Postprandial Responses to Animal Products with Distinct Fatty Acid and Amino Acid Composition Are Diet-Dependent
Abstract
1. Introduction
2. Materials and Methods
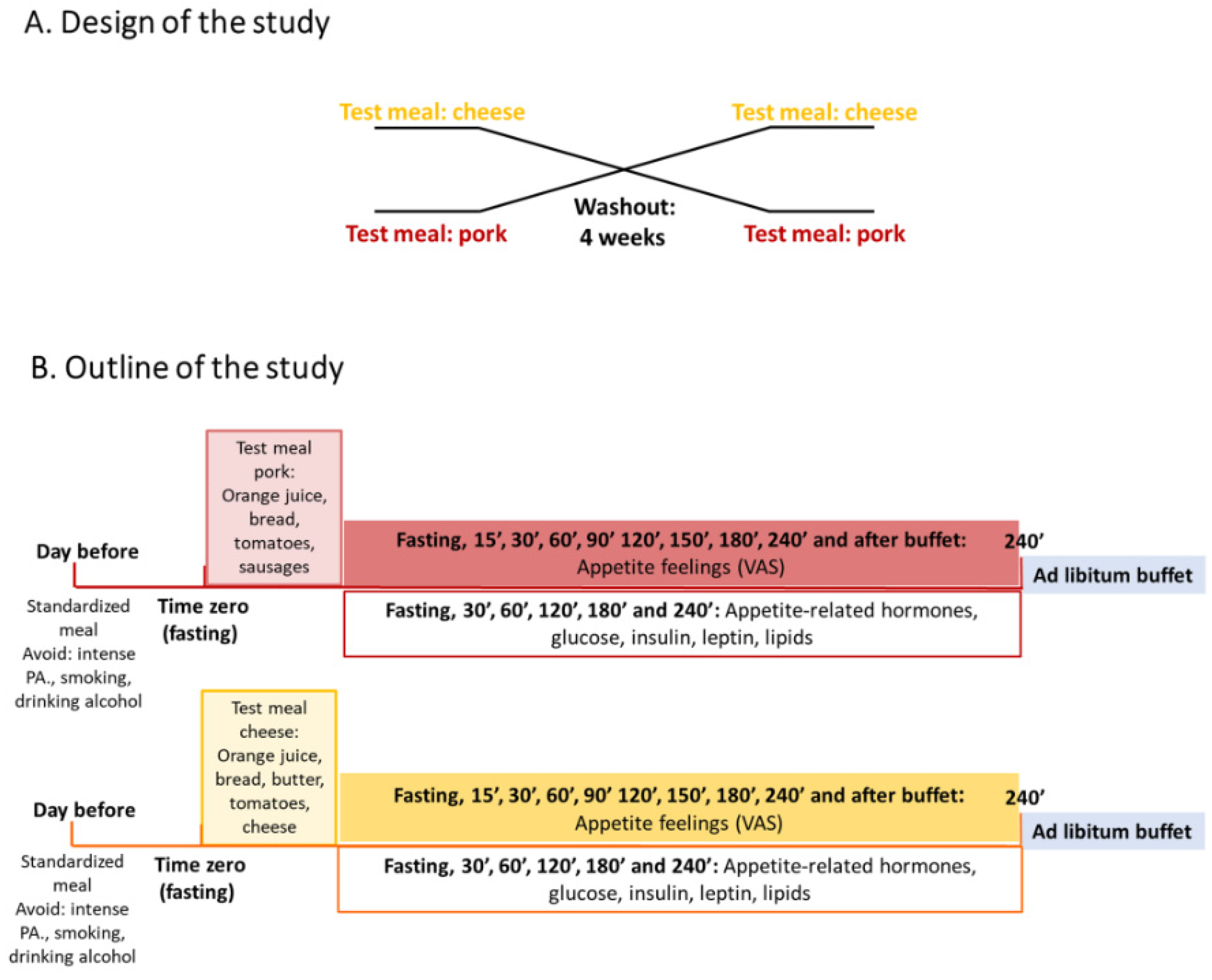
2.1. Study Design
2.2. Participants
2.3. Breakfast Test Meals
2.4. Fatty Acids and Amino Acids Analysis of the Test Products
2.5. Anthropometric Measurements
2.6. Outcome Variables
2.7. Power of Study
2.8. Statistical Analysis
3. Results
3.1. Blood Markers
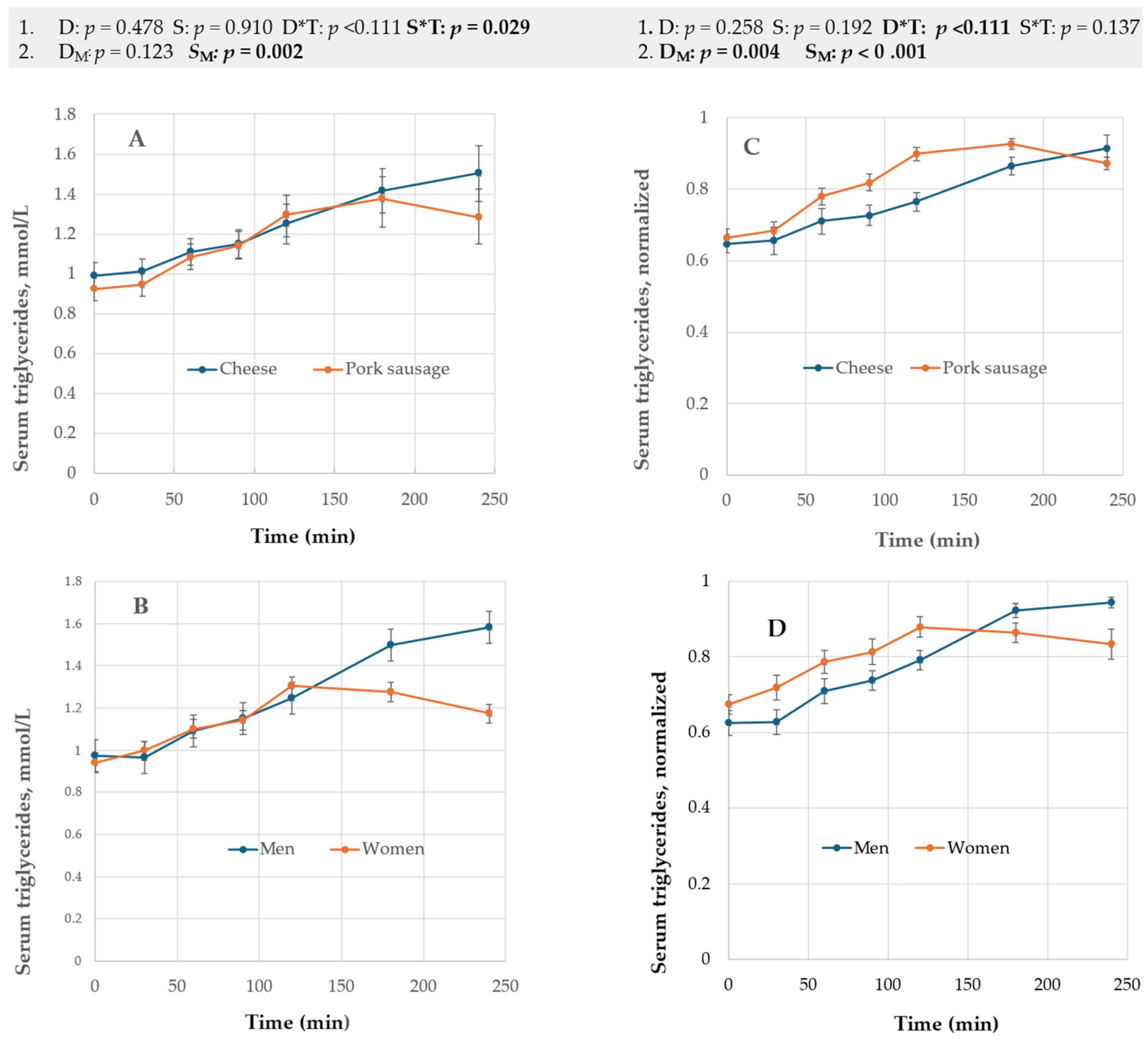
