Retinoids: Biology, Chemistry, and Medicine, 2nd; Sporn, M.B., Roberts, A.B., Goodman, D.S., Eds.; Raven Press: New York, NY, USA, 1994; pp. 443–520. [Google Scholar]
- Harvat, B.; Jetten, A. Growth control by retinoids: regulation of cell cycle progression and apoptosis. In Handbook of Experimental Pharmacology, vol. 139, Retinoids, The Biochemical and Molecular Basis of Vitamin A and Retinoid Action; Nau, H., Blaner, W.S., Eds.; Springer-Verlag: Heidelberg, Germany, 1999; pp. 240–276. [Google Scholar]
- Ross, S.A.; McCaffery, P.J.; Drager, U.C.; DeLuca, L.M. Retinoids in embryonal development. Physiol. Rev. 2000, 80, 1021–1054. [Google Scholar]
- Balmer, J.E.; Blomhoff, R. Gene expression regulation by retinoic acid. J. Lipid Res. 2002, 43, 1773–1808. [Google Scholar]
- Clagett-Dame, M.; DeLuca, H.F. The role of vitamin A in mammalian reproduction and embryonic development. Annu. Rev. Nutr. 2002, 22, 347–381. [Google Scholar]
- Manolescu, D.-C.; Sima, A.; Bhat, P.V. All-trans-retinoic acid lowers serum retinol-binding protein 4 concentrations and increases insulin sensitivity in diabetic mice. J. Nutr. 2010, 140, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Piedrafita, F.J.; Pfahl, M. Nuclear retinoid receptors and mechanisms of action. In Handbook of Experimental Pharmacology, vol.139, Retinoids, The Biochemical and Molecular Basis of Vitamin A and Retinoid Action; Nau, H., Blaner, W.S., Eds.; Springer-Verlag: Heidelberg, Germany, 1999; pp. 154–184. [Google Scholar]
- Bastien, J.; Rochette-Egly, C. Nuclear retinoid receptors and the transcription of retinoid target genes. Gene 2004, 328, 1–16. [Google Scholar]
- Rochette-Egly, C.; Germain, P. Dynamic and combinatorial control of gene expression by nuclear retinoic acid receptors (RARs). Nuclear Receptor Signaling 2009, 7, e005. [Google Scholar]
- Dolle, P. Developmental expression of retinoic acid receptors (RARs). Nuclear Receptor Signaling 2009, 7, e006. [Google Scholar]
- Mark, M.; Ghyselinck, N.B.; Chambon, P. Function of retinoic acid receptors during embryonic development. Nuclear Receptor Signaling 2009, 7, e002. [Google Scholar]
- Mascrez, B.; Gyselinck, N.B.; Chambon, P.; Mark, M. A transcriptionally silent RXRα supports early embryonic morphogenesis and heart development. Proc. Natnl. Acad. Sci. USA. 2009, 106, 4272–4277. [Google Scholar]
- Shaw, N.; Elholm, M.; Noy, N. Retinoic acid is a high affinity selective ligand for the peroxisome proliferator-activated receptor. J. Biol. Chem. 2003, 278, 41589–41592. [Google Scholar]
- Schug, T.T.; Berry, D.C.; Shaw, N.S.; Travis, S.N.; Noy, N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell 2007, 129, 723–733. [Google Scholar]
- Berry, D.C.; Noy, N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor β/* and retinoic acid receptor. Mol. Cellular Biol. 2009, 29, 3286–3296. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, Y.C.; Na, S.Y.; Jung, D.; Lee, S.K. Transcriptional coregulators of the nuclear receptor superfamily: coactivators and corepressors. Cell Mol. Life Sci. 2001, 58, 289–297. [Google Scholar]
- Gupta, P.; Ho, P.-C.; Huq, M.D.M.; Ha, S.G.; Park, S.W.; Khan, A.A.; Tsai, N.-P.; Wei, L.-N. Retinoic acid-stimulated sequential phosphorylation, PML recruitment, and SUMOylation of nuclear receptor TR2 to suppress Oct4 expression. Proc. Natnl. Acad. Sci. USA. 2008, 105, 11424–11429. [Google Scholar]
- Laserna, E.J.; Valero, M.L.; Sanz, L.; Sanchez del Pino, M.M.; Calvete, J.J.; Barettino, D. Proteomic analysis of phosphorylated nuclear proteins underscores novel roles for rapid actions of retinoic acid in the regulation of mRNA splicing and translation. Mol. Endocrinol. 2009, 23, 1799–1814. [Google Scholar]
