The Genistein Supply and Elemental Composition of Rat Kidneys in an Induced Breast Cancer Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Animals
2.2. Elemental Level Testing
2.3. Sampling
2.4. Instrumental Analysis
2.5. Statistical Analysis
3. Results
3.1. Comparison of Kidney Weights of Rats Fed Different Diets (Standard and Supplemented with Macrogenistein, Microgenistein and Nanogenistein) in the Conditions of the Neoplastic Process
- -
- There were no differences in the body weight of the rats;
- -
- In groups of rats supplemented with macrogenistein, microgenistein, and nanogenistein, there was an increase in relative kidney weights of 17%, 9% and 12%, respectively, compared to the standard group (Table 1).
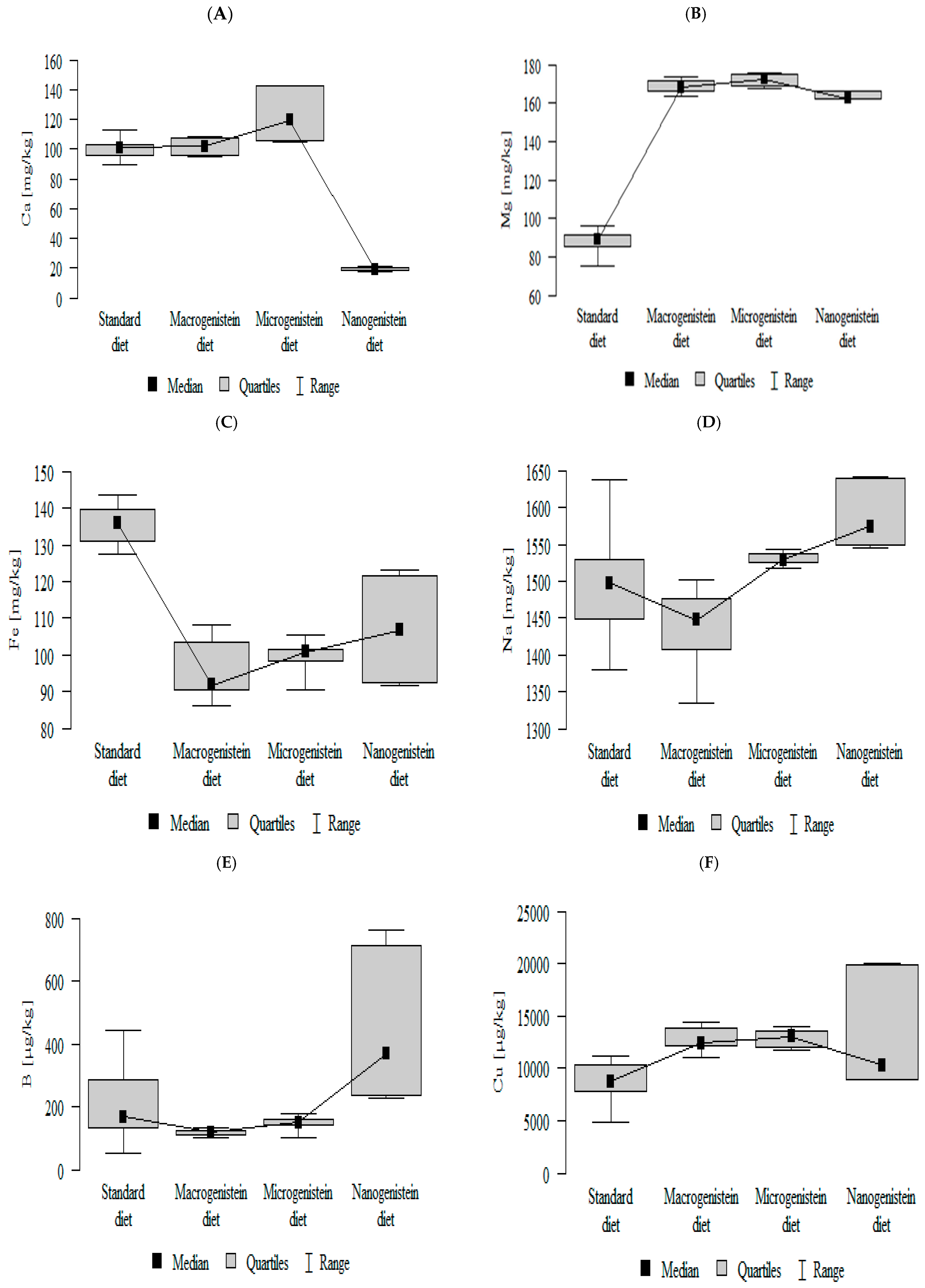
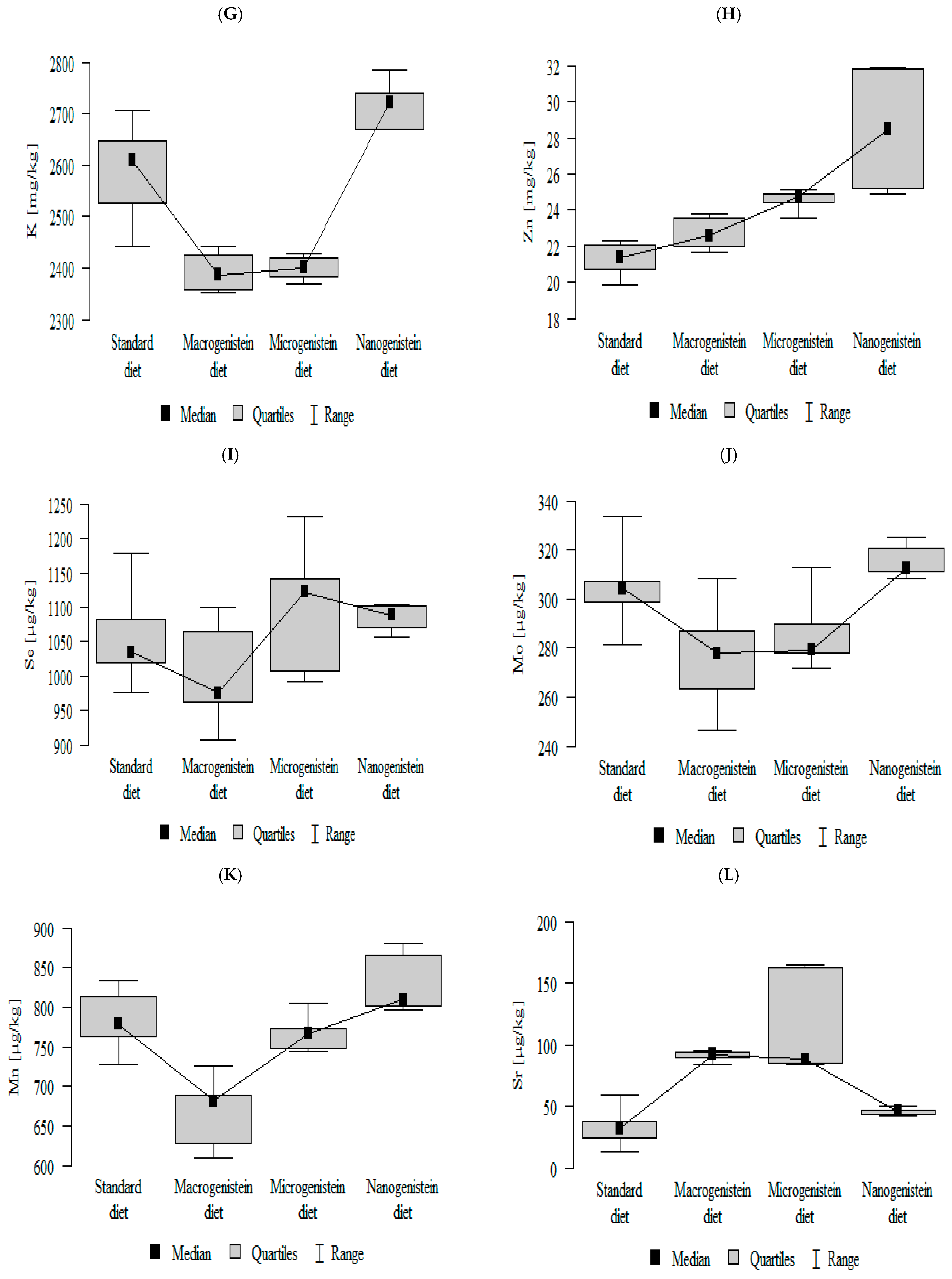
3.2. Comparison of Mineral Content in Kidney Tissue of Rats Receiving Different Forms of Genistein—Macrogenistein, Microgenistein, and Nanogenistein—Without Supplementation (Standard Diet)
- -
- -
- -
- -
- -
- -
- -
- -
- -
- The contents of molybdenum and manganese were significantly higher in the group with nanogenistein than in the groups receiving micro- and macrogenistein. The level of molybdenum was higher in the control group compared to the group supplemented with macrogenistein. The level of manganese was higher in the control group (standard) and the group receiving microgenistein compared to the group receiving macrogenistein (Figure 1J,K; Table S1).
- -
- -
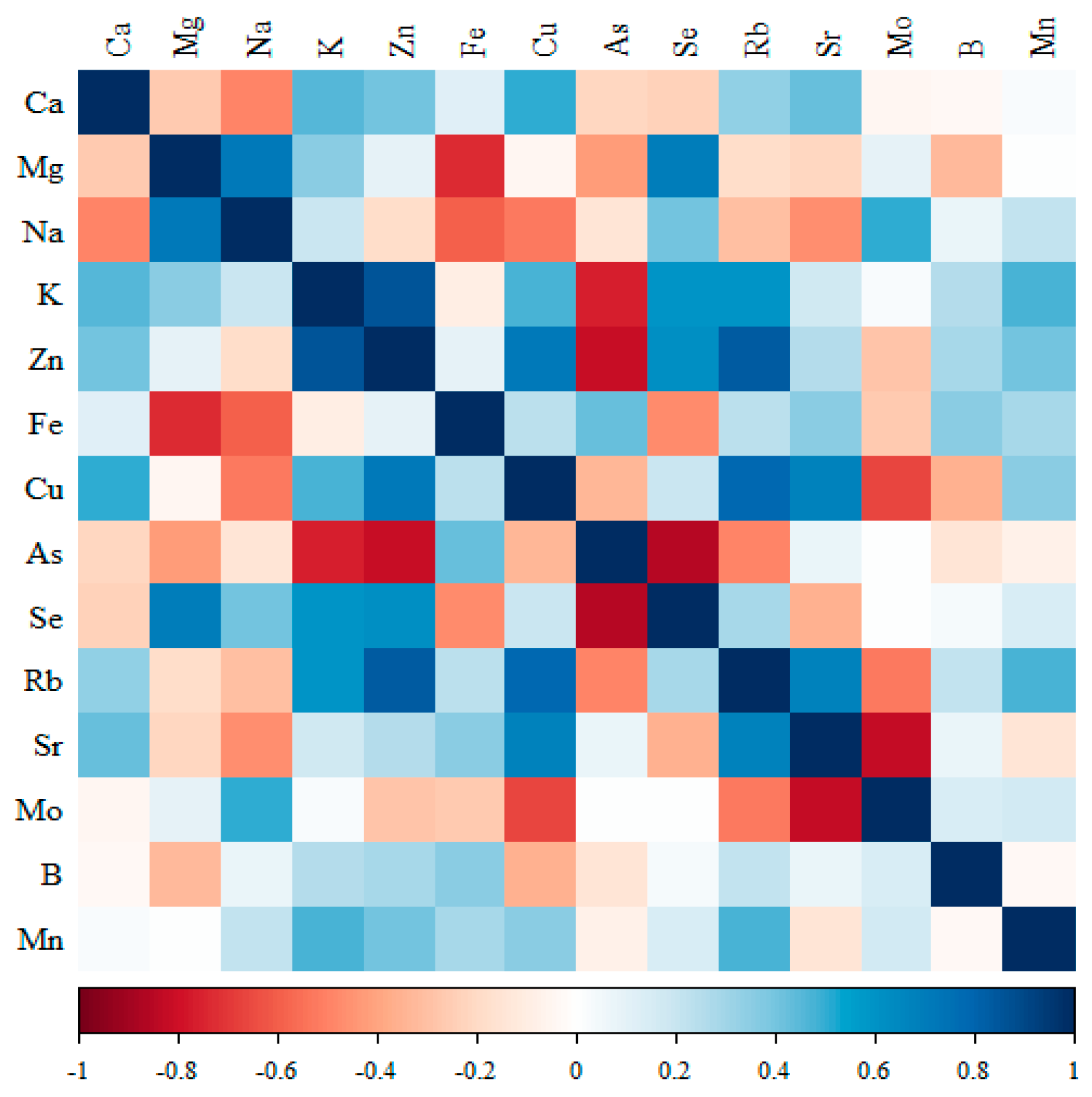
3.3. Analysis of Data on the Levels of Minerals in the Kidneys of Rats Receiving Different Forms of Genistein Under the Conditions of the Neoplastic Process (Figure 2, Figure 3, Figure 4, Figure 5 and Tables S2–S5 (Supplementary Materials))
3.3.1. Comparison of the Correlation of 14 Minerals in the Kidneys of the Control Group (Standard Diet)
3.3.2. Comparison of the Correlation of 14 Minerals in the Kidneys of the Group Receiving Macrogenistein
3.3.3. Comparison of the Correlation of 14 Minerals in the Kidneys of the Group Receiving Microgenistein
- -
- Significant negative correlations were observed for Ca with Cu; Mg with Fe; Na with Se; K with Cu; Fe with As and Mo; and Cu with Sr.
- -
3.3.4. Comparison of the Correlation of 14 Minerals in the Kidneys of the Group Receiving Nanogenistein
- -
- Significant negative correlations were observed for Mg with Fe, Cu, Sr and Mn; Fe with Se, Rb and B; Cu with Se, Rb and B; Se with Mn; Rb with Sr and Mn; Sr with Rb and B; and B with Mn.
- -
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banys, K.; Giebultowicz, J.; Sobczak, M.; Wyrebiak, R.; Bielecki, W.; Wrzesien, R.; Bobrowska-Korczak, B. Effect of Genistein Supplementation on the Progression of Neoplasms and the Level of the Modified Nucleosides in Rats With Mammary Cancer. In Vivo 2021, 35, 2059–2072. [Google Scholar]
- Ramos, S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J. Nutr. Biochem. 2007, 18, 427–442. [Google Scholar] [PubMed]
- Gan, M.; Chen, X.; Chen, Z.; Chen, L.; Zhang, S.; Zhao, Y.; Niu, L.; Li, X.; Shen, L.; Zhu, L. Genistein Alleviates High-Fat Diet-Induced Obesity by Inhibiting the Process of Gluconeogenesis in Mice. Nutrients 2022, 14, 1551. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Fazel Nabavi, S.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and Cancer: Current Status, Challenges, and Future Directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar]
