Effects of Lactobacillus paracei JY062 Postbiotic on Intestinal Barrier, Immunity, and Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
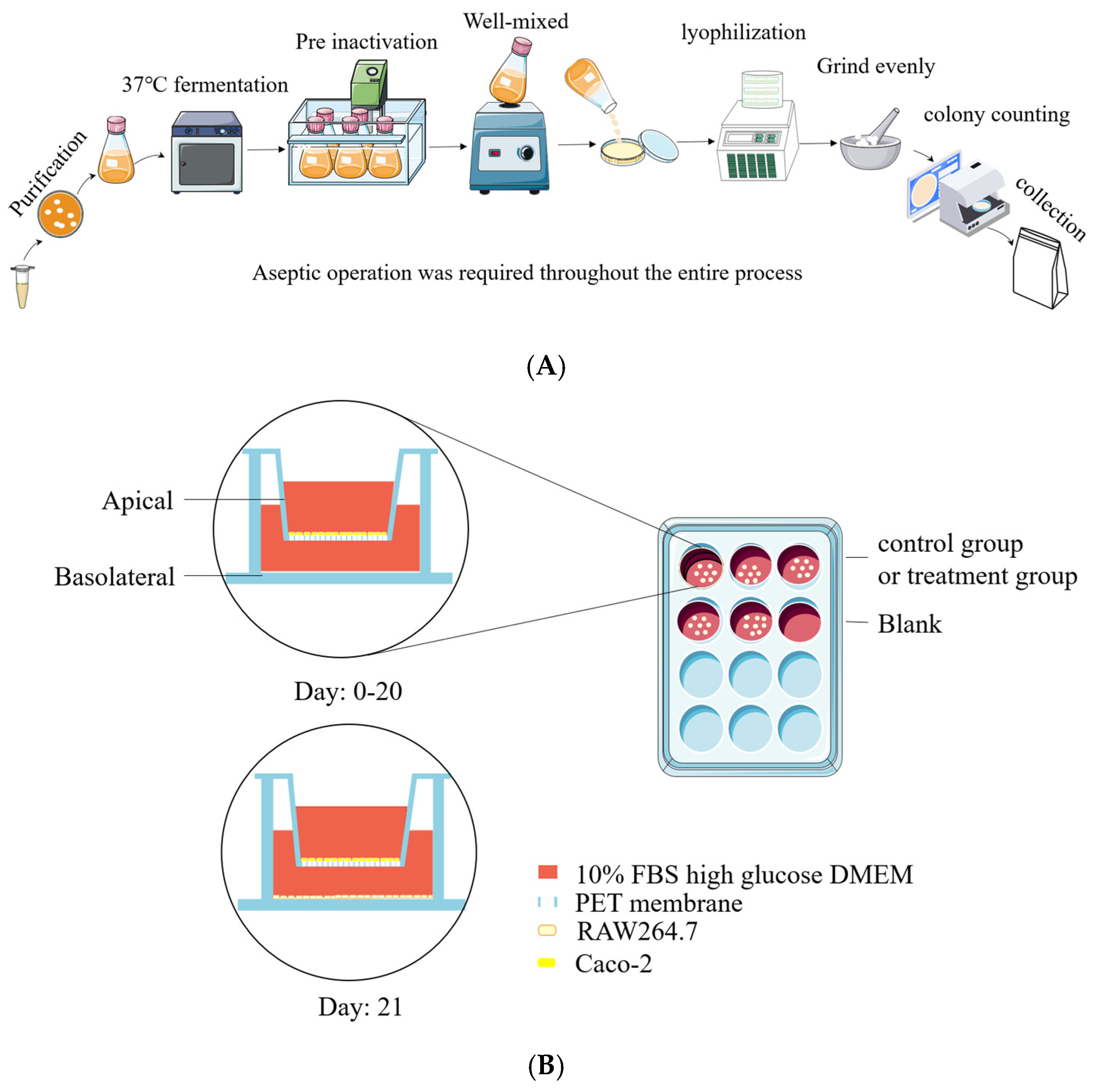
2.2. Bacterial Strains and Production of Postbiotic Powder
2.3. Cell Culture and Cell Viability Assay
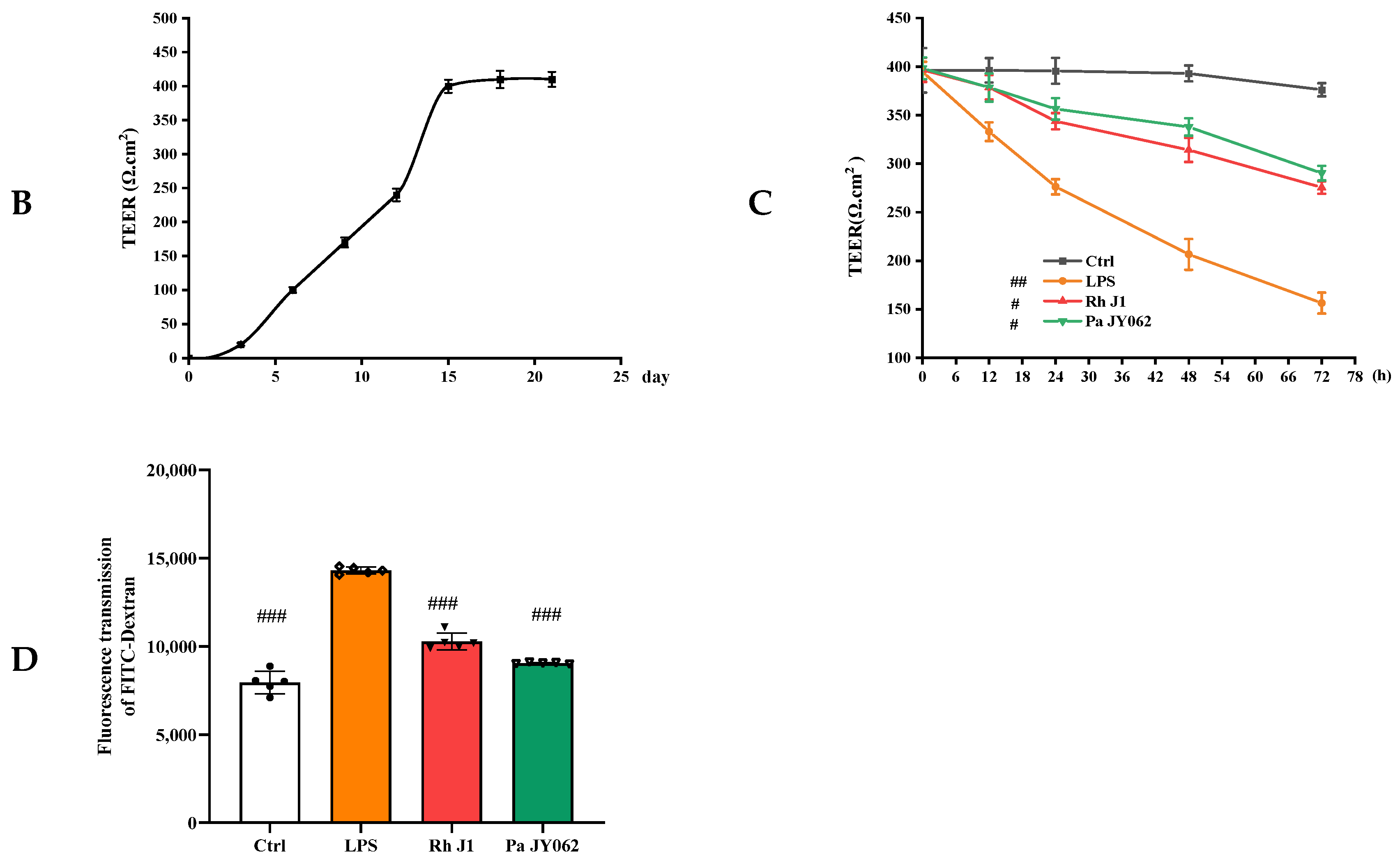
2.4. Co-Culture System of Caco-2/RAW264.7 Cell
2.5. In Vitro Paracellular Permeability Assay
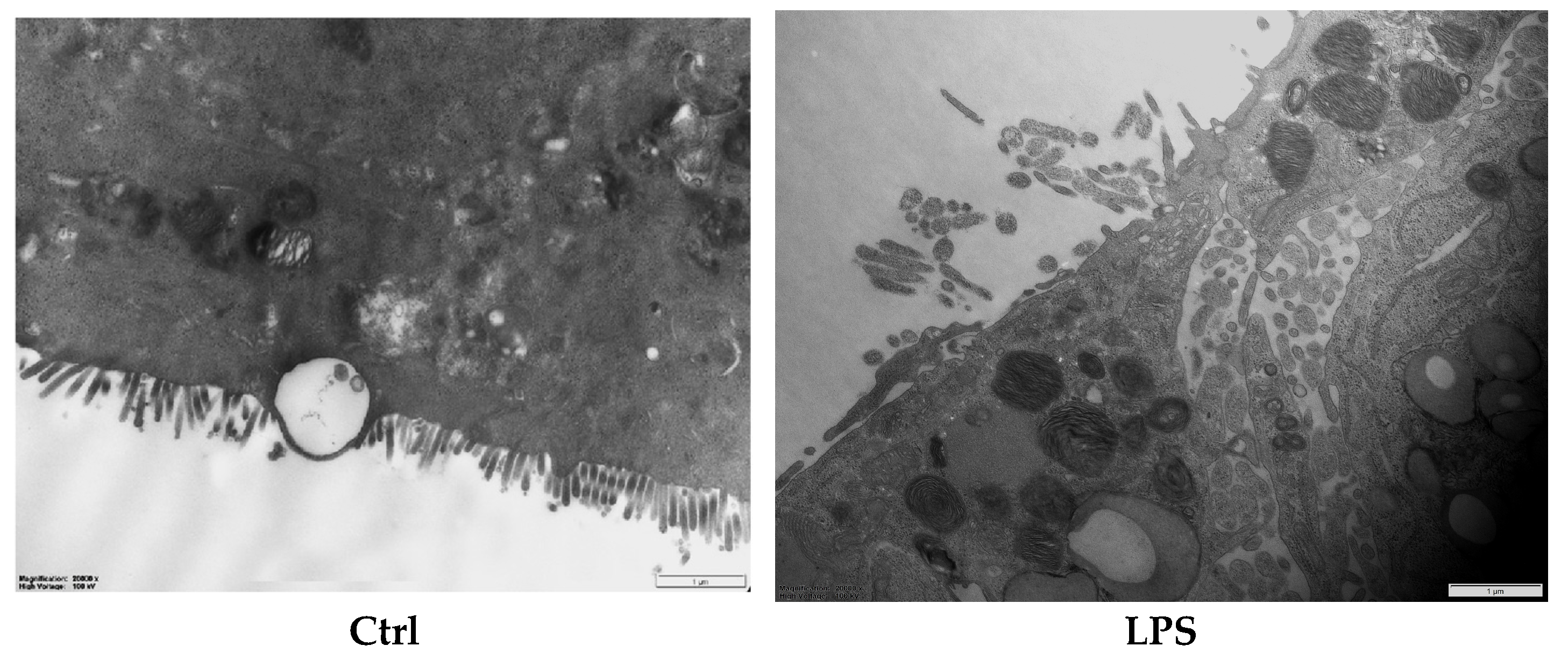
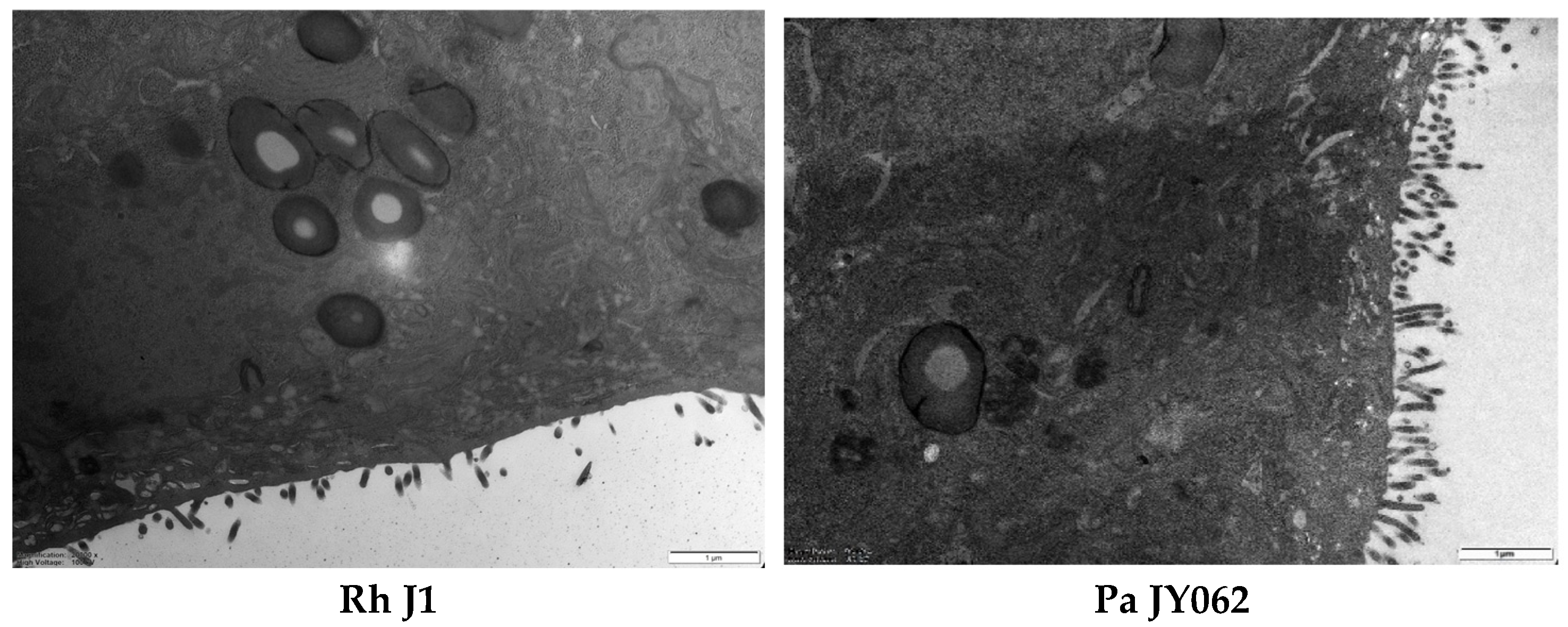
2.6. Determining the Ultrastructure of the Intestinal Barrier Using Transmission Electron Microscopy
2.7. Neutral Red Phagocytosis and NO Assay
2.8. Cellular Inflammatory Factor Assay
2.9. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction
2.10. Static Simulation of Gastrointestinal Digestive Processes In Vitro
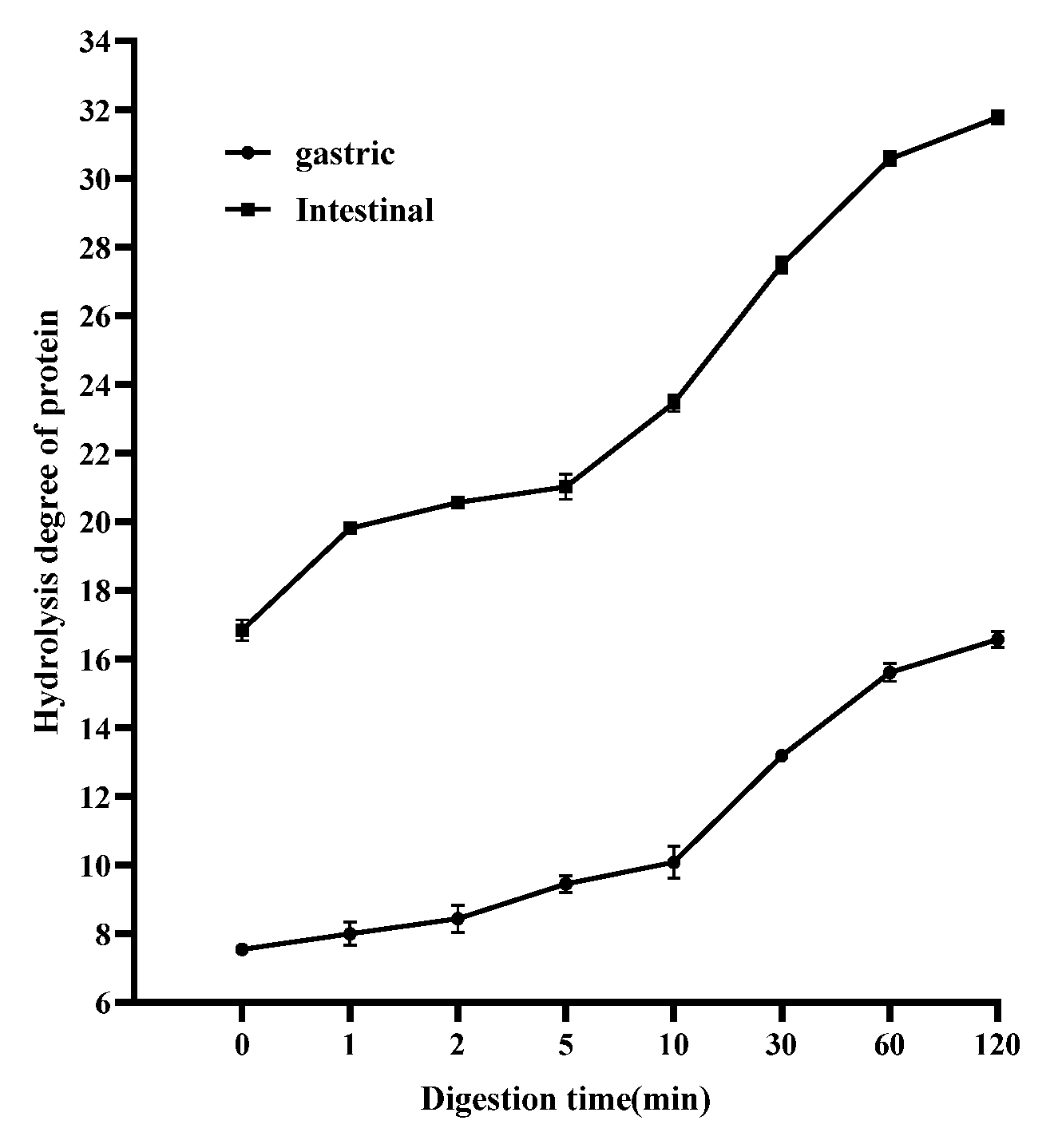
2.11. Degree of Protein Hydrolysis
2.12. Simulated Gut Fermentation In Vitro
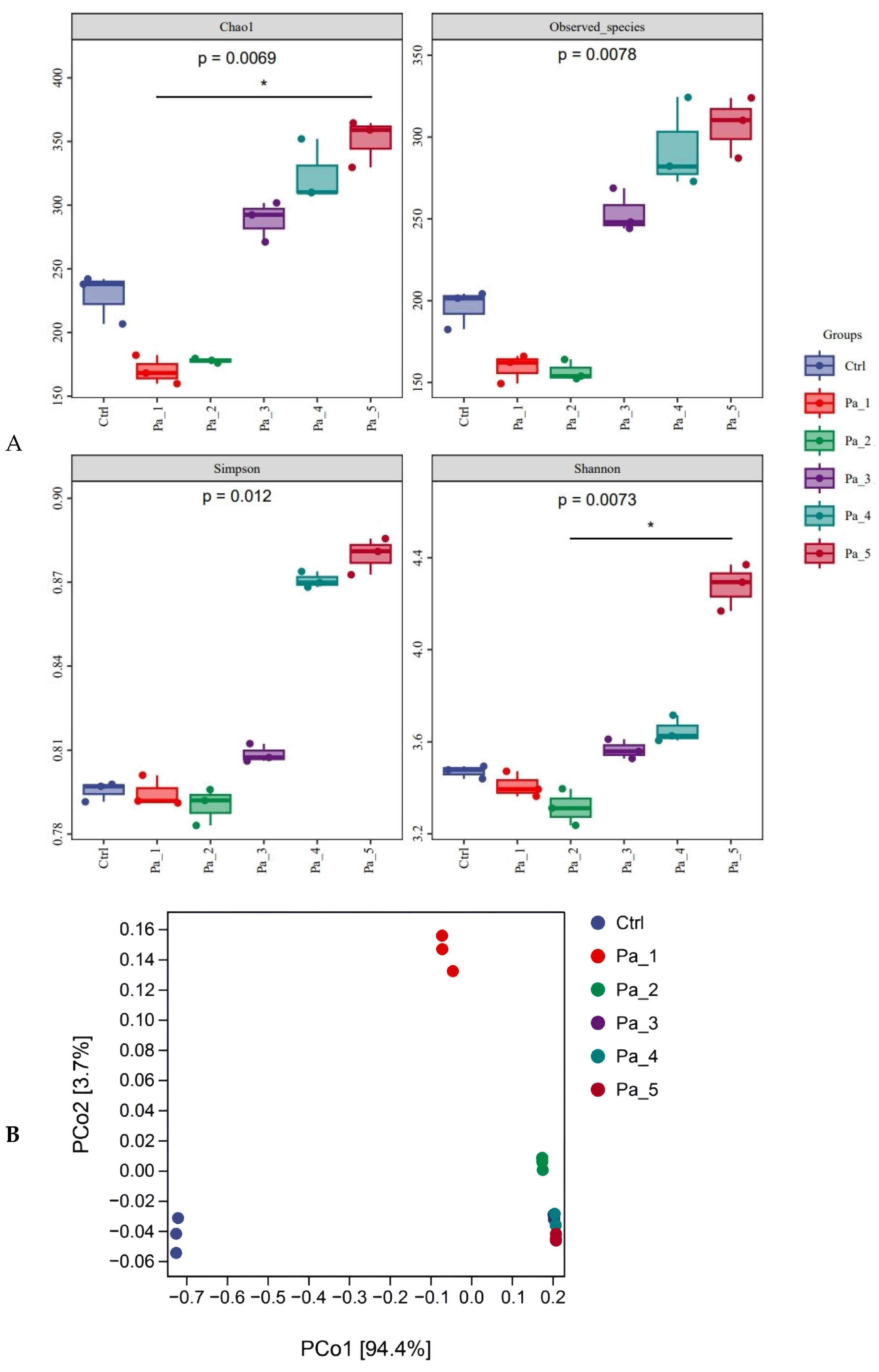
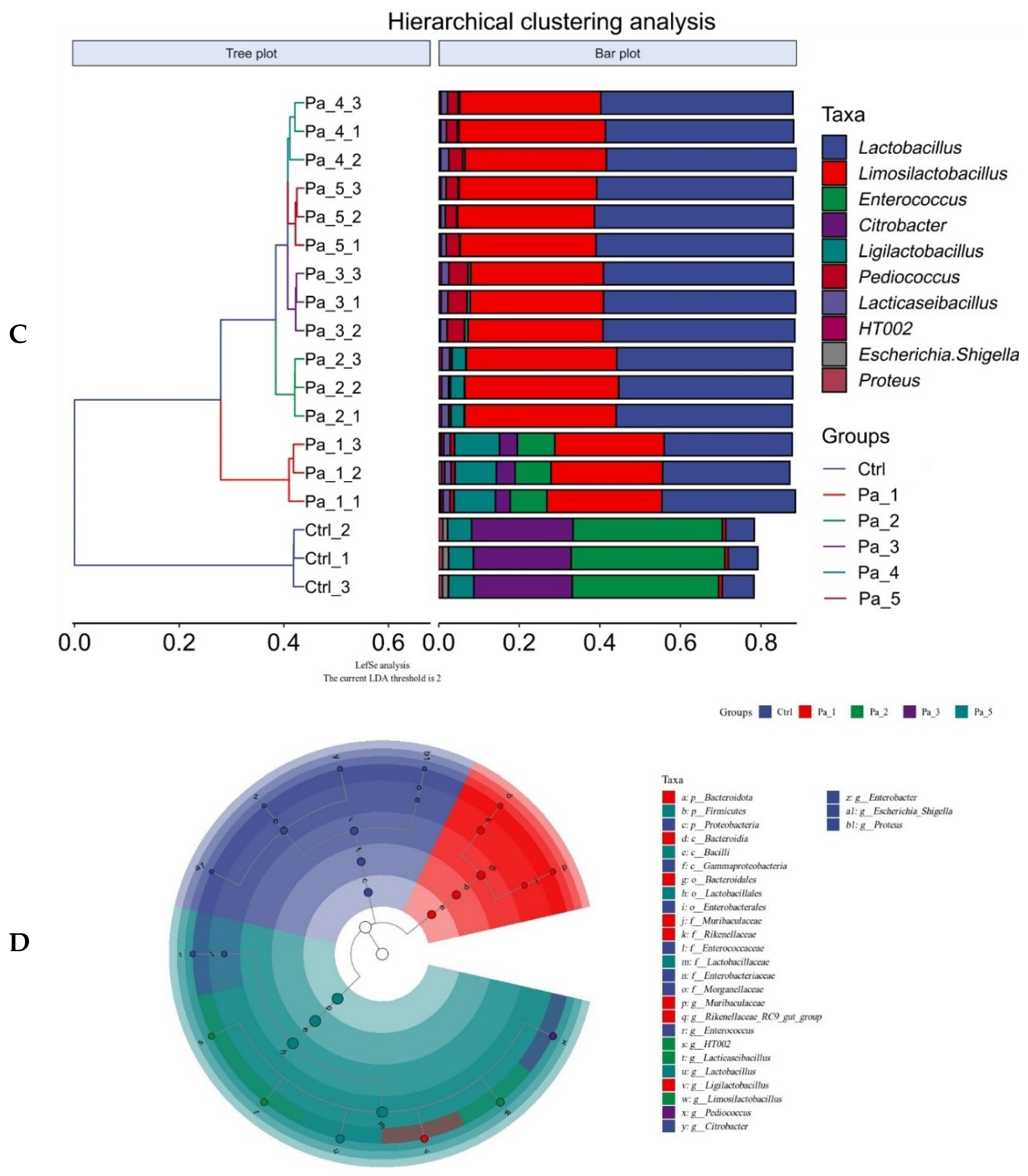
2.13. Analysis of 16S rRNA in Gut Microbes
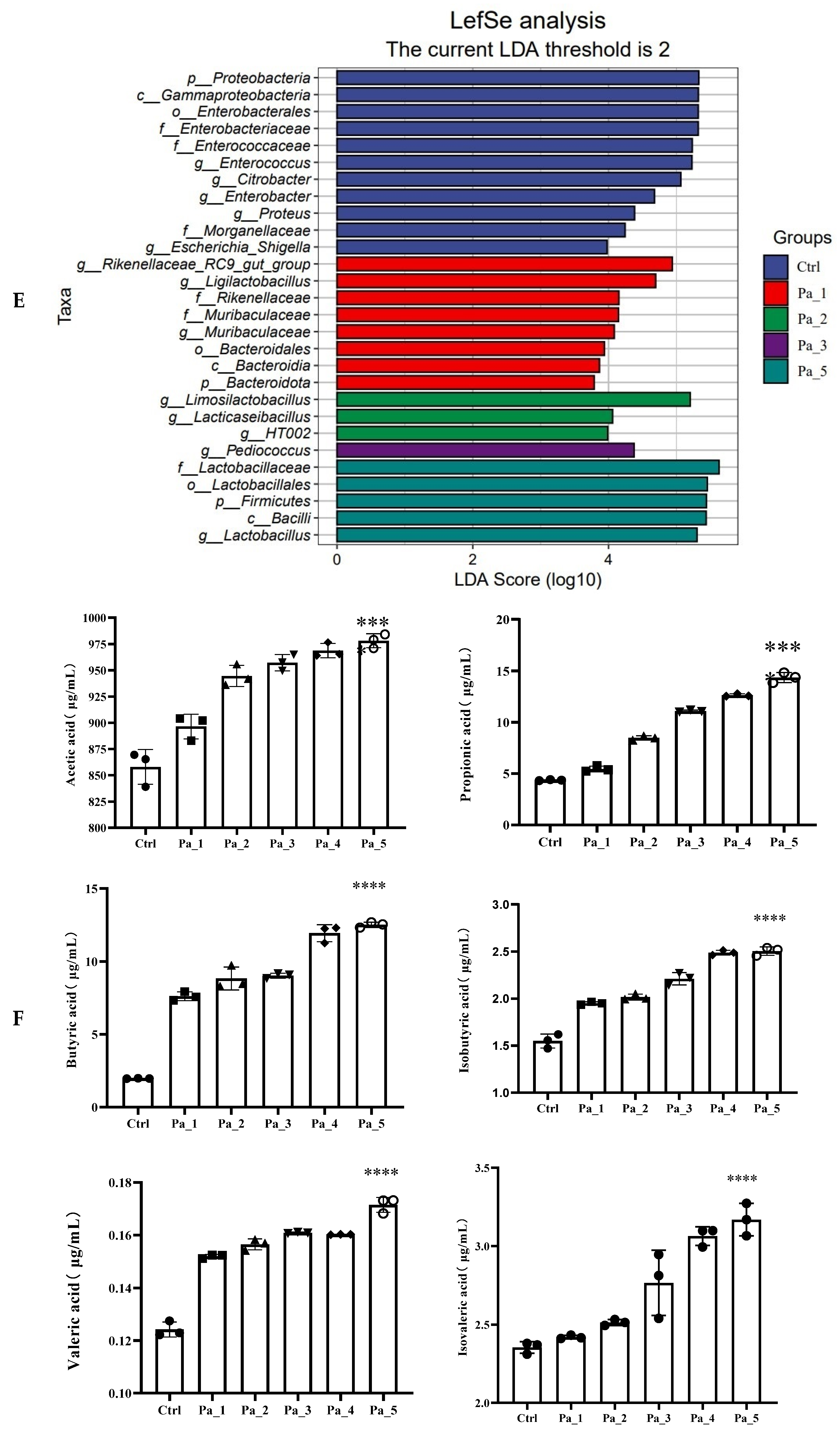
2.14. Short Chain Fatty Acids in Gut Microbes
2.15. The Analysis of Pa JY062 Ingredients
2.16. Statistical Analysis
3. Results
3.1. Postbiotics Promoted Cell Viability in a Manner Dependent on Dosage
3.2. The Effect of Postbiotics on Intestinal Barrier Integrity
3.3. The Effect of Postbiotics on the Intestinal Barrier Microstructure
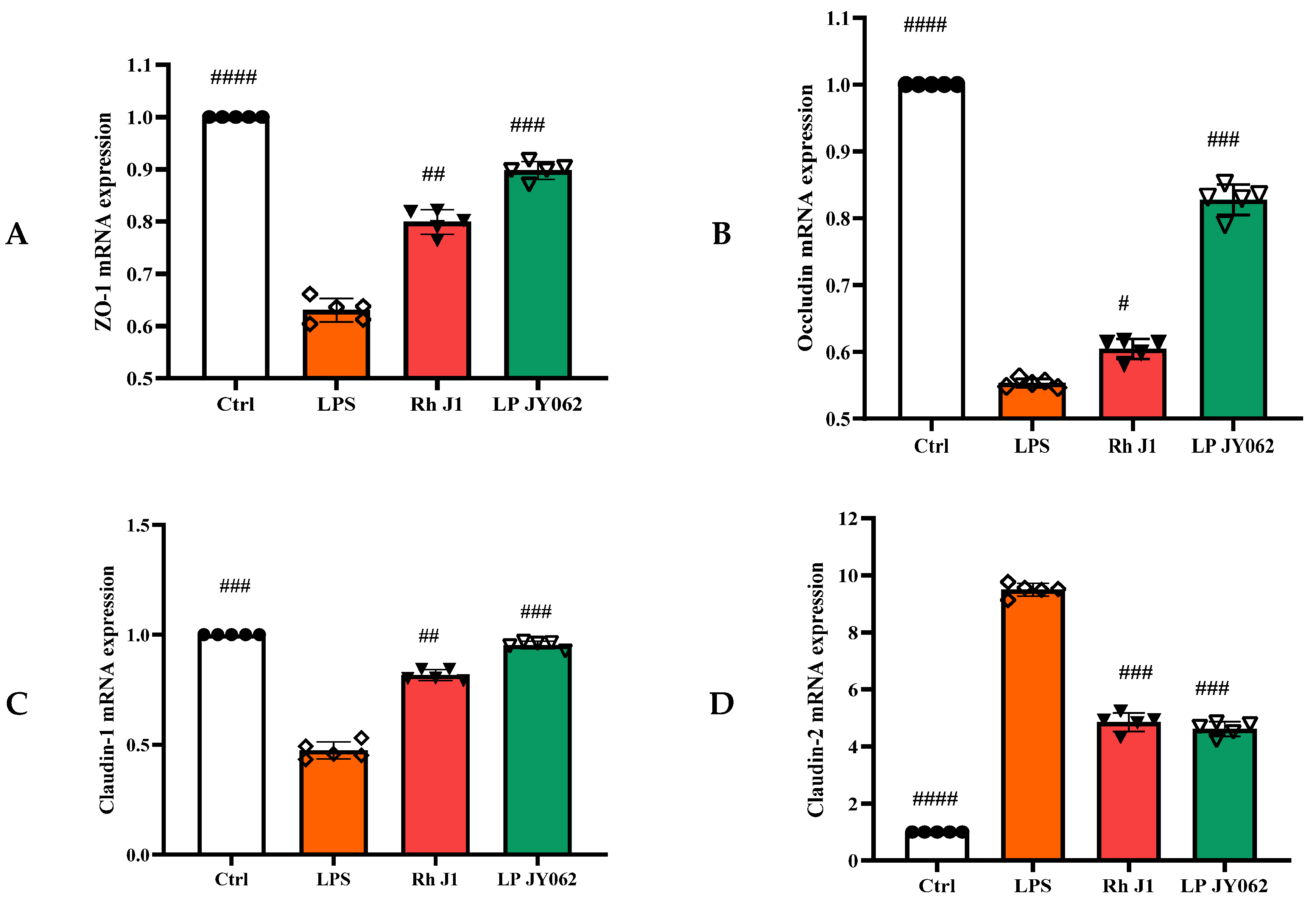
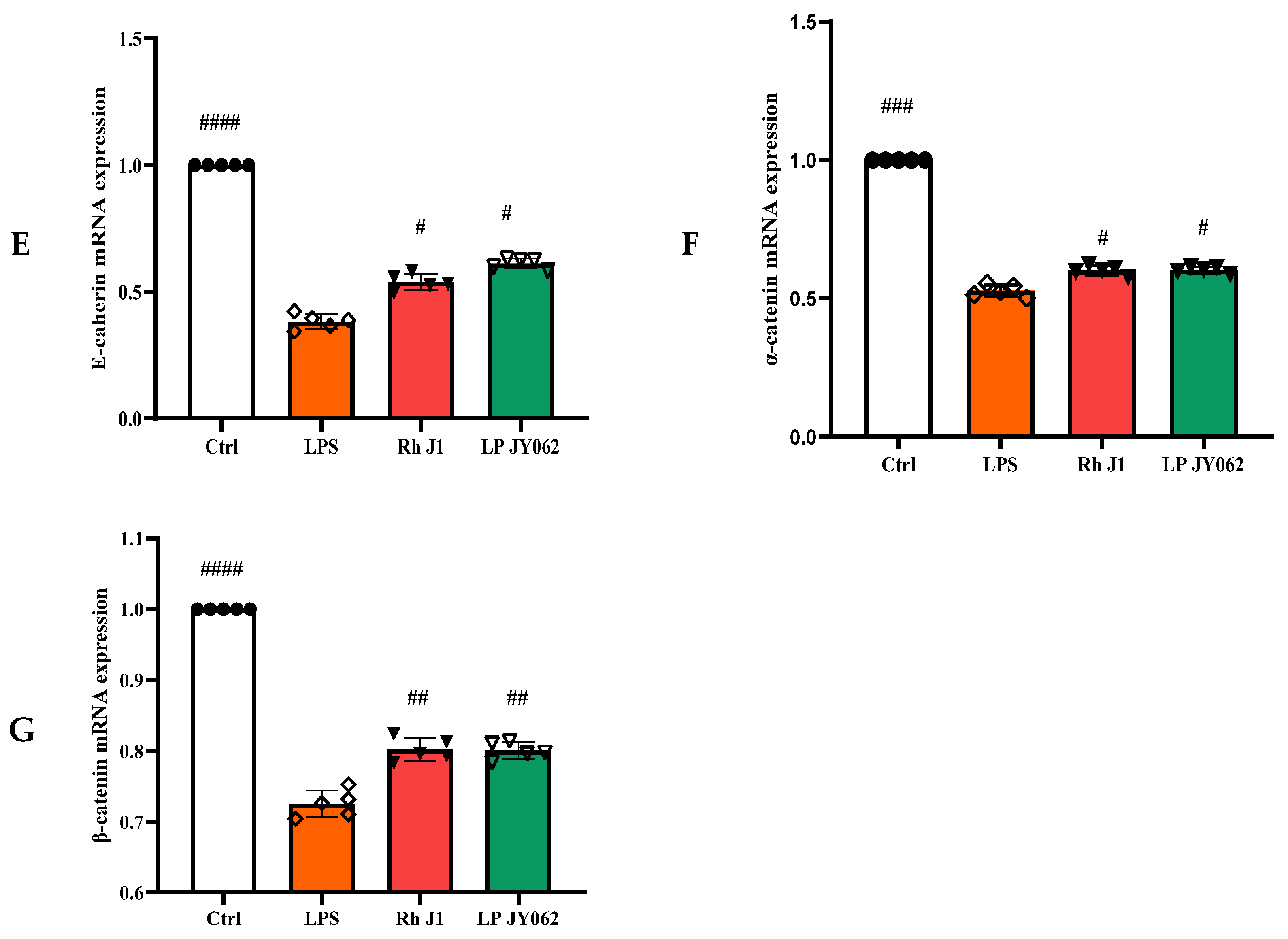
3.4. The Influence of Postbiotics on the mRNA Expression of Intestinal Barrier Protein
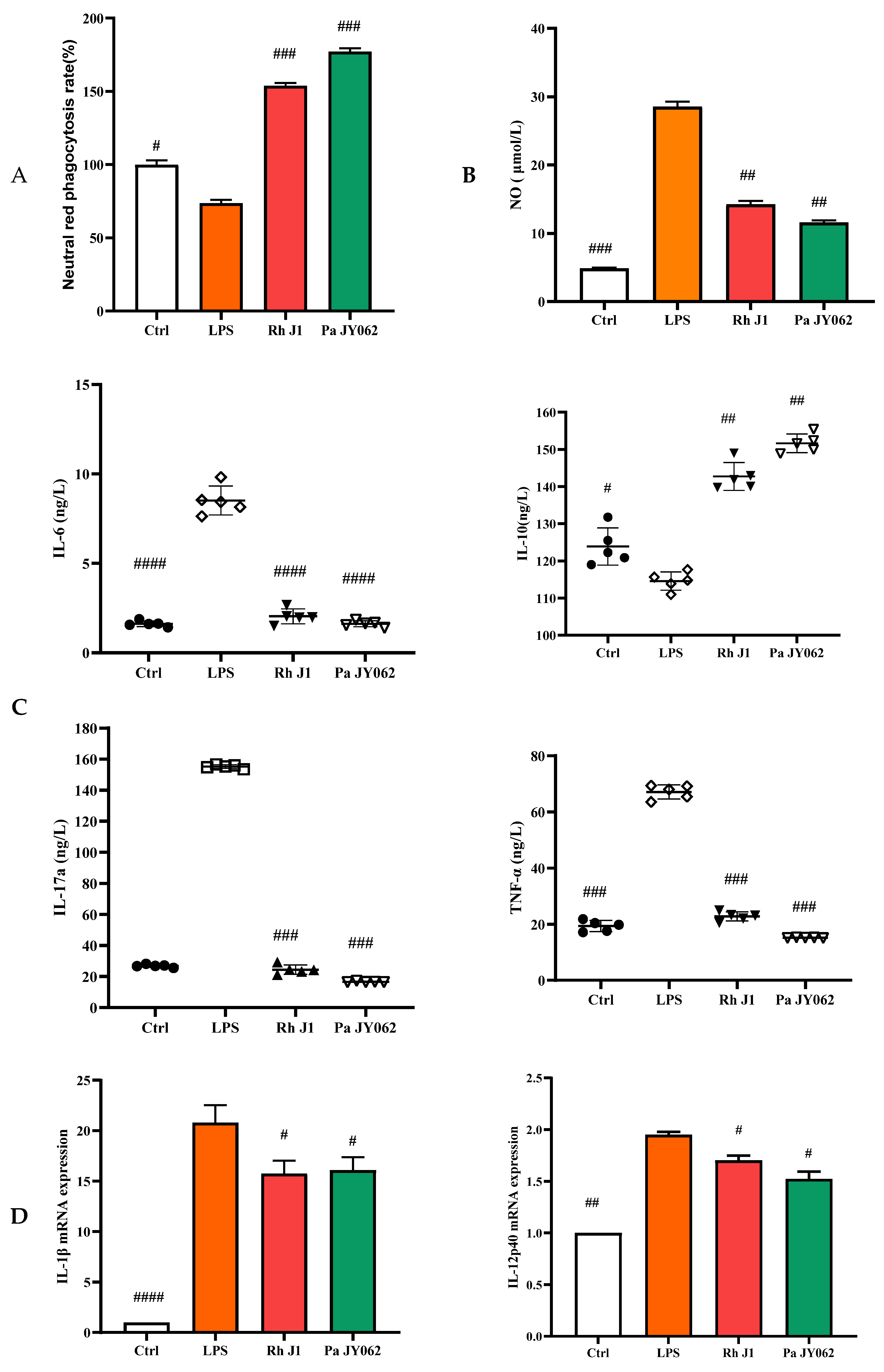
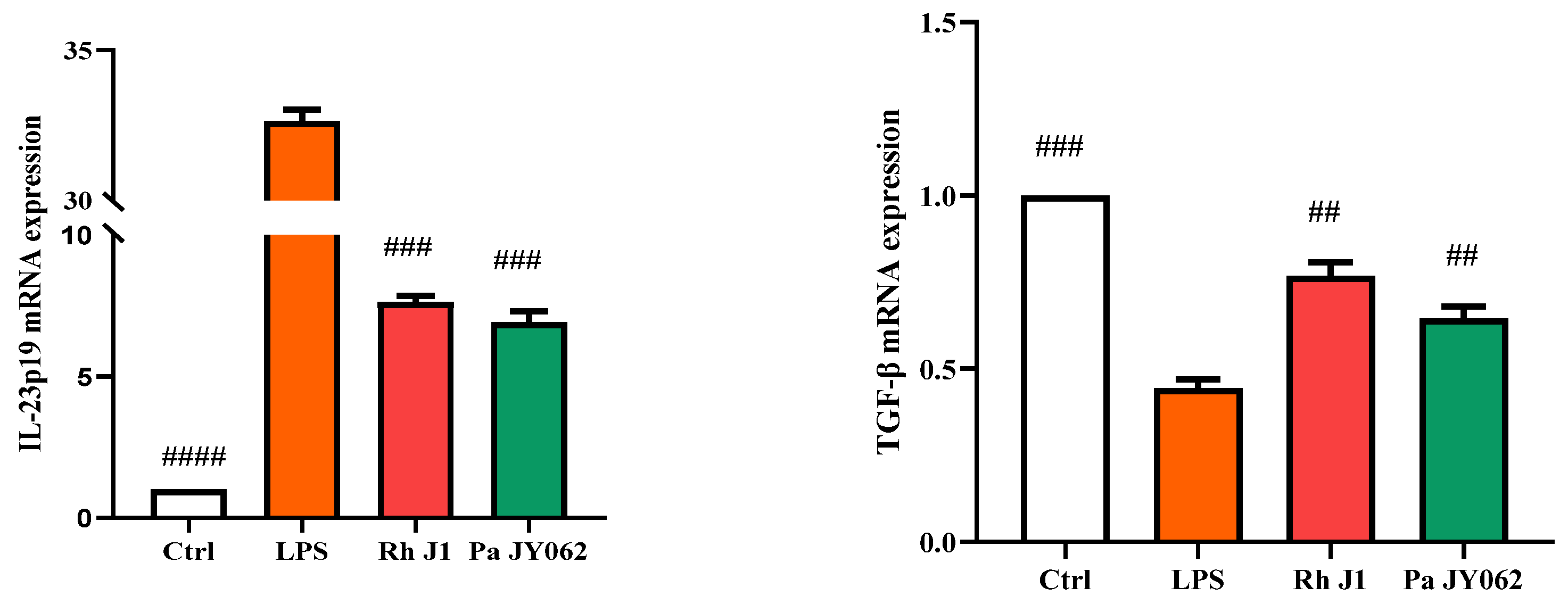
3.5. The Influence of Postbiotics on Intestinal Inflammation
3.6. Effects of PaJY062 on Protein Digestion in In Vitro Simulated Digestion Model
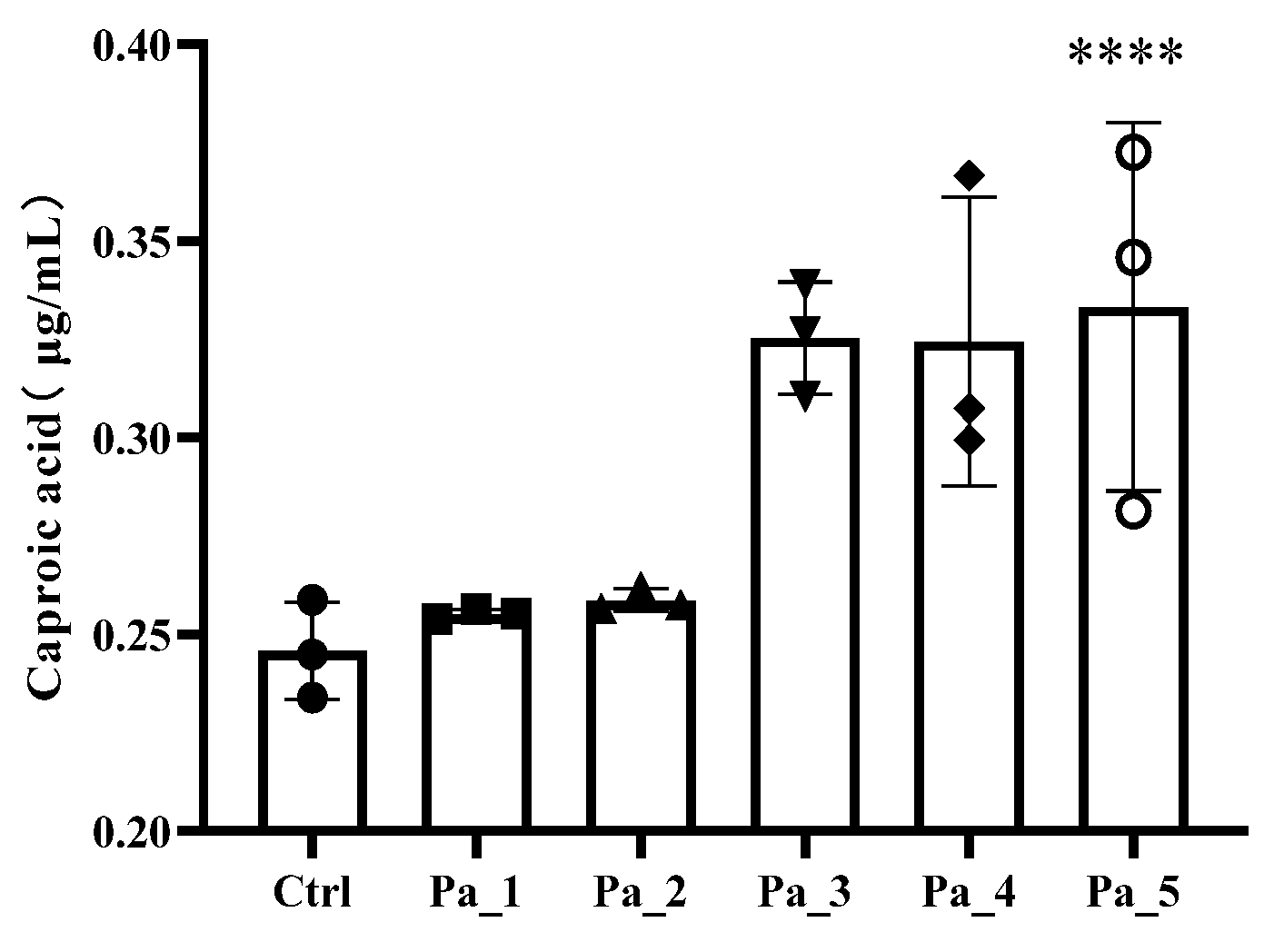
3.7. Effect of PaJY062 on Gut Microbiota and SCFAs
3.8. PaJY062 Contained a Variety of Beneficial Ingredients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LPS | Lipopolysaccharide |
| Pa JY062 | Lactobacillus paracei JY062 postbiotics |
| Re JL-1 | Lactobacillus rhamnosus JL-1 postbiotics |
| VB6 | Pyridoxal hydrochloride |
| GABA | γ-aminobutyric acid |
| Rh J1 | Lactobacillus reuteri J1 postbiotics |
| IL-6 | Interleukin-6 |
| IL-17a | Interleukin-17a |
| TNF-α | Tumor necrosis factor-α |
| IL-10 | Interleukin-10 |
| NO | Nitric oxide |
| TEER | Transmembrane electrical resistance |
| FITC | Fluorescein 5-isothiocyanate |
| mRNA | Messenger RNA |
| ZO-1 | Zonula occludens-1 |
| MUC2 | Mucin 2 |
| DSS | Dextran sodium sulfate |
| SCFAs | Short-chain fatty acids |
| kDa | Kilodalton |
| PBS | Phosphate-buffered saline |