- Wilson, J.G.; Roth, C.B.; Warkany, J. An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am. J. Anat. 1953, 92, 189–217. [Google Scholar] [CrossRef] [PubMed]
- Hale, F. Relation of maternal vitamin A deficiency to microphthalmia in pigs. Texas State J. Med. 1937, 33, 228–232. [Google Scholar]
- Mason, K.E. Fetal death, prolonged gestation, and difficult parturition in the rat as a result of vitamin A deficiency. Am. J. Anat. 1935, 57, 303–349. [Google Scholar] [CrossRef]
- Dong, D.; Zile, M.H. Endogenous retinoids in the early avian embryo. Biochem. Biophys. Res. Commun. 1995, 217, 1026–1031. [Google Scholar]
- Swindell, E.C.; Thaller, C.; Sockanathan, S.; Petkovich, M.; Jessel, T.M.; Eichele, G. Complementary domains of retinoic acid production and degradation in the early chick embryo. Dev. Biol. 1999, 216, 282–296. [Google Scholar]
- Ulven, S.M.; Gundersen, T.E.; Weedon, M.S.; Landaas, V.Q.; Sakhi, A.K.; Fromm, S.H.; Geronimo, B.A.; Moskaug, J.O.; Blomhoff, R. Identification of endogenous retinoids, enzymes, binding proteins, and receptors during early postimplantation development in mouse: important role of retinal dehydrogenase type 2 in synthesis of all-trans- retinoic acid. Dev. Biol. 2000, 220, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Marletaz, F.; Holland, L.Z.; Laudet, V.; Schubert, M. Retinoic acid signaling and the evolution of chordates. Int. J. Biol. Sci. 2006, 2, 38–47. [Google Scholar]
- Campo-Paysaa, F.; Marletaz, F.; Laudet, V.; Schubert, M. Retinoic acid signaling in development: tissue-specific functions and evolutionary origins. Genesis 2008, 46, 640–656. [Google Scholar]
- Zile, M.H. Avian embryo as model for retinoid function in early development. In Handbook of Experimental Pharmacology, Retinoids, The Biochemical and Molecular Basis of Vitamin A and Retinoid Action; Nau, H., Blaner, W.S., Eds.; Springer-Verlag: Heidelberg, Germany, 1999; pp. 443–464. [Google Scholar]
- Zile, M.H. Vitamin A requirement for early cardiovascular morphogenesis specification in the vertebrate embryo: Insights from the avian embryo. Exp. Biol. Med. 2004, 229, 598–606. [Google Scholar]
- Mark, M.; Gyselinck, N.B.; Chambon, P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathways during mouse embryogenesis. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 451–480. [Google Scholar]
- Pan, J.; Baker, K.M. Retinoic acid and the heart. Vitam. Horm. 2007, 75, 257–283. [Google Scholar]
- See, A. W.-M.; Kaiser, M.E.; White, J.C.; Clagett-Dame, M. A nutritional model of late embryonic vitamin A deficiency produces defects in organogenesis at a high penetrance and reveals new roles for the vitamin in skeletal development. Dev. Biol. 2008, 316, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Collins, M.D. All-trans-retinoic acid-induced ectopic limb and caudal structures: murine strain sensitivities and pathogenesis. Dev. Dyn. 2008, 237, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- See, A.W.; Clagett-Dame, M. The temporal requirement for vitamin A in the developing eye: mechanism of action in optic fissure closure and new roles for the vitamin in regulating cell proliferation and adhesion in the embryonic retina. Dev. Biol. 2009, 325, 94–105. [Google Scholar]
- Waxman, J.S.; Yelon, D. Increased Hox activity mimics the teratogenic effects of excess retinoic acid signaling. Dev. Dyn. 2009, 238, 1207–1213. [Google Scholar]
- Shenefelt, R.E. Morphogenesis of malformations in hamsters caused by retinoic acid: relation to dose and stage at treatment. Teratology 1972, 5, 103–118. [Google Scholar]
- Osmond, M.K.; Butler, A.J.; Voon, F.C.T.; Bellairs, R. The effects of retinoic acid on heart formation in the early chick embryo. Development 1991, 113, 1405–1417. [Google Scholar]