- Chen, X.; Xie, J.; Tan, Q.; Li, H.; Lu, J.; Zhang, X. Genistein improves systemic metabolism and enhances cold resistance by promoting adipose tissue beiging. Biochem. Biophys. Res. Commun. 2021, 18, 154–160. [Google Scholar]
- Jiang, T.; Dong, Y.; Zhu, W.; Wu, T.; Chen, L.; Cao, Y.; Yu, X.; Peng, Y.; Wang, L.; Xiao, Y.; et al. Underlying mechanisms and molecular targets of genistein in the management of type 2 diabetes mellitus and related complications. Crit. Rev. Food Sci. Nutr. 2024, 64, 11543–11555. [Google Scholar] [CrossRef]
- Eustache, F.; Bennani Smires, B.; Moison, D.; Bergès, R.; Canivenc-Lavier, M.C.; Vaiman, D.; Auger, J. Different exposure windows to low doses of genistein and/or vinclozolin result in contrasted disorders of testis function and gene expression of exposed rats and their unexposed progeny. Environ. Res. 2020, 190, 109975. [Google Scholar] [PubMed]
- Walker, C.; Ghazisaeidi, S.; Collet, B.; Boisvert, A.; Culty, M. In utero exposure to low doses of genistein and di-(2-ethylhexyl) phthalate (DEHP) alters innate immune cells in neonatal and adult rat testes. Andrology 2020, 8, 943–964. [Google Scholar]
- Lv, Z.; Fan, H.; Song, B.; Li, G.; Liu, D.; Guo, Y. Supplementing Genistein for Breeder Hens Alters the Fatty Acid Metabolism and Growth Performance of Offsprings by Epigenetic Modification. Oxid. Med. Cell Longev. 2019, 2019, 9214209. [Google Scholar]
- McClain, R.; Wolz, E.; Davidovich, A.; Bausch, J. Genetic toxicity studies with genistein. Food Chem. Toxicol. Int. J. 2006, 44, 42–55. [Google Scholar]
- Klein, C.B.; King, A.A. Genistein genotoxicity: Critical considerations of in vitro exposure dose. Toxicol. Appl. Pharmacol. 2007, 224, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Shinohara, M.; Nagai, T.; Konishi, Y. Transport mechanisms for soy isoflavones and microbial metabolites dihydrogenistein and dihydrodaidzein across monolayers and membranes. Biosci. Biotechnol. Biochem. 2013, 77, 2210–2217. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 30, 118642. [Google Scholar] [CrossRef]
- Patel, V.R.; Agrawal, Y.K. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81–87. [Google Scholar] [PubMed]
- Li, M.; Azad, M.; Davé, R.; Bilgili, E. Nanomilling of Drugs for Bioavailability Enhancement: A Holistic Formulation-Process Perspective. Pharmaceutics 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Langasco, R.; Fancello, S.; Rassu, G.; Cossu, M.; Cavalli, R.; Galleri, G.; Giunchedi, P.; Migheli, R.; Gavini, E. Increasing protective activity of genistein by loading into transfersomes: A new potential adjuvant in the oxidative stress-related neurodegenerative diseases? Int. J. Phytother. Phytopharm. 2019, 52, 23–31. [Google Scholar] [CrossRef]
- Kim, J.T.; Barua, S.; Kim, H.; Hong, S.C.; Yoo, S.Y.; Jeon, H.; Cho, Y.; Gil, S.; Oh, K.; Lee, J. Absorption Study of Genistein Using Solid Lipid Microparticles and Nanoparticles: Control of Oral Bioavailability by Particle Sizes. Biomol. Ther. 2017, 25, 452–459. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Ye, T.; Chen, F.; Sun, X.; Kong, J.; Yang, X.; Pan, W.; Li, S. Design, characterization, and in vitro cellular inhibition and uptake of optimized genistein-loaded NLC for the prevention of posterior capsular opacification using response surface methodology. Int. J. Pharm. 2013, 454, 354–366. [Google Scholar] [CrossRef]
