Effect of Lactiplantibacillus plantarum DSW3805 Isolated from Kimchi for Gut Health Attenuating Colonic Inflammation in a Dextran Sulfate Sodium-Induced Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparations of LAB Samples
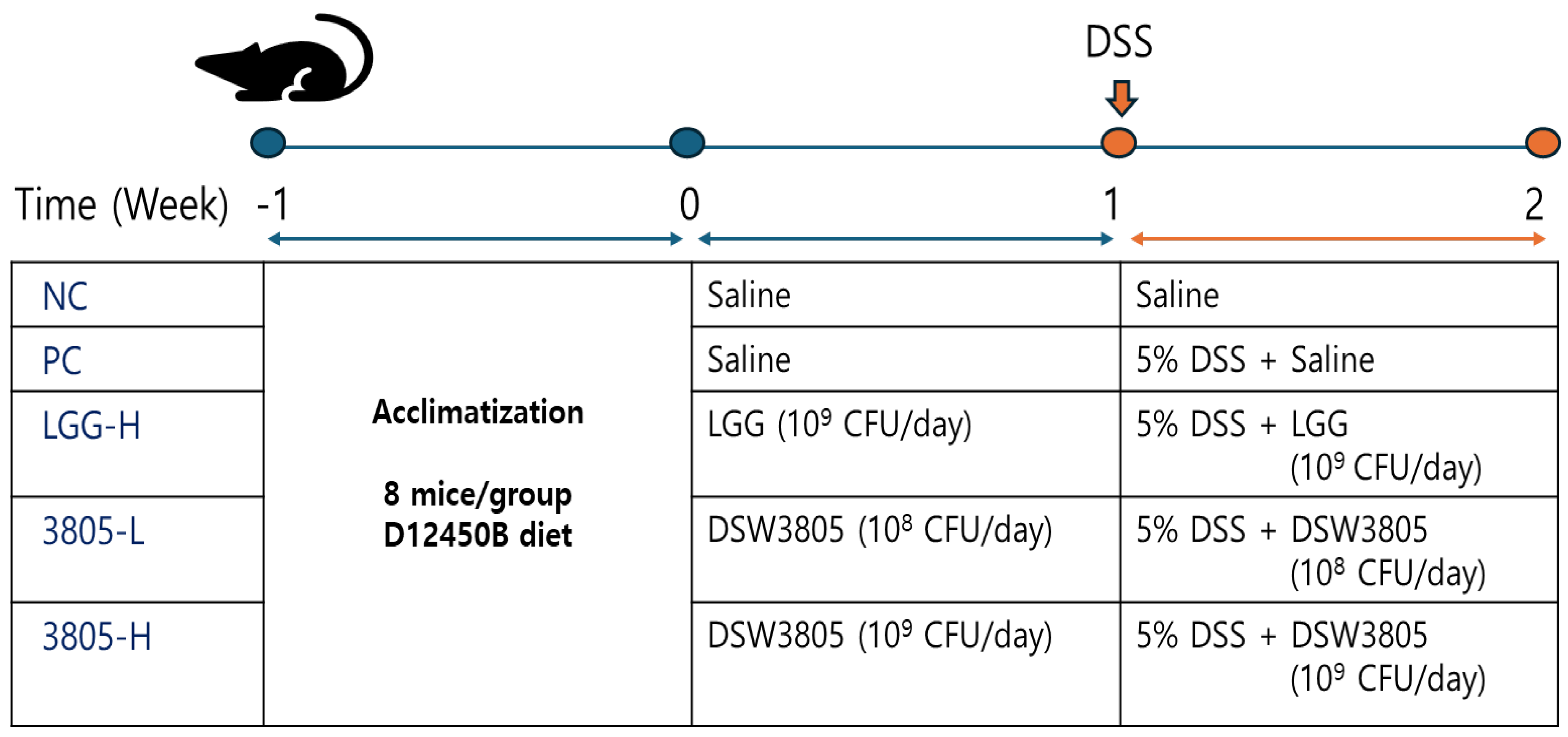
2.2. Animal Groups and Experimental Design
2.3. Clinical Evaluation
2.4. Colon Tissue Observation Using Eye and Microscope
2.5. Quantification of Cytokine, Immunoglobulin, and Inflammatory Factors Using Enzyme-Linked Immunosorbent Assays
2.6. Microbiome Analysis, Beneficial, and Harmful Bacteria
2.7. Analysis of Short Chain Fatty Acid, Calprotectin, and β-Glucuronidase
2.8. Data Analysis
3. Results
3.1. Clinical Evaluation and Intestinal Damage in DSS-Induced Mouse Models
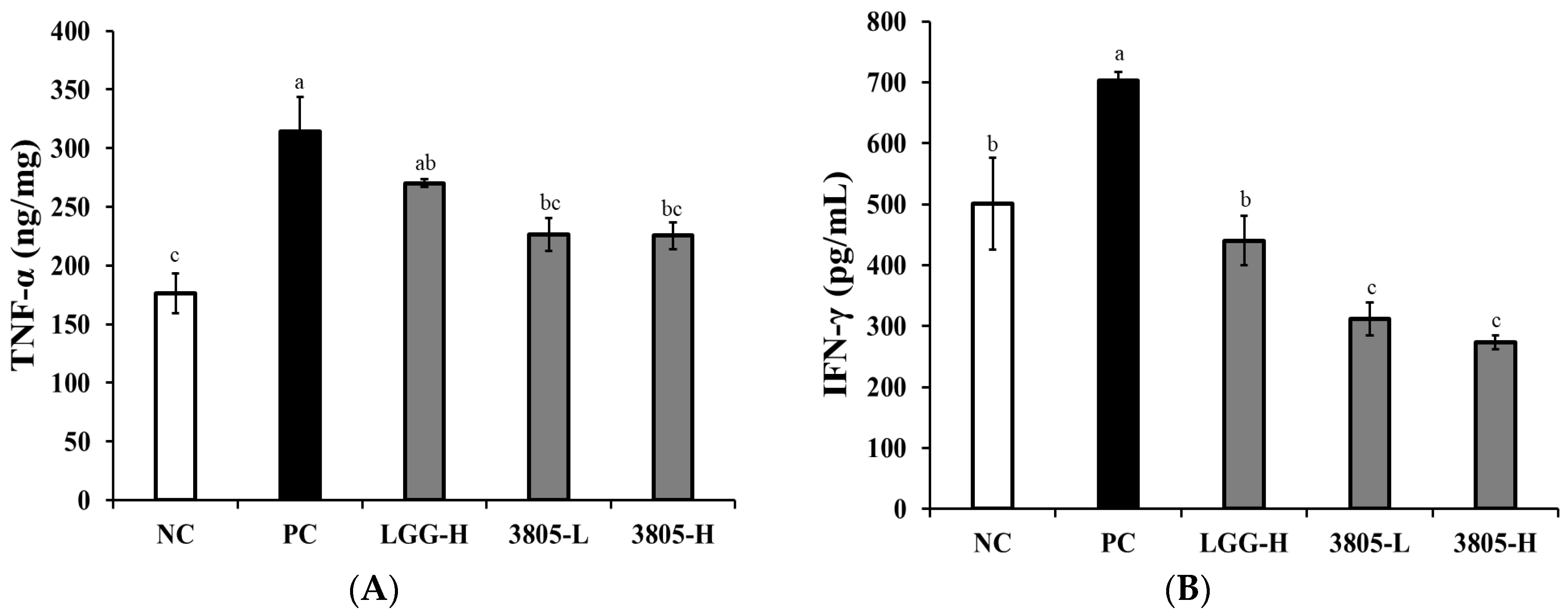
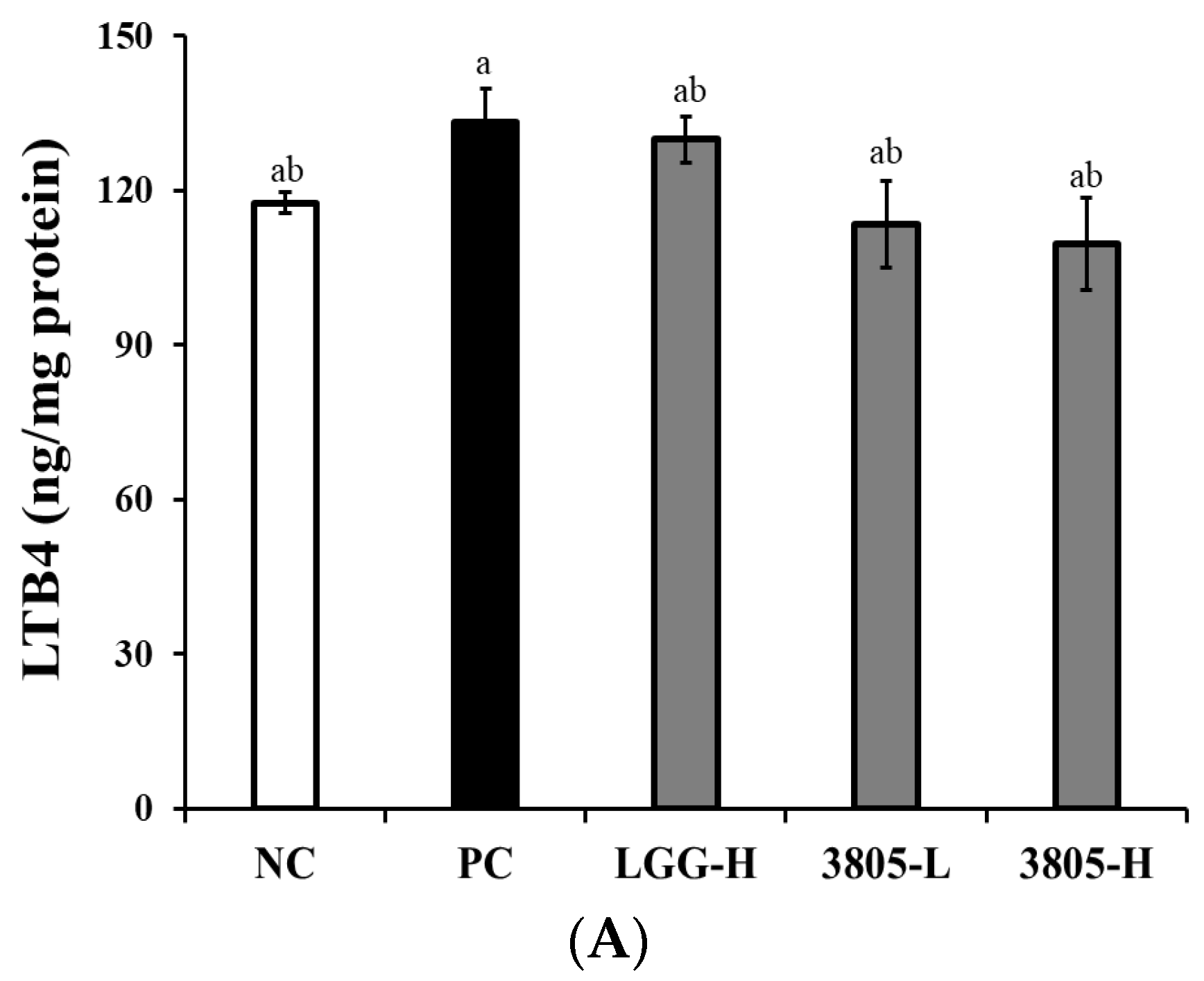
3.2. Immune Regulation Effect in Intestinal Tissue
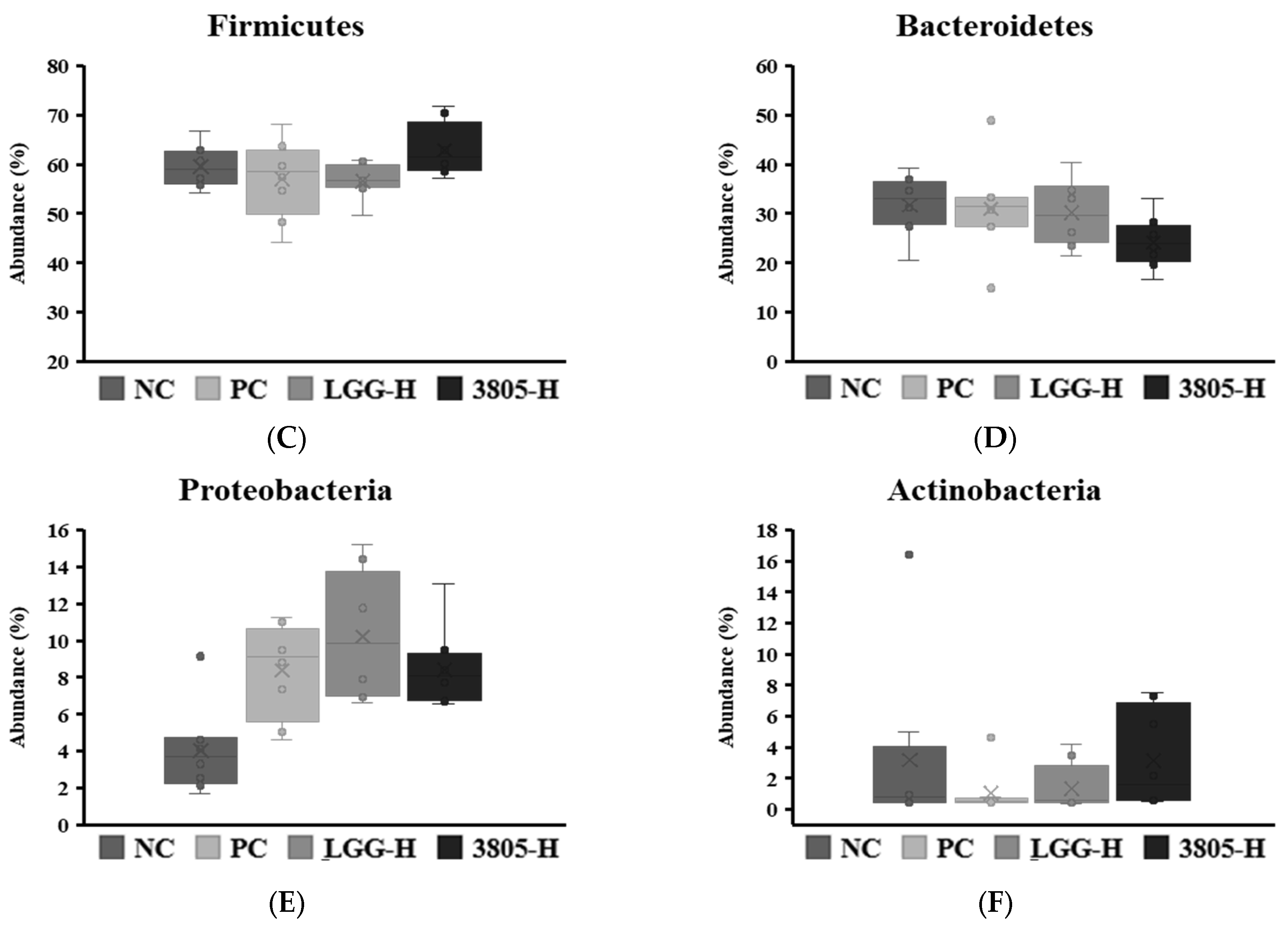
3.3. Microbiome Analysis and Beneficial and Harmful Bacteria in Feces
3.4. SCFAs, Calprotectin, and β-Glucuronidase Analysis in Feces
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Enck, P.; Aziz, Q.; Barbara, G.; Farmer, A.D.; Fukudo, S.; Mayer, E.A.; Niesler, B.; Quigley, E.M.M.; Rajilić-Stojanović, M.; Schemann, M.; et al. Irritable bowel syndrome. Nat. Rev. Dis. Prim. 2016, 2, 16014. [Google Scholar] [CrossRef] [PubMed]
- Goodoory, V.C.; Guthrie, E.A.; Ng, C.E.; Black, C.J.; Ford, A.C. Factors associated with lower disease-specific and generic health-related quality of life in Rome IV irritable bowel syndrome. Aliment. Pharmacol. Ther. 2023, 57, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Goodoory, V.C.; Ng, C.E.; Black, C.J.; Ford, A.C. Impact of Rome IV irritable bowel syndrome on work and