The Relationship between Ultraviolet Radiation Exposure and Vitamin D Status
Abstract
1. Introduction
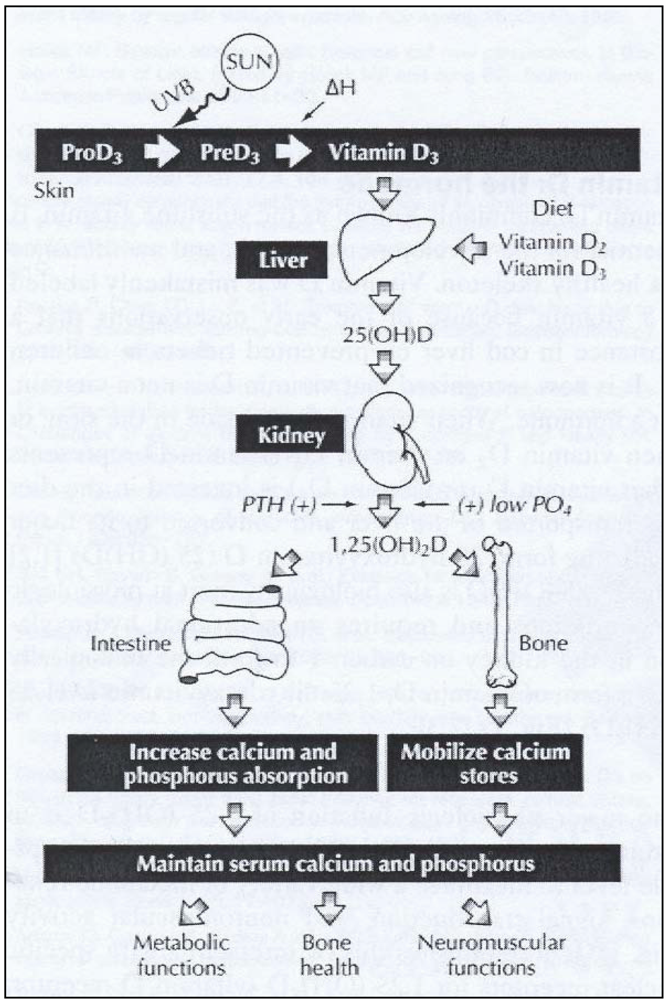
2. Ultraviolet Radiation Exposure and Effects on Cutaneous Vitamin D Synthesis
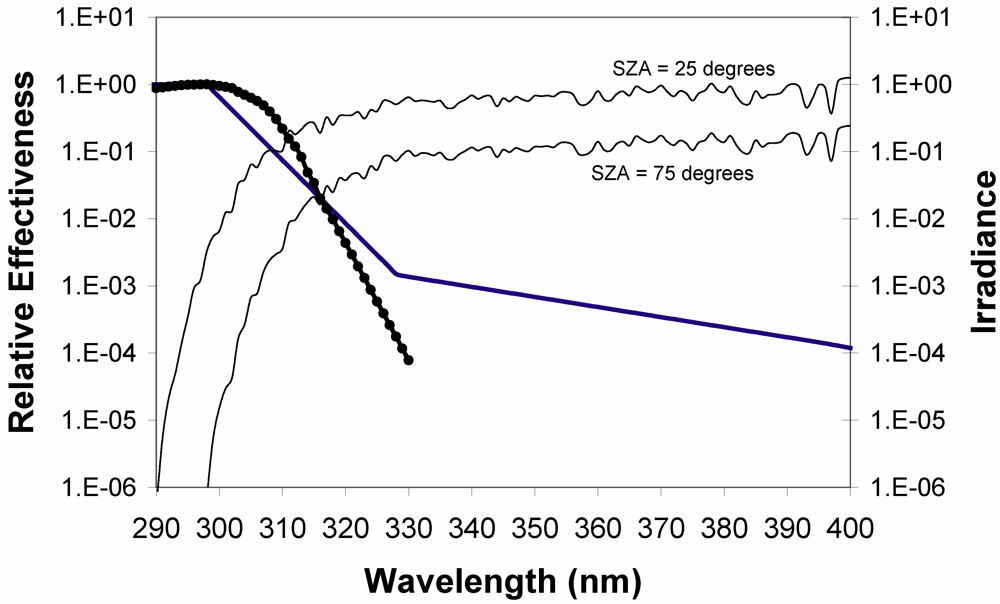
- Solar zenith angle (season and latitude) have a substantial impact on UVB radiation. At low solar zenith angles, photons must travel longer distances through the ozone layer, increasing the probability of absorption. There is also an enhanced possibility of interaction with air molecules, leading to absorption or scattering back into space, thus effectively attenuating UV radiation (Figure 2). Because the atmosphere attenuates UV radiation differently for various wavelengths, the UV spectrum varies with solar elevations. The vitamin D effective radiation is described in terms of its action spectrum (i.e., the efficiency of each wavelength to synthesize vitamin D in skin) [37]. In broad terms, the action spectrum covers the UVB spectral range with a maximum at about 295 nm (Figure 2).