| DMEM | Dulbecco’s modified Eagle medium |
| FBS | Fetal bovine serum |
| ELISA | Enzyme-linked immunosorbent assay |
| MRS | Man, Rogosa, and Sharpe |
| AP | Apical side |
| BA | Basal side |
| TEM | Transmission electron microscopy |
| OD | Optical density |
| cDNA | Complementary DNA |
| RT-qPCR | Real-time quantitative polymerase chain reaction |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| SGF | Simulated gastric fluid |
| SIF | Simulated intestinal fluid |
| OPA | O-phthalaldehyde |
| ASV | Amplicon sequence variant |
| GC-MS | Gas chromatograohy-mass spectrometry |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| ANOVA | One-way analysis of variance |
| Ctrl | Control |
| TGF -β | Transforming growth factor-β |
| IL-1β | Interleukin-1β |
| IL-12p40 | Interleukin-12 p40 subunit |
| IL-23p19 | Interleukin-23 p19 subunit |
| Th1 | helper T cell 1 |
| Th2 | helper T cell 2 |
| LDA | linear discriminant analysis |
| LEfSe | LDA effect size |
| IBD | Inflammatory bowel disease |
References
- Dong, Y.; Fan, H. Berberine ameliorates DSS-induced intestinal mucosal barrier dysfunction through microbiota-dependence and Wnt/β-catenin pathway. Int. J. Biol. Sci. 2022, 18, 1381–1397. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, J. Gut dysbiosis in rheumatic diseases: A systematic review and meta-analysis of 92 observational studies. EBioMedicine 2022, 80, 104055. [Google Scholar] [CrossRef]
- Kwak, S.Y.; Jang, W.I.; Park, S.; Cho, S.S.; Lee, S.B.; Kim, M.J.; Park, S.; Shim, S.; Jang, H. Metallothionein 2 activation by pravastatin reinforces epithelial integrity and ameliorates radiation-induced enteropathy. EBioMedicine 2021, 73, 103641. [Google Scholar] [CrossRef]
- Dai, Z.; Li, S.; Meng, Y.; Zhao, Q.; Zhang, Y.; Suonan, Z.; Sun, Y.; Shen, Q.; Liao, X.; Xue, Y. Capsaicin Ameliorates High-Fat Diet-Induced Atherosclerosis in ApoE(−/−) Mice via Remodeling Gut Microbiota. Nutrients 2022, 14, 4334. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Sun, W.; Pei, X.; Jin, Y.; Wang, H.; Tao, W.; Xiao, Z.; Liu, L.; Wang, M. Galactooligosaccharide pretreatment alleviates damage of the intestinal barrier and inflammatory responses in LPS-challenged mice. Food Funct. 2021, 12, 1569–1579. [Google Scholar] [CrossRef]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 2018, 6, 121. [Google Scholar] [CrossRef]