activities of daily living. Aliment. Pharmacol. Ther. 2022, 56, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Hu, M.; Ding, Y.; Meng, Y.; Zhao, Y. Efficacy in bowel movement and change of gut microbiota on adult functional constipation patients treated with probiotics-containing products: A systematic review and meta-analysis. BMJ Open 2024, 14, e074557. [Google Scholar] [CrossRef]
- Bennet, S.M.; Ohman, L.; Simren, M. Gut microbiota as potential orchestrators of irritable bowel syndrome. Gut Liver 2015, 9, 318–331. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Chénard, T.; Prévost, K.; Dubé, J.; Massé, E. Immune system modulations by products of the gut microbiota. Vaccines 2020, 8, 461. [Google Scholar] [CrossRef]
- Tong, K.; Nicandro, J.P.; Shringarpure, R.; Chuang, E.; Chang, L. A 9-year evaluation of temporal trends in alosetron postmarketing safety under the risk management program. Ther. Adv. Gastroenterol. 2013, 6, 344–357. [Google Scholar] [CrossRef]
- Lacy, B.E. Diagnosis and treatment of diarrhea-predominant irritable bowel syndrome. Int. J. Gen. Med. 2016, 9, 7–17. [Google Scholar]
- Zhang, Y.; Huang, S.; Zhang, S.; Hao, Z.; Shen, J. Pomegranate peel extract mitigates diarrhea-predominant irritable bowel syndromes via MAPK and NF-κB pathway modulation in rats. Nutrients 2024, 16, 3854. [Google Scholar] [CrossRef]
- Matsuura, N.; Kanayama, M.; Watanabe, Y.; Yamada, H.; Lili, L.; Torill, A. Effect of personalized prebiotic and probiotic supplements on the symptoms of irritable bowel syndrome: An open-label, single-arm, multicenter clinical trial. Nutrients 2024, 16, 3333. [Google Scholar] [CrossRef] [PubMed]