- Clouds can both attenuate and enhance UVB radiation, although attenuation is generally the case. Completely overcast clouds always attenuate UVB rays, even up to 99% of UVB radiation in extreme cases [38]. Up to 50% enhancement of UVB radiation can occur from broken clouds [39] or at elevated sites above clouds [40].
- Ozone effectively absorbs UVB radiation, particularly at shorter wavelengths [41]. Besides clouds, it is the most important atmospheric modulator of vitamin D synthesis.
- Surface reflection, from snow in particular, reflects up to 95% of UVB radiation [42].
- Altitude. Solar UVB radiation increases by about 7% every km in altitude under clear sky conditions, and more if the subject is in or above clouds or a turbid atmosphere.
- Sunscreen blocks UVB radiation effectively [43,44]. However, it is questionable whether sunscreen in practise causes any vitamin D deficiency. Absolute full-body coverage of sunscreen is uncommon. Some areas of the skin are always left out. At times and locations where the sun is intense and the temperature is high enough to make the population use sunscreen, its vitamin D status is generally very satisfactory.
- Outdoor behavior. There is an ongoing trend towards less outdoor exposure, either through work or preferences in leisure activities. For instance, children in the USA now only spend half-an-hour outdoors a day during week-ends, and only minutes during week-days [45]. Furthermore, the orientation of the skin with respect to the sun has a great impact on the personal UV exposure. Nearby objects can obstruct both direct and diffuse UV rays [46], and thus affect UV synthesis. The best way to obtain precise personal UV exposure is dosimeters [47].
- Obesity. Overweight individuals have reduced capacity of vitamin D synthesis [55].
