A Randomized, Placebo-Controlled Trial Evaluating Multi-Species Synbiotic Supplementation for Bloating, Gas, and Abdominal Discomfort
Abstract
1. Introduction
2. Methods
2.1. Clinical Trial Design
2.2. Study Participants
2.3. Multi-Species Synbiotic Intervention
2.4. Outcomes: Efficacy and Safety
2.5. Statistical Analysis
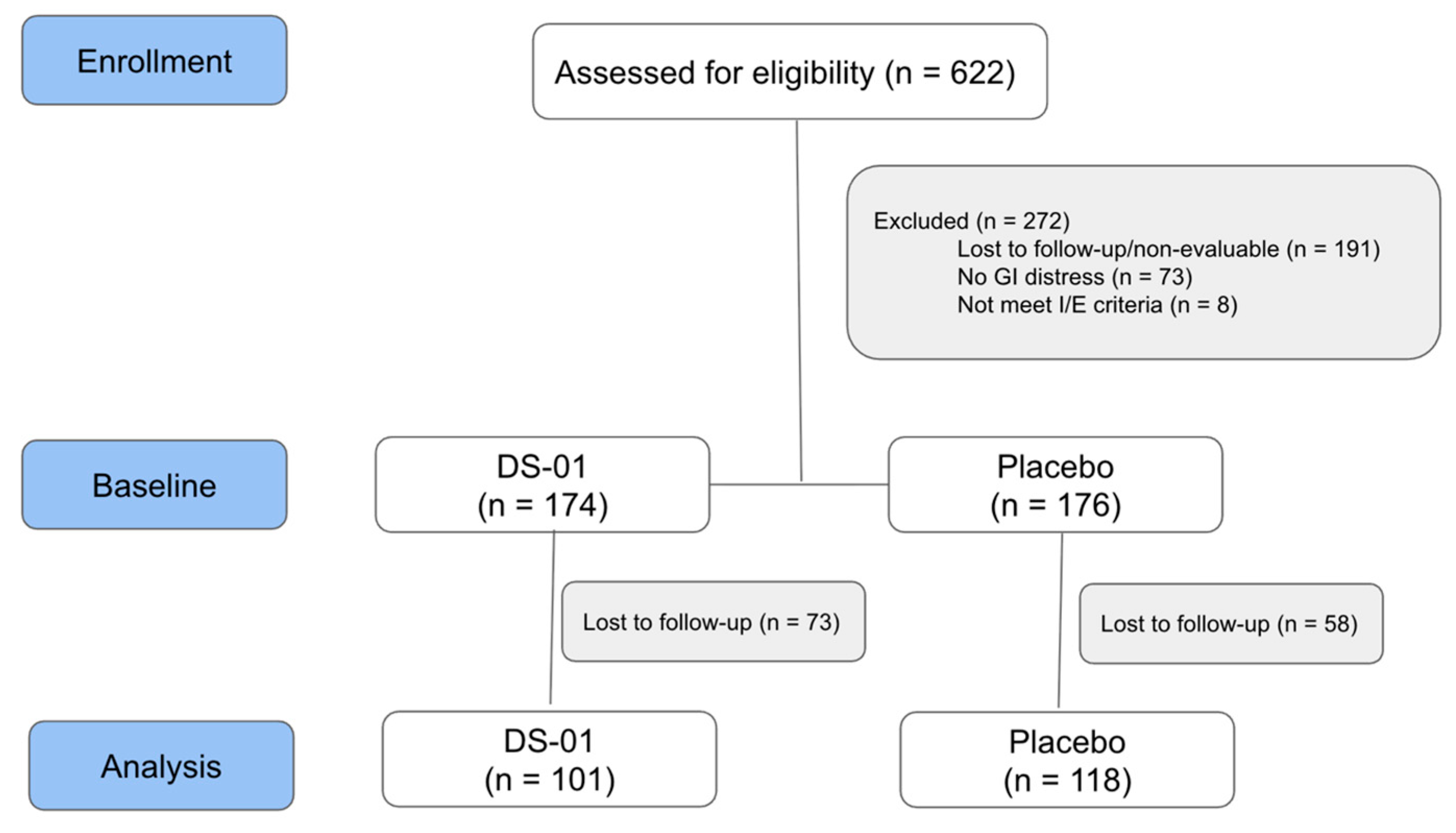
3. Results
3.1. Participant Demographics
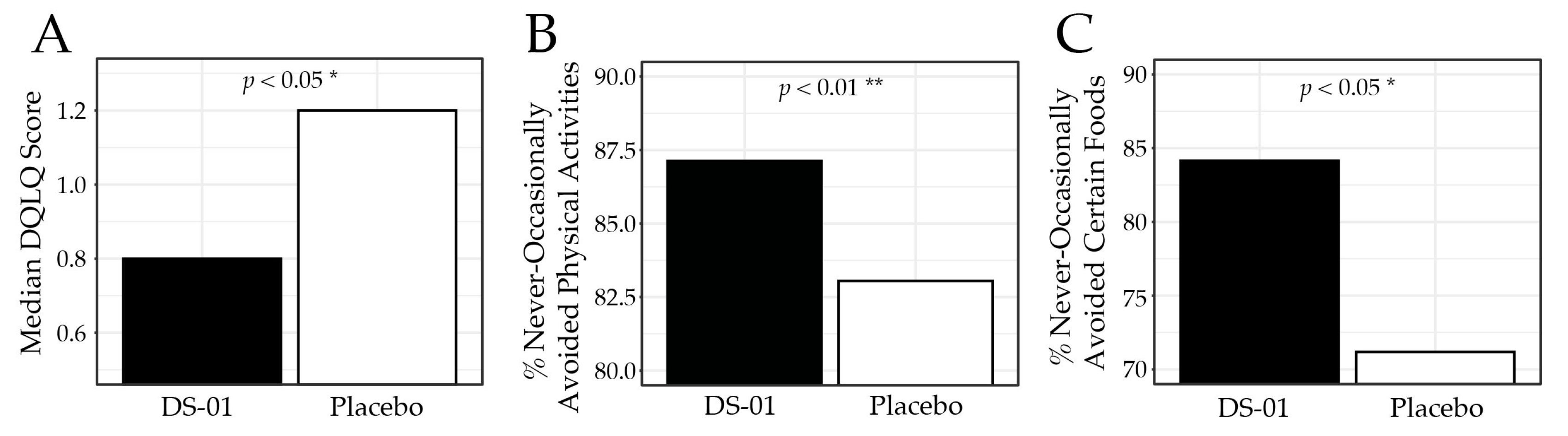
3.2. Gastrointestinal Quality-of-Life
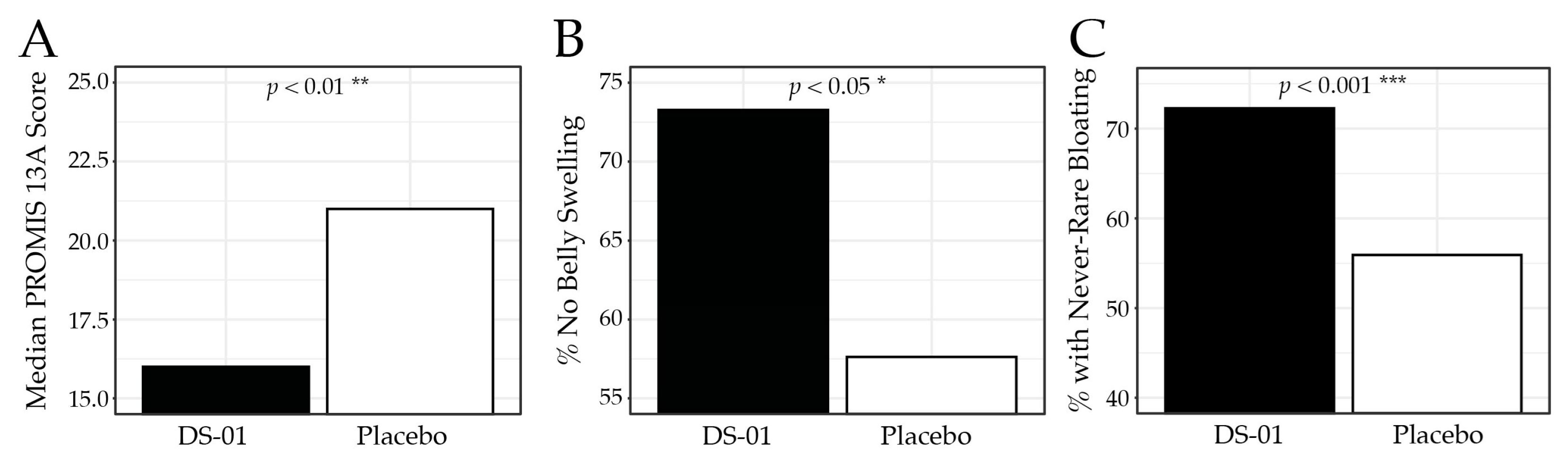
3.3. Bloating and Gas
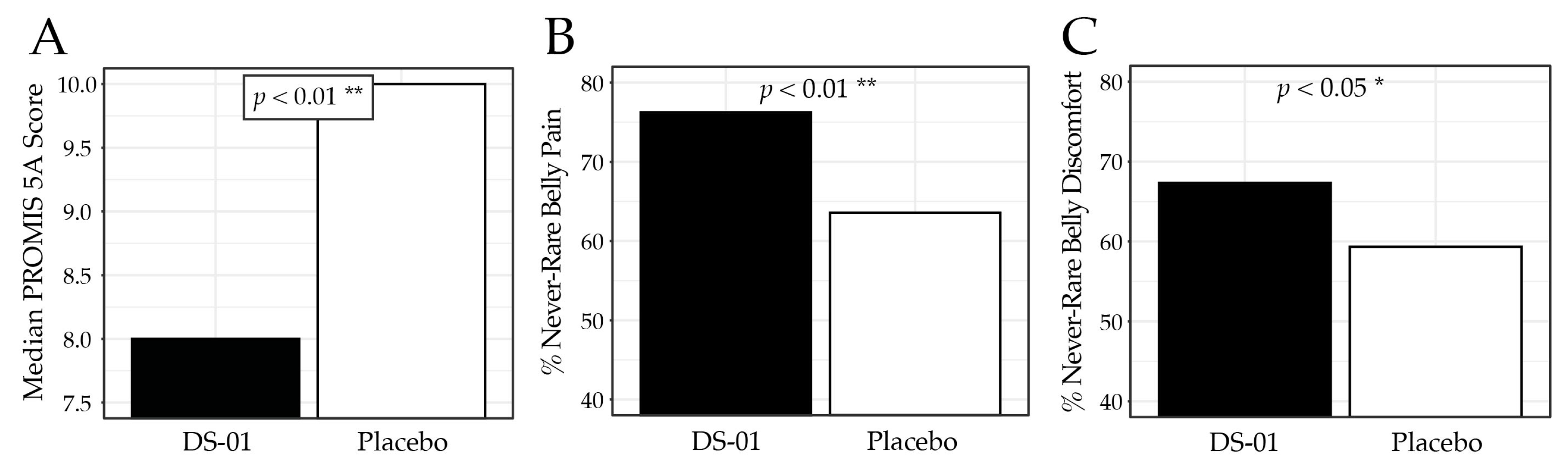
3.4. Abdominal Discomfort/Pain
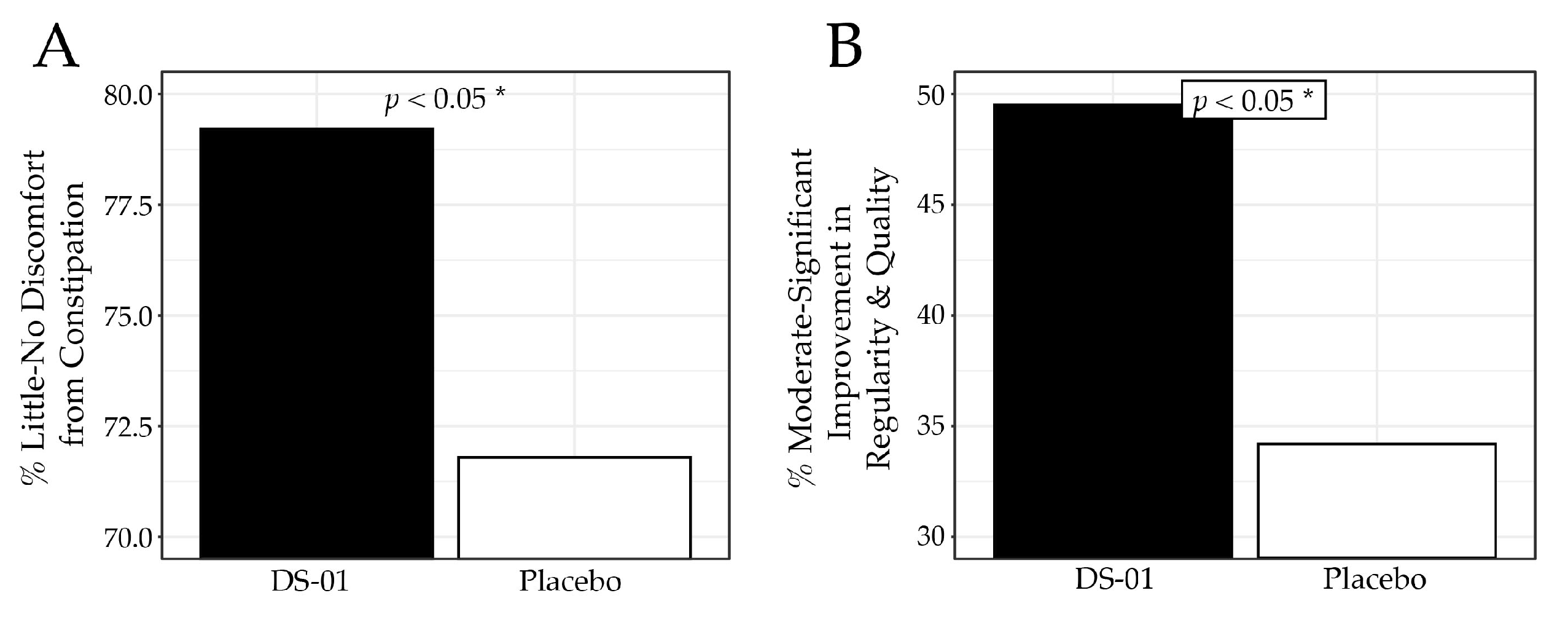
3.5. Constipation and Regularity
3.6. Mood
3.7. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballou, S.; Singh, P.; Nee, J.; Rangan, V.; Iturrino, J.; Geeganage, G.; Löwe, B.; Bangdiwala, S.I.; Palsson, O.S.; Sperber, A.D.; et al. Prevalence and Associated Factors of Bloating: Results from the Rome Foundation Global Epidemiology Study. Gastroenterology 2023, 165, 647–655.e4. [Google Scholar] [CrossRef]
- Oh, J.E.; Chey, W.D.; Spiegel, B. Abdominal Bloating in the United States: Results of a Survey of 88,795 Americans Examining Prevalence and Healthcare Seeking. Clin. Gastroenterol. Hepatol. 2023, 21, 2370–2377. [Google Scholar] [CrossRef]
- Palsson, O.S.; Drossman, D.A.; Jan, T.; Le Nevé, B.; Quinquis, L.; Hassouna, R.; Ruddy, J.; Schmulson, M.J.; Morris, C.B.; Bangdiwala, S.I.; et al. Gas-Related Symptoms in the General Population: Prevalence, Impact and Associated Factors in a Survey of the United States, the United Kingdom, and Mexico. Neurogastroenterol. Motil. 2025, 20, e70076. [Google Scholar] [CrossRef]