- Javed, Z.; Khan, K.; Herrera-Bravo, J.; Naeem, S.; Iqbal, M.J.; Sadia, H.; Qadri, Q.R.; Raza, S.; Irshad, A.; Akbar, A.; et al. Genistein as a regulator of signaling pathways and microRNAs in different types of cancers. Cancer Cell Int. 2021, 21, 388. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, G.; Zeng, X.; Wu, Y.; Yang, C.; Mei, L.; Wang, Z.; Huand, L. Fabrication of genistein-loaded biodegradable TPGS-b-PCL nanoparticles for improved therapeutic effects in cervical cancer cells. Int. J. Nanomed. 2015, 10, 2461–2473. [Google Scholar]
- Dev, A.; Sardoiwala, M.N.; Kushwaha, A.C.; Karmakar, S.; Choudhury, S.R. Genistein nanoformulation promotes selective apoptosis in oral squamous cell carcinoma through repression of 3PK-EZH2 signalling pathway. Int. J. Phytother. Phytopharm. 2021, 80, 153386. [Google Scholar]
- Rudrapal, M.; Mishra, A.K.; Rani, L.; Sarwa, K.K.; Zothantluanga, J.H.; Khan, J.; Kamal, M.; Palai, S.; Bendale, A.R.; Talele, S.G.; et al. Nanodelivery of Dietary Polyphenols for Therapeutic Applications. Molecules 2022, 27, 8706. [Google Scholar] [CrossRef] [PubMed]
- Kumah, E.A.; Fopa, R.D.; Harati, S.; Boadu, P.; Zohoori, F.V.; Pak, T. Human and environmental impacts of nanoparticles: A scoping review of the current literature. BMC Public Health 2023, 23, 1059. [Google Scholar]
- Chang, E.C.; Charn, T.H.; Park, S.H.; Helferich, W.G.; Komm, B.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Estrogen Receptors alpha and beta as determinants of gene expression: Influence of ligand, dose, and chromatin binding. Mol. Endocrinol. 2008, 22, 1032–1043. [Google Scholar]
- Helferich, W.G.; Andrade, J.E.; Hoagland, M.S. Phytoestrogens and breast cancer: A complex story. Inflammopharmacology 2008, 16, 219–226. [Google Scholar]
- Anderson, B.M.; MacLennan, M.B.; Hillyer, L.M.; Ma, D.W.L. Lifelong exposure to n-3 PUFA affects pubertal mammary gland development. Appl. Physiol. Nutr. Metab. 2014, 39, 699–706. [Google Scholar]
- Krisanits, B.; Randise, J.F.; Burton, C.E.; Findlay, V.J.; Turner, D.P. Pubertal mammary development as a “susceptibility window” for breast cancer disparity. Adv. Cancer Res. 2020, 146, 57–82. [Google Scholar]
- Peng, Q.; Li, Y.; Shang, J.; Huang, H.; Zhang, Y.; Ding, Y.; Liang, Y.; Xie, Z.; Chen, C. Effects of Genistein on Common Kidney Diseases. Nutrients 2022, 14, 3768. [Google Scholar] [CrossRef]
- Palit, S.; Kendrick, J. Vascular Calcification in Chronic Kidney Disease: Role of Disordered Mineral Metabolism. Curr. Pharm. Des. 2014, 20, 5829–5833. [Google Scholar]
- Savic, I.; Farver, C.; Milovanovic, P. Pathogenesis of Pulmonary Calcification and Homologies with Biomineralization in Other Tissues. Am. J. Pathol. 2022, 192, 1496–1505. [Google Scholar]
- Živanović, J.; Jarić, I.; Ajdžanović, V.; Miler, M.; Stanković, S.; Milošević, V.; Filipovic, B. Genistein regulates calcium and phosphate homeostasis without activation of MEK 1/2 signalling pathway in an animal model of the andropause. Anat. Anz. Off. Organ. Anat. Ges. 2022, 239, 151836. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Okada, K.; Soma, M. Mineral metabolic abnormalities and mortality in dialysis patients. Nutrients 2013, 5, 1023. [Google Scholar]