- Age. Elderly people have thinner skin, and consequently are less capable of synthesizing vitamin D in their skin [56].
- Clothing (temperature). At cold temperatures the population wears more clothes for comfort, exposing less skin area to UVB radiation, and thereby inhibiting vitamin D synthesis [58,59]. At moderate and high latitudes, face, neck and hands are generally exposed at best. During freezing temperatures, only the face is usually exposed.
3. Comparative Effect of Diet and UV Exposure on Serum 25-hydroxyvitamin D (25(OH)D) Level
| Vit. D > | 400 IU | 1000 IU | 4000 IU | |||
|---|---|---|---|---|---|---|
| Skin Type> | 2 | 5 | 2 | 5 | 2 | 5 |
| Area | ||||||
| F,N,H (11.5%) | 0.15 (0.21) | 0.35 (0.21) | 0.36 (0.54) | 0.89 (0.54) | 1.49 (2.16) | 3.95 (2.16) |
| F,N,H,A (25.5%) | 0.07 (0.09) | 0.16 (0.09) | 0.17 (0.24) | 0.40 (0.24) | 0.67 (0.97) | 1.62 (0.97) |
| F,N,H,A,L (57.5%) | 0.03 (0.04) | 0.07 (0.04) | 0.07 (0.10) | 0.18 (0.10) | 0.29 (0.43) | 0.70 (0.43) |
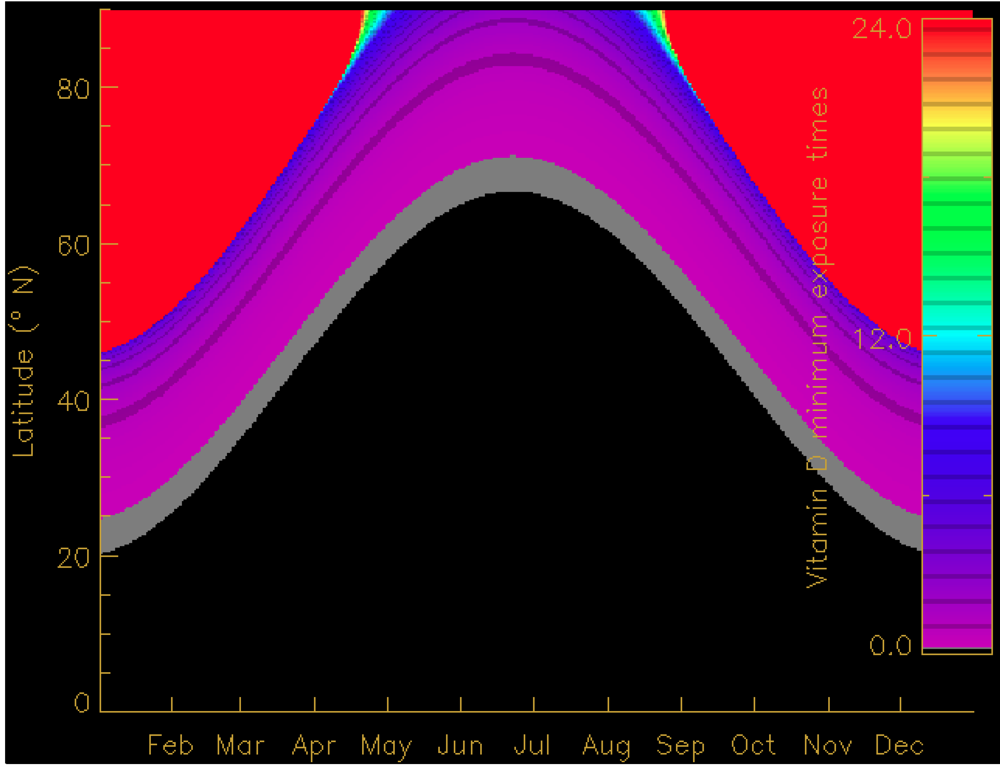
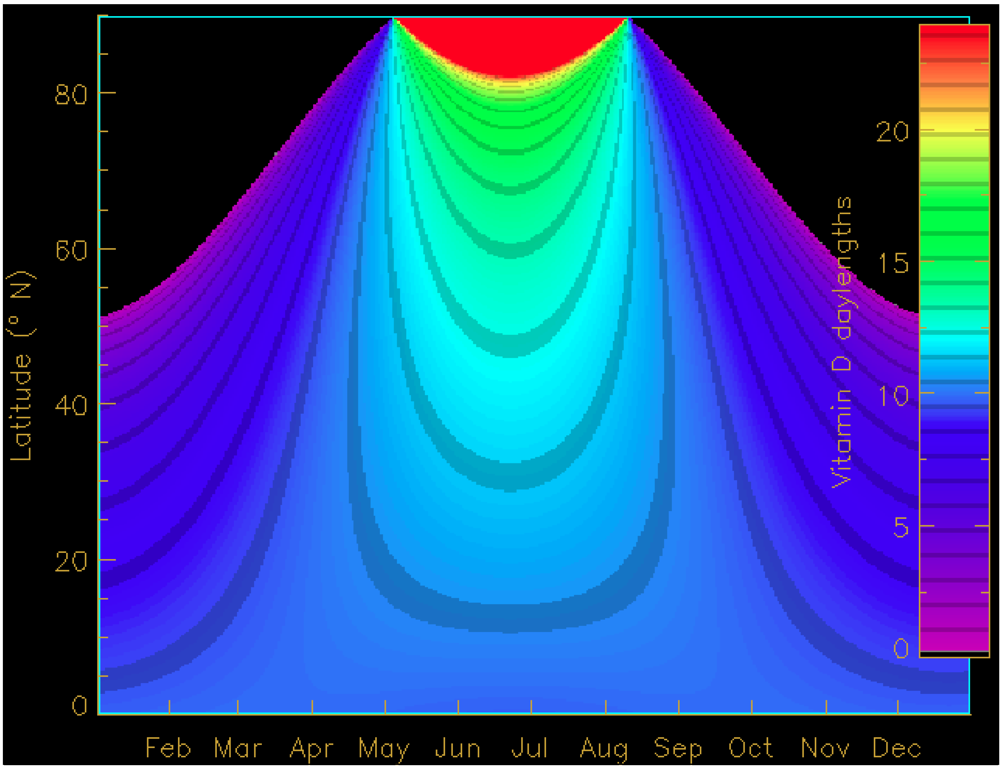
4. Seasonal and Latitudinal Effects on Vitamin D Synthesis and the Vitamin D Winter
5. A Note on Online Vitamin D Calculator Facilities
- Vitamin D winter duration [83] (http://nadir.nilu.no/~olaeng/fastrt/VitD.html).
- UV exposure times to substitute dietary intake [53] (http://nadir.nilu.no/~olaeng/fastrt/VitD_quartMEDandMED.html).
- Vitamin D effective doses [85] (http://nadir.nilu.no/~olaeng/fastrt/fastrt.html).
6. Conclusions and Suggestions for Additional Research
- There is a good qualitative understanding of underlying processes, but still cutaneous UV synthesis is inadequately understood for practical purposes. Quantitative modeling is possible, but it is incomplete and is based on very limited cohort experiments.