- Wood, H.; Gurman, P.; Morriss-Kay, G. Exposure to retinoic acid before or after the onset of somitogenesis reveals separate effects on rhombomeric segmentation and 3' HoxB gene expression domains. Development 1994, 120, 2279–2285. [Google Scholar] [PubMed]
- Zhang, Z.; Balmer, J.E.; Lovlie, A.; Fromm, S.H.; Blomhoff, R. Specific teratogenic effects of different retinoic acid isomers and analogs in the developing anterior central nervous system of zebrafish. Dev. Dyn. 1996, 206, 73–86. [Google Scholar]
- Avantaggiato, V.; Acampora, D.; Tuorto, F.; Simeone, A. Retinoic acid induces stage- specific repatterning of the rostral central nervous system. Dev. Biol. 1996, 175, 347–357. [Google Scholar]
- Colon-Teicher, L.S.; Dugyala, R.R.; Sharma, R.P. Temporal expression of retinoic acid receptors in hamster fetus during organogenesis and alteration by retinoic acid treatment. Comp. Biochem. Physiol. 1996, 114C, 71–78. [Google Scholar]
- Luo, J.; Sucov, H.M.; Bader, J.; Evans, R.M.; Giguere, V. Compound mutants for retinoic acid receptor RARβ and RARα1 reveal developmental functions for multiple RARβ isoforms. Mech. Dev. 1996, 55, 33–44. [Google Scholar]
- Duester, G. Retinoic acid synthesis and signaling during early organogenesis. Cell 2008, 134, 921–931. [Google Scholar]
- Niederreither, K.; Subbarayan, V.; Dolle, P.; Chambon, P. Embryonic retinoic acid synthesis is essential for early mouse postimplantation development. Nat. Gen. 1999, 21, 444–448. [Google Scholar]
- Niederreither, K.; Vermot, J.; Messaddeq, N.; Schuhbaur, B.; Chambon, P.; Dolle, P. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development 2001, 128, 1019–1031. [Google Scholar]
- Molotkov, A.; Duester, G. Genetic evidence that retinaldehyde dehydrogenase Raldh1 (Aldh1a1) functions downstream of alcohol dehydrogoenase Adh1 in metabolism of retinol to retinoic acid. J. Biol Chem. 2003, 278, 36085–36090. [Google Scholar] [CrossRef] [PubMed]
- Molotkova, N.; Molotkov, A.; Duester, G. Role of retinoic acid during forebrain development begins late when Raldh3 generates retinoic acid in the ventral subventricular zone. Dev. Biol. 2007, 303, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sirbu, I.O.; Mic, F.A.; Molotkova, N.; Molotkov, A.; Kumar, S.; Duester, G. Retinoic acid promotes limb induction through effects on body axis extension but is unnecessary for limb patterning. Current Biol. 2009, 19, 1050–1057. [Google Scholar]
- Uehara, M.; Yashiro, K.; Takaoka, K.; Yamamoto, M.; Hamada, H. Removal of maternal retinoic acid by embryonic CYP26 is required for correct Nodal expression during early embryonic patterning. Genes Develop. 2009, 23, 1689–1698. [Google Scholar] [CrossRef]
- Mclean, G.; Li, H.; Metzger, D.; Chambon, P.; Petkovich, M. Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology 2007, 148, 4560–4567. [Google Scholar]
- Mclean, G.; Dolle, P.; Petkovich, M. Genetic disruption of cyp26b1 severly affects development of neural crest derived head structures, but does not compromise hindbrain patterning. Dev. Dyn. 2009, 238, 732–745. [Google Scholar]
- Niederreither, K.; Abu-Abed, S.; Schuhbaur, B.; Petkovich, M.; Chambon, P.; Dolle, P. Genetic evidence that oxidative derivatives of retinoic acid are not involved in retinoid signaling during mouse development. Nat. Genet. 2002, 31, 84–88. [Google Scholar] [PubMed]
- Pennimpede, T.; Cameron, D.A.; MacLean, G.A.; Petkovich, M. Analysis of Cyp26b1/Rarg compound-null mice reveals two genetically separable effects of retinoic acid on limb outgrowth. Dev. Biol. 2010, 339, 179–186. [Google Scholar]
- Spoorendonk, K.M.; Peterson-Maduro, J.; Renn, J.; Trowe, T.; Kranenbarg, S.; Winkler, C.; Schulte-Merker, S. Retinoic acid and Cyp26b1 are critical regulators of osteogenesis in the axial skeleton. Development 2008, 135, 3766–3774. [Google Scholar]