- Mejía-Caballero, A.; Marco, M.L. Lactobacilli biology, applications and host interactions. Nat. Rev. Microbiol. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef]
- Mutuyemungu, E.; Motta-Romero, H.A.; Yang, Q.; Liu, S.; Liu, S.; Singh, M.; Rose, D.J. Megasphaera elsdenii, a commensal member of the gut microbiota, is associated with elevated gas production during in vitro fermentation. Gut Microbiome 2023, 5, e1. [Google Scholar] [CrossRef]
- Soret, R.; Chevalier, J.; De Coppet, P.; Poupeau, G.; Derkinderen, P.; Segain, J.P.; Neunlist, M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 2010, 138, 1772–1782. [Google Scholar] [CrossRef]
- Zheng, Z.; Tang, J.; Hu, Y.; Zhang, W. Role of gut microbiota-derived signals in the regulation of gastrointestinal motility. Front. Med. 2022, 9, 961703. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, B.; Verne, G.N. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 2009, 146, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Takagi, T.; Asaeda, K.; Hashimoto, H.; Kajiwara, M.; Azuma, Y.; Kitae, H.; Hirai, Y.; Mizushima, K.; Doi, T.; et al. Urolithin A-mediated augmentation of intestinal barrier function through elevated secretory mucin synthesis. Sci. Rep. 2024, 14, 15706. [Google Scholar] [CrossRef]
- Goodoory, V.C.; Khasawneh, M.; Black, C.J.; Quigley, E.M.M.; Moayyedi, P.; Ford, A.C. Efficacy of Probiotics in Irritable Bowel Syndrome: Systematic Review and Meta-analysis. Gastroenterology 2023, 165, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Ringel-Kulka, T.; McRorie, J.; Ringel, Y. Multi-Center, Double-Blind, Randomized, Placebo-Controlled, Parallel-Group