- Individuals risk sun burn if high doses of vitamin D should be obtained by normal skin exposure (face, neck, hands).
- Unrealistically long exposure times are sometimes required to obtain recommended vitamin D doses through skin.
- Desirable vitamin D doses and erythemal doses are more similar for low solar elevations.
Notes Added in Proof
Acknowledgements
References
- Holick, M.F.; Vitamin , D. Modern Nutrition in Health and Disease; Shils, M., Olson, J.A., Shike, M., Eds.; Lea &Febiger: Malvern, PA, USA, 1994; pp. 308–325. [Google Scholar]
- Grant, W.B.; Garland , C.F.; Holick, M.F. Comparisons of estimated economic burdens due to insufficient solar ultraviolet irradiance and vitamin D and excess solar UV irradiance for the United States. Photochem. Photobiol. 2005, 81, 1276–1286. [Google Scholar]
- Ponsonby, A.L.; Lucas, R.M.; van der Mei, I.A. UVR, vitamin D and three autoimmune diseases-multiple sclerosis, type 1 diabetes, rheumatoid arthritis. Photochem. Photobiol. 2005, 81, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Tomaschitz, A.; Ritz, E.; Pieber, T.R. Medscape. Vitamin D status and arterial hypertension: a systematic review. Nat. Rev. Cardiol. 2009, 6, 621–630. [Google Scholar]
- Yamshchikov, A.V.; Desai, N.S.; Blumberg, H.M.; Ziegler, T.R.; Tangpricha, V. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr. Pract. 2009, 15, 438–449. [Google Scholar]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; Kamen, D.L.; Wagner, M.; Bals, R.; Steinmeyer, A.; Zügel, U.; Gallo, R.L.; Eisenberg, D.; Hewison, M.; Hollis, B.W.; Adams, J.S.; Bloom, B.R.; Modlin, R.L. Toll-Like receptor triggering of a vitamin D-Mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [PubMed]
- Holick, M.F. Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Multihormonal systems disorders. Curr. Opin. Endocrinol. Diabetes. 2002, 9, 87–98. [Google Scholar] [CrossRef]
- Hill, A.B. The environment and disease: Association or causation? Proc. R. Soc. Med. 1965, 58, 295–300. [Google Scholar] [PubMed]
- Potischman, N.; Weed, D.L. Causal criteria in nutritional epidemiology. Am. J. Clin. Nutr. 1999, 69, 1309S–1314S. [Google Scholar]
- Weed, D.L. Environmental epidemiology: basics and proof of cause-effect. Toxicology 2002, 181/182, 399–403. [Google Scholar] [CrossRef]
- Lucas, R.M.; McMichael, A.J.; Armstrong, B.K.; Smith, W.T. Estimating the global disease burden due to ultraviolet radiation exposure. Int. J. Epidemiol. 2008, 37, 654–667. [Google Scholar]
- Holick, M.F. Sunlight, UV-radiation, vitamin D and skin cancer: how much sunlight do we need? Ad. Exp. Med. Biol. 2008, 624, 1–15. [Google Scholar] [CrossRef]
- Lucas, R.M.; Ponsonby, A.L. Considering the potential benefits as well as adverse effects of sun exposure: can all the potential benefits be provided by oral vitamin D supplementation? Prog. Biophys. Mol. Biol. 2006, 92, 140–149. [Google Scholar]
- Reichrath, J. The challenge resulting from positive and negative effects of sunlight: how much solar UV exposure is appropriate to balance between risks of vitamin D deficiency and skin cancer? Prog. Biophys. Mol. Biol. 2006, 92, 9–16. [Google Scholar]
- Webb, A.R.; Engelsen, O. Ultraviolet exposure scenarios: risks of erythema from recommendations on cutaneous vitamin D synthesis. Adv. Exp. Med. Biol. 2008, 624, 72–85. [Google Scholar]
- Davies, P.S.; Bates, C.J.; Cole, T.J.; Prentice, A.; Clark, P.C. Vitamin D: seasonal and regional differences in preschool children in Great Britain. Eur. J. Clin. Nutr. 1999, 53, 195–198. [Google Scholar]
- Guillemant, J. Wintertime vitamin D deficiency in male adolescents: effect on parathyroid function and response to vitamin D supplements. Osteoporosis Intl. 2001, 12, 875–879. [Google Scholar]