- Antipatis, C.; Ashworth, C.J.; Grant, G.; Lea, R.G.; Hay, S.M.; Rees, W.D. Effects of maternal vitamin A status on fetal heart and lung: changes in expression of key developmental genes. Am. J. Physiol. 1998, 275, L1184–L1191. [Google Scholar]
- Smith, S.M.; Dickman, E.D.; Power, S.C.; Lancman, J. Retinoids and their receptors in vertebrate embryogenesis. J. Nutr. 1998, 128, 467S–470S. [Google Scholar]
- White, J.C.; Highland, M.; Kaiser, M.; Clagett-Dame, M. Vitamin A deficiency results in the dose- dependent acquisition of anterior character and shortening of the caudal hindbrain of the rat embryo. Dev. Biol. 2000, 220, 263–284. [Google Scholar]
- Kaiser, M.E.; Merrill, R.A.; Stein, A.C.; Breburda, E.; Clagett-Dame, M. Vitamin A deficiency in the late gastrula stage rat embryo results in a one to two vertebral anteriorization that extends throughout the axial skeleton. Dev. Biol. 2003, 257, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Dupé, V.; Lumsden, A. Hindbrain patterning involves graded responses to retinoic acid signaling. Development 2001, 128, 2199–2208. [Google Scholar]
- Xavier-Neto, J.; Rosenthal, N.; Silva, F.A.; Matos, T.G.F.; Hochgreb, T.; Linhares, V.L.F. Retinoid signaling and cardiac anteroposterior segmentation. Genesis 2001, 31, 97–104. [Google Scholar]
- Iulianella, A.; Lohnes, D. Chimeric analysis of retinoic acid receptor function during cardiac looping. Dev. Biol. 2002, 247, 62–75. [Google Scholar]
- Mark, M.; Chambon, P. Functions of RARs and RXRs in vivo: genetic dissection of the retinoid signaling pathway. Pure Appl. Chem. 2003, 75, 1709–1732. [Google Scholar]
- Zile, M.H.; Kostetskii, I.; Yuan, S.; Kostetskaia, E.; St.Amand, T.R.; Chen, Y.; Jiang, W. Retinoid signaling is required to complete the vertebrate cardiac left/right asymmetry pathway. Dev. Biol. 2000, 223, 323–338. [Google Scholar]
- Kostetskii, I.; Yuan, S.-Y.; Kostetskaia, E.; Linask, K.K.; Blanchet, S.; Seleiro, E.; Michaille, J.-J.; Brickell, P.; Zile, M. Initial retinoid requirement for early avian development coincides with retinoid receptor coexpression in the precardiac fields and induction of normal cardiovascular development. Dev. Dyn. 1998, 213, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Kostetskii, I.; Jiang, Y.; Kostetskaia, E.; Yuan, S.; Evans, S.; Zile, M. Retinoid signaling required for normal heart development regulates GATA-4 in a pathway distinct from cardiomyocyte differentiation. Dev. Biol. 1999, 206, 206–218. [Google Scholar]
- Maden, M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat. Rev. Neurosci. 2007, 8, 755–765. [Google Scholar]
- Hoffman, J.I.E. Incidence of congenital heart disease: I. Postnatal incidence. Pediatr. Cardiol. 1995, 16, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Argao, E.A.; Kern, M.J.; Branford, W.; Scott, W.J., Jr.; Potter, S.S. Malformations of the heart, kidney, palate, and skeleton in alpha MHC-Hoxb-7 transgenic mice. Mech. Dev. 1995, 52, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D. Congenital heart defects: trapping the genetic culprits. Circ. Res. 2000, 86, 917–918. [Google Scholar]
- Srivastava, D. Genetic assembly of the heart: implications for congential heart disease. Annu. Rev. Physiol. 2001, 63, 451–469. [Google Scholar]
- Sommer, A.; Tarwotjo, I.; Djunaedi, E.; West, K.P., Jr.; Loeden, A.A. Impact of vitamin A supplementation on childhood mortality: a randomized controlled community trial. Lancet 1986, 1, 1169–1173. [Google Scholar]
- Micronutrient Deficiency Information System, Global prevalence of vitamin A deficiency, Paper No.2; World Health Organization: Geneva, Switzerland, 1995; Okonofua, F. Vitamin A supplementation during pregnancy. In The WHO Reproductive Health Library; RHL Commentary, World Health Organization, Geneva, Switzerland, 2003.