- Liu, C.; Qi, X.; Li, D.; Zhao, L.; Li, Q.; Mao, K.; Shen, G.; Ma, Y.; Wang, R. Limosilactobacillus fermentum HF06-derived paraprobiotic and postbiotic alleviate intestinal barrier damage and gut microbiota disruption in mice with ulcerative colitis. J. Sci. Food Agric. 2024, 104, 1702–1712. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.; Wan, Y.; Hu, T.; Liu, L.; Yang, S.; Gong, Z.; Zeng, Q.; Wei, Y.; Yang, W.; et al. A Novel Postbiotic From Lactobacillus rhamnosus GG with a Beneficial Effect on Intestinal Barrier Function. Front. Microbiol. 2019, 10, 477. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Feng, C.; Kwok, L.-Y.; He, Q.; Sun, Z. Stronger gut microbiome modulatory effects by postbiotics than probiotics in a mouse colitis model. NPJ Sci. Food 2022, 6, 53. [Google Scholar] [CrossRef]
- Batista, V.L.; De Jesus, L.C.L.; Tavares, L.M.; Barroso, F.L.A.; Fernandes, L.; Freitas, A.D.S.; Americo, M.F.; Drumond, M.M.; Mancha-Agresti, P.; Ferreira, E.; et al. Paraprobiotics and Postbiotics of Lactobacillus delbrueckii CIDCA 133 Mitigate 5-FU-Induced Intestinal Inflammation. Microorganisms 2022, 10, 1418. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Li, H.; Ding, Y.; Huo, J.; Zheng, Y.; Jiang, Y.; Zhang, Y.; Man, C. The Probiotic Combination of Lacticaseibacillus paracasei JY062 and Lactobacillus gasseri JM1 Alleviates Gastrointestinal Motility Disorder via Improving Gut Microbiota. Nutrients 2023, 15, 839. [Google Scholar] [CrossRef]
- Li, X.; Hu, D.; Tian, Y.; Song, Y.; Hou, Y.; Sun, L.; Zhang, Y.; Man, C.; Zhang, W.; Jiang, Y. Protective effects of a novel Lactobacillus rhamnosus strain with probiotic characteristics against lipopolysaccharide-induced intestinal inflammation in vitro and in vivo. Food Funct. 2020, 11, 5799–5814. [Google Scholar] [CrossRef]
- Zhang, C.; Fang, R.; Lu, X.; Zhang, Y.; Yang, M.; Su, Y.; Jiang, Y.; Man, C. Lactobacillus reuteri J1 prevents obesity by altering the gut microbiota and regulating bile acid metabolism in obese mice. Food Funct. 2022, 13, 6688–6701. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Guo, F.; Pei, M.; Tsao, R.; Wang, X.; Jiang, L.; Sun, Y.; Xiong, H. Anti-inflammatory effect of lentil hull (Lens culinaris) extract via MAPK/NF-κB signaling pathways and effects of digestive products on intestinal barrier and inflammation in Caco-2 and Raw264.7 co-culture. J. Funct. Foods 2022, 92, 105044. [Google Scholar] [CrossRef]
- Wang, Z.; Litterio, M.C.; Müller, M.; Vauzour, D.; Oteiza, P.I. (-)-Epicatechin and NADPH oxidase inhibitors prevent bile acid-induced Caco-2 monolayer permeabilization through ERK1/2 modulation. Redox Biol. 2020, 28, 101360. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Guo, Y.; Zhao, J.; Xu, X.; Wang, N.; Liu, Q. Ferulic Acid Ameliorates Lipopolysaccharide-Induced Barrier Dysfunction via MicroRNA-200c-3p-Mediated Activation of PI3K/AKT Pathway in Caco-2 Cells. Front. Pharmacol. 2020, 11, 376. [Google Scholar] [CrossRef]
- Jia, K.; Wei, M.; He, Y.; Wang, Y.; Wei, H.; Tao, X. Characterization of Novel Exopolysaccharides from Enterococcus hirae WEHI01 and Its Immunomodulatory Activity. Foods 2022, 11, 3538. [Google Scholar] [CrossRef]
