Safety and Efficacy of High-Dose Folinic Acid in Children with Autism: The Impact of Folate Metabolism Gene Polymorphisms
Abstract
1. Introduction
2. Materials and Methods
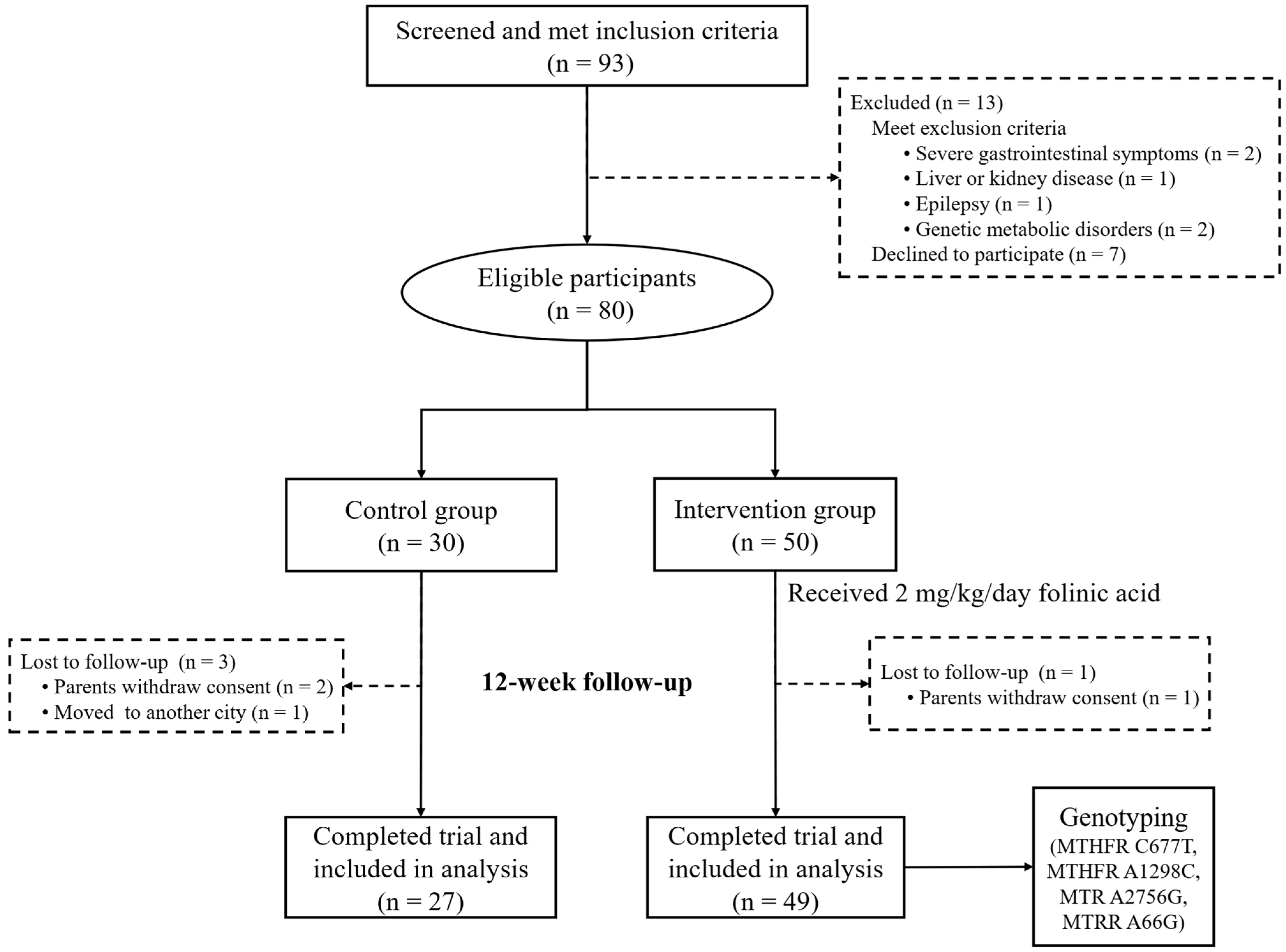
2.1. Participants and Study Design
2.2. Interventions
2.3. Outcome Measures
2.4. Adverse Effects
2.5. Genotyping
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Children with ASD
3.2. Safety and Efficacy of High-Dose Folinic Acid Intervention in Chinese Children with ASD
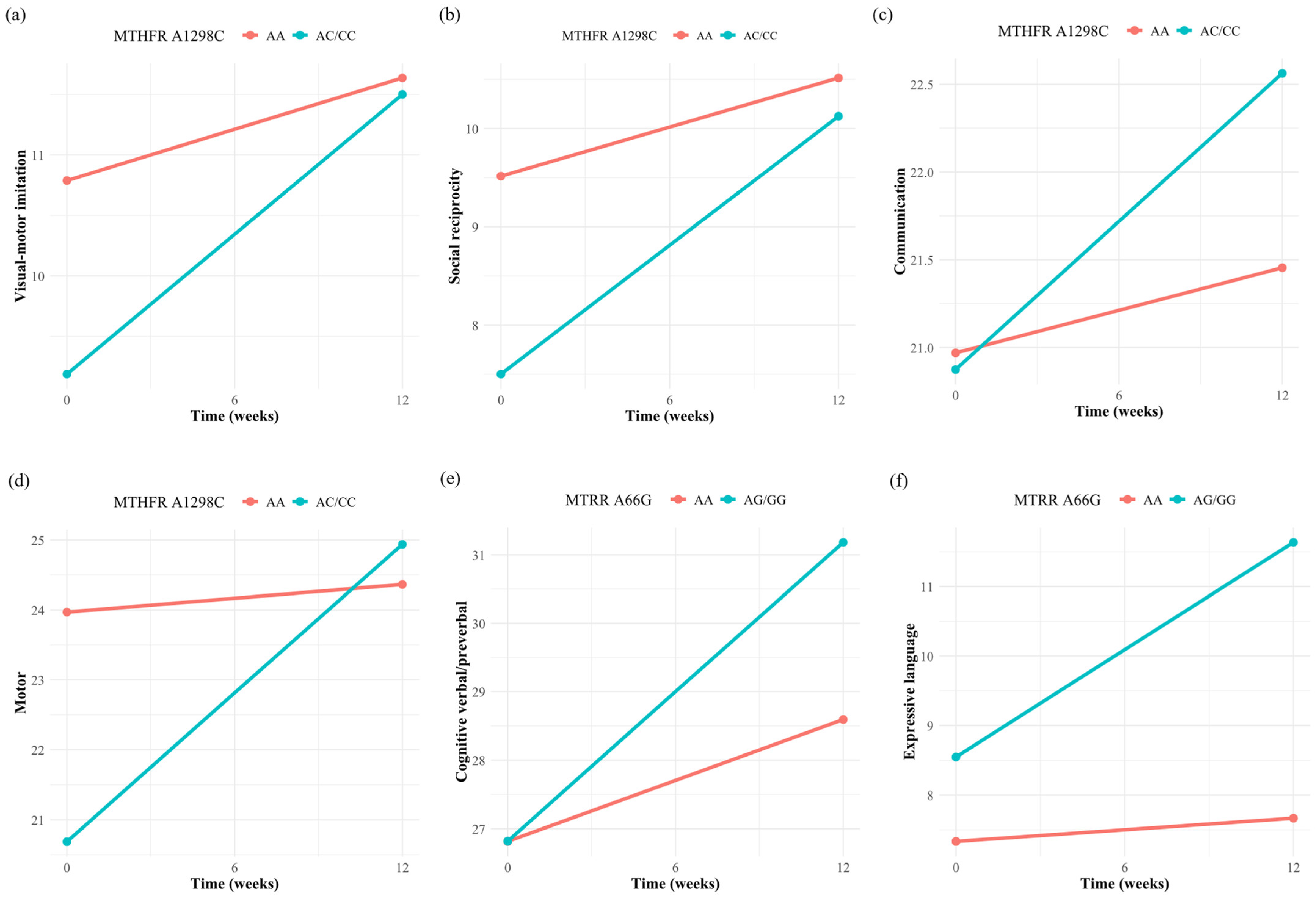
3.3. Association of Single Folate Metabolism Gene Polymorphism with the Efficacy of Folinic Acid
3.4. Association of Folate Metabolism Gene Polymorphism Combination with Efficacy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | Autism Behavior Checklist |
| ASD | Autism Spectrum Disorder |
| CARS | Childhood Autism Rating Scale |
| MTHFR | Methylenetetrahydrofolate reductase |
| MTR | Methionine synthase |
| MTRR | Methionine synthase reductase |
| PEP-3 | Psycho-Educational Profile, Third Edition |
| PCR | Polymerase chain reaction |
| RCT | Randomized controlled trial |
References
- Wang, S.-H.; Zhang, H.-T.; Zou, Y.-Y.; Cheng, S.-M.; Zou, X.-B.; Chen, K.-Y. Efficacy and Moderating Factors of the Early Start Denver Model in Chinese Toddlers with Autism Spectrum Disorder: A Longitudinal Study. World J. Pediatr. 2023, 19, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.A. Early Identification of Autism Spectrum Disorder Among Children Aged 4 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR Surveill Summ 2023, 72, 1–15. [Google Scholar] [CrossRef]
- Wang, S.; Deng, H.; You, C.; Chen, K.; Li, J.; Tang, C.; Ceng, C.; Zou, Y.; Zou, X. Sex Differences in Diagnosis and Clinical Phenotypes of Chinese Children with Autism Spectrum Disorder. Neurosci. Bull. 2017, 33, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Dudley, C.; Emery, J.C.H. The Value of Caregiver Time: Costs of Support and Care for Individuals Living with Autism Spectrum Disorder. 2014. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2379633 (accessed on 15 January 2014).