3.1.1. Serum Triglycerides, Serum Cholesterols, and Plasma Leptin Hormone
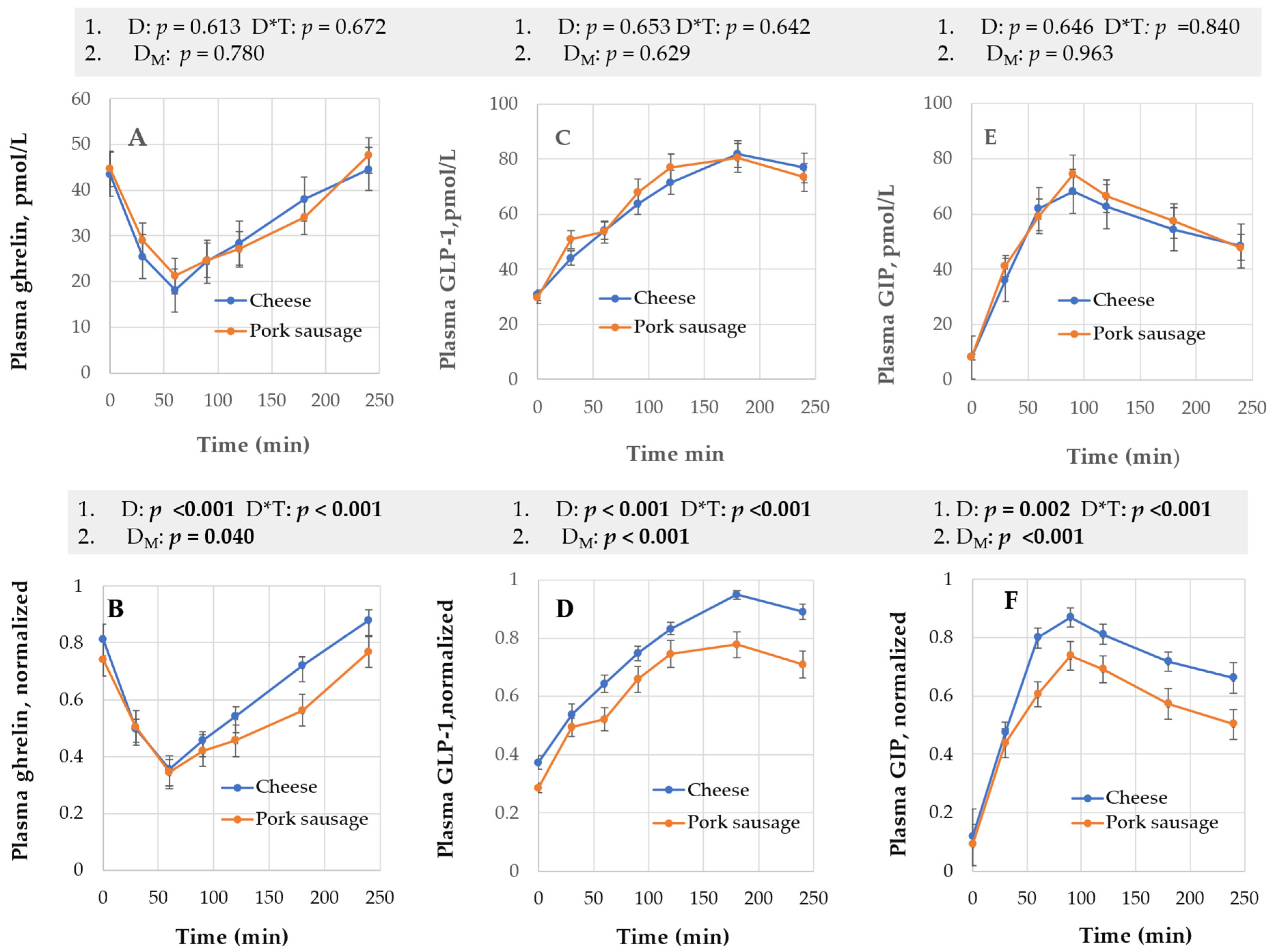
3.1.2. Plasma Ghrelin, Active GLP-1, and GIP
3.1.3. Serum Insulin, Glucose, and Plasma CCK
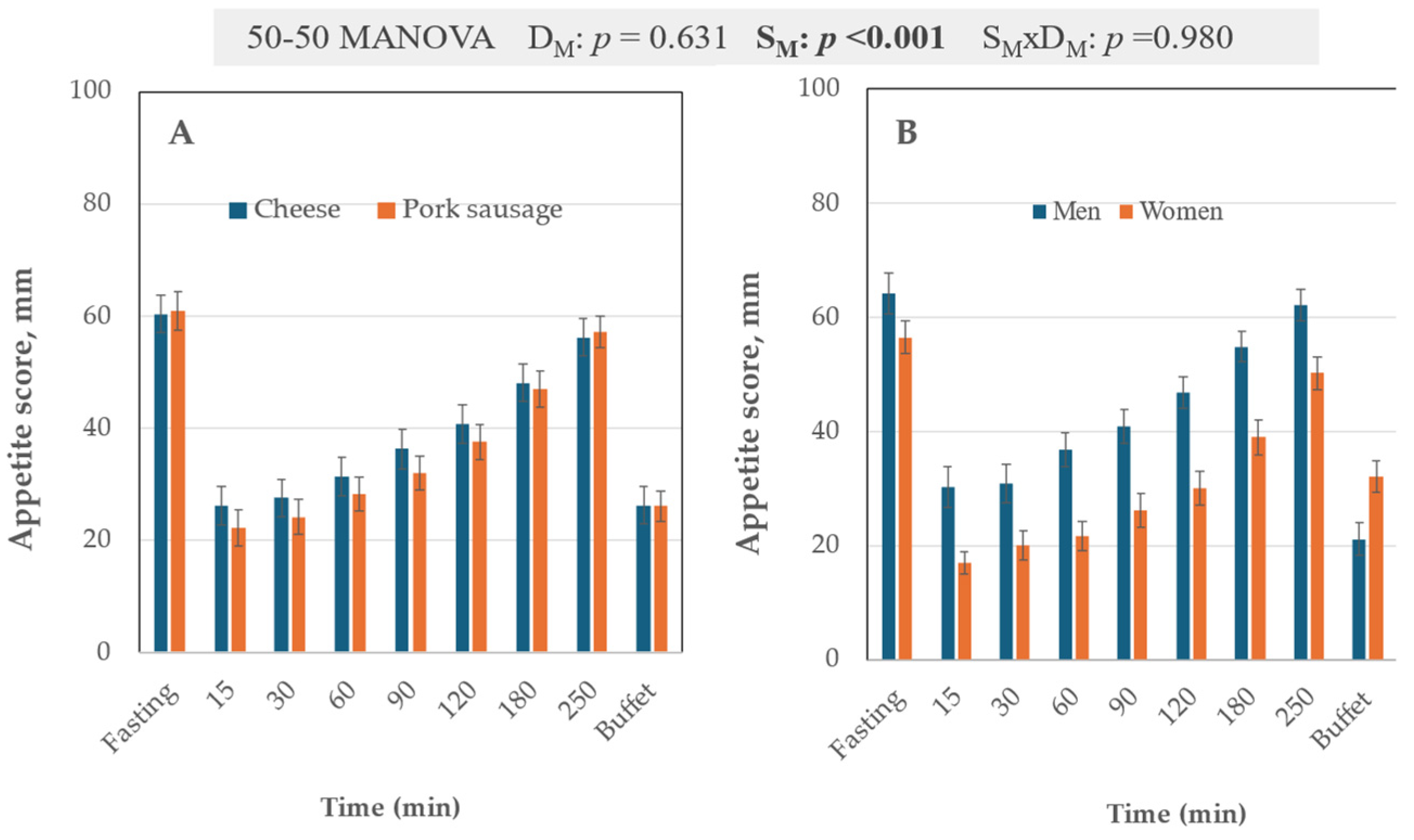
3.2. VAS Scale
3.3. Post-Buffet VAS and Buffet Energy Intake
4. Discussion
4.1. Diet Effect
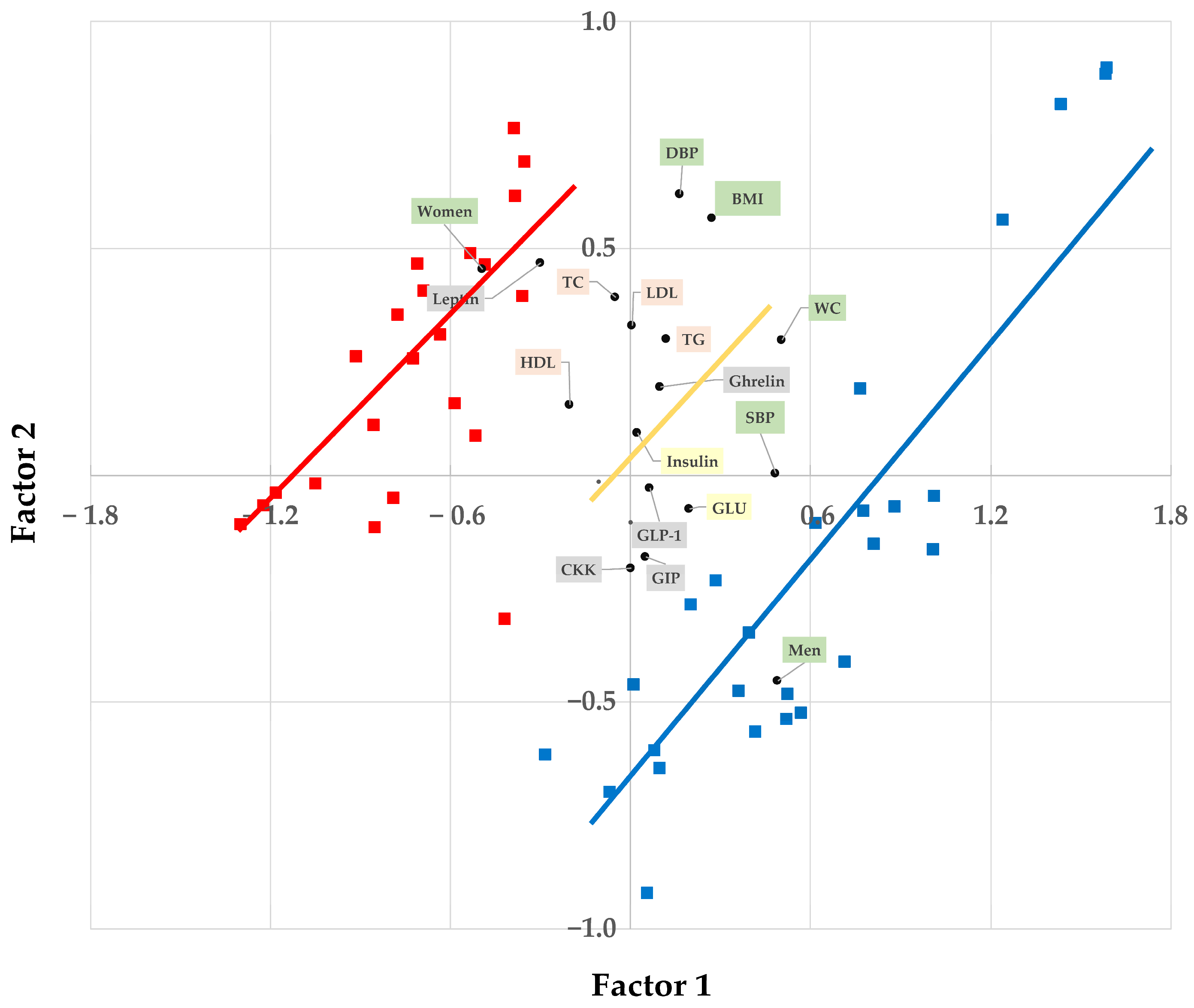
4.2. The Sex Factor
4.3. Methodology
4.4. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| CCK | Cholecystokinin |
| GLP-1 | Glucagon-like peptide-1 |
| GLM | Generalized linear model |
| GIP | Glucose-dependent insulinotropic polypeptide |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| MANOVA | Multivariate analysis of variance |
| MN | Max normalized |
| MUFA | Monounsaturated fatty acid |
| PCA | Principal component analysis |
| PLSR | Partial least squares regression |
| PUFA | Polyunsaturated fatty acid |
| PYY | Peptide YY |
| RM-ANOVA | Repeated measure analysis of variance |
| SFA | Saturated fatty acids |
| VAS | Visual analogue scale |
| TC | Total cholesterol |
| TG | Triglycerides |
References
- Valenzuela, P.L.; Carrera-Bastos, P.; Castillo-García, A.; Lieberman, D.E.; Santos-Lozano, A.; Lucia, A. Obesity and the risk of cardiometabolic diseases. Nat. Rev. Cardiol. 2023, 20, 475–494. [Google Scholar] [CrossRef] [PubMed]
- Lobstein, T.; Jackson-Leach, R.; Powis, J.; Brinsden, H.; Gray, M. World Obesity Atlas 2023. Available online: https://data.worldobesity.org/publications/?cat=19/ (accessed on 30 March 2025).