- Cremon, C.; Carini, G.; Wang, B.; Vaxina, V.; Cogliandro, R.F.; De Giorgio, R.; Stanghellini, V.; Grundy, D.; Tonini, M.; De Ponti, F.; et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am. J. Gastroenterol. 2011, 106, 1290–1298. [Google Scholar] [PubMed]
- Lee, N.K.; Lee, Y.J.; Park, J.Y.; Park, E.J.; Paik, H.D. Heat-killed Lactococcus lactis KC24 ameliorates scopolamine-induced memory impairment in ICR mice. Probiotics Antimicrob. Proteins 2024. [Google Scholar] [CrossRef]
- Kang, S.; You, H.J.; Ju, Y.; Kim, H.J.; Jeong, Y.J.; Johnston, T.V.; Ji, G.E.; Ku, S.; Park, M.S. Butyl-fructooligosaccharides modulate gut microbiota in healthy mice and ameliorate ulcerative colitis in a DSS-induced model. Food Funct. 2022, 13, 1834–1845. [Google Scholar] [CrossRef]
- Kim, M.; Chung, K.-S.; Hwang, S.-J.; Yoon, Y.S.; Jang, Y.P.; Lee, J.K.; Lee, K.T. Protective effect of Cicer arietinum L. (Chickpea) ethanol extract in the dextran sulfate sodium-induced mouse model of ulcerative colitis. Nutrients 2020, 12, 456. [Google Scholar] [CrossRef]
- Xiong, T.; Zheng, X.; Zhang, K.; Wu, H.; Gan, W.; Zhang, L.; Li, Y.; Li, J.; Li, Y.; Li, P.; et al. Ganluyin ameliorates DSS-induced ulcerative colitis by inhibiting the enteric-origin LPS/TLR4/NF-κB pathway. J. Ethnopharmacol. 2022, 289, 115001. [Google Scholar]
- Kodani, M.; Fukui, H.; Tomita, T.; Oshima, T.; Watari, J.; Miwa, H. Association between gastrointestinal motility and macrophage/mast cell distribution in mice during the healing stage after DSS-induced colitis. Med. Rep. 2018, 17, 8167–8172. [Google Scholar]
- Prakash, T.; Janadri, S. Anti-inflammatory effect of wedelolactone on DSS-induced colitis in rats: IL-6/STAT3 signaling pathway. J. Ayurveda Integr. Med. 2023, 14, 100544. [Google Scholar]