- Zhao, Y.; Lu, F.; Wang, X.; Luo, Y.; Zhang, R.; He, P.; Zheng, X. The Economic Burden of Autism Spectrum Disorder with and without Intellectual Disability in China: A Nationwide Cost-of-Illness Study. Asian J. Psychiatry 2024, 92, 103877. [Google Scholar] [CrossRef]
- Frye, R.E.; Rossignol, D.A.; Scahill, L.; McDougle, C.J.; Huberman, H.; Quadros, E.V. Treatment of Folate Metabolism Abnormalities in Autism Spectrum Disorder. Semin. Pediatr. Neurol. 2020, 35, 100835. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Rossignol, D.A. Treatments for Biomedical Abnormalities Associated with Autism Spectrum Disorder. Front. Pediatr. 2014, 2, 66. [Google Scholar] [CrossRef]
- Sobral, A.F.; Cunha, A.; Silva, V.; Gil-Martins, E.; Silva, R.; Barbosa, D.J. Unveiling the Therapeutic Potential of Folate-Dependent One-Carbon Metabolism in Cancer and Neurodegeneration. Int. J. Mol. Sci. 2024, 25, 9339. [Google Scholar] [CrossRef]
- Lam, N.S.K.; Long, X.X.; Li, X.; Saad, M.; Lim, F.; Doery, J.C.; Griffin, R.C.; Galletly, C. The Potential Use of Folate and Its Derivatives in Treating Psychiatric Disorders: A Systematic Review. Biomed. Pharmacother. 2022, 146, 112541. [Google Scholar] [CrossRef]
- Vasconcelos, C.; Schweigert Perry, I.; Gottfried, C.; Riesgo, R.; Castro, K. Folic Acid and Autism: Updated Evidences. Nutr. Neurosci. 2025, 28, 273–307. [Google Scholar] [CrossRef]
- Frye, R.E.; Slattery, J.; Delhey, L.; Furgerson, B.; Strickland, T.; Tippett, M.; Sailey, A.; Wynne, R.; Rose, S.; Melnyk, S.; et al. Folinic Acid Improves Verbal Communication in Children with Autism and Language Impairment: A Randomized Double-Blind Placebo-Controlled Trial. Mol. Psychiatry 2018, 23, 247–256. [Google Scholar] [CrossRef]
- James, S.J.; Melnyk, S.; Jernigan, S.; Cleves, M.A.; Halsted, C.H.; Wong, D.H.; Cutler, P.; Bock, K.; Boris, M.; Bradstreet, J.J.; et al. Metabolic Endophenotype and Related Genotypes Are Associated with Oxidative Stress in Children with Autism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006, 141B, 947–956. [Google Scholar] [CrossRef] [PubMed]
- James, S.J.; Cutler, P.; Melnyk, S.; Jernigan, S.; Janak, L.; Gaylor, D.W.; Neubrander, J.A. Metabolic Biomarkers of Increased Oxidative Stress and Impaired Methylation Capacity in Children with Autism. Am. J. Clin. Nutr. 2004, 80, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Batebi, N.; Moghaddam, H.S.; Hasanzadeh, A.; Fakour, Y.; Mohammadi, M.R.; Akhondzadeh, S. Folinic Acid as Adjunctive Therapy in Treatment of Inappropriate Speech in Children with Autism: A Double-Blind and Placebo-Controlled Randomized Trial. Child. Psychiatry Hum. Dev. 2021, 52, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Melnyk, S.; Fuchs, G.; Reid, T.; Jernigan, S.; Pavliv, O.; Hubanks, A.; Gaylor, D.W.; Walters, L.; James, S.J. Effectiveness of Methylcobalamin and Folinic Acid Treatment on Adaptive Behavior in Children with Autistic Disorder Is Related to Glutathione Redox Status. Autism Res. Treat. 2013, 2013, 609705. [Google Scholar] [CrossRef]