- Thomsen, C.; Rasmussen, O.; Lousen, T.; Holst, J.J.; Fenselau, S.; Schrezenmeir, J.; Hermansen, K. Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am. J. Clin. Nutr. 1999, 69, 1135–1143. [Google Scholar] [CrossRef]
- Dehnavi, Z.; Barghchi, H.; Esfehani, A.J.; Barati, M.; Khorasanchi, Z.; Farsi, F.; Ostad, A.N.; Ranjbar, G.; Rezvani, R.; Gorgani, M.R.; et al. Animal and plant-based proteins have different postprandial effects on energy expenditure, glycemia, insulinemia, and lipemia: A review of controlled clinical trials. Food Sci. Nutr. 2023, 11, 4398–4408. [Google Scholar] [CrossRef] [PubMed]
- Grom, L.C.; Rocha, R.S.; Balthazar, C.F.; Guimaraes, J.T.; Coutinho, N.M.; Barros, C.P.; Pimentel, T.C.; Venancio, E.L.; Collopy Junior, I.; Maciel, P.M.C.; et al. Postprandial glycemia in healthy subjects: Which probiotic dairy food is more adequate? J. Dairy. Sci. 2020, 103, 1110–1119. [Google Scholar] [CrossRef]
- Gibbons, C.; Caudwell, P.; Finlayson, G.; Webb, D.L.; Hellstrom, P.M.; Naslund, E.; Blundell, J.E. Comparison of postprandial profiles of ghrelin, active GLP-1, and total PYY to meals varying in fat and carbohydrate and their association with hunger and the phases of satiety. J. Clin. Endocrinol. Metab. 2013, 98, E847–E855. [Google Scholar] [CrossRef]
- Maher, T.; Clegg, M.E. Dietary lipids with potential to affect satiety: Mechanisms and evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 1619–1644. [Google Scholar] [CrossRef] [PubMed]
- Marmonier, C.; Chapelot, D.; Louis-Sylvestre, J. Effects of macronutrient content and energy density of snacks consumed in a satiety state on the onset of the next meal. Appetite 2000, 34, 161–168. [Google Scholar] [CrossRef]
- Pan, A.; Hu, F.B. Effects of carbohydrates on satiety: Differences between liquid and solid food. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 385–390. [Google Scholar] [CrossRef]
- Kaviani, S.; Cooper, J.A. Appetite responses to high-fat meals or diets of varying fatty acid composition: A comprehensive review. Eur. J. Clin. Nutr. 2017, 71, 1154–1165. [Google Scholar] [CrossRef]
- Strik, C.M.; Lithander, F.E.; Mcgill, A.T.; Macgibbon, A.K.; Mcardle, B.H.; Poppitt, S.D. No evidence of differential effects of SFA, MUFA or PUFA on post-ingestive satiety and energy intake: A randomised trial of fatty acid saturation. Nutr. J. 2010, 9, 24. [Google Scholar] [CrossRef]
- Rust, B.M.; Raatz, S.K.; Casperson, S.L.; Duke, S.E.; Picklo, M.J. Dietary Fat Chain Length, Saturation, and PUFA Source Acutely Affect Diet-Induced Thermogenesis but Not Satiety in Adults in a Randomized, Crossover Trial. Nutrients 2021, 13, 2615. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Koh, G. Clinical Evidence and Mechanisms of High-Protein Diet-Induced Weight Loss. J. Obes. Metab. Syndr. 2020, 29, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Paddon-Jones, D.; Westman, E.; Mattes, R.D.; Wolfe, R.R.; Astrup, A.; Westerterp-Plantenga, M. Protein, weight management, and satiety. Am. J. Clin. Nutr. 2008, 87, 1558S–1561S. [Google Scholar] [CrossRef]
- Gonzalez, J.T.; Green, B.P.; Brown, M.A.; Rumbold, P.L.; Turner, L.A.; Stevenson, E.J. Calcium ingestion suppresses appetite and produces acute overcompensation of energy intake independent of protein in healthy adults. J. Nutr. 2015, 145, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Teegarden, D.; Gunther, C.W. Can the controversial relationship between dietary calcium and body weight be mechanistically explained by alterations in appetite and food intake? Nutr. Rev. 2008, 66, 601–605. [Google Scholar] [CrossRef]
- Anjom-Shoae, J.; Fitzgerald, P.C.; Horowitz, M.; Mohammadpour, Z.; Hall, G.V.; Holst, J.J.; Rehfeld, J.F.; Veedfald, S.; Feinle-Bisset, C. Intraduodenal calcium enhances the effects of L-tryptophan to stimulate gut hormone secretion and suppress energy intake in healthy males: A randomized, crossover, clinical trial. Am. J. Clin. Nutr. 2024, 120, 528–539. [Google Scholar] [CrossRef]
- Anjom-Shoae, J.; Fitzgerald, P.C.E.; Horowitz, M.; Holst, J.J.; Rehfeld, J.F.; Veedfald, S.; Feinle-Bisset, C. Dose-related effects of calcium to enhance the effects of L-tryptophan on gut hormones and energy intake in obesity. J. Clin. Endocrinol. Metab. 2025, dgaf008. [Google Scholar] [CrossRef]
- Monfort-Pires, M.; Lamichhane, S.; Alonso, C.; Egelandsdal, B.; Oresic, M.; Jordahl, V.O.; Skjolsvold, O.; Perez-Ruiz, I.; Blanco, M.E.; Skeie, S.; et al. Classification of Common Food Lipid Sources Regarding Healthiness Using Advanced Lipidomics: A Four-Arm Crossover Study. Int. J. Mol. Sci. 2023, 24, 4941. [Google Scholar] [CrossRef]
- Veldhorst, M.; Smeets, A.; Soenen, S.; Hochstenbach-Waelen, A.; Hursel, R.; Diepvens, K.; Lejeune, M.; Luscombe-Marsh, N.; Westerterp-Plantenga, M. Protein-induced satiety: Effects and mechanisms of different proteins. Physiol. Behav. 2008, 94, 300–307. [Google Scholar] [CrossRef]
- Braden, M.L.; Gwin, J.A.; Leidy, H.J. Protein Source Influences Acute Appetite and Satiety but Not Subsequent Food Intake in Healthy Adults. J. Nutr. 2023, 153, 1825–1833. [Google Scholar] [CrossRef]
- Abou-Samra, R.; Keersmaekers, L.; Brienza, D.; Mukherjee, R.; Mace, K. Effect of different protein sources on satiation and short-term satiety when consumed as a starter. Nutr. J. 2011, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Ndla. Kostholdsplanleggeren. Available online: https://ndla.no/ (accessed on 15 March 2025). (In Norwegian).
- Norstat. Dette Spiser Nordmenn til Frokost. Available online: https://www.godt.no/aktuelt/i/K3RAkX/dette-spiser-nordmenn-til-frokost) (accessed on 15 June 2024). (In Norwegian).
