Nutritional Risk Score (NRS-2002) as a Predictor of In-Hospital Mortality in COVID-19 Patients: A Retrospective Single-Center Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. In-Hospital Mortality Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DASH | Dietary Approaches to Stop Hypertension |
| IUC | Intensive Care Unit |
| NRS-2002 | Nutritional Risk Score 2002 |
| COPD | chronic obstructive pulmonary disease |
| ADL | Activities of Daily Living |
| CRP | C-reactive protein |
| WBC | white blood cells |
| PCT | procalcitonin |
| HR | hazard ratio |
| CI | confidence interval |
| AICc | corrected Akaike criterion |
References
- Flisiak, R.; Rzymski, P.; Zarębska-Michaluk, D.; Ciechanowski, P.; Dobrowolska, K.; Rogalska, M.; Jaroszewicz, J.; Szymanek-Pasternak, A.; Rorat, M.; Kozielewicz, D.; et al. Variability in the Clinical Course of COVID-19 in a Retrospective Analysis of a Large Real-World Database. Viruses 2023, 15, 149. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, M.; Ribeiro Dos Santos, G.; Wang, L.; Cummings, D.A.T.; Azman, A.S.; Paireau, J.; Fontanet, A.; Cauchemez, S.; Salje, H. Age-Specific Mortality and Immunity Patterns of SARS-CoV-2. Nature 2021, 590, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-J.; Dong, X.; Liu, G.-H.; Gao, Y.-D. Risk and Protective Factors for COVID-19 Morbidity, Severity, and Mortality. Clin. Rev. Allergy Immunol. 2023, 64, 90–107. [Google Scholar] [CrossRef]
- Bauer, J.M.; Morley, J.E. Editorial: COVID-19 in Older Persons: The Role of Nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Larrazabal, R.B.; Chiu, H.H.C.; Palileo-Villanueva, L.A.M. Outcomes of Nutritionally At-Risk Coronavirus Disease 2019 (COVID 19) Patients Admitted in a Tertiary Government Hospital: A Follow-up Study of the MalnutriCoV Study. Clin. Nutr. ESPEN 2021, 43, 239–244. [Google Scholar] [CrossRef]
- Tassakos, A.; Kloppman, A.; Louie, J.C.Y. The Impact of Diet Quality on COVID-19 Severity and Outcomes-A Scoping Review. Curr. Nutr. Rep. 2025, 14, 27. [Google Scholar] [CrossRef]
- Boaz, M.; Kaufman-Shriqui, V. Systematic Review and Meta-Analysis: Malnutrition and In-Hospital Death in Adults Hospitalized with COVID-19. Nutrients 2023, 15, 1298. [Google Scholar] [CrossRef]
- Abate, S.M.; Chekole, Y.A.; Estifanos, M.B.; Abate, K.H.; Kabthymer, R.H. Prevalence and Outcomes of Malnutrition among Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis. Clin. Nutr. ESPEN 2021, 43, 174–183. [Google Scholar] [CrossRef]