- Conley, J.M.; Lambright, C.S.; Evans, N.; McCord, J.; Strynar, M.J.; Hill, D.; Medlock-Kakaley, E.; Wilson, V.S.; Gray, L.E., Jr. Hexafluoropropylene oxide-dimer acid (HFPO-DA or GenX) alters maternal and fetal glucose and lipid metabolism and produces neonatal mortality, low birthweight, and hepatomegaly in the Sprague-Dawley rat. Environ. Int. 2021, 146, 106204. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Guo, J.; Yin, M.; Han, X.; You, Y.; Huang, W.; Zhan, J. The influence of oxygen on the metabolites of phenolic blueberry extract and the mouse microflora during in vitro fermentation. Food Res. Int. 2020, 136, 109610. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Davidson, E.A.; Shaikh, M.W.; Forsyth, C.B.; Prenni, J.E.; Broeckling, C.D. Quantitative analysis of short-chain fatty acids in human plasma and serum by GC-MS. Anal. Bioanal. Chem. 2022, 414, 4391–4399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, H.; Zhu, M.-J. A sensitive GC/MS detection method for analyzing microbial metabolites short chain fatty acids in fecal and serum samples. Talanta 2019, 196, 249–254. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Chen, C.C.; Lin, Y.T.; Wu, W.K.; Chang, L.C.; Lai, C.H.; Wu, M.S.; Kuo, C.H. Evaluation and Optimization of Sample Handling Methods for Quantification of Short-Chain Fatty Acids in Human Fecal Samples by GC-MS. J. Proteome Res. 2019, 18, 1948–1957. [Google Scholar] [CrossRef]
- Aherne, C.M.; Collins, C.B.; Masterson, J.C.; Tizzano, M.; Boyle, T.A.; Westrich, J.A.; Parnes, J.A.; Furuta, G.T.; Rivera-Nieves, J.; Eltzschig, H.K. Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis. Gut 2012, 61, 695–705. [Google Scholar] [CrossRef]
- Mu, Y.; Yin, T.L.; Zhang, Y.; Yang, J.; Wu, Y.T. Diet-induced obesity impairs spermatogenesis: The critical role of NLRP3 in Sertoli cells. Inflamm. Regen. 2022, 42, 24. [Google Scholar] [CrossRef] [PubMed]
- Blaconà, G.; Raso, R.; Castellani, S.; Pierandrei, S.; Del Porto, P.; Ferraguti, G.; Ascenzioni, F.; Conese, M.; Lucarelli, M. Downregulation of epithelial sodium channel (ENaC) activity in cystic fibrosis cells by epigenetic targeting. Cell Mol. Life Sci. 2022, 79, 257. [Google Scholar] [CrossRef] [PubMed]
- Guilloteau, E.; Coll, P.; Lu, Z.; Djouina, M.; Cazaunau, M.; Waxin, C.; Bergé, A.; Caboche, S.; Gratien, A.; Al Marj, E.; et al. Murine in utero exposure to simulated complex urban air pollution disturbs offspring gut maturation and microbiota during intestinal suckling-to-weaning transition in a sex-dependent manner. Part Fibre. Toxicol. 2022, 19, 41. [Google Scholar] [CrossRef]