- Renard, E.; Leheup, B.; Guéant-Rodriguez, R.-M.; Oussalah, A.; Quadros, E.V.; Guéant, J.-L. Folinic Acid Improves the Score of Autism in the EFFET Placebo-Controlled Randomized Trial. Biochimie 2020, 173, 57–61. [Google Scholar] [CrossRef]
- Pu, D.; Shen, Y.; Wu, J. Association between MTHFR Gene Polymorphisms and the Risk of Autism Spectrum Disorders: A Meta-Analysis: Association of MTHFR Polymorphisms with Autism. Autism Res. 2013, 6, 384–392. [Google Scholar] [CrossRef]
- Tisato, V.; Silva, J.A.; Longo, G.; Gallo, I.; Singh, A.V.; Milani, D.; Gemmati, D. Genetics and Epigenetics of One-Carbon Metabolism Pathway in Autism Spectrum Disorder: A Sex-Specific Brain Epigenome? Genes 2021, 12, 782. [Google Scholar] [CrossRef]
- Mohammad, N.S.; Jain, J.M.N.; Chintakindi, K.P.; Singh, R.P.; Naik, U.; Akella, R.R.D. Aberrations in Folate Metabolic Pathway and Altered Susceptibility to Autism. Psychiatr. Genet. 2009, 19, 171. [Google Scholar] [CrossRef]
- Ni, W.; Li, H.; Wu, A.; Zhang, P.; Yang, H.; Yang, X.; Huang, X.; Jiang, L. Lack of Association between Genetic Polymorphisms in Three Folate-Related Enzyme Genes and Male Infertility in the Chinese Population. J. Assist. Reprod. Genet. 2015, 32, 369–374. [Google Scholar] [CrossRef]
- Li, W.-X.; Dai, S.-X.; Zheng, J.-J.; Liu, J.-Q.; Huang, J.-F. Homocysteine Metabolism Gene Polymorphisms (MTHFR C677T, MTHFR A1298C, MTR A2756G and MTRR A66G) Jointly Elevate the Risk of Folate Deficiency. Nutrients 2015, 7, 6670–6687. [Google Scholar] [CrossRef]
- Frosst, P.; Blom, H.J.; Milos, R.; Goyette, P.; Sheppard, C.A.; Matthews, R.G.; Boers, G.J.H.; den Heijer, M.; Kluijtmans, L.a.J.; van den Heuve, L.P.; et al. A Candidate Genetic Risk Factor for Vascular Disease: A Common Mutation in Methylenetetrahydrofolate Reductase. Nat. Genet. 1995, 10, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Solehdin, F.; Cohen, I.L.; Gonzalez, M.G.; Jenkins, E.C.; Lewis, M.E.S.; Holden, J.J.A. Population- and Family-Based Studies Associate the MTHFR Gene with Idiopathic Autism in Simplex Families. J. Autism Dev. Disord. 2011, 41, 938–944. [Google Scholar] [CrossRef]
- van der Put, N.M.J.; Gabreëls, F.; Stevens, E.M.B.; Smeitink, J.A.M.; Trijbels, F.J.M.; Eskes, T.K.A.B.; van den Heuvel, L.P.; Blom, H.J. A Second Common Mutation in the Methylenetetrahydrofolate Reductase Gene: An Additional Risk Factor for Neural-Tube Defects? Am. J. Hum. Genet. 1998, 62, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- De Mattia, E.; Toffoli, G. C677T and A1298C MTHFR Polymorphisms, a Challenge for Antifolate and Fluoropyrimidine-Based Therapy Personalisation. Eur. J. Cancer 2009, 45, 1333–1351. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Tancredi, D.J.; Ozonoff, S.; Hansen, R.L.; Hartiala, J.; Allayee, H.; Schmidt, L.C.; Tassone, F.; Hertz-Picciotto, I. Maternal Periconceptional Folic Acid Intake and Risk of Autism Spectrum Disorders and Developmental Delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) Case-Control Study123. Am. J. Clin. Nutr. 2012, 96, 80–89. [Google Scholar] [CrossRef]
- Adams, J.B.; Bhargava, A.; Coleman, D.M.; Frye, R.E.; Rossignol, D.A. Ratings of the Effectiveness of Nutraceuticals for Autism Spectrum Disorders: Results of a National Survey. J. Pers. Med. 2021, 11, 878. [Google Scholar] [CrossRef] [PubMed]