- Bedock, D.; Bel Lassen, P.; Mathian, A.; Moreau, P.; Couffignal, J.; Ciangura, C.; Poitou-Bernert, C.; Jeannin, A.-C.; Mosbah, H.; Fadlallah, J.; et al. Prevalence and Severity of Malnutrition in Hospitalized COVID-19 Patients. Clin. Nutr. ESPEN 2020, 40, 214–219. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, D.; Sacco, G.; Gautier, J.; Brière, O.; Annweiler, C. Effects of Malnutrition on Mortality in Oldest-Old Inpatients with COVID-19 in the GERIA-COVID Cohort. Maturitas 2022, 161, 40–43. [Google Scholar] [CrossRef]
- Feng, X.; Liu, Z.; He, X.; Wang, X.; Yuan, C.; Huang, L.; Song, R.; Wu, Y. Risk of Malnutrition in Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 5267. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Hu, K.; Cai, S.; Deng, X.; Shao, X.; Liang, Y.; Wang, J.; Zhong, T.; Hu, Z.; Lei, M. Hypoproteinemia Is an Independent Risk Factor for the Prognosis of Severe COVID-19 Patients. J. Clin. Biochem. Nutr. 2020, 67, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Mancin, S.; Bertone, A.; Cattani, D.; Morenghi, E.; Passadori, L.; Donizzetti, D.; Sökeland, F.; Azzolini, E.; Mazzoleni, B. Malnutrition Risk as a Negative Prognostic Factor in COVID-19 Patients. Clin. Nutr. ESPEN 2021, 45, 369–373. [Google Scholar] [CrossRef]
- Mureșan, A.V.; Hălmaciu, I.; Arbănași, E.M.; Kaller, R.; Arbănași, E.M.; Budișcă, O.A.; Melinte, R.M.; Vunvulea, V.; Filep, R.C.; Mărginean, L.; et al. Prognostic Nutritional Index, Controlling Nutritional Status (CONUT) Score, and Inflammatory Biomarkers as Predictors of Deep Vein Thrombosis, Acute Pulmonary Embolism, and Mortality in COVID-19 Patients. Diagnostics 2022, 12, 2757. [Google Scholar] [CrossRef]
- Zhang, P.; He, Z.; Yu, G.; Peng, D.; Feng, Y.; Ling, J.; Wang, Y.; Li, S.; Bian, Y. The Modified NUTRIC Score Can Be Used for Nutritional Risk Assessment as Well as Prognosis Prediction in Critically Ill COVID-19 Patients. Clin. Nutr. 2021, 40, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Recinella, G.; Marasco, G.; Serafini, G.; Maestri, L.; Bianchi, G.; Forti, P.; Zoli, M. Prognostic Role of Nutritional Status in Elderly Patients Hospitalized for COVID-19: A Monocentric Study. Aging Clin. Exp. Res. 2020, 32, 2695–2701. [Google Scholar] [CrossRef]
- Zou, S.; Lin, P.; Chen, X.; Xia, L.; Liu, X.; Su, S.; Zhou, Y.; Li, Y. Comparative Analysis of Six Nutritional Scores in Predicting Prognosis of COVID-19 Patients. Front. Nutr. 2024, 11, 1501132. [Google Scholar] [CrossRef]