Study to Evaluate the Benefit of the Probiotic Bifidobacterium infantis 35624 in Non-Patients with Symptoms of Abdominal Discomfort and Bloating. Am. J. Gastroenterol. 2017, 112, 145–151. [Google Scholar] [CrossRef]
- Diop, L.; Guillou, S.; Durand, H. Probiotic food supplement reduces stress-induced gastrointestinal symptoms in volunteers: A double-blind, placebo-controlled, randomized trial. Nutr. Res. 2008, 28, 1–5. [Google Scholar] [CrossRef]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 2018, 6, 121. [Google Scholar] [CrossRef]
- Napier, B.A.; Allegretti, J.R.; Feuerstadt, P.; Kelly, C.R.; Van Hise, N.W.; Jäger, R.; Kassam, Z.; Reid, G. Multi-Species Synbiotic Supplementation Enhances Gut Microbial Diversity, Increases Urolithin A and Butyrate Production, and Reduces Inflammation in Healthy Adults: A Randomized, Placebo-Controlled Trial. Nutrients 2025, 17, 2734. [Google Scholar] [CrossRef]
- Tierney, B.T.; Van den Abbeele, P.; Al-Ghalith, G.A.; Verstrepen, L.; Ghyselinck, J.; Calatayud, M.; Marzorati, M.; Gadir, A.A.; Daisley, B.; Reid, G.; et al. Capacity of a Microbial Synbiotic To Rescue the In Vitro Metabolic Activity of the Gut Microbiome following Perturbation with Alcohol or Antibiotics. Appl. Environ. Microbiol. 2023, 89, e0188022. [Google Scholar] [CrossRef]
- Paxton, A.E.; Strycker, L.A.; Toobert, D.J.; Ammerman, A.S.; Glasgow, R.E. Starting the conversation performance of a brief dietary assessment and intervention tool for health professionals. Am. J. Prev. Med. 2011, 40, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Del Piano, M.; Carmagnola, S.; Anderloni, A.; Andorno, S.; Ballarè, M.; Balzarini, M.; Montino, F.; Orsello, M.; Pagliarulo, M.; Sartori, M.; et al. The use of probiotics in healthy volunteers with evacuation disorders and hard stools: A double-blind, randomized, placebo-controlled study. J. Clin. Gastroenterol. 2010, 44 (Suppl. S1), S30–S34. [Google Scholar] [CrossRef] [PubMed]
- Beke, M.; Burns, A.M.; Weir, S.; Solch, R.J.; Judkins, T.C.; Nieves, C., Jr.; Langkamp-Henken, B. Validation of a novel quality of life questionnaire: The Digestion-associated Quality of Life Questionnaire (DQLQ). Health Qual. Life Outcomes 2022, 20, 53. [Google Scholar] [CrossRef]
- Spiegel, B.M.; Hays, R.D.; Bolus, R.; Melmed, G.Y.; Chang, L.; Whitman, C.; Khanna, P.P.; Paz, S.H.; Hays, T.; Reise, S.; et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am. J. Gastroenterol. 2014, 109, 1804–1814. [Google Scholar] [CrossRef]
- Whorwell, P.J.; Altringer, L.; Morel, J.; Bond, Y.; Charbonneau, D.; O’Mahony, L.; Kiely, B.; Shanahan, F.; Quigley, E.M. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am. J. Gastroenterol. 2006, 101, 1581–1590. [Google Scholar] [CrossRef]
- La Monica, M.B.; Raub, B.; Hartshorn, S.; Gustat, A.L.; Grdic, J.; Kirby, T.O.; Townsend, J.R.; Sandrock, J.; Ziegenfuss, T.N. The effects of AG1® supplementation on the gut microbiome of healthy adults: A randomized, double-blind, placebo-controlled clinical trial. J. Int. Soc. Sports Nutr. 2024, 21, 2409682. [Google Scholar] [CrossRef] [PubMed]