- Fletcher-Campbell, F. Treatment and Education of Autistic and Related Communication Handicapped Children (TEACCH). In Review of the Research Literature on Educational Interventions for Pupils with Autism Spectrum Disorders; National Foundation for Educational Research: Slough, UK, 2003; pp. 11–15. [Google Scholar]
- Schopler, E.; Lansing, M.D.; Reichler, R.J.; Marcus, L.M. Examiner’s Manual of Psychoeducational Profile; Pro-Ed Inc.: Austin, TX, USA, 2005; Volume 3. [Google Scholar]
- Ghanatghestani, L.M.; Ahmadi, M.S.; Tajali, P. The Role of Tests and Tools in Accurate Diagnosis and Severity in Children with Autism Spectrum Disorders. Appl. Fam. Ther. J. (AFTJ) 2024, 5, 76–83. [Google Scholar] [CrossRef]
- Nilsson, M.E.; Koke, S.C. Defining Treatment-Emergent Adverse Events with the Medical Dictionary for Regulatory Activities (MedDRA). Drug Inf. J. 2001, 35, 1289–1299. [Google Scholar] [CrossRef]
- Elhawary, N.A. Methylenetetrahydrofolate Reductase Gene and Potential Risk to Autism Spectrum Disorder. In New Horizons in Medicine and Medical Research; Donmez, S., Ed.; Book Publisher International (a part of SCIENCEDOMAIN International): London, UK, 2022; Volume 7, pp. 158–173. ISBN 978-93-5547-630-2. [Google Scholar]
- Montella, A.; Cantalupo, S.; D’Alterio, G.; Damiano, V.; Iolascon, A.; Capasso, M. Improving Single Nucleotide Polymorphisms Genotyping Accuracy for Dihydropyrimidine Dehydrogenase Testing in Pharmacogenetics. Explor. Target. Antitumor Ther. 2024, 5, 374–383. [Google Scholar] [CrossRef]
- Ramaekers, V.T.; Sequeira, J.M.; DiDuca, M.; Vrancken, G.; Thomas, A.; Philippe, C.; Peters, M.; Jadot, A.; Quadros, E.V. Improving Outcome in Infantile Autism with Folate Receptor Autoimmunity and Nutritional Derangements: A Self-Controlled Trial. Autism Res. Treat. 2019, 2019, e7486431. [Google Scholar] [CrossRef]
- Bettencourt, C.; Garret-Gloanec, N.; Pellerin, H.; Péré, M.; Squillante, M.; Roos-Weil, F.; Ferrand, L.; Pernel, A.-S.; Apter, G.; Cohen, D. Migration Is Associated with Baseline Severity and Progress over Time in Autism Spectrum Disorder: Evidence from a French Prospective Longitudinal Study. PLoS ONE 2022, 17, e0272693. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Sharawat, I.K.; Saha, S.; Gupta, D.; Palayullakandi, A.; Meena, K. Efficacy of Oral Folinic Acid Supplementation in Children with Autism Spectrum Disorder: A Randomized Double-Blind, Placebo-Controlled Trial. Eur. J. Pediatr. 2024, 183, 4827–4835. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.M.; Tan, C.S.; Koh, H.C.; Gan, X.; Hie, S.L.; Saffari, S.E.; Yeo, J.G.; Lam, J.C.M. Folinic Acid as a Treatment for Autism in Children: A within-Subjects Open-Label Study on Safety and Efficacy. Int. J. Dev. Neurosci. 2025, 85, e10402. [Google Scholar] [CrossRef] [PubMed]
- Bent, S.; Chen, Y.; McDonald, M.G.; Widjaja, F.; Wahlberg, J.; Hendren, R.L. An Examination of Changes in Urinary Metabolites and Behaviors with the Use of Leucovorin Calcium in Children with Autism Spectrum Disorder (ASD). Adv. Neurodev. Disord. 2020, 4, 241–246. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Cerebral Folate Deficiency, Folate Receptor Alpha Autoantibodies and Leucovorin (Folinic Acid) Treatment in Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 1141. [Google Scholar] [CrossRef]