- Haskey, N.; Ye, J.; Estaki, M.; Verdugo Meza, A.A.; Barnett, J.A.; Yousefi, M.; Birnie, B.W.; Gruenheid, S.; Ghosh, S.; Gibson, D.L. A Mediterranean-like fat blend protects against the development of severe colitis in the mucin-2 deficient murine model. Gut Microbes 2022, 14, 2055441. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Zhang, C.; Chi, H.; Liu, C.; Li, A.; Yu, W. Postbiotics derived from Lactobacillus plantarum 1.0386 ameliorate lipopolysaccharide-induced tight junction injury via MicroRNA-200c-3p mediated activation of the MLCK-MLC pathway in Caco-2 cells. Food Funct. 2022, 13, 11008–11020. [Google Scholar] [CrossRef]
- Popović, N.; Djokić, J.; Brdarić, E.; Dinić, M.; Terzić-Vidojević, A.; Golić, N.; Veljović, K. The influence of heat-killed Enterococcus faecium BGPAS1-3 on the tight junction protein expression and immune function in differentiated Caco-2 cells infected with Listeria monocytogenes ATCC 19111. Front. Microbiol. 2019, 10, 412. [Google Scholar] [CrossRef]
- Shi, J.; Du, P.; Xie, Q.; Wang, N.; Li, H.; Smith, E.E.; Li, C.; Liu, F.; Huo, G.; Li, B. Protective effects of tryptophan-catabolizing Lactobacillus plantarum KLDS 1.0386 against dextran sodium sulfate-induced colitis in mice. Food Funct. 2020, 11, 10736–10747. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Lu, W.; Ding, L.; Bao, Y.; Hong, F.; Chen, Y.; Gao, H.; Xu, X.; Wang, G.; Wang, W.; et al. PEDF promotes the repair of bone marrow endothelial cell injury and accelerates hematopoietic reconstruction after bone marrow transplantation. J. Biomed. Sci. 2020, 27, 91. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, J.; Xu, M.; Zheng, Y.; Wang, M.; Yang, S.; Qin, B.; Li, S.; Dong, L.; Dai, F. DDX3 acts as a tumor suppressor in colorectal cancer as loss of DDX3 in advanced cancer promotes tumor progression by activating the MAPK pathway. Int. J. Biol. Sci. 2022, 18, 3918–3933. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.C.; Huang, S.C. The Pivotal Role of Aryl Hydrocarbon Receptor-Regulated Tight Junction Proteins and Innate Immunity on the Synergistic Effects of Postbiotic Butyrate and Active Vitamin D3 to Defense against Microbial Invasion in Salmonella Colitis. Nutrients 2023, 15, 305. [Google Scholar] [CrossRef]
- Bruno, C.; Paparo, L.; Pisapia, L.; Romano, A.; Cortese, M.; Punzo, E.; Berni Canani, R. Protective effects of the postbiotic deriving from cow’s milk fermentation with L. paracasei CBA L74 against Rotavirus infection in human enterocytes. Sci. Rep. 2022, 12, 6268. [Google Scholar] [CrossRef]
- Xu, X.; Wu, J.; Jin, Y.; Huang, K.; Zhang, Y.; Liang, Z. Both Saccharomyces boulardii and Its Postbiotics Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice, Association with Modulating Inflammation and Intestinal Microbiota. Nutrients 2023, 15, 1484. [Google Scholar] [CrossRef]