- Rzymski, P.; Pazgan-Simon, M.; Kamerys, J.; Moniuszko-Malinowska, A.; Sikorska, K.; Wernik, J.; Zarębska-Michaluk, D.; Supronowicz, Ł.; Sobala-Szczygieł, B.; Skrzat-Klapaczyńska, A.; et al. Severe Breakthrough COVID-19 Cases during Six Months of Delta Variant (B.1.617.2) Domination in Poland. Vaccines 2022, 10, 557. [Google Scholar] [CrossRef]
- Sheikh, A.; McMenamin, J.; Taylor, B.; Robertson, C. SARS-CoV-2 Delta VOC in Scotland: Demographics, Risk of Hospital Admission, and Vaccine Effectiveness. Lancet 2021, 397, 2461–2462. [Google Scholar] [CrossRef]
- Erkihun, M.; Ayele, B.; Asmare, Z.; Endalamaw, K. Current Updates on Variants of SARS-CoV- 2: Systematic Review. Health Sci. Rep. 2024, 7, e70166. [Google Scholar] [CrossRef]
- Katz, S. Assessing Self-Maintenance: Activities of Daily Living, Mobility, and Instrumental Activities of Daily Living. J. Am. Geriatr. Soc. 1983, 31, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Pokorska-Śpiewak, M.; Jackowska, T.; Kuchar, E.; Nitsch-Osuch, A.; Pawłowska, M.; Babicki, M.; Jaroszewicz, J.; Szenborn, L.; Wysocki, J.; et al. Key Considerations during the Transition from the Acute Phase of the COVID-19 Pandemic: A Narrative Review. Vaccines 2023, 11, 1502. [Google Scholar] [CrossRef] [PubMed]
- Hersberger, L.; Bargetzi, L.; Bargetzi, A.; Tribolet, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; et al. Nutritional Risk Screening (NRS 2002) Is a Strong and Modifiable Predictor Risk Score for Short-Term and Long-Term Clinical Outcomes: Secondary Analysis of a Prospective Randomised Trial. Clin. Nutr. 2020, 39, 2720–2729. [Google Scholar] [CrossRef]
- Barbosa, A.A.D.O.; Vicentini, A.P.; Langa, F.R. Comparison of NRS-2002 Criteria with Nutritional Risk in Hospitalized Patients. Ciênc. Saúde Coletiva 2019, 24, 3325–3334. [Google Scholar] [CrossRef]
- Alikiaii, B.; Heidari, Z.; Fazeli, A.; Rahimi Varposhti, M.; Moradi Farsani, D.; Fattahpour, S.; Rafiee, S.; Bagherniya, M. Evaluation of the Effectiveness of the Nutritional Risk Screening System 2002 (NRS-2002) in COVID-19 Patients Admitted to the Intensive Care Unit. Int. J. Clin. Pract. 2021, 75, e14934. [Google Scholar] [CrossRef] [PubMed]
- Fatemeh, G.; Fotsing, G.; Marques-Vidal, P.; Kopp, P.; Barigou, M. Predictive Value of Multiple Variable Models Including Nutritional Risk Score (NRS 2002) on Mortality and Length of Stay of Patients with Covid-19 Infections. The INCOVO Study. Clin. Nutr. ESPEN 2023, 55, 357–363. [Google Scholar] [CrossRef]