- Kubalak, S.W.; Sucov, H.M. Retinoids in heart development. In Heart Development; Harvey, R.P., Ed.; Academic Press: San Diego, CA, USA, 1999; pp. 209–219. [Google Scholar]
- Harvey, R.P. Patterning the vertebrate heart. Nature Genetics 2002, 3, 544–556. [Google Scholar]
- Hoover, L.L.; Burton, E.G.; Brooks, B.A.; Kubalak, S.W. The expanding role for retinoid signaling in heart development. Sci. World J. 2008, 8, 194–211. [Google Scholar]
- Dersch, H.; Zile, M.H. Induction of normal cardiovascular development in the vitamin A- deprived quail embryo by natural retinoids. Dev. Biol. 1993, 160, 424–433. [Google Scholar]
- Heine, U.I.; Roberts, A.B.; Munoz, E.F.; Roche, N.S.; Sporn, M.B. Effects of retinoid deficiency on the development of the heart and vascular system of the quail embryo. Virchows Arch. Cell Pathol. 1985, 50, 135–152. [Google Scholar]
- Ghatpande, S.; Brand, T.; Zile, M.; Evans, T. Bmp2 and GATA4 function additively to rescue heart tube development in the absence of retinoids. Dev. Dyn. 2006, 235, 2030–2039. [Google Scholar]
- Romeih, M.; Cakstina, I.; Zile, M.H. Retinoic acid is a negative physiological regulator of N-cadherin during early avian heart morphogenesis. Dev. Growth Differ. 2009, 51, 763–767. [Google Scholar]
- Ghatpande, S.K.; Zhou, H.-R.; Cakstina, I.; Carlson, C.; Rondini, E.A.; Romeih, M.; Zile, M.H. Transforming growth factor β2 is negatively regulated by endogenous retinoic acid during early heart morphogenesis. Dev. Growth Differ. 2010, 52, 433–455. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Michaille, J.-J.; Jiang, W.; Zile, M.H. Retinoid receptors and vitamin A deficiency: differential patterns of transcription during early avian development and the rapid induction of RARs by retinoic acid. Dev. Biol. 2003, 260, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Romeih, M.H.; Cui, J.; Michaille, J.-J.; Jiang, W.; Zile, M.H. Function of RARgamma and RARalpha2 at the initiation of retinoid signaling is essential for avian embryo survival and for distinct events in cardiac morphogenesis. Dev. Dyn. 2003, 228, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Kostetskii, I.; Linask, K.K.; Zile, M.H. Vitamin A deficiency and the expression of retinoic acid receptors during early cardiogenesis in quail embryo. Roux’s Arch. Dev. Biol. 1996, 205, 260–271. [Google Scholar] [CrossRef]
- Yutzey, K.E.; Kirby, M.L. Wherefore heart thou? Embryonic origins of cardiogenic mesoderm. Dev. Dyn. 2002, 223, 307–320. [Google Scholar]
- Linask, K.K. Regulation of heart morphology : current molecular and cellular perspectives on the coordinated emergence of cardiac form and function. Birth Defects Res. (Part C) 2003, 69, 14–24. [Google Scholar] [CrossRef]
- LaRue, A.C.; Argraves, W.S.; Zile, M.H.; Drake, C.J. Critical role for retinol in the generation/differentiation of angioblasts required for embryonic blood vessel formation. Dev. Dyn. 2004, 230, 666–674. [Google Scholar]
- Yutzey, K.E.; Rhee, J.T.; Bader, D. Expression of the atrial-specific mysin heavy chain AMHC1 and the establishment of anteroposterior polarity in the developing chicken heart. Development 1994, 120, 871–883. [Google Scholar]
- Xavier-Neto, J.; Shapiro, M.D.; Houghton, L.; Rosenthal, N. Sequential programs of retinoic acid synthesis in the myocardial and epicardial layers of the developing avian heart. Dev. Biol. 2000, 219, 129–141. [Google Scholar]