- Dahlin, M.; Singleton, S.S.; David, J.A.; Basuchoudhary, A.; Wickström, R.; Mazumder, R.; Prast-Nielsen, S. Higher levels of Bifidobacteria and tumor necrosis factor in children with drug-resistant epilepsy are associated with anti-seizure response to the ketogenic diet. eBioMedicine 2022, 80, 104061. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, T.; Wang, X.; Lü, X. The Protective Effect of Heat-Inactivated Companilactobacillus crustorum on Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Nutrients 2023, 15, 2746. [Google Scholar] [CrossRef]
- Lee, H.; Jung, K.B.; Kwon, O.; Son, Y.S.; Choi, E.; Yu, W.D.; Son, N.; Jeon, J.H.; Jo, H.; Yang, H.; et al. Limosilactobacillus reuteri DS0384 promotes intestinal epithelial maturation via the postbiotic effect in human intestinal organoids and infant mice. Gut Microbes 2022, 14, 2121580. [Google Scholar] [CrossRef]
- Feng, C.; Peng, C.; Zhang, W.; Zhang, T.; He, Q.; Kwok, L.-Y.; Zhang, H. Postbiotic Administration Ameliorates Colitis and Inflammation in Rats Possibly through Gut Microbiota Modulation. J. Agric. Food Chem. 2024, 72, 9054–9066. [Google Scholar] [CrossRef]
- Mu, Z.; Yang, Y.; Xia, Y.; Wang, F.; Sun, Y.; Yang, Y.; Ai, L. Probiotic yeast BR14 ameliorates DSS-induced colitis by restoring the gut barrier and adjusting the intestinal microbiota. Food Funct. 2021, 12, 8386–8398. [Google Scholar] [CrossRef] [PubMed]
- Warda, A.K.; de Almeida Bettio, P.H.; Hueston, C.M.; Di Benedetto, G.; Clooney, A.G.; Hill, C. Oral Administration of Heat-Treated Lactobacilli Modifies the Murine Microbiome and Reduces Citrobacter Induced Colitis. Front. Microbiol. 2020, 11, 69. [Google Scholar] [CrossRef]
- Fang, F.; Li, Y.; Lu, X.; Wu, K.; Zhou, L.; Sun, Y.; Wu, J.; Gao, J. Effect of potential postbiotics derived from food-isolated Lactobacillus parabuchneri on different enterotypes of human gut microbiome. LWT 2023, 182, 114782. [Google Scholar] [CrossRef]
- Guo, S.; Ma, T.; Kwok, L.Y.; Quan, K.; Li, B.; Wang, H.; Zhang, H.; Menghe, B.; Chen, Y. Effects of postbiotics on chronic diarrhea in young adults: A randomized, double-blind, placebo-controlled crossover trial assessing clinical symptoms, gut microbiota, and metabolite profiles. Gut Microbes 2024, 16, 2395092. [Google Scholar] [CrossRef]
- Li, M.; Han, X.; Sun, L.; Liu, X.; Zhang, W.; Hao, J. Indole-3-acetic acid alleviates DSS-induced colitis by promoting the production of R-equol from Bifidobacterium pseudolongum. Gut Microbes 2024, 16, 2329147. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Xiang, H.; Guo, M.; Li, S.; Liu, M.; Yao, J. The Tryptophan Metabolite Indole-3-Carboxaldehyde Alleviates Mice with DSS-Induced Ulcerative Colitis by Balancing Amino Acid Metabolism, Inhibiting Intestinal Inflammation, and Improving Intestinal Barrier Function. Molecules 2023, 28, 3704. [Google Scholar] [CrossRef]