- Barretta, F.; Uomo, F.; Fecarotta, S.; Albano, L.; Crisci, D.; Verde, A.; Fisco, M.G.; Gallo, G.; Dottore Stagna, D.; Pricolo, M.R.; et al. Contribution of Genetic Test to Early Diagnosis of Methylenetetrahydrofolate Reductase (MTHFR) Deficiency: The Experience of a Reference Center in Southern Italy. Genes 2023, 14, 980. [Google Scholar] [CrossRef]
- Ulvik, A.; Ueland, P.M.; Fredriksen, Å.; Meyer, K.; Vollset, S.E.; Hoff, G.; Schneede, J. Functional Inference of the Methylenetetrahydrofolate Reductase 677 C > T and 1298A > C Polymorphisms from a Large-Scale Epidemiological Study. Hum. Genet. 2007, 121, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Sun, C.; Yang, X.; Zhang, Z.; Li, N.; Hou, L. Association of Methylenetetrahydrofolate Reductase in Gene C677T and A1298C Polymorphism among Children with Autism. Chin. J. Sch. Health 2013, 34, 52–58. [Google Scholar]
- Guo, T.; Chen, H.; Liu, B.; Ji, W.; Yang, C. Methylenetetrahydrofolate Reductase Polymorphisms C677T and Risk of Autism in the Chinese Han Population. Genet. Test. Mol. Biomark. 2012, 16, 968–973. [Google Scholar] [CrossRef]
- Haghiri, R.; Mashayekhi, F.; Bidabadi, E.; Salehi, Z. Analysis of Methionine Synthase (Rs1805087) Gene Polymorphism in Autism Patients in Northern Iran. Acta Neurobiol. Exp. 2016, 76, 318–323. [Google Scholar] [CrossRef]
| Characteristic | Total (n = 76) | Control Group (n = 27) | Intervention Group (n = 49) | p |
|---|---|---|---|---|
| Gender | 0.745 | |||
| Female | 12 (15.8%) | 5 (18.5%) | 7 (14.3%) | |
| Male | 64 (84.2%) | 22 (81.5%) | 42 (85.7%) | |
| Age at enrollment, months | 51.28 (10.90) | 51.30 (12.94) | 51.27 (9.75) | 0.991 |
| Ethnicity | 0.650 | |||
| Han | 71 (93.4%) | 26 (96.3%) | 45 (91.8%) | |
| Other | 5 (6.6%) | 1 (3.7%) | 4 (8.2%) | |
| Preterm birth | 0.999 | |||
| No | 69 (90.8%) | 25 (92.6%) | 44 (89.8%) | |
| Yes | 7 (9.2%) | 2 (7.4%) | 5 (10.2%) | |
| Birth weight, kg | 3.28 (0.43) | 3.42 (0.43) | 3.21 (0.42) | 0.041 |
| Mode of delivery | 0.328 | |||
| Vaginal delivery | 46 (60.5%) | 14 (51.9%) | 32 (65.3%) | |
| Cesarean section | 30 (39.5%) | 13 (48.1%) | 17 (34.7%) | |
| Paternal age at birth, years | 31.51 (5.57) | 31.19 (6.14) | 31.69 (5.28) | 0.718 |
| Maternal age at birth, years | 29.33 (4.89) | 28.89 (5.34) | 29.57 (4.65) | 0.580 |
| Father’s education level | 0.355 | |||
| Less than high school | 13 (17.1%) | 7 (25.9%) | 6 (12.2%) | |
| High school | 15 (19.7%) | 5 (18.5%) | 10 (20.4%) | |
| College degree or higher | 48 (63.2%) | 15 (55.6%) | 33 (67.3%) | |
| Mother’s education level | 0.027 | |||
| Less than high school | 11 (14.5%) | 8 (29.6%) | 3 (6.1%) | |
| High school | 25 (32.9%) | 8 (29.6%) | 17 (34.7%) | |
| College degree or higher | 40 (52.6%) | 11 (40.7%) | 29 (59.2%) | |
| ABC scores | 59.42 (14.18) | 60.44 (14.04) | 58.86 (14.36) | 0.642 |
| CARS scores | 36.24 (4.00) | 35.96 (4.59) | 36.39 (3.68) | 0.681 |