- Opplysningskontoret-Melk-Og-Meieriprodukter. Disse Ostene kan du Spise Hver Dag. Available online: https://www.melk.no/Kosthold-og-helse/Kosthold-for-voksne/Disse-ostene-kan-du-spise-hver-dag (accessed on 15 September 2024). (In Norwegian).
- Blomhoff, R.; Andersen, R.; Arnesen, E.K.; Christensen, J.J.; Eneroth, H.; Erkkola, M.; Gudanaviciene, I.; Halldorsson, Y.I.; Høyer-Lund, A.; Lemming, E.W.; et al. Nordic Nutrition Recommendations 2023—Integrating Environmental Aspects; Nordic Council of Ministers: Copenhagen, Denmark, 2023. [Google Scholar]
- Mattilsynet. Cheese, Hard, Norvegia. Available online: https://www.matvaretabellen.no/en/cheese-hard-norvegia/#mineraler (accessed on 9 September 2024).
- Mattilsynet. Pork, Minced Meat. Available online: https://www.matvaretabellen.no/en/pork-minced-meat-9-fat-raw/ (accessed on 9 September 2024).
- Egelandsdal, B.; Grabez-Ågren, V.; Mydland, L.T.; Haug, A.; Prestløkken, E. Animal breeding and feeding tools may close human nutrition gaps. Front. Anim. Sci. 2024, 5, 1426044. [Google Scholar] [CrossRef]
- European Commision, E.-L. Commission Regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed. Off. J. Eur. Union 2009, 54, 2–54. [Google Scholar]
- Hou, Y.; Wu, G. Nutritionally Essential Amino Acids. Adv. Nutr. 2018, 9, 849–851. [Google Scholar] [CrossRef]
- Fao/Who/Una. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation. Available online: https://iris.who.int/bitstream/handle/10665/43411/WHO_TRS_935_eng.pdf?ua=1 (accessed on 15 March 2025).
- Rehfeld, J.F. Accurate measurement of cholecystokinin in plasma. Clin. Chem. 1998, 44, 991–1001. [Google Scholar] [CrossRef]
- Stubbs, R.J.; Hughes, D.A.; Johnstone, A.M.; Rowley, E.; Reid, C.; Elia, M.; Stratton, R.; Delargy, H.; King, N.; Blundell, J.E. The use of visual analogue scales to assess motivation to eat in human subjects: A review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br. J. Nutr. 2002, 84, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.H.; Catherine, N.L.; Woodend, D.M.; Wolever, T.M. Inverse association between the effect of carbohydrates on blood glucose and subsequent short-term food intake in young men. Am. J. Clin. Nutr. 2002, 76, 1023–1030. [Google Scholar] [CrossRef]
- Blom, W.A.; Lluch, A.; Stafleu, A.; Vinoy, S.; Holst, J.J.; Schaafsma, G.; Hendriks, H.F. Effect of a high-protein breakfast on the postprandial ghrelin response. Am. J. Clin. Nutr. 2006, 83, 211–220. [Google Scholar] [CrossRef]
- Harris, W.S.; Connor, W.E.; Alam, N.; Illingworth, D.R. Reduction of postprandial triglyceridemia in humans by dietary n-3 fatty acids. J. Lipid Res. 1988, 29, 1451–1460. [Google Scholar] [CrossRef]
- Harper, A.; James, A.; Flint, A.; Astrup, A. Increased satiety after intake of a chocolate milk drink compared with a carbonated beverage, but no difference in subsequent ad libitum lunch intake. Br. J. Nutr. 2007, 97, 579–583. [Google Scholar] [CrossRef]
- Diester, C.M.; Banks, M.L.; Neigh, G.N.; Negus, S.S. Experimental design and analysis for consideration of sex as a biological variable. Neuropsychopharmacology 2019, 44, 2159–2162. [Google Scholar] [CrossRef] [PubMed]
- Langsrud, Ø. Fifty-Fifty MANOVA: Multivariate Analysis of Variance for Collinear Responses. Proc. Ind. Stat. Action 2000, 2000, 250–264. [Google Scholar]
- R-Core-Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 15 March 2025).
- Singh, D.; Singh, B. Investigating the impact of data normalization on classification performance. Appl. Soft Comput. 2020, 97, e105524. [Google Scholar] [CrossRef]
- Keirns, B.H.; Sciarrillo, C.M.; Koemel, N.A.; Emerson, S.R. Fasting, non-fasting and postprandial triglycerides for screening cardiometabolic risk. J. Nutr. Sci. 2021, 10, e75. [Google Scholar] [CrossRef]
- Neumann, H.F.; Egert, S. Impact of Meal Fatty Acid Composition on Postprandial Lipemia in Metabolically Healthy Adults and Individuals with Cardiovascular Disease Risk Factors: A Systematic Review. Adv. Nutr. 2022, 13, 193–207. [Google Scholar] [CrossRef]
- Hansson, P.; Holven, K.B.; Oyri, L.K.L.; Brekke, H.K.; Biong, A.S.; Gjevestad, G.O.; Raza, G.S.; Herzig, K.H.; Thoresen, M.; Ulven, S.M. Meals with Similar Fat Content from Different Dairy Products Induce Different Postprandial Triglyceride Responses in Healthy Adults: A Randomized Controlled Cross-Over Trial. J. Nutr. 2019, 149, 422–431. [Google Scholar] [CrossRef]
- Muesing, R.A.; Griffin, P.; Mitchell, P. Corn oil and beef tallow elicit different postprandial responses in triglycerides and cholesterol, but similar changes in constituents of high-density lipoprotein. J. Am. Coll. Nutr. 1995, 14, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.; Marckmann, P.; Sandstrom, B. Postprandial lipoprotein, glucose and insulin responses after two consecutive meals containing rapeseed oil, sunflower oil or palm oil with or without glucose at the first meal. Br. J. Nutr. 1999, 82, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Tholstrup, T.; Sandstrom, B.; Bysted, A.; Holmer, G. Effect of 6 dietary fatty acids on the postprandial lipid profile, plasma fatty acids, lipoprotein lipase, and cholesterol ester transfer activities in healthy young men. Am. J. Clin. Nutr. 2001, 73, 198–208. [Google Scholar] [CrossRef]
- Kendall, M. Optimal Amino Acid Intakes for Satiety and Weight Loss. Available online: https://optimisingnutrition.com/amino-acids-weight-loss/#h-decoding-the-role-of-protein-and-amino-acids-in-weight-loss (accessed on 1 June 2024).