- Pereira, S.R.; Pereira, R.; Figueiredo, I.; Freitas, V.; Dinis, T.C.P.; Almeida, L.M. Comparison of anti-inflammatory activities of an anthocyanin-rich fraction from Portuguese blueberries (Vaccinium corymbosum L.) and 5-aminosalicylic acid in a TNBS-induced colitis rat model. PLoS ONE 2017, 12, e0174116. [Google Scholar]
- Shaikh, S.D.; Sun, N.; Canakis, A.; Park, W.Y.; Weber, H.C. irritable bowel syndrome and the gut microbiome: A comprehensive review. J. Clin. Med. 2023, 12, 2558. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental models of inflammatory bowel disease. Cell. Mol. Gastroentol. Hepatol. 2015, 22, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Amara, J.; Itani, T.; Hajal, J.; Bakhos, J.J.; Saliba, Y.; Aboushanab, S.A.; Kovaleva, E.G.; Fares, N.; Mondragon, A.C.; Miranda, J.M. Circadian rhythm perturbation aggravates gut microbiota dysbiosis in dextran sulfate sodium-induced colitis in mice. Nutrients 2024, 16, 247. [Google Scholar] [CrossRef]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The microbiome and irritable bowel syndrome—A review on the pathophysiology, current research and future therapy. Front. Microbiol. 2019, 10, 1136. [Google Scholar]
- Hyun, H.J.; Yu, H.S.; Woo, I.K.; Lee, G.W.; Lee, N.K.; Paik, H.D. Anti-inflammatory activities of Levilactobacillus brevis KU15147 in RAW 264.7 cells stimulated with lipopolysaccharide on attenuating NF-B, AP-1, and MAPK signaling pathways. Food Sci. Biotechnol. 2023, 33, 2179–2187. [Google Scholar] [CrossRef]
- Jung, H.S.; Lee, H.W.; Kim, K.T.; Lee, N.K.; Paik, H.D. Anti-inflammatory, antioxidant effects, and antimicrobial effect of Bacillus subtilis P223. Food Sci. Biotechnol. 2024, 33, 2179–2187. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Santos, A.R.O.D.; Carvalho, A.C.A.; Bechara, M.D.; Guiguer, E.L.; Goulart, R.A.; Vargas Sinatora, R.; Araújo, A.C.; Barbalho, S.M. Phytochemicals and regulation of NF-kB in inflammatory bowel diseases: An overview of in vitro and in vivo effects. Metabolites 2023, 13, 96. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, X.; He, B.; Hoang, T.K.; Taylor, C.M.; Blanchard, E.; Freeborn, J.; Park, S.; Luo, M.; Couturier, J.; et al. Lactobacillus reuteri DSM 17938 feeding of healthy newborn mice regulates immune responses while modulating gut microbiota and boosting beneficial metabolites. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G824–G838. [Google Scholar] [CrossRef]
- Yokota, Y.; Shikano, A.; Kuda, T.; Takei, M.; Takahashi, H.; Kimura, B. Lactobacillus plantarum AN1 cells increase caecal L. reuteri in an ICR mouse model of dextran sodium sulphate-induced inflammatory bowel disease. Int. Immunopharmacol. 2018, 56, 119–127. [Google Scholar] [CrossRef]
- Chen, H.; Ou, R.; Tang, N.; Su, W.; Yang, R.; Yu, X.; Zhang, G.; Jiao, J.; Zhou, X. Alternation of the gut microbiota in irritable bowel syndrome: An integrated analysis based on multicenter amplicon sequencing data. J. Transl. Med. 2023, 21, 117. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Tun, H.M.; Liu, Q.; Yeoh, Y.K.; Mak, J.W.Y.; Chan, F.K.; Ng, S.C. Gut microbiome signatures reflect different subtypes of irritable bowel syndrome. Gut Microbes 2023, 15, 2157697. [Google Scholar] [CrossRef] [PubMed]
- Shaidullove, I.F.; Sorokina, D.M.; Sitdikove, F.G.; Hermann, A.; Abdulkhakov, S.R.; Sitdikova, G.F. Short chain fatty acids and colon motility in a mouse model of irritable bowel syndrome. BMC Gastrienterol. 2021, 21, 37. [Google Scholar] [CrossRef]