- Tangpricha, V.; Pearce, E.N.; Chen , T.C.; Holick, M.F. Vitamin D insufficiency among free-living healthy young adults. Am. J. Med. 2002, 112, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Burgaz, A.; Akesson, A.; Oster, A.; Michaëlsson, K.; Wolk, A. Associations of diet, supplement use, and ultraviolet B radiation exposure with vitamin D status in Swedish women during winter. Am.J. Clin. Nutr. 2007, 86, 1399–1404. [Google Scholar] [PubMed]
- Brustad, M.; Alsaker, E.; Engelsen, O.; Aksnes, L.; Lund, E. Vitamin D status in middle-aged women at 65-71 ºN in relation to dietary intake and exposure to ultraviolet radiation. Publ. Health Nutr. 2004, 7, 327–335. [Google Scholar]
- Lamberg-Allardt, C.J.; Outila, T.A.; Karkkainen, M.U.; Rita, H.J.; Valsta, L.M. Vitamin D deficiency and bone health in healthy adults in Finland: could this be a concern in other parts of Europe? J. Bone Miner Res. 2001, 16, 2066–2073. [Google Scholar] [CrossRef] [PubMed]
- Ovesen, L.; Andersen, R.; Jakobsen, J. Geographical differences in vitamin D status, with particular reference to European countries. Proc. Nutr. Soc. 2002, 62, 813–821. [Google Scholar]
- Rucker, D.; Allan, J.A.; Fick, G.H.; Hanley, D.A. Vitamin D insufficiency in a population of healthy western Canadians. CMAJ 2002, 166, 1517–1524. [Google Scholar]
- Arya, V.; Bhambri, R.; Godbole, M.M.; Mithal, A. Vitamin D status and its relationship with bone mineral density in healthy Asian Indians. Osteoporos Int. 2004, 15, 56–61. [Google Scholar]
- Shaw, N.J.; Pal, B.R. Vitamin D deficiency in UK Asian families: activating a new concern. Arch. Dis. Child. 2002, 86, 147–149. [Google Scholar]
- Holvik, K.; Meyer, H.E.; Haug, E.; Brunvand, L. Prevalence and predictors of vitamin D deficiency in five immigrant groups living in Oslo, Norway: the Oslo Immigrant Health Study. Eur J Clin Nutr. 2005, 59, 57–63. [Google Scholar] [PubMed]
- Stellinga-Boelen, A.A.M.; Wiegersma, P.A.; Storm, H.; Bijleveld, C.M.A.; Verkade, H.J. Vitamin D levels in children of asylum seekers in The Netherlands in relation to season and dietary intake. Eur. J. Pediatr. 2007, 166, 201–206. [Google Scholar]
- Townsend, K.; Evans, K.N.; Campbell, M.J.; Colston, K.W.; Adams, J.S.; Hewison, M. Biological actions of extra-renal 25-hydroxyvitamin D-1 -hydroxylase and implications for chemoprevention and treatment. J. Ster. Biochem. Molec. Biol. 2005, 97, 103–109. [Google Scholar]
- Galkin, O.N.; Terenetskaya, I.P. Vitamin D’ biodosimeter: basic characteristics and potential applications. J. Photochem. Photobiol. B: Biol. 1999, 53, 12–19. [Google Scholar] [CrossRef]
- Lehmann, B.; Genehr, T.; Knuschke, P.; Pietzsch, J.; Meurer, M. UVB-Induced Conversion of 7-Dehydrocholesterol to 1α,25-Dihydroxyvitamin D3 in an In Vitro Human Skin Equivalent Model. J. Investig. Dermatol. 2001, 117, 1179–1185. [Google Scholar] [CrossRef]
- Olds, W.J.; McKinley, A.R.; Moore, M.R.; Kimlin, M.G. In vitro model of vitamin D3 (cholecalciferol) synthesis by UV radiation: dose-response relationships. J. Photochem. Photobiol. B. 2008, 93, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Obi-Tabot, E.T.; Tian, X.Q.; Chen, T.C.; Holick, M.F. A human skin equivalent model that mimics the photoproduction of vitamin D3 in human skin. In vitro Cell. Dev. Biol. –Animal. 2000, 36, 201–204. [Google Scholar]
- Matsuoka, L.Y.; Wortsman, J.; Haddad, J.G.; Hollis, B.W. In vivo threshold for cutaneous synthesis of vitamin D3. J. Lab. Clin. Med. 1989, 114, 301–305. [Google Scholar] [PubMed]
- Holick, M.F. Sunlight, Vitamin D and Human Health. In Biologic Effects of Light; Holick, M.F., Jung, E.G., Eds.; Walter de Gruyter & Co.: Berlin, Germany, 1994; pp. 3–15. [Google Scholar]
- Holick, M.F. Environmental factors that influence the cutaneous production of vitamin D. Am. J. Clin. Nutr. 1995, 61, 638S–645S. [Google Scholar]
- Webb, A.R. Who, what, where and when – influences on cutaneous vitamin D synthesis. Prog. Biophys. Mol. Biol. 2006, 92, 17–25. [Google Scholar] [CrossRef] [PubMed]
- CIE 174, Action Spectrum for the Production of Previtamin D3 in Human Skin.; CIE publication 174, Publisher: CIE, Vienna, Austria, 2006; ISBN 3 901 906 50 9.