- Guo, Y.; Zhang, A.; Ding, Y.; Wang, Y.; Yuan, W. Genistein ameliorates parathyroid hormone-induced epithelial-to-mesenchymal transition and inhibits expression of connective tissue growth factor in human renal proximal tubular cells. Arch. Med. Sci. 2013, 9, 724–730. [Google Scholar] [PubMed]
- Skrajnowska, D.; Bielecki, W.; Szterk, A.; Ofiara, K.; Bobrowska-Korczak, B. Genistein Supplementation and Bone Health in Breast Cancer in Rats. Nutrients 2024, 21, 912. [Google Scholar]
- Guo, T.L.; Germolec, D.R.; Zheng, J.F.; Kooistra, L.; Auttachoat, W.; Smith, M.J.; White, K.L.; Elmore, S.A. Genistein Protects Female Nonobese Diabetic Mice from Developing Type 1 Diabetes When Fed a Soy- and Alfalfa-free Diet. Toxicol. Pathol. 2015, 43, 435–448. [Google Scholar]
- Hirata, H.; Ueno, K.; Nakajima, K.; Tabatabai, Z.L.; Hinoda, Y.; Ishii, N.; Dahiya, R. Genistein downregulates onco-miR-1260b and inhibits Wnt-signalling in renal cancer cells. Br. J. Cancer 2013, 108, 2070–2078. [Google Scholar] [CrossRef]
- Palanisamy, N.; Viswanathan, P.; Anuradha, C.V. Effect of Genistein, a Soy Isof lavone, on Whole Body Insulin Sensitivity and Renal Damage Induced by a High-Fructose Diet. Ren. Fail. 2008, 30, 645–654. [Google Scholar]
- Pantelic, J.; Ajdzanovic, V.; Medigovic, I.; Mojic, M.; Trifunovic, S.; Milosevic, V.; Filipovic, B. Genistein affects parathyroid gland and NaPi 2a cotransporter in an animal model of the andropause. J. Physiol. Pharmacol. 2013, 64, 361–368. [Google Scholar]
- Couchourel, D.; Leclerc, M.; Filep, J.; Brunette, M.G. Testosterone enhances calcium reabsorption by the kidney. Mol. Cell. Endocrinol. 2004, 222, 71–81. [Google Scholar] [CrossRef]
- Iavicoli, I.; Fontana, L.; Nordberg, G. The effects of nanoparticles on the renal system. Crit. Rev. Toxicol. 2016, 46, 490–560. [Google Scholar] [CrossRef]
- Fontana, L.; Leso, V.; Marinaccio, A.; Cenacchi, G.; Papa, V.; Leopold, K.; Schindl, R.; Bocca, B.; Alimonti, A.; Iavicoli, I. The effects of palladium nanoparticles on the renal function of female Wistar rats. Nanotoxicol 2015, 9, 843–851. [Google Scholar]
- Oberdörster, G. Toxicokinetics and effects of fibrous and nonfibrous particles. Inhal. Toxicol. 2002, 14, 29–56. [Google Scholar] [PubMed]
- Khalili Fard, J.; Jafari, S.; Eghbal, M.A. A Review of Molecular Mechanisms Involved in Toxicity of Nanoparticles. Adv. Pharm. Bull. 2015, 5, 447–454. [Google Scholar]
- Fruijtier-Pölloth, C. The toxicological mode of action and the safety of synthetic amorphous silica-a nanostructured material. Toxicology 2012, 294, 61–79. [Google Scholar] [CrossRef]
- Ryabova, Y.V.; Minigalieva, I.A.; Sutunkova, M.P.; Klinova, S.V.; Tsaplina, A.K.; Valamina, I.E.; Petrunina, E.M.; Tsatsakis, A.M.; Mamoulakis, C.; Stylianou, K.; et al. Toxic Kidney Damage in Rats Following Subchronic Intraperitoneal Exposure to Element Oxide Nanoparticles. Toxics 2023, 11, 791. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, Y.; Watari, A.; Hayata, Y.; Li, X.; Kondoh, M.; Yoshioka, Y.; Tsutsumi, Y.; Yagi, K. Acute and chronic nephrotoxicity of platinum nanoparticles in mice. Nanoscale Res. Lett. 2013, 8, 395. [Google Scholar]