- García Mansilla, M.J.; Rodríguez Sojo, M.J.; Lista, A.R.; Ayala Mosqueda, C.V.; Ruiz Malagón, A.J.; Gálvez, J.; Rodríguez Nogales, A.; Rodríguez Sánchez, M.J. Exploring gut microbiota imbalance in irritable bowel syndrome: Potential therapeutic effects of probiotics and their metabolites. Nutrients 2025, 17, 155. [Google Scholar] [CrossRef]
- Wang, L.; Cen, S.; Wang, G.; Lee, Y.K.; Zhao, J.; Zhang, H.; Chen, W. Acetic acid and butyric acid released in large intestine play different roles in the alleviation of constipation. J. Funct. Foods 2020, 69, 103953. [Google Scholar]
- Melchior, C.; Aziz, M.; Aubry, T.; Gourcerol, G.; Quillard, M.; Zalar, A.; Coëffier, M.; Dechelotte, P.; Leroi, A.M.; Ducrotté, P. Does calprotectin level identify a subgroup among patients suffering from irritable bowel syndrome? Results of a prospective study. United Eur. Gastroenterol. J. 2017, 5, 261–269. [Google Scholar]
- Chang, M.H.; Chou, J.W.; Chen, S.M.; Tsai, M.C.; Sun, Y.S.; Lin, C.C.; Lin, C.P. Faecal calprotectin as a novel biomarker for differentiating between inflammatory bowel disease and irritable bowel syndrome. Mol. Med. Rep. 2014, 10, 522–526. [Google Scholar]
- Kim, H.Y.; Park, E.S.; Choi, Y.S.; Park, S.J.; Kim, J.H.; Chang, H.K.; Park, K.Y. Kimchi improves irritable bowel syndrome: Results of a randomized, double-blind placebo-controlled study. Food Nutr. Res. 2022, 66, 8628. [Google Scholar]





| NC | PC | LGG-H | 3805-L | 3805-H | |
|---|---|---|---|---|---|
| Body weight (g) | 33.6 ± 1.3 a | 29.9 ± 0.9 b | 32.2 ± 0.8 ab | 30.5 ± 0.9 b | 31.0 ± 0.9 ab |
| Spleen weight (g) | 0.08 ± 0.01 c | 0.15 ± 0.01 a | 0.12 ± 0.01 ab | 0.11 ± 0.01 b | 0.13 ± 0.01 ab |
| Disease activity index of 7 day (Score) | 0.0 ± 0.0 c | 13.3 ± 0.6 a | 11.4 ± 0.7 b | 12.2 ± 0.7 ab | 10.9 ± 0.7 b |