- Estupiñán, J.G.; Raman, S.; Crescenti, G.; Streicher, J.J.; Barnard, W.F. Effects of clouds and haze on UV-B radiation. J. Geophys. Res. 1996, 101, 16807–16908. [Google Scholar]
- Sabburg, J.; Calbó, J. Five years of cloud enhanced surface UV radiation measurements at two sites (in the Northern and Southern Hemispheres). Atm. Res. 2009, 93, 902–912. [Google Scholar] [CrossRef]
- McKenzie, R.L.; Johnston, P.V; Smale, D.; Bodhaine, B.A.; Madronich, S. Altitude effects on UV spectral irradiance deduced from measurements at Lauder, New Zealand, and at Mauna Loa Observatory, Hawaii. J. Geophys. Res. 2001, 106, 22,845–22,860. [Google Scholar]
- Bass, A.M.; Paur, R.J. The ultraviolet cross-sections of ozone, I, The measurements. In Atmospheric Ozone; Zerefos, C.S., Ghazi, A., Eds.; D. Reide: Norwell, MA, USA, 1985; pp. 606–610. [Google Scholar]
- Blumthaler, M.; Ambach, W. Solar UVB-Albedo of various surfaces. Photochem. Photobiol. 1988, 48, 85–88. [Google Scholar]
- Matsuoka, L.Y.; Wortsman, J.; Hanifan, N.; Holick, M.F. Chronic sunscreen use decreases circulating concentrations of 25-hydroxyvitamin D. A preliminary study. Arch. Dermatol. 1988, 124, 1802–1804. [Google Scholar] [PubMed]
- Matsuoka, L.Y.; Wortsman, J.; Hollis, B.W. Use of topical sunscreen for the evaluation of regional synthesis of vitamin D3. J. Am. Acad. Dermatol. 1990, 22, 772–775. [Google Scholar]
- EPA, Chapter 15: Activity Factors. In Exposure Factors Handbook; Environmental Protection Agency: Boston, MA, USA, 1997.
- Turnbull, D.J.; Parisi, A.V.; Kimlin, M.G. Vitamin D effective ultraviolet wavelengths due to scattering in shade. J. Steroid. Biochem. Mol. Biol. 2005, 96, 431–436. [Google Scholar]
- McCarty, C.A. Sunlight exposure assessment: can we accurately assess vitamin D exposure from sunlight questionnaires? Am. J. Clin. Nutr. 2008, 87, 1097S–1101S. [Google Scholar] [PubMed]
- Fitzpatrick, T.B. The validity and practicality of sun-reactive skin types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar]
- Clemens, T.L.; Adams, J.S.; Henderson, S.L.; Holick, M.F. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1982, 1, 74–76. [Google Scholar]
- Lo, C.W.; Paris, P.W.; Holick, M.F. Indian and Pakistani immigrants have the same capacity as Caucasians to produce vitamin D in response to ultraviolet radiation. Am. J. Clin. Nutr. 1986, 44, 683–685. [Google Scholar]
- Armas, L.A.G.S.; Dowell, R.N.; Akhter, M.; Duthuluru, S.; Huerter, C.; Hollis, B.W.; Lund, R.; Heaney, R.P. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: The effect of UVB dose and skin color. J. Am. Ac. Dermatol. 2007, 57, 588–593. [Google Scholar] [CrossRef]
- Matsuoka, L.Y,; Wortsman, J.; Haddad, J.G.; Kolm, P.; Hollis, B.W. Racial pigmentation and the cutaneous synthesis of vitamin D. Arch. Dermatol. 1991, 127, 536–538. [Google Scholar] [PubMed]
- Webb, A.R.; Engelsen, O. Calculated Ultraviolet Exposure Levels for a Healthy Vitamin D Status. Photochem. Photobiol. 2006, 82, 1697–1703. [Google Scholar]
- Chen, T.C.; Chimeh, F.; Lu, Z.; Mathieu, J.; Person, K.S.; Zhang, A.; Kohn, N.; Martinello, S.; Berkowitz, R.; Holick, M.F. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch. Biochem. Biophys. 2007, 460, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar]
- MacLaughlin, J.; Holick, M.F. Aging decreases the capacity of human skin to produce vitamin D3. J. Clin. Invest. 1985, 76, 1536–1538. [Google Scholar]
- Tangpricha, V.; Turner, A.; Spina, C.; Decastro, S; Chen, T.C.; Holick, M.F. Tanning is associated with optimal vitamin D status (serum 25-hydroxyvitamin D concentration) and higher bone mineral density. Am. J. Clin. Nutr. 2004, 80, 1645–1649. [Google Scholar] [PubMed]