- Stevenson, J.L.; Clevenger, H.C.; Cooper, J.A. Hunger and satiety responses to high-fat meals of varying fatty acid composition in women with obesity. Obesity (Silver Spring) 2015, 23, 1980–1986. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Jamialahmadi, T.; Moallem, S.A.; Sahebkar, A. Boosting GLP-1 by Natural Products. Adv. Exp. Med. Biol. 2021, 1328, 513–522. [Google Scholar] [PubMed]
- Hilkens, L.; Praster, F.; Van Overdam, J.; Nyakayiru, J.; Singh-Povel, C.M.; Bons, J.; Van Loon, L.J.; Van Dijk, J.W. Graded Replacement of Carbohydrate-Rich Breakfast Products with Dairy Products: Effects on Postprandial Aminoacidemia, Glycemic Control, Bone Metabolism, and Satiety. J. Nutr. 2024, 154, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Marsset-Baglieri, A.; Fromentin, G.; Nau, F.; Airinei, G.; Piedcoq, J.; Remond, D.; Barbillon, P.; Benamouzig, R.; Tome, D.; Gaudichon, C. The satiating effects of eggs or cottage cheese are similar in healthy subjects despite differences in postprandial kinetics. Appetite 2015, 90, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Van Der Klaauw, A.A.; Keogh, J.M.; Henning, E.; Trowse, V.M.; Dhillo, W.S.; Ghatei, M.A.; Farooqi, I.S. High protein intake stimulates postprandial GLP1 and PYY release. Obesity (Silver Spring) 2013, 21, 1602–1607. [Google Scholar] [CrossRef]
- Watkins, J.D.; Koumanov, F.; Gonzalez, J.T. Protein- and Calcium-Mediated GLP-1 Secretion: A Narrative Review. Adv. Nutr. 2021, 12, 2540–2552. [Google Scholar] [CrossRef]
- Mace, O.J.; Schindler, M.; Patel, S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J. Physiol. 2012, 590, 2917–2936. [Google Scholar] [CrossRef]
- Vien, S.; Fard, S.; El Khoury, D.; Luhovyy, B.; Fabek, H.; Anderson, G.H. Age and Sex Interact to Determine the Effects of Commonly Consumed Dairy Products on Postmeal Glycemia, Satiety, and Later Meal Food Intake in Adults. J. Nutr. 2021, 151, 2161–2174. [Google Scholar] [CrossRef]
- Watkins, J.D.; Smith, H.A.; Hengist, A.; Nielsen, S.B.; Mikkelsen, U.R.; Saunders, J.; Koumanov, F.; Betts, J.A.; Gonzalez, J.T. Effects of physical form of beta-lactoglobulin and calcium ingestion on GLP-1 secretion, gastric emptying and energy intake in humans: A randomised crossover trial. Br. J. Nutr. 2024, 131, 1730–1739. [Google Scholar]
- Chang, C.Y.; Kanthimathi, M.S.; Tan, A.T.; Nesaretnam, K.; Teng, K.T. The amount and types of fatty acids acutely affect insulin, glycemic and gastrointestinal peptide responses but not satiety in metabolic syndrome subjects. Eur. J. Nutr. 2018, 57, 179–190. [Google Scholar] [CrossRef]
- Lardinois, C.K.; Starich, G.H.; Mazzaferri, E.L. The postprandial response of gastric inhibitory polypeptide to various dietary fats in man. J. Am. Coll. Nutr. 1988, 7, 241–247. [Google Scholar] [CrossRef]
- Wong, G.; Shafat, A.; Clegg, M.; Jackson, K.G.; Lovegrove, J.A. The acute effects of meals rich in saturated or unsaturated fatty acids on postprandial lipaemia and satiety responses in healthy men (CocoHeart Study). Proc. Nutr. Soc. 2022, 81, e229. [Google Scholar] [CrossRef]
- Holst, J.J.; Rosenkilde, M.M. GIP as a Therapeutic Target in Diabetes and Obesity: Insight From Incretin Co-agonists. J. Clin. Endocrinol. Metab. 2020, 105, e2710–e2716. [Google Scholar] [CrossRef]
- Acosta, A.; Cifuentes, L.; Anazco, D.; O’connor, T.; Hurtado, M.; Ghusn, W.; Campos, A.; Fansa, S.; Mcrae, A.; Madhusudhan, S.; et al. Unraveling the Variability of Human Satiation: Implications for Precision Obesity Management. Res. Sq. 2024, rs.3.rs-4402499, [Version 1]. [Google Scholar]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Appelman, Y.; Van Rijn, B.B.; Ten Haaf, M.E.; Boersma, E.; Peters, S.A. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis 2015, 241, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Tollin, C.; Ericsson, M.; Johnson, O.; Backman, C.; Jacobsson, K.A.; Osterman, G.; Billström, Å. Decreased removal of triglycerides from the blood—A mechanism for the hypertriglyceridemia in male patients with coronary artery disease. Int. J. Cardiol. 1984, 5, 185–192. [Google Scholar] [CrossRef]