| Characteristic | Control Group (n = 27) | Intervention Group (n = 49) | Between-Group Difference in Change | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12-Weeks | Change from Baseline | Baseline | 12-Weeks | Change from Baseline | Mean Difference (95% CI) | p | |
| Subtest | ||||||||
| Cognitive verbal/preverbal | 29.07 (15.11) | 33.96 (15.72) | 4.89 (7.03) | 26.82 (12.72) | 29.76 (13.30) | 2.94 (4.36) | −1.95 (−4.55, 0.65) | 0.140 |
| Expressive language | 10.89 (12.08) | 14.00 (13.09) | 3.11 (4.39) | 7.88 (9.97) | 9.45 (11.97) | 1.57 (3.76) | −1.54 (−3.45, 0.37) | 0.112 |
| Receptive language | 14.52 (11.05) | 18.89 (10.92) | 4.37 (5.43) | 13.22 (9.67) | 15.65 (11.00) | 2.43 (3.61) | −1.94 (−4.01, 0.13) | 0.066 |
| Fine motor | 27.22 (7.24) | 28.74 (7.00) | 1.52 (3.95) | 26.65 (6.57) | 28.41 (6.49) | 1.76 (2.77) | 0.24 (−1.31, 1.78) | 0.761 |
| Gross motor | 22.41 (5.72) | 23.96 (5.85) | 1.56 (2.81) | 23.06 (5.78) | 24.49 (5.01) | 1.43 (3.40) | −0.13 (−1.66, 1.40) | 0.869 |
| Visual-motor imitation | 11.00 (4.81) | 12.22 (4.53) | 1.22 (2.03) | 10.27 (4.73) | 11.59 (4.61) | 1.33 (2.42) | 0.10 (−0.99, 1.20) | 0.850 |
| Affective expression | 10.33 (3.46) | 10.26 (2.85) | −0.07 (2.06) | 9.61 (2.56) | 9.92 (2.57) | 0.31 (1.88) | 0.38 (−0.55, 1.31) | 0.418 |
| Social reciprocity | 10.19 (3.56) | 10.52 (3.18) | 0.33 (1.71) | 8.86 (3.07) | 10.39 (3.43) | 1.53 (1.98) | 1.20 (0.29, 2.10) | 0.010 |
| Characteristic motor behaviors | 17.15 (4.15) | 17.70 (4.67) | 0.56 (3.09) | 15.94 (4.45) | 15.73 (4.64) | −0.20 (3.24) | −0.76 (−2.28, 0.76) | 0.324 |
| Characteristic verbal behaviors | 6.56 (5.92) | 7.89 (5.70) | 1.33 (2.57) | 4.29 (4.50) | 5.10 (5.19) | 0.82 (2.19) | −0.52 (−1.63, 0.6) | 0.357 |
| Composites | ||||||||
| Communication | 23.15 (8.27) | 23.93 (9.38) | 0.78 (4.29) | 20.94 (7.38) | 21.82 (7.70) | 0.88 (1.91) | 0.10 (−1.32, 1.52) | 0.889 |
| Motor | 24.74 (7.82) | 24.78 (9.87) | 0.04 (6.30) | 22.90 (8.98) | 24.55 (8.91) | 1.65 (4.53) | 1.62 (−0.88, 4.11) | 0.200 |
| Maladaptive behaviors | 27.85 (9.13) | 27.44 (8.87) | −0.41 (5.57) | 24.39 (7.57) | 25.14 (7.82) | 0.76 (3.70) | 1.16 (−0.96, 3.29) | 0.279 |
| Caregiver report | ||||||||
| Problem behavior | 8.70 (3.34) | 9.22 (4.08) | 0.52 (2.31) | 8.63 (3.57) | 9.15 (4.06) | 0.50 (2.90) | −0.02 (−1.31, 1.28) | 0.977 |
| Personal self-care | 16.41 (4.75) | 17.30 (4.43) | 0.89 (2.14) | 16.08 (4.80) | 17.25 (4.52) | 1.33 (3.39) | 0.44 (−1.00, 1.89) | 0.541 |
| Adaptive behaviors | 17.33 (5.72) | 18.41 (5.49) | 1.07 (3.30) | 15.71 (6.18) | 17.23 (6.84) | 1.52 (3.41) | 0.45 (−1.17, 2.07) | 0.584 |
| Variables | MTHFR A1298C | MTHFR C677T | MTR A2756G | MTRR A66G | ||||
|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | p | Estimate (95% CI) | p | Estimate (95% CI) | p | Estimate (95% CI) | p | |
| Subtest | ||||||||
| Cognitive verbal/preverbal | 1.20 (−1.22, 3.63) | 0.370 | −1.20 (−3.51, 1.10) | 0.348 | −2.02 (−4.63, 0.60) | 0.166 | 2.59 (0.37, 4.80) | 0.038 |
| Expressive language | 0.73 (−1.25, 2.71) | 0.514 | −0.22 (−2.11, 1.68) | 0.839 | −1.86 (−3.99, 0.26) | 0.124 | 2.76 (1.02, 4.50) | 0.006 |