- Liao, M.H.; Tai, Y.T.; Cherng, Y.G.; Liu, S.H.; Chang, Y.A.; Lin, P.I.; Chen, R.M. Genistein induces oestrogen receptor-α gene expression in osteoblasts through the activation of mitogen-activated protein kinases/NF-κB/activator protein-1 and promotes cell mineralisation. Br. J. Nutr. 2014, 111, 55–63. [Google Scholar]
- Poulsen, R.C.; Kruger, M.C. Soy phytoestrogens: Impact on postmenopausal bone loss and mechanisms of action. Nutr. Rev. 2008, 66, 359–374. [Google Scholar]
- Cepeda, S.B.; Sandoval, M.J.; Crescitelli, M.C.; Rauschemberger, M.B.; Massheimer, V.L. The isoflavone genistein enhances osteoblastogenesis: Signaling pathways involved. J. Physiol. Biochem. 2020, 76, 99–110. [Google Scholar]
- Habas, E.; Eledrisi, M.; Khan, F.; Elzouki, A.N.Y. Secondary Hyperparathyroidism in Chronic Kidney Disease: Pathophysiology and Management. Cureus 2021, 3, e16388. [Google Scholar]
- Suki, W.N.; Moore, L.W. Phosphorus Regulation in Chronic Kidney Disease. Cardiovasc. J. 2016, 12, 6–9. [Google Scholar]
- Shen, Y. Mini review: A reevaluation of nutritional vitamin D in the treatment of chronic kidney disease. Medicine 2023, 102, e35811. [Google Scholar]
- Lavi-Moshayoff, V.; Wasserman, G.; Meir, T.; Silver, J.; Naveh-Many, T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: A bone parathyroid feedback loop. Am. J. Physiol. 2010, 299, 882–889. [Google Scholar]
- Jeon, U.S. Kidney and Calcium Homeostasis. EBP 2008, 6, 68–76. [Google Scholar]
- Hoenderop, J.G.J.; van Leeuwen, J.P.T.M.; van der Eerden, B.C.J.; Kersten, F.F.J.; van der Kemp, A.W.C.M.; Mérillat, A.M.; Waarsing, J.H.; Rossier, B.C.; Vallon, V.; Hummler, E.; et al. Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J. Clin. Investig. 2003, 112, 1906–1914. [Google Scholar] [PubMed]
- Landauer, M.R.; Harvey, A.J.; Kaytor, M.D.; Day, R.M. Mechanism and therapeutic window of a genistein nanosuspension to protect against hematopoietic-acute radiation syndrome. J. Radiat. Res. 2019, 60, 308–317. [Google Scholar] [PubMed]
- Nilsson, S.; Gustafsson, J.Å. Estrogen receptors: Therapies targeted to receptor subtypes. Clin. Pharmacol. Ther. 2011, 89, 44–55. [Google Scholar]
- Brzezinski, A.; Debi, A. Phytoestrogens: The “natural” selective estrogen receptor modulators? Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 85, 47–51. [Google Scholar]
- Sareddy, G.R.; Li, X.; Liu, J.; Viswanadhapalli, S.; Garcia, L.; Gruslova, A.; Cavazos, D.; Garcia, M.; Strom, A.M.; Gustafsson, J.A.; et al. Selective Estrogen Receptor β Agonist LY500307 as a Novel Therapeutic Agent for Glioblastoma. Sci. Rep. 2016, 6, 24185. [Google Scholar]
- Gong, P.; Madak-Erdogan, Z.; Li, J.; Cheng, J.; Greenlief, C.M.; Helferich, W.; Katzenellenbogen, J.A.; Ketzenellenbogen, B.S. Transcriptomic Analysis Identifies Gene Networks Regulated by Estrogen Receptor α (ERα) and ERβ that Control Distinct Effects of Different Botanical Estrogens. Nucl. Recept. Signal 2014, 12, 12001. [Google Scholar]
- Warner, M.; Huang, B.; Gustafsson, J.A. Estrogen Receptor β as a Pharmaceutical Target. Trends Pharmacol. Sci. 2017, 38, 92–99. [Google Scholar] [PubMed]
- Liu, X.; Wang, Q.; Liu, B.; Zheng, X.; Li, P.; Zhao, T.; Jin, X.; Ye, F.; Zhang, P.; Chen, W.; et al. Genistein inhibits radiation-induced invasion and migration of glioblastoma cells by blocking the DNA-PKcs/Akt2/Rac1 signaling pathway. J. Eur. Soc. Ther. Radiol. Oncol. 2021, 155, 93–104. [Google Scholar]