- Scott, S.A.; Fu, J.; Chang, P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 19376–19387. [Google Scholar] [CrossRef]
- Selhub, J.; Byun, A.; Liu, Z.; Mason, J.B.; Bronson, R.T.; Crott, J.W. Dietary vitamin B6 intake modulates colonic inflammation in the IL10−/− model of inflammatory bowel disease. J. Nutr. Biochem. 2013, 24, 2138–2143. [Google Scholar] [CrossRef]
- Baier, J.; Gänsbauer, M.; Giessler, C.; Arnold, H.; Muske, M.; Schleicher, U.; Lukassen, S.; Ekici, A.; Rauh, M.; Daniel, C.; et al. Arginase impedes the resolution of colitis by altering the microbiome and metabolome. J. Clin. Investig. 2020, 130, 5703–5720. [Google Scholar] [CrossRef]


| Lactobacillus reuteri J1 (CFU/mL) | Lactobacillus rhamnosus JL-1 (CFU/mL) | Lactobacillus paracasei JY062 (CFU/mL) | |
|---|---|---|---|
| 65 °C 30 min before | 1.36 × 1012 | 1.32 × 1013 | 2.42 × 1012 |
| 65 °C 30 min after | 100 | 3400 | 35,000 |
| 70 °C 15 min before | 1.32 × 1012 | 1.35 × 1013 | 2.45 × 1012 |
| 70 °C 15 min after | 60 | 350 | 1500 |
| 70 °C 30 min before | 1.31 × 1012 | 1.36 × 1013 | 2.61 × 1012 |
| 70 °C 30 min after | 0 | 120 | 320 |
| 75 °C 15 min before | 1.39 × 1012 | 1.44 × 1013 | 2.51 × 1012 |
| 75 °C 15 min after | 0 | 40 | 50 |
| 75 °C 30 min before | - | 1.35 × 1013 | 2.51 × 1012 |
| 75 °C 30 min after | - | 0 | 0 |
| lyophilization before | 1.36 × 1012 | 1.32 × 1013 | 2.42 × 1012 |
| lyophilization after | 500 | 3800 | 1500 |
| 70 °C 15 min and lyophilization before | 1.31 × 1012 | 1.36 × 1013 | 2.61 × 1012 |
| 70 °C 15 min and lyophilization after | 0 | 20 | 86 |
| 70 °C 30 min and lyophilization before | - | 1.33 × 1013 | 2.52 × 1012 |
| 70 °C 30 min and lyophilization after | - | 0 | 0 |
| Primer Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| ZO-1 | TGAGGCAGCTCACATAATGC | GGTCTCTGCTGGCTTGTTTC |
| Occludin | AAAGGGCATTGCTCATCCTGA | ACAATGGCAATGGCAATTCATC |
| Claudin-1 | CCAGTCAATGCCAGGTACGAAT | GGCCTTGGTGTTGGGTAAGA |
| Claudin-2 | CCATGGTCAACACAACAGCA | GGCATCTAGAAGACCTGAATGG |
| E-cadherin | CCCAAACGTAACGAGGGTATC | GGCAGCTTGAAGTGGTAGAAGT |
| α-catenin | CAACCCTTGTAAACACCAAT | ACTGAACCTGACCGTACACCTTCTCCAAGAAATTCTCA |
| β-catenin | CGCTTGGCTGAACCATCACA | AGCAGCTTTATTAACTACCACCT |
| IL-1β | GGACAGGATATGGAGCAACAAGTGG | TCATCTTTCAACACGCAGGACAGG |
| IL-12 p40 | AGACCCTGCCCATTGAACTG | CAGGAGTCAGGGTACTCCCA |
| IL-23 p19 | AGAGCCAGCCAGATYTGAGAAG | CTGCTCCRTGGGCAAAGA |
| TGF-β | TACAGCAACAATTCCTGGCGATACC | CTCAACCACTGCCGCACAACTC |
| GAPDH | F: GAGAAGGCTGGGGCTCATTT | TAAGCAGTTGGTGGTGCAGG |
| Substance | Substance Type | Chemical Formula | Content (ng/mL) |