- de Wouters d’Oplinter, A.; Huwart, S.J.P.; Cani, P.D.; Everard, A. Gut microbes and food reward: From the gut to the brain. Front. Neurosci. 2022, 16, 947240. [Google Scholar] [CrossRef] [PubMed]
- Kalman, D.S.; Schwartz, H.I.; Alvarez, P.; Feldman, S.; Pezzullo, J.C.; Krieger, D.R. A prospective, randomized, double-blind, placebo-controlled parallel-group dual site trial to evaluate the effects of a Bacillus coagulans-based product on functional intestinal gas symptoms. BMC Gastroenterol. 2009, 9, 85. [Google Scholar] [CrossRef]
- Vanhoutvin, S.A.; Troost, F.J.; Kilkens, T.O.; Lindsey, P.J.; Hamer, H.M.; Jonkers, D.M.; Venema, K.; Brummer, R.J. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol. Motil. 2009, 21, 952-e76. [Google Scholar] [CrossRef]
- Cheng, J.; Gao, C.; Ala-Jaakkola, R.; Forssten, S.D.; Saarinen, M.; Hibberd, A.; Ouwehand, A.C.; Ibarra, A.; Li, D.; Nordlund, A.; et al. Eight-Week Supplementation with Bifidobacterium lactis HN019 and Functional Constipation: A Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e2436888. [Google Scholar] [CrossRef]
- Ibarra, A.; Latreille-Barbier, M.; Donazzolo, Y.; Pelletier, X.; Ouwehand, A.C. Effects of 28-day Bifidobacterium animalis subsp. lactis HN019 supplementation on colonic transit time and gastrointestinal symptoms in adults with functional constipation: A double-blind, randomized, placebo-controlled, and dose-ranging trial. Gut Microbes 2018, 9, 236–251. [Google Scholar] [CrossRef]