| Weight loss | 0.00 ± 0.00 b | 3.00 ± 0.24 a | 2.67 ± 0.29 a | 3.20 ± 0.20 a | 2.89 ± 0.26 a |
| Diarrhea | 0.00 ± 0.00 b | 3.33 ± 0.17 a | 3.22 ± 0.15 a | 3.05 ± 0.23 a | 2.89 ± 0.18 a |
| Hematochezia | 0.00 ± 0.00 c | 3.22 ± 0.15 a | 3.11 ± 0.11 ab | 2.90 ± 0.10 b | 3.00 ± 0.00 ab |
| Rectal bleeding | 0.00 ± 0.00 d | 3.78 ± 0.15 a | 2.75 ± 0.25 c | 3.33 ± 0.17 ab | 3.17 ± 0.17 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, N.-K.; Lee, Y.; Shin, D.-S.; Choi, Y.-M.; Lee, J.; Park, E.; Paik, H.-D. Effect of Lactiplantibacillus plantarum DSW3805 Isolated from Kimchi for Gut Health Attenuating Colonic Inflammation in a Dextran Sulfate Sodium-Induced Mouse Model. Nutrients 2025, 17, 1259. https://doi.org/10.3390/nu17071259
Lee N-K, Lee Y, Shin D-S, Choi Y-M, Lee J, Park E, Paik H-D. Effect of Lactiplantibacillus plantarum DSW3805 Isolated from Kimchi for Gut Health Attenuating Colonic Inflammation in a Dextran Sulfate Sodium-Induced Mouse Model. Nutrients. 2025; 17(7):1259. https://doi.org/10.3390/nu17071259
Chicago/Turabian StyleLee, Na-Kyoung, Yunjung Lee, Da-Soul Shin, Yong-Min Choi, Jinhyeuk Lee, Eunju Park, and Hyun-Dong Paik. 2025. "Effect of Lactiplantibacillus plantarum DSW3805 Isolated from Kimchi for Gut Health Attenuating Colonic Inflammation in a Dextran Sulfate Sodium-Induced Mouse Model" Nutrients 17, no. 7: 1259. https://doi.org/10.3390/nu17071259
APA StyleLee, N.-K., Lee, Y., Shin, D.-S., Choi, Y.-M., Lee, J., Park, E., & Paik, H.-D. (2025). Effect of Lactiplantibacillus plantarum DSW3805 Isolated from Kimchi for Gut Health Attenuating Colonic Inflammation in a Dextran Sulfate Sodium-Induced Mouse Model. Nutrients, 17(7), 1259. https://doi.org/10.3390/nu17071259