- Matsuoka, L.Y.; Wortsman, J.; Dannenberg, M.J.; Hollis, B.W.; Lu, Z.; Holick, M.F. Clothing Prevents Ultraviolet-B Radiation-Dependent Photosynthesis of Vitamin D3. J. Clin. Endocrinol. Metab. 1992, 75, 1999–1103. [Google Scholar]
- Parisi, A.V.; Wilson, C.A. Pre-vitamin D effective ultraviolet transmission through clothing during simulated wear. Photodermatol. Photoimmunol Photomed. 2005, 21, 303–310. [Google Scholar]
- MacKinley, A.F.; Diffey, B.L. A reference action spectrum for ultraviolet induced erythema in human skin. CIE J. 1987, 6, 17–22. [Google Scholar]
- Holick, M.F. Sunlight, Vitamin D and Human Health. In Biological Effects of Light; Walter de Gruyter & Co: Berlin, Germany, 1993. [Google Scholar]
- Lo, C.W.; Paris, P.W.; Clemens, T.L.; Nolan, J.; Holick, M.F. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am. J. Clin. Nutr. 1985, 42, 644–649. [Google Scholar]
- Adams, J.S.; Clemens, T.L.; Parrish, J.A.; Holick, M.D. Vitamin-D synthesis and metabolism after ultraviolet irradiation of normal and vitamin-D-deficient subjects. N. Engl. J. Med. 1982, 306, 722–725. [Google Scholar]
- Holick, M.F. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease and osteoporosis. Am. J. Clin. Nutr. 2004, 79, 362–371. [Google Scholar] [PubMed]
- Holick, M.F. The Vitamin D Advantage; iBooks: New York, NY, USA, 2004. [Google Scholar]
- Samanek, A.J.; Croager, E.J.; Milne, E.; Prince, R.; McMichael, A.J.; Lucas, R.M.; Slevin, T. Estimates of beneficial and harmful sun exposure times during the year for major Australian population centres. Med. J. Australia 2006, 184, 338–341. [Google Scholar]
- McKenzie, R.L.; Liley, J.B.; Björn, L.O. UV Radiation: Balancing Risks and Benefits. Photochem. Photobiol. 2008, 85, 88–98. [Google Scholar] [PubMed]
- Fioletov, V.E.; McArthur, L.J.; Mathews, T.W.; Marrett, L. On the relationship between erythemal and vitamin D action spectrum weighted ultraviolet radiation. J. Photochem. Photobiol. B. 2009, 95, 9–16. [Google Scholar]
- Vieth, R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am. J. Clin. Nutr. 1999, 69, 842–856. [Google Scholar] [PubMed]
- Stamp, T.C.; Haddad, J.G.; Twigg, C.A. Comparison of oral 25-hydroxycholecalciferol, vitamin D, and ultraviolet light as determinants of circulating 25-hydroxyvitamin D. Lancet 1977, 1, 1341–1343. [Google Scholar] [PubMed]
- Davie, M.W.; Lawson, D.E.; Emberson, C.; Barnes, J.L.; Roberts, G.E.; Barnes, N.D. Vitamin D from skin: contribution to vitamin D status compared with oral vitamin D in normal and anticonvulsant-treated subjects. Clin. Sci. 1982, 63, 461–472. [Google Scholar]
- Chel, V.G.; Ooms, M.E.; Popp-Snijders, C.; Pavel, S.; Schothorst, A.A.; Meulemans, C.C.; Lips, P. Ultraviolet irradiation corrects vitamin D deficiency and suppresses secondary hyperparathyroidism in the elderly. J. Bone Miner. Res. 1998, 13, 1238–1242. [Google Scholar]
- Vieth, R. The Pharmacology of Vitamin D, Including Fortification Strategies. In Vitamin D; Feldman, D., Pike, J.W., Glorieux, F.H., Eds.; Elsevier: Amsterdam, The Netherland, 2005. [Google Scholar]
- Chuck, A.; Todd, J.; Diffey, B. Subliminal ultraviolet-B irradiation for the prevention of vitamin D deficiency in the elderly: a feasibility study. Photodermatol. Photoimmunol. Photomed. 2001, 17, 168–171. [Google Scholar]
- Prystowsky, J.H.; Muzio, P.J.; Sevran, S.; Clemens, T.L. Effect of UVB phototherapy and oral calcitriol (1,25- dihydroxyvitamin D3) on vitamin D photosynthesis in patients with psoriasis. J. Am. Acad. Dermatol. 1996, 35, 690–695. [Google Scholar]