- Ha, C.T.; Li, X.H.; Fu, D.; Xiao, M.; Landauer, M.R. Genistein Nanoparticles Protect Mouse Hematopoietic System and Prevent Proinflammatory Factors after Gamma Irradiation. Radiat. Res. 2013, 180, 316–325. [Google Scholar]
- Zhu, Q.; Zhang, W.; Mu, D.; Zhou, H.; Wu, S.; Zou, H. Effects of genistein on lipopolysaccharide-induced injury of mouse alveolar epithelial cells and its mechanism. Biosci. Biotechnol. Biochem. 2020, 84, 544–551. [Google Scholar]
- Jiang, H.; Fan, J.; Cheng, L.; Hu, P.; Liu, R. The anticancer activity of genistein is increased in estrogen receptor beta 1-positive breast cancer cells. OncoTargets Ther. 2018, 11, 8153–8163. [Google Scholar]
- Zhang, Z.; Jin, F.; Lian, X.; Li, M.; Wang, G.; Lan, B.; He, H.; Liu, G.-D.; Wu, Y.; Sun, G.; et al. Genistein promotes ionizing radiation-induced cell death by reducing cytoplasmic Bcl-xL levels in non-small cell lung cancer. Sci. Rep. 2018, 8, 328. [Google Scholar]
- Yan, H.; Jiang, J.; Du, A.; Gao, J.; Zhang, D.; Song, L. Genistein Enhances Radiosensitivity of Human Hepatocellular Carcinoma Cells by Inducing G2/M Arrest and Apoptosis. Radiat. Res. 2020, 193, 286–300. [Google Scholar] [PubMed]
- Kaytor, M.D.; Serebrenik, A.A.; Lapanowski, K.; McFall, D.; Jones, M.; Movsas, B.; Simone, C.B.; Brown, S.L. The radioprotectant nano-genistein enhances radiotherapy efficacy of lung tumors in mice. Transl. Lung Cancer Res. 2023, 12, 999–1010. [Google Scholar]
- Bertho, A.; Dos Santos, M.; Braga-Cohen, S.; Buard, V.; Paget, V.; Guipaud, O.; Tarlet, G.; Millat, F.; Francois, A. Preclinical Model of Stereotactic Ablative Lung Irradiation Using Arc Delivery in the Mouse: Is Fractionation Worthwhile? Front. Med. 2021, 8, 794324. [Google Scholar]
- Jackson, I.L.; Zodda, A.; Gurung, G.; Pavlovic, R.; Kaytor, M.D.; Kuskowski, M.A.; Vujaskovic, Z. BIO 300, a nanosuspension of genistein, mitigates pneumonitis/fibrosis following high-dose radiation exposure in the C57L/J murine model. Br. J. Pharmacol. 2017, 174, 4738–4750. [Google Scholar]
- Fischer, N.; Seo, E.J.; Efferth, T. Prevention from radiation damage by natural products. Int. J. Phytother. Phytopharm. 2018, 47, 192–200. [Google Scholar]
- Singh, V.K.; Fatanmi, O.O.; Santiago, P.T.; Simas, M.; Hanlon, B.K.; Garcia, M.; Wise, S.Y. Current Status of Radiation Countermeasures for Acute Radiation Syndrome Under Advanced Development. J. Radiat. Cancer Res. 2018, 9, 13. [Google Scholar]
- Canyilmaz, E.; Uslu, G.H.; Bahat, Z.; Kandaz, M.; Mungan, S.; Haciislamoglu, E.; Mentese, A.; Yoney, A. Comparison of the effects of melatonin and genistein on radiation-induced nephrotoxicity: Results of an experimental study. Biomed. Rep. 2016, 4, 45–50. [Google Scholar]
- Cohen, E.P.; Robbins, M.E.C. Radiation nephropathy. Semin. Nephrol. 2003, 5, 486–499. [Google Scholar]
- Malyszko, J.; Tesarova, P.; Capasso, G.; Capasso, A. The link between kidney disease and cancer: Complications and treatment. Lancet 2020, 396, 277–287. [Google Scholar]
- Lowrance, W.T.; Ordoñez, J.; Udaltsova, N.; Russo, P.; Go, A.S. CKD and the risk of incident cancer. J. Am. Soc. Nephrol. 2014, 25, 2327–2334. [Google Scholar]
- Janus, N.; Launay-Vacher, V.; Byloos, E.; MacHiels, J.P.; Duck, L.; Kerger, J.; Wynendaele, W.; Canon, J.L.; Lybaert Nortier, J.W.; Deray, G.; et al. Cancer and renal insufficiency results of the BIRMA study. Br. J. Cancer 2010, 103, 1815–1821. [Google Scholar]