- Ojetti, V.; Ianiro, G.; Tortora, A.; D’Angelo, G.; Di Rienzo, T.A.; Bibbò, S.; Migneco, A.; Gasbarrini, A. The effect of Lactobacillus reuteri supplementation in adults with chronic functional constipation: A randomized, double-blind, placebo-controlled trial. J. Gastrointestin Liver Dis. 2014, 23, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Madempudi, R.S.; Ahire, J.J.; Neelamraju, J.; Tripathi, A.; Nanal, S. Randomized clinical trial: The effect of probiotic Bacillus coagulans Unique IS2 vs. placebo on the symptoms management of irritable bowel syndrome in adults. Sci. Rep. 2019, 9, 122. [Google Scholar] [CrossRef]
- Firoozi, D.; Masoumi, S.J.; Mohammad-Kazem, H.; Asl, S.; Labbe, A.; Razeghian-Jahromi, I.; Fararouei, M.; Lankarani, K.B.; Dara, M. Effects of short-chain fatty acid-butyrate supplementation on expression of circadian-clock genes, sleep quality, and inflammation in patients with active ulcerative colitis: A double-blind randomized controlled trial. Lipids Health Dis. 2024, 23, 216. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, S.; Chan, A.W.; Collins, G.S.; Hróbjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. CONSORT 2025 Statement: Updated guideline for reporting randomised trials. BMJ 2025, 388, e081123. [Google Scholar] [CrossRef] [PubMed]
| Measure | DS-01 (n = 174) | Placebo (n = 176) | |||
|---|---|---|---|---|---|
| Mean/N | SD/% | Mean/N | SD/% | p-Value | |
| Age (years) | 45.2 | 12.2 | 45.1 | 11.7 | 0.90 |
| Sex (% Male) | 56 | 32.2% | 57 | 32.4% | 1.00 |
| BMI (kg/m2) | 29.4 | 7.8 | 29.2 | 8.4 | 0.78 |
| Race | 0.088 | ||||
| American Indian/Alaska Native | 0 | 0.0% | 5 | 2.8% | |
| Asian | 14 | 8.0% | 8 | 4.5% | |
| Black | 24 | 13.8% | 22 | 12.5% | |
| Multi-racial | 15 | 8.6% | 18 | 10.2% | |
| Native Hawaiian/Pacific Islander | 1 | 0.6% | 2 | 1.1% | |
| White | 117 | 67.2% | 110 | 62.5% | |