|---|---|---|---|
| Dihydroxyacetone phosphate | Phosphoric acids | C3H7O6P | 1,199,244 |
| N-Acetyl-D-Galactosamine | Amines | C8H15NO6 | 457,323.6 |
| α-Ketoglutaric Acid | Organic acid and its derivatives | C5H6O5 | 384,721.2 |
| rac Normetanephrine Hydrochloride | Hormones and hormone-related compunds | C9H14ClNO3 | 275,776.8 |
| N-Acetylneuraminic Acid | Amino acid derivatives | C11H19NO9 | 274,082.4 |
| 2-Methyllactic acid | Organic acid and its derivatives | C4H8O3 | 248,861.4 |
| Pantothenic acid | Sugars | C9H17NO5 | 245,755.8 |
| L-Pyroglutamic acid | Amino acids | C5H7NO3 | 157,103.4 |
| L-Glutamic acid | Amino acids | C5H9NO4 | 150,073.8 |
| Choline alfoscerate | LPC | C8H20NO6P | 140,098.2 |
| Glutaric acid | Organic acid and its derivatives | C5H8O4 | 118,312.2 |
| L-Leucic acid | Amino acids | C6H12O3 | 117,873 |
| L-Alanine | Amino acids | C3H7NO2 | 107,682 |
| N-Acetyl-L-glutamic acid | Amino acid derivatives | C7H11NO5 | 104,548.2 |
| Indolelactic acid | Indole and its derivatives | C11H11NO3 | 3846.162 |
| Indole-3-Carboxaldehyde | Indole and its derivatives | C9H7NO | 18.5718 |
| Tryptophol | Indole and its derivatives | C10H11NO | 438.7266 |
| Pyridoxal hydrochloride | CoEnzyme and vitamins | C8H10ClNO3 | 2699.274 |
| Arginine | Amino acids | C6H14N4O2 | 19,382.1 |
| γ-Aminobutyric acid | Organic acid and its derivatives | C4H9NO2 | 59,846.52 |
| L-Glutamic acid | Amino acids | C5H9NO4 | 150,073.80 |
| Lysine | Amino acids | C6H14N2O2 | 13,724.82 |
| Tyrosine | Amino acids | C9H11NO3 | 77,773.2 |
| Phenylalanine | Amino acids | C9H11NO2 | 81,640.2 |
| Histidine | Amino acids | C6H9N3O2 | 85,330.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Zhao, Y.; Guo, W.; Sun, Y.; Zhang, W.; Zhao, Q.; Zhang, Y.; Jiang, Y. Effects of Lactobacillus paracei JY062 Postbiotic on Intestinal Barrier, Immunity, and Gut Microbiota. Nutrients 2025, 17, 1272. https://doi.org/10.3390/nu17071272
Guo J, Zhao Y, Guo W, Sun Y, Zhang W, Zhao Q, Zhang Y, Jiang Y. Effects of Lactobacillus paracei JY062 Postbiotic on Intestinal Barrier, Immunity, and Gut Microbiota. Nutrients. 2025; 17(7):1272. https://doi.org/10.3390/nu17071272
Chicago/Turabian StyleGuo, Jinfeng, Ying Zhao, Wenqian Guo, Yilin Sun, Wei Zhang, Qianyu Zhao, Yu Zhang, and Yujun Jiang. 2025. "Effects of Lactobacillus paracei JY062 Postbiotic on Intestinal Barrier, Immunity, and Gut Microbiota" Nutrients 17, no. 7: 1272. https://doi.org/10.3390/nu17071272
APA StyleGuo, J., Zhao, Y., Guo, W., Sun, Y., Zhang, W., Zhao, Q., Zhang, Y., & Jiang, Y. (2025). Effects of Lactobacillus paracei JY062 Postbiotic on Intestinal Barrier, Immunity, and Gut Microbiota. Nutrients, 17(7), 1272. https://doi.org/10.3390/nu17071272