- Izzedine, H.; Perazella, M.A. Onco-nephrology: An appraisal of the cancer and chronic kidney disease links. Nephrol. Dial. Transplant. 2015, 30, 1979–1988. [Google Scholar]
- Sayer, J.A.; Carr, G.; Simmons, N.L. Nephrocalcinosis: Molecular insights into calcium precipitation within the kidney. Clin. Sci. Lond. Engl. 2004, 106, 549–561. [Google Scholar]
- Van Laecke, S. Hypomagnesemia and hypermagnesemia. Acta Clin. Belg. 2019, 74, 41–47. [Google Scholar]
- Swaminathan, R. Magnesium Metabolism and its Disorders. Clin. Biochem. Rev. 2003, 24, 47–66. [Google Scholar] [PubMed]
- Salem, S.; Bruck, H.; Bahlmann, F.H.; Peter, M.; Passlick-Deetjen, J.; Kretschmer, A.; Steppan, S.; Volsek, M.; Kribben, A.; Nierhaus, M.; et al. Relationship between magnesium and clinical biomarkers on inhibition of vascular calcification. Am. J. Nephrol. 2012, 35, 31–39. [Google Scholar] [CrossRef]
- Floege, J. Magnesium in CKD: More than a calcification inhibitor? J. Nephrol. 2015, 28, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Hamano, T.; Isaka, Y. Magnesium and Progression of Chronic Kidney Disease: Benefits Beyond Cardiovascular Protection? Adv. Chronic Kidney Dis. 2018, 25, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.C.; Tomasi, C.D.; Constantino, L.; Giombelli, V.; Candal, R.; Bristot, M.; Topanotti, M.F.; Burdmann, E.A.; Dal-Pizzol, F.; Fraga, C.M.; et al. Hypomagnesemia as a risk factor for the non-recovery of the renal function in critically ill patients with acute kidney injury. Nephrol. Dial. Transplant. 2013, 28, 910–916. [Google Scholar]
- Vormann, J. Magnesium and Kidney Health—More on the “Forgotten Electrolyte”. Am. J. Nephrol. 2016, 44, 379–380. [Google Scholar] [CrossRef]
- Macías Ruiz, C.; Cuenca Bermejo, L.; Veronese, N.; Fernández Villalba, E.; González Cuello, A.M.; Kublickiene, K.; Raparelli, V.; Norris, C.M.; Kautzky-Willer, A.; Pilote, L.; et al. Magnesium in Kidney Function and Disease—Implications for Aging and Sex—A Narrative Review. Nutrients 2023, 1710. [Google Scholar] [CrossRef]
- Cunningham, J.; Rodríguez, M.; Messa, P. Magnesium in chronic kidney disease Stages 3 and 4 and in dialysis patients. Clin. Kidney J. 2012, 5, 39–51. [Google Scholar] [CrossRef]
- Kanbay, M.; Goldsmith, D.; Uyar, M.E.; Turgut, F.; Covic, A. Magnesium in chronic kidney disease: Challenges and opportunities. Blood Purif. 2010, 29, 280–292. [Google Scholar] [CrossRef]
- Contiguglia, S.R.; Alfrey, A.C.; Miller, N.; Butkus, D. Total-body magnesium excess in chronic renal failure. Lancet 1972, 299, 1300–1302. [Google Scholar] [CrossRef]
- Rossaint, J.; Zarbock, A. Acute kidney injury: Definition, diagnosis and epidemiology. J. Urol. Nephrol. 2016, 68, 49–57. [Google Scholar]
- Wang, H.; Nishiya, K.; Ito, H.; Hosokawa, T.; Hashimoto, K.; Moriki, T. Iron deposition in renal biopsy specimens from patients with kidney diseases. Am. J. Kidney Dis. 2001, 38, 1038–1044. [Google Scholar]
- Shah, S. Role of iron in progressive renal disease. Am. J. Kidney Dis. 2001, 37, 30–33. [Google Scholar]
- Dev, S.; Babitt, J.L. Overview of iron metabolism in health and disease. Hemodial. Int. Int. Symp. 2017, 21, 6–20. [Google Scholar]
- Ribeiro, S.; Belo, L.; Reis, F.; Santos-Silva, A. Iron therapy in chronic kidney disease: Recent changes, benefits and risks. Blood Rev. 2016, 30, 65–72. [Google Scholar]