- Lyons, I.; Parsons, L.M.; Hartley, L.; Li, R.; Andrews, J.E.; Robb, L.; Harvey, R.P. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeobox gene Nkx2-5. Genes Dev. 1995, 9, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Doevendans, P.A.; VanBilsen, M. Transcription factors and the cardiac gene programme. Int. J. Bioch. Cell Biol. 1996, 28, 387–403. [Google Scholar]
- Jiang, Y.M.; Tarzami, S.; Burch, J.B.E.; Evans, T. Common role for each of the GATA-4/5/6 genes in the regulation of cardiac morphogenesis. Dev. Genet. 1998, 22, 263–277. [Google Scholar]
- Takeichi, M.; Nakagawa, S.; Aono, S.; Usui, T.; Uemura, T. Patterning of cell assemblies regulated by adhesion receptors of the cadherin superfamily. Phil. Trans. R. Soc. Lond. B. 2000, 355, 885–890. [Google Scholar]
- Juliano, R.L. Signal transduction by cell adhesion receptors and the cytoskeleton: Functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 283–323. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Takeichi, M. N-cadherin is crucial for heart formation in the chick embryo. Dev. Growth Differ. 1997, 39, 451–455. [Google Scholar]
- Linask, K.K.; Knudsen, K.A.; Gui, Y.-H. N-cadherin-catenin interaction: Necessary component of cardiac cell compartmentalization during early vertebrate heart development. Dev. Biol. 1997, 185, 148–164. [Google Scholar]
- Luo, Y.; High, F.A.; Epstein, J.A.; Radice, G.L. N-cadherin is required for neural crest remodeling of the cardiac outflow tract. Dev. Biol. 2006, 299, 517–528. [Google Scholar]
- Garcia-Castro, M.I.; Vielmetter, E.; Bronner-Fraser, M. N-cadherin, a cell adhesion molecule involved in establishment of embryonic left-right asymmetry. Science 2000, 288, 1047–1051. [Google Scholar]
- Chen, Y.-P.; Solursh, M. Mirror–image duplication of the primary axis and heart in Xenopusembryos by overexpression of Msx-1 gene. J. Exp. Zool. 1995, 273, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Ishii, M.; Sun, J.; Sucov, H.M.; Maxon, R.E., Jr. Msx1 and Msx2 regulate survival of secondary heart field precursors and post-migratory proliferation of cardiac neural crest in the outflow tract. Dev. Biol. 2007, 308, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kostetskii, I.; Zile, M.H.; Solursh, M. A comparative study of Msx-1 expression in early normal and vitamin A-deficient avian embryos. J. Exptl. Zool. 1995, 272, 299–310. [Google Scholar]
- Lincecum, J.M.; Fannon, A.; Song, K.; Wang, Y.; Sassoon, D.A. Msh homeobox genes regulate cadherin-mediated cell adhesion and cell-cell sorting. J. Cell Biochem. 1998, 70, 22–28. [Google Scholar]
- Shen, R.; Chen, Y.-P.; Huang, L.; Vitale, E.; Solursh, M. Characterization of the human MSX-1 promoter and an enhancer responsible for retinoic acid induction. Cellular Mol. Biol. Res. 1994, 40, 297–312. [Google Scholar]
- Kuzuoka, M.; Takahashi, T.; Guron, C.; Ragow, R. Murine homeobox-containing gene, Msx-1: analysis of genomic organization, promoter structure, and potential autoregulatory cis-acting elements. Genomics 1994, 21, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.M.; Ferland, L.H.; Robert, B.; Abdelhay, E. Structural and functional analysis of mouse Msx1 gene promoter: sequence conservation with human MSX1 promoter points at potential regulatory elements. DNA Cell Biol. 1998, 17, 561–572. [Google Scholar]