| Some other race | 1 | 0.6% | 5 | 2.8% | |
| Prefer not to say | 2 | 1.1% | 6 | 3.4% | |
| Hispanic, LatinX, or Spanish | 20 | 11.5% | 29 | 16.5% | 0.38 |
| Education | 0.34 | ||||
| Less than high school | 0 | 0.0% | 1 | 0.6% | |
| High school diploma | 27 | 15.5% | 17 | 9.7% | |
| Some college, no degree | 30 | 17.2% | 43 | 24.4% | |
| Bachelors or associate degree | 78 | 44.8% | 81 | 46.0% | |
| Masters or professional degree | 26 | 14.9% | 23 | 13.1% | |
| Trade/technical/vocational degree | 12 | 6.9% | 9 | 5.1% | |
| Prefer not to say | 1 | 0.6% | 2 | 1.1% | |
| DQLQ | 4.5 | 1.9 | 4.5 | 1.9 | 0.98 |
| PROMIS 13A Bloating and Gas | 34.9 | 11.7 | 36.0 | 10.9 | 0.34 |
| PROMIS 5A Belly Pain | 15.7 | 4.5 | 16.1 | 4.7 | 0.45 |
| Study Week | Median | p-Value | ||
|---|---|---|---|---|
| DS-01 | Placebo | Difference | ||
| Baseline | 4.30 | 4.30 | 0.00 | 0.928 |
| Week 1 | 1.40 | 1.75 | −0.35 | 0.547 |
| Week 2 | 1.30 | 1.40 | −0.10 | 0.759 |
| Week 3 | 1.00 | 1.40 | −0.40 | <0.05 * |
| Week 4 | 0.90 | 1.20 | −0.30 | <0.05 * |
| Week 5 | 0.85 | 1.10 | −0.25 | 0.077 |
| Week 6 | 0.80 | 1.20 | −0.40 | <0.05 * |
| Study Week | Median | p-Value | ||
|---|---|---|---|---|
| DS-01 | Placebo | Difference | ||
| Baseline | 35.0 | 36.5 | −1.5 | 0.415 |
| Week 1 | 22.0 | 24.5 | −2.5 | 0.143 |
| Week 2 | 20.0 | 20.0 | 0.0 | 0.432 |
| Week 3 | 19.0 | 19.0 | 0.0 | 0.254 |
| Week 4 | 16.0 | 22.0 | −6.0 | <0.001 *** |
| Week 5 | 18.0 | 21.0 | −3.0 | <0.05 * |
| Week 6 | 16.0 | 21.0 | −5.0 | <0.01 ** |
| Study Week | Median | p-Value | ||
|---|---|---|---|---|
| DS-01 | Placebo | Difference | ||