| Receptive language | 0.76 (−1.16, 2.67) | 0.485 | 0.64 (−1.19, 2.47) | 0.536 | −0.68 (−2.77, 1.41) | 0.566 | 0.38 (−1.43, 2.18) | 0.711 |
| Fine motor | 1.01 (−0.42, 2.45) | 0.214 | −1.09 (−2.46, 0.28) | 0.161 | −1.11 (−2.67, 0.45) | 0.211 | 1.35 (0.01, 2.70) | 0.078 |
| Gross motor | 0.85 (−0.91, 2.60) | 0.393 | −0.71 (−2.39, 0.97) | 0.454 | 0.98 (−0.94, 2.89) | 0.367 | 0.62 (−1.04, 2.29) | 0.506 |
| Visual-motor imitation | 1.46 (0.29, 2.64) | 0.030 | −0.04 (−1.20, 1.12) | 0.952 | 0.34 (−0.98, 1.66) | 0.649 | 0.40 (−0.74, 1.54) | 0.538 |
| Affective expression | 0.10 (−0.91, 1.11) | 0.858 | 0.52 (−0.44, 1.48) | 0.342 | 0.48 (−0.63, 1.59) | 0.446 | −0.14 (−1.10, 0.82) | 0.792 |
| Social reciprocity | 1.63 (0.66, 2.59) | 0.004 | −0.03 (−1.01, 0.95) | 0.953 | −0.15 (−1.27, 0.97) | 0.811 | −0.39 (−1.35, 0.58) | 0.480 |
| Characteristic motor behaviors | 0.03 (−1.68, 1.73) | 0.980 | 0.50 (−1.13, 2.13) | 0.587 | 0.93 (−0.92, 2.78) | 0.373 | −0.62 (−2.22, 0.98) | 0.492 |
| Characteristic verbal behaviors | −0.93 (−2.12, 0.25) | 0.163 | 1.13 (0.01, 2.24) | 0.076 | 0.02 (−1.31, 1.36) | 0.976 | 0.09 (−1.07, 1.24) | 0.893 |
| Composites | ||||||||
| Communication | 1.20 (0.20, 2.21) | 0.037 | −0.12 (−1.12, 0.88) | 0.828 | −0.72 (−1.85, 0.41) | 0.261 | 0.39 (−0.60, 1.37) | 0.486 |
| Motor | 3.86 (1.62, 6.09) | 0.003 | −0.67 (−2.97, 1.63) | 0.606 | 0.02 (−2.62, 2.66) | 0.990 | 0.13 (−2.14, 2.41) | 0.917 |
| Maladaptive behaviors | 1.57 (−0.48, 3.62) | 0.166 | 1.70 (−0.20, 3.59) | 0.115 | 1.32 (−0.92, 3.55) | 0.288 | −1.21 (−3.13, 0.72) | 0.261 |
| Caregiver report | ||||||||
| Problem behavior | 0.10 (−1.44, 1.65) | 0.906 | 0.69 (−0.78, 2.15) | 0.409 | −0.77 (−2.46, 0.92) | 0.420 | −0.40 (−1.86, 1.07) | 0.631 |
| Personal self-care | −0.39 (−2.15, 1.37) | 0.696 | −0.30 (−1.99, 1.40) | 0.756 | 0.57 (−1.35, 2.49) | 0.601 | −1.04 (−2.70, 0.63) | 0.272 |
| Adaptive behaviors | 1.15 (−0.70, 3.00) | 0.273 | −0.67 (−2.45, 1.11) | 0.508 | −1.08 (−3.08, 0.92) | 0.342 | 1.18 (−0.57, 2.93) | 0.235 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Chen, Y.; Hou, F.; Li, Y.; Wang, W.; Guo, L.; Zhang, C.; Li, L.; Lu, C. Safety and Efficacy of High-Dose Folinic Acid in Children with Autism: The Impact of Folate Metabolism Gene Polymorphisms. Nutrients 2025, 17, 1602. https://doi.org/10.3390/nu17091602
Zhang C, Chen Y, Hou F, Li Y, Wang W, Guo L, Zhang C, Li L, Lu C. Safety and Efficacy of High-Dose Folinic Acid in Children with Autism: The Impact of Folate Metabolism Gene Polymorphisms. Nutrients. 2025; 17(9):1602. https://doi.org/10.3390/nu17091602
Chicago/Turabian StyleZhang, Caiyun, Yanlin Chen, Fang Hou, Yanzhi Li, Wanxin Wang, Lan Guo, Caixia Zhang, Li Li, and Ciyong Lu. 2025. "Safety and Efficacy of High-Dose Folinic Acid in Children with Autism: The Impact of Folate Metabolism Gene Polymorphisms" Nutrients 17, no. 9: 1602. https://doi.org/10.3390/nu17091602
APA StyleZhang, C., Chen, Y., Hou, F., Li, Y., Wang, W., Guo, L., Zhang, C., Li, L., & Lu, C. (2025). Safety and Efficacy of High-Dose Folinic Acid in Children with Autism: The Impact of Folate Metabolism Gene Polymorphisms. Nutrients, 17(9), 1602. https://doi.org/10.3390/nu17091602