- Sümer, A.; Uzun, L.N.; Özbek, Y.D.; Tok, H.H.; Altınsoy, C. Nutrition Improves COVID-19 Clinical Progress. Ir. J. Med. Sci. 2022, 191, 1967–1972. [Google Scholar] [CrossRef]
- Ahmadi, S.; Firoozi, D.; Dehghani, M.; Zare, M.; Mehrabi, Z.; Ghaseminasab-Parizi, M.; Masoumi, S.J. Evaluation of Nutritional Status of Intensive Care Unit COVID-19 Patients Based on the Nutritional Risk Screening 2002 Score. Int. J. Clin. Pract. 2022, 2022, 2448161. [Google Scholar] [CrossRef]
- Can, B.; Senturk Durmus, N.; Olgun Yıldızeli, S.; Kocakaya, D.; Ilhan, B.; Tufan, A. Nutrition Risk Assessed by Nutritional Risk Screening 2002 Is Associated with In-Hospital Mortality in Older Patients with COVID-19. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2022, 37, 605–614. [Google Scholar] [CrossRef]
- Polidoro, R.B.; Hagan, R.S.; de Santis Santiago, R.; Schmidt, N.W. Overview: Systemic Inflammatory Response Derived From Lung Injury Caused by SARS-CoV-2 Infection Explains Severe Outcomes in COVID-19. Front. Immunol. 2020, 11, 1626. [Google Scholar] [CrossRef]
- Weatherhead, J.E.; Clark, E.; Vogel, T.P.; Atmar, R.L.; Kulkarni, P.A. Inflammatory Syndromes Associated with SARS-CoV-2 Infection: Dysregulation of the Immune Response across the Age Spectrum. J. Clin. Investig. 2020, 130, 6194–6197. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Bischoff, S.C.; Breda, J.; Wickramasinghe, K.; Krznaric, Z.; Nitzan, D.; Pirlich, M.; Singer, P.; ESPEN Council. ESPEN Expert Statements and Practical Guidance for Nutritional Management of Individuals with SARS-CoV-2 Infection. Clin. Nutr. 2020, 39, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Hinkelmann, J.V.; de Oliveira, N.A.; Marcato, D.F.; Costa, A.R.R.O.; Ferreira, A.M.; Tomaz, M.; Rodrigues, T.J.; Mendes, A.P. Nutritional Support Protocol for Patients with COVID-19. Clin. Nutr. ESPEN 2022, 49, 544–550. [Google Scholar] [CrossRef]
- Melchers, M.; Hubertine Hermans, A.J.; Hulsen, S.B.; Kehinde Kouw, I.W.; Hubert van Zanten, A.R. Individualised Energy and Protein Targets Achieved during Intensive Care Admission Are Associated with Lower Mortality in Mechanically Ventilated COVID-19 Patients: The COFEED-19 Study. Clin. Nutr. 2023, 42, 2486–2492. [Google Scholar] [CrossRef]
- Cereda, E.; Guzzardella, A.; Klersy, C.; Belliato, M.; Pellegrini, A.; Sciutti, F.; Mongodi, S.; Masi, S.; Crotti, S.; Savioli, M.; et al. Early Caloric Deficit Is Associated with a Higher Risk of Death in Invasive Ventilated COVID-19 Patients. Clin. Nutr. 2022, 41, 3096–3099. [Google Scholar] [CrossRef]