| Baseline | 15.0 | 16.0 | −1.0 | 0.444 |
| Week 1 | 10.0 | 12.0 | −2.0 | 0.148 |
| Week 2 | 10.0 | 11.0 | −1.0 | 0.121 |
| Week 3 | 9.0 | 11.0 | −2.0 | <0.01 ** |
| Week 4 | 8.0 | 11.0 | −3.0 | <0.05 * |
| Week 5 | 9.0 | 10.0 | −1.0 | 0.089 |
| Week 6 | 8.0 | 10.0 | −2.0 | <0.01 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Allegretti, J.R.; Kassam, Z.; Kelly, C.R.; Grinspan, A.; El-Nachef, N.; Van Den Elzen, C.; Jäger, R.; Feuerstadt, P. A Randomized, Placebo-Controlled Trial Evaluating Multi-Species Synbiotic Supplementation for Bloating, Gas, and Abdominal Discomfort. Nutrients 2026, 18, 255. https://doi.org/10.3390/nu18020255
Allegretti JR, Kassam Z, Kelly CR, Grinspan A, El-Nachef N, Van Den Elzen C, Jäger R, Feuerstadt P. A Randomized, Placebo-Controlled Trial Evaluating Multi-Species Synbiotic Supplementation for Bloating, Gas, and Abdominal Discomfort. Nutrients. 2026; 18(2):255. https://doi.org/10.3390/nu18020255
Chicago/Turabian StyleAllegretti, Jessica R., Zain Kassam, Colleen R. Kelly, Ari Grinspan, Najwa El-Nachef, Courtney Van Den Elzen, Ralf Jäger, and Paul Feuerstadt. 2026. "A Randomized, Placebo-Controlled Trial Evaluating Multi-Species Synbiotic Supplementation for Bloating, Gas, and Abdominal Discomfort" Nutrients 18, no. 2: 255. https://doi.org/10.3390/nu18020255
APA StyleAllegretti, J. R., Kassam, Z., Kelly, C. R., Grinspan, A., El-Nachef, N., Van Den Elzen, C., Jäger, R., & Feuerstadt, P. (2026). A Randomized, Placebo-Controlled Trial Evaluating Multi-Species Synbiotic Supplementation for Bloating, Gas, and Abdominal Discomfort. Nutrients, 18(2), 255. https://doi.org/10.3390/nu18020255