- Schule, R.; Rangarajan, P.; Yang, N.; Kliewer, S.; Ransone, L.J.; Bolado, J.; Verma, I.M.; Evans, R.M. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc. Natl. Acad. Sci. USA 1991, 88, 6092–6096. [Google Scholar]
- Tsai, L.N.; Ku, T.K.S.; Salib, N.K.; Crowe, D.L. Extracellular signals regulate rapid coactivator recruitment at AP-1 sites by altered phosphorylation of both CREB binding protein and c-jun. Mol.Cell. Biol. 2008, 28, 4240–4250. [Google Scholar]
- Li, J.; Molkentin, J.D.; Colbert, M.C. Retinoic acid inhibits cardiac neural crest migration by blocking c-Jun N-terminal kinase activation. Dev. Biol. 2001, 232, 351–361. [Google Scholar]
- Chan-Thomas, P.S.; Thompson, R.P.; Robert, B.; Yacoub, M.H.; Barton, P.J.R. Expression of homeobox genes Msx-1 (Hox-7) and Msx-2 (Hox-8) during cardiac development in the chick. Dev.Dyn. 1993, 197, 203–216. [Google Scholar]
- Davidson, D. The function and evolution of Msx genes: pointers and paradoxes. Trends Genet. 1995, 11, 405–411. [Google Scholar]
- Marazzi, G.; Wang, Y.; Sassoon, D. Msx-2 is a transcriptional regulator in the Bmp4-mediated programmed cell death pathway. Dev. Biol. 1997, 186, 127–138. [Google Scholar]
- van den Hoff, M.J.B.; Van den Eijnde, S.M.; Viragh, S.; Moorman, A.F.M. Programmed cell death in the developing heart. Cardiovascular Res. 2000, 45, 603–620. [Google Scholar]
- Carlson, C.S.; Zile, M.H. Vitamin A in avian vascular development. FASEB J. 2001, 7, A6972. [Google Scholar]
- Maden, M.; Gale, E.; Kostetskii, I.; Zile, M. Vitamin A-deficient quail embryos have half a hindbrain and other neural defects. Curr. Biol. 1996, 6, 417–426. [Google Scholar]
- Maden, M.; Graham, A.; Gale, E.; Rollinson, C.; Zile, M.H. Positional apoptosis during vertebrate CNS development in the absence of endogenous retinoids. Development 1997, 124, 2799–2805. [Google Scholar]
- Ghatpande, S.; Ghatpande, A.; Zile, M.; Evans, T. Anterior endoderm is sufficient to rescue foregut apoptosis and heart tube morphogenesis in an embryo lacking retinoic acid. Dev. Biol. 2000, 219, 59–70. [Google Scholar]
- Roberts, A.B.; Sporn, M.B. Mechanistic interrelationships between two superfamilies: the steroid/retinoid receptors and transforming factor-β. Cancer Surveys 1992, 14, 205–220. [Google Scholar]
- Mahmood, R.; Flanders, K.C.; Morriss-Kay, G.M. Interactions between retinoids and TGFβs in mouse morphogenesis. Development 1992, 115, 67–74. [Google Scholar]
- Mahmood, R.; Flanders, K.C.; Morriss-Kay, G.M. The effects of retinoid status on TGFβ expression during mouse embryogenesis. Anat. Embryol. 1995, 192, 21–33. [Google Scholar]
- Salbert, G.; Fanjul, A.; Piedrafita, F.J.; Lu, X.P.; Kim, S.J.; Tran, P.; Pfahl, M. Retinoic acid receptors and retinoid X receptor-α down-regulate the transforming growth factor-β1 promoter by antagonizing AP-1 activity. Mol. Endocrinol. 1993, 7, 1347–1356. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walkley, C.R.; Olsen, G.H.; Dworkin, S.; Fabb, S.A.; Swann, J.; McArthur, G.A.; Westmoreland, S.V.; Chambon, P.; Scadden, D.T.; Purton, L.E. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor deficiency. Cell 2007, 129, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Glick, A.B.; McCune, B.K.; Abdulkarem, N.; Flanders, K.C.; Lumadue, J.A.; Smith, J.M.; Sporn, M.B. Complex regulation of TGFβ expression by retinoic acid in the vitamin A- deficient rat. Development 1991, 111, 1081–1086. [Google Scholar]