- Leone, A.; De Amicis, R.; Pellizzari, M.; Bertoli, S.; Ravella, S.; Battezzati, A. Appetite ratings and ghrelin concentrations in young adults after administration of a balanced meal. Does sex matter? Biol. Sex. Differ. 2022, 13, 25. [Google Scholar] [CrossRef]
- Vollmer, K.; Holst, J.J.; Baller, B.; Ellrichmann, M.; Nauck, M.A.; Schmidt, W.E.; Meier, J.J. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 2008, 57, 678–687. [Google Scholar] [CrossRef]

| Test Meal | Kcal * | Orange Juice (g) | Bread (g) | Tomatoes (g) | Butter (g) | Test Product (g) | Macronutrients (E%) ** | ||
|---|---|---|---|---|---|---|---|---|---|
| Fat | Carb. | Protein | |||||||
| Cheese | 598 | 184 | 69 | 23 | 4.8 *** | 104 (27% fat) | 46.5 | 32.0 | 21.5 |
| Pork sausage | 596 | 184 | 69 | 23 | - | 152 (21% fat) | |||
| Carbohydrates (g) | Protein (g) # | Fat (g) # | Energy (kJ/kcal) # | |
|---|---|---|---|---|
| Cheese | 0 | 27 | 27 | 1458/351 |
| Sausage | 0 | 20 | 21 | 1127/269 |
| Fat Source | C4:0 (g) | C6:0–C12:0 (g) | >C12:0 (g) | Sum SFA (g) | Sum * MUFA (g) | Sum C18:1tr (g) | Sum CLA (g) | Sum PUFA (g) | Unidentified (g) | n6:n3 Ratio |
|---|---|---|---|---|---|---|---|---|---|---|
| Pork fat | <LOQ ** | 0.03 ± 0.02 | 10.31 ± 0.06 | 10.34 ± 0.06 | 14.22 ± 0.27 | 0.07 ± 0.009 | <LOQ | 5.17 ± 0.23 | 0.88 ± 0.10 | 7.9 ± 0.3 |
| Dairy fat *** | 1.15 ± 0.02 | 3.13 ± 0.003 | 16.73 ± 0.003 | 21.02 ± 0.033 | 6.54 ± 0.006 | 0.38 ± 0.003 | 0.10 ± 0.003 | 0.69 ± 0.009 | 1.78 ± 0.009 | 3.05 ± 0.02 |
| Amino Acid | Wheat and Rye Bread (g/Test Diet) | Pork Sausage (g/Test Diet) | Gouda Cheese (g/Test Diet) | p *** |
|---|---|---|---|---|
| Tryptophan | 0.085 ± 0.003 | 0.334 ± 0.007 | 0.289 ± 0.008 | 0.053 |
| Phenylalanine | 0.401 ± 0.006 | 0.985 ± 0.002 | 1.327 ± 0.056 | 0.026 |
| Arginine | 0.334 ± 0.001 | 1.832 ± 0.028 | 1.750 ± 0.004 | <0.001 |
| Glutamate and Glutamine | 3.252 ± 0.036 | 4.553 ± 0.067 | 6.389 ± 0.015 | <0.001 **** |
| Anthropometric Variables | Values |
|---|---|
| Sex (number of men/women) | 13/11 |
| BMI (kg/m2) | 24.5 ± 2.6 |
| WC (cm) | 82.4 ± 8.8 |
| SBP (mmHg) | 125.5 ± 11.8 |
| DBP (mmHg) | 79.0 ± 6.7 |
| Appetite markers | Values |
| Ghrelin (pmol/L) | 44.2 ± 21.2 |
| GIP (pmol/L) | 8.3 ± 6.7 |
| GLP-1 (pmol/L) | 30.0 ± 10.2 |
| CCK (pmol/L) | 0.89 ±0.67 |
| Leptin (pmol/L) | 2354 ± 2159 |
| Clinical markers | Values |
| Insulin (pmol/L) | 46.4 ± 17.7 |
| LDL (mmol/L) | 2.62 ± 0.63 |
| HDL (mmol/L) | 1.48 ± 0.35 |
| Total cholesterol (mmol/L) | 4.35 ± 0.72 |
| Triglycerides (mmol/L) | 0.96 ± 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egelandsdal, B.; Haug, A.; Rehfeld, J.F.; Coutinho, S.; Roglà Ricart, L.; Martens, H.; Monfort-Pires, M.; Martins, C. Postprandial Responses to Animal Products with Distinct Fatty Acid and Amino Acid Composition Are Diet-Dependent. Nutrients 2025, 17, 1581. https://doi.org/10.3390/nu17091581
Egelandsdal B, Haug A, Rehfeld JF, Coutinho S, Roglà Ricart L, Martens H, Monfort-Pires M, Martins C. Postprandial Responses to Animal Products with Distinct Fatty Acid and Amino Acid Composition Are Diet-Dependent. Nutrients. 2025; 17(9):1581. https://doi.org/10.3390/nu17091581
Chicago/Turabian StyleEgelandsdal, Bjørg, Anna Haug, Jens F. Rehfeld, Sílvia Coutinho, Lledó Roglà Ricart, Harald Martens, Milena Monfort-Pires, and Catia Martins. 2025. "Postprandial Responses to Animal Products with Distinct Fatty Acid and Amino Acid Composition Are Diet-Dependent" Nutrients 17, no. 9: 1581. https://doi.org/10.3390/nu17091581
APA StyleEgelandsdal, B., Haug, A., Rehfeld, J. F., Coutinho, S., Roglà Ricart, L., Martens, H., Monfort-Pires, M., & Martins, C. (2025). Postprandial Responses to Animal Products with Distinct Fatty Acid and Amino Acid Composition Are Diet-Dependent. Nutrients, 17(9), 1581. https://doi.org/10.3390/nu17091581