| Variable | Total (n = 222) | Non-Survivors (n = 31) | Survivors (n = 191) | p-Value |
|---|---|---|---|---|
| Gender: male | 116 (52.2%) | 15 (48.4%) | 101 (52.9%) | 0.7005 |
| Age: over 65 years | 141 (63.5%) | 30 (96.8%) | 111 (58.1%) | <0.0001 |
| Data from the medical history at admission | ||||
| Hypertension | 118 (53.1%) | 18 (58.1%) | 100 (52.4%) | 0.5682 |
| Cardiovascular diseases (congestive heart failure, ischemic heart disease, arrhythmia) | 64 (28.8%) | 11(35.5%) | 53 (27.7%) | 0.3965 |
| Renal failure | 18 (8.1%) | 4 (12.9%) | 14 (7.39%) | 0.2898 |
| Neurodegenerative disease | 32 (14.4%) | 8 (25.8%) | 24 (12.6%) | 0.0926 |
| Stroke/TIA | 38 (17.1%) | 8 (25.8%) | 30 (15.7%) | 0.1972 |
| Diabetes | 59 (26.6%) | 11 (35.5%) | 48 (25.1%) | 0.2727 |
| Asthma or COPD | 25 (11.3%) | 2 (6.5%) | 23 (12.0%) | 0.5427 |
| Anti-SARS-CoV-2 vaccination | 45 (20.3%) | 2 (6.5%) | 43 (22.5%) | 0.0432 |
| Clinical and laboratory data at admission | ||||
| ADL (Katz Index < 5) | 116 (52.3%) | 29 (93.6%) | 87 (45.6%) | <0.0001 |
| Cognitive impairment | 67 (30.2%) | 22 (71.0%) | 45 (23.6%) | <0.0001 |
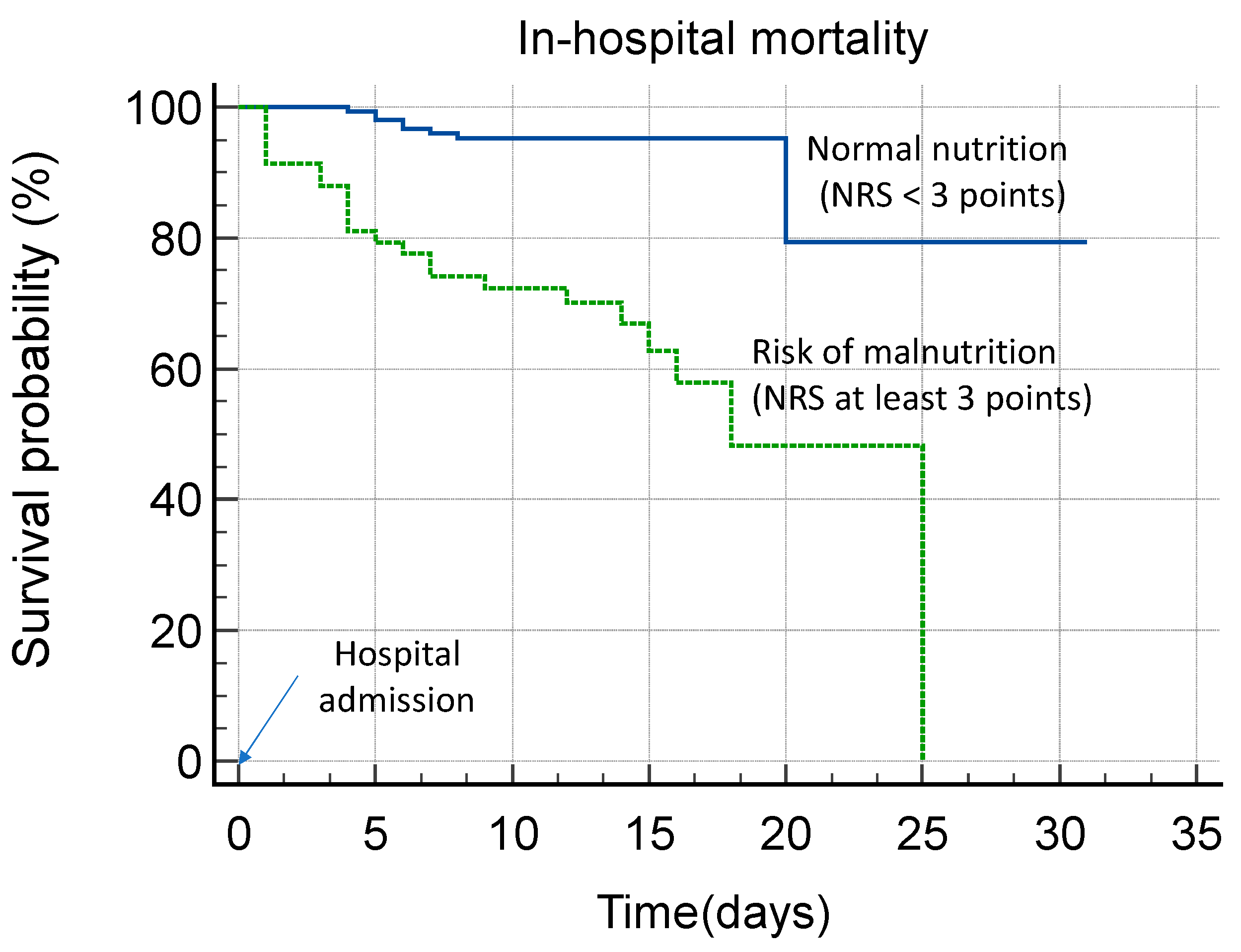
| Increased risk of malnutrition (NRS ≥ 3) | 58 (26.1%) | 23 (74.2%) | 35 (18.3%) | <0.0001 |
| Increased risk of pressure sores (Norton Scale < 14) | 81 (35.5%) | 23 (74.2%) | 58 (30.4%) | <0.0001 |
| Anemia | 77 (34.7%) | 15 (48.8%) | 62 (32.5%) | 0.1037 |
| Increased serum CRP (above 20 mg/L) | 177 (79.7%) | 28 (90.3%) | 149 (78.0%) | 0.1492 |
| Increased serum procalcitonin (above 0.5 ng/mL) | 43 (19.4%) | 18 (58.1%) | 25 (13.1%) | <0.0001 |
| Increased WBC count (above 103/μL3) | 84 (37.8%) | 19 (62.3%) | 65 (34.0%) | 0.0050 |
| Increased serum D-dimer (above 0.5 ng/mL) | 200 (90.1%) | 30 (96.8%) | 170 (89.0%) | 0.3268 |
| In hospital therapy | ||||
| Antiviral treatment against SARS-CoV-2 | 109 (49.1%) | 12 (38.7%) | 97 (50.8%) | 0.2478 |
| Antibiotic treatment | 116 (52.2%) | 19 (61.3%) | 97 (50.8%) | 0.3342 |
| Heparin in therapeutic doses | 33 (14.9%) | 5 (16.1%) | 28 (14.7%) | 0.7887 |
| Oxygen therapy needed | 199 (89.6%) | 31 (100.0%) | 168 (88.0%) | 0.0510 |
| Analyzed Variables | Hazard Ratio (95% CI) | p-Value |
|---|---|---|
| Age: at least 65 years old | 13.50 (1.35–99.48) | 0.0106 |
| Anti-SARS-CoV-2 vaccination | 0.26 (0.06–1.08) | 0.0635 |
| ADL at admission (Katz Index < 5) | 10.4 (2.5–43.9) | 0.0015 |
| Cognitive impairment | 5.08 (2.31–11.14) | <0.0001 |