- Holick, M.F.; Tian, X.Q.; Allen, M. Evolutionary importance for the membrane enhancement of the production of vitamin D3 in the skin of poikilothermic animals. Proc. Natl. Acad. Sci.USA 1995, 92, 3124–3126. [Google Scholar]
- Heaney, R.P.; Davies, K.M.; Chen, T.C.; Holick, M.F.; Barger-Lux, M.J. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am. J. Clin. Nutr. 2003, 77, 204–210. [Google Scholar]
- Snell, A.P.; MacLennan, W.J.; Hamilton, J.C. Ultra-Violet irradiation and 25-hydroxy-vitamin D levels in sick old people. Age Ageing 1978, 7, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Mawer, E.B.; Berry, J.L.; Sommer-Tsilenis, E.; Beykirch, W.; Kuhlwein, A.; Rohde, B.T. Ultraviolet irradiation increases serum 1,25-dihydroxyvitamin D in vitamin-D-replete adults. Miner. Electrol. Metab. 1984, 10, 117–121. [Google Scholar]
- Webb, A.R.; Kline, L.; Holick, M.F. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J. Clin. Endocrinol. Metab. 1988, 67, 373–378. [Google Scholar]
- Chen, T.C. Photobiology of vitamin D. In Vitamin D – Physiology, Molecular Biology, and Clinical Applications; Holick, M.F., Totawa, N.J., Eds.; Humana Press: Totowa, NJ, USA, 1999; pp. 17–37. [Google Scholar]
- Pettifor, J.M.; Moodley, G.P.; Hough, F.S.; Koch, H.; Chen, T.; Lu , Z.; Holick, M.F. The effect of season and latitude on in vitro vitamin D formation by sunlight in South Africa. S. Afr. Med. J. 1996, 86, 1270–1272. [Google Scholar] [PubMed]
- Engelsen, O.; Brustad, M.; Aksnes, L; E. Lund, E. Daily duration of vitamin D synthesis in human skin with relation to latitude, total ozone, altitude, ground cover, aerosols and cloud thickness. Photochem. Photobiol. 2005, 81, 1287–1290. [Google Scholar] [CrossRef] [PubMed]
- Kimlin, M.G.; Schallhorn, K.A. Estimations of the human 'vitamin D' UV exposure in the USA. Photochem. Photobiol. Sci. 2004, 3, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Engelsen, O.; Kylling, A. Fast simulation tool for ultraviolet radiation at the earth’s surface. Opt. Eng. 2005, 44, 041012.1–041012.7. [Google Scholar]
- Brustad, M.; Edvardsen, K.; Wilsgaard, T.; Engelsen, O.; Aksnes, L.; Lund, E. Seasonality of UV-radiation and vitamin D status at 69 degrees north. Photochem. Photobiol. Sci. 2007, 6, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Scharla, S.H. Epidemiology of vitamin-D-deficiency/insufficiency in different European countries. J. Für Menopause 2000, 7, 29–33. [Google Scholar]
- Moan, J.; Porojnicu, A.C.; Robsahm, T.E.; Dahlback, A.; Juzeniene, A.; Tretli, S.; Grant, W. Solar radiation, vitamin D and survival rate of colon cancer in Norway. J. Photochem. Photobiol. B. 2005, 78, 189–193. [Google Scholar]
- Grant, W.B. Geographic variation of prostate cancer mortality rates in the United States: implications for prostate cancer risk related to vitamin D. Int. J. Cancer 2004, 111, 470–471. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Engelsen, O. The Relationship between Ultraviolet Radiation Exposure and Vitamin D Status. Nutrients 2010, 2, 482-495. https://doi.org/10.3390/nu2050482
Engelsen O. The Relationship between Ultraviolet Radiation Exposure and Vitamin D Status. Nutrients. 2010; 2(5):482-495. https://doi.org/10.3390/nu2050482
Chicago/Turabian StyleEngelsen, Ola. 2010. "The Relationship between Ultraviolet Radiation Exposure and Vitamin D Status" Nutrients 2, no. 5: 482-495. https://doi.org/10.3390/nu2050482
APA StyleEngelsen, O. (2010). The Relationship between Ultraviolet Radiation Exposure and Vitamin D Status. Nutrients, 2(5), 482-495. https://doi.org/10.3390/nu2050482