- Noma, T.; Glick, A.B.; Geiser, A.G.; O’Reilly, M.A.; Miller, J.; Roberts, A.B.; Sporn, M.B. Molecular cloning and structure of the human transforming growth factor-β2 gene promoter. Growth Factors 1991, 4, 247–255. [Google Scholar]
- Akhurst, R.J. A sweet link between TGFβ and vascular disease? Nature Genetics 2006, 38, 400–401. [Google Scholar] [CrossRef] [PubMed]
- August, P.; Suthanthiran, M. Transforming growth factor β signaling, vascular remodeling, and hypertension. N. Engl. J. Med. 2006, 354, 2721–3723. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Stewart, C.P. Maternal micronutrient deficiency, fetal development, and the risk of chronic disease. J. Nutr. 2010, 140, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.B.; Xavier-Neto, J.; Shapiro, M.D.; Nayeem, S.M.; McCaffery, P.; Drager, U.C.; Rosenthal, N. Dynamic patterns of retinoic acid synthesis and response in the developing mammalian heart. Dev. Biol. 1998, 199, 55–71. [Google Scholar]
- Kubalak, S.W.; Hutson, D.R.; Scott, K.K.; Shannon, R.A. Elevated transforming growth factor β2 enhances apoptosis and contributes to abnormal outflow tract and aortic sac development in retinoic X receptor alpha knockout embryos. Development 2002, 129, 733–746. [Google Scholar]
- Lai, L.; Bohnsack, B.L.; Niederreither, K.; Hirschi, K.K. Retinoic acid regulates endothelial cell proliferation during vasculogenesis. Development 2003, 130, 6465–6474. [Google Scholar]
- Bohnsack, B.L.; Lai, L.; Dolle, P.; Hirschi, K.K. Signaling hierarchy downstream of retinoic acid that independently regulates vascular remodeling and endothelial cell proliferation. Genes Dev. 2004, 18, 1345–1358. [Google Scholar]
- Sirbu, I.O.; Zhao, X.; Duester, G. Retinoic acid controls heart aneteroposterior patterning by down-regulating Isl1 through the Fgf8 pathway. Dev. Dyn. 2008, 237, 1627–1635. [Google Scholar]
- McCann, J.C.; Ames, B.N. Vitamin K, an example of triage theory: is micronutrient inadequacy linked to diseases of aging? Am. J. Clin. Nutr. 2009, 90, 889–907. [Google Scholar] [CrossRef] [PubMed]
- Chmurzynska, A. Fetal programming: link between early nutrition, DNA methylation, and complex diseases. Nutr. Revs. 2010, 68, 87–98. [Google Scholar]
- Symposium: Nutritional experiences in early life as determinants of adult metabolic phenotype. J. Nutr. 2010, 140, 648–666.
- Heller, L.C.; Li, Y.; Abrams, K.L.; Rogers, M.B. Transcriptional regulation of the Bmp2 gene. Retinoic acid induction in F9 embryonal carcinoma cells and Saccharomyces cerevisiae. J. Biol. Chem. 1999, 274, 1394–1400. [Google Scholar] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zile, M.H. Vitamin A–Not for Your Eyes Only: Requirement for Heart Formation Begins Early in Embryogenesis. Nutrients 2010, 2, 532-550. https://doi.org/10.3390/nu2050532
Zile MH. Vitamin A–Not for Your Eyes Only: Requirement for Heart Formation Begins Early in Embryogenesis. Nutrients. 2010; 2(5):532-550. https://doi.org/10.3390/nu2050532
Chicago/Turabian StyleZile, Maija H. 2010. "Vitamin A–Not for Your Eyes Only: Requirement for Heart Formation Begins Early in Embryogenesis" Nutrients 2, no. 5: 532-550. https://doi.org/10.3390/nu2050532
APA StyleZile, M. H. (2010). Vitamin A–Not for Your Eyes Only: Requirement for Heart Formation Begins Early in Embryogenesis. Nutrients, 2(5), 532-550. https://doi.org/10.3390/nu2050532