| Increased risk of malnutrition (NRS ≥ 3) | 7.48 (3.32–16.83) | <0.0001 |
| Increased risk of pressure sores (Notron scale < 14) | 4.35 (1.94–9.77) | 0.0004 |
| Increased serum procalcitonin (above 0.5 ng/mL) | 5.39 (2.55–11.40) | <0.0001 |
| Increased WBC count (above 103/μL3) | 2.25 (1.05–4.83) | 0.0377 |
| Variables Included in the Model Additionally to Malnutrition | HR (95% CI) | p-Value for Malnutrition | R2 (Nagelkerke) | AICc (Corrected Akaike Criterion) | p-Value for the Model |
|---|---|---|---|---|---|
| B, C, D | 5.27 (1.92–14.50) | 0.0013 | 0.71 | 262 | <0.0001 |
| B, C, E | 4.04 (1.56–10.46) | 0.0041 | 0.77 | 245 | <0.0001 |
| B, C, F | 4.44 (1.67–11.83) | 0.0029 | 0.70 | 254 | <0.0001 |
| B, D, E | 3.19 (1.36–7.48) | 0.0076 | 0.77 | 245 | <0.0001 |
| B, D, F | 3.38 (1.42–8.02) | 0.0057 | 0.71 | 252 | <0.0001 |
| C, D, E | 5.31 (1.87–15.12) | 0.0017 | 0.75 | 248 | <0.0001 |
| C, D, F | 5.88 (1.99–17.38) | 0.0014 | 0.66 | 257 | <0.0001 |
| B, E, F | 3.53 (1.50–8.26) | 0.0037 | 0.77 | 245 | <0.0001 |
| C, E, F | 4.97 (1.85–13.36) | 0.0015 | 0.72 | 251 | <0.0001 |
| D, E, F | 4.48 (1.89–10.64) | 0.0007 | 0.75 | 247 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilkowski, J.; Guzik, P.; Kaluźniak-Szymanowska, A.; Rzymski, P.; Chudek, J.; Wieczorowska-Tobis, K. Nutritional Risk Score (NRS-2002) as a Predictor of In-Hospital Mortality in COVID-19 Patients: A Retrospective Single-Center Cohort Study. Nutrients 2025, 17, 1278. https://doi.org/10.3390/nu17071278
Ilkowski J, Guzik P, Kaluźniak-Szymanowska A, Rzymski P, Chudek J, Wieczorowska-Tobis K. Nutritional Risk Score (NRS-2002) as a Predictor of In-Hospital Mortality in COVID-19 Patients: A Retrospective Single-Center Cohort Study. Nutrients. 2025; 17(7):1278. https://doi.org/10.3390/nu17071278
Chicago/Turabian StyleIlkowski, Jan, Przemysław Guzik, Aleksandra Kaluźniak-Szymanowska, Piotr Rzymski, Jerzy Chudek, and Katarzyna Wieczorowska-Tobis. 2025. "Nutritional Risk Score (NRS-2002) as a Predictor of In-Hospital Mortality in COVID-19 Patients: A Retrospective Single-Center Cohort Study" Nutrients 17, no. 7: 1278. https://doi.org/10.3390/nu17071278
APA StyleIlkowski, J., Guzik, P., Kaluźniak-Szymanowska, A., Rzymski, P., Chudek, J., & Wieczorowska-Tobis, K. (2025). Nutritional Risk Score (NRS-2002) as a Predictor of In-Hospital Mortality in COVID-19 Patients: A Retrospective Single-Center Cohort Study. Nutrients, 17(7), 1278. https://doi.org/10.3390/nu17071278