- Mullens, W.; Nijst, P. Cardiac Output and Renal Dysfunction. J. Am. Coll. Cardiol. 2016, 67, 2209–2212. [Google Scholar]
- Zhang, D.; Meyron-Holtz, E.; Rouault, T.A. Renal Iron Metabolism: Transferrin Iron Delivery and the Role of Iron Regulatory Proteins. J. Am. Soc. Nephrol. 2007, 18, 401. [Google Scholar] [PubMed]
- Scindia, Y.; Leeds, J.; Swaminathan, S. Iron Homeostasis in Healthy Kidney and its Role in Acute Kidney Injury. Semin. Nephrol. 2019, 39, 76–84. [Google Scholar]
- Agarwal, R.; Kusek, J.W.; Pappas, M.K. A randomized trial of intravenous and oral iron in chronic kidney disease. Kidney Int. 2015, 88, 905–914. [Google Scholar]
- Agarwal, R.; Vasavada, N.; Sachs, N.G.; Chase, S. Oxidative stress and renal injury with intravenous iron-in patients with chronic kidney disease. Kidney Int. 2004, 65, 2279–2289. [Google Scholar]
- Singh, B.; Arora, S.; Agrawal, P.; Gupta, S.K. Hepcidin: A novel peptide hormone regulating iron metabolism. Int. J. Clin. Chem. 2011, 412, 823–830. [Google Scholar]
- Al-Timimi, D.J.; Sulieman, D.M.; Hussen, K.R. Zinc status in type 2 diabetic patients: Relation to the progression of diabetic nephropathy. J. Clin. Diagn. Res. 2014, 8, 4–8. [Google Scholar]
- Teslariu, O.; Pasca, A.S.; Mititelu-Tartau, L.; Schiriac, C.E.; Gales, C.; Saftencu, P.M.; Nechifor, M. The protective effects of zinc in experimental gentamicin induced acute renal failure in rats. J. Physiol. Pharmacol. 2016, 67, 751–757. [Google Scholar]
- Abdollahi, A.; Ghahramani, A.; Ghahramani, N. Zinc and Kidney Disease: A Review. Iran. J. Kidney Dis. 2022, 16, 79–87. [Google Scholar] [PubMed]
- Batista, M.N.; Cuppari, L.; de Fátima Campos Pedrosa, L.; Almeida, M.; Almeida, J.B.; Medeiros, A.C.Q.; Canziani, M.E.F. Effect of end-stage renal disease and diabetes on zinc and copper status. Biol. Trace Elem. Res. 2006, 112, 1–12. [Google Scholar]
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 17, 624. [Google Scholar]
- Soussi, A.; Gargouri, M.; El Feki, A. Effects of co-exposure to lead and zinc on redox status, kidney variables, and histopathology in adult albino rats. Toxicol. Ind. Health 2018, 34, 469–480. [Google Scholar]
- Lenartowicz, M.; Windak, R.; Tylko, G.; Kowal, M.; Styrna, J. Effects of copper supplementation on the structure and content of elements in kidneys of Mosaic mutant mice. Biol. Trace Elem. Res. 2010, 136, 204–220. [Google Scholar]
- Guo, F.; Lin, Y.; Meng, L.; Peng, L.; Zhang, H.; Zhang, X.; Jin, M.; Wang, J.; Zhang, Y.; Tang, M.; et al. Association of copper exposure with prevalence of chronic kidney disease in older adults. Clin. Nutr. 2022, 41, 2720–2728. [Google Scholar]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar]
- Fang, J.; Tang, S.; Zhou, J.; Zhou, J.; Cui, L.; Kong, F.; Gao, Y.; Shen, Y.; Deng, F.; Zhang, Y.; et al. Associations between Personal PM2.5 Elemental Constituents and Decline of Kidney Function in Older Individuals: The China BAPE Study. Environ. Sci. Technol. 2020, 54, 13167–13174. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kalita, J.; Bora, H.K.; Misra, U.K. Relationship of antioxidant and oxidative stress markers in different organs following copper toxicity in a rat model. Toxicol. Appl. Pharmacol. 2016, 293, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zhang, Y.; Zhu, Z.; Zhang, X.; Liu, X.; Zhu, S.; Song, Y.; Jin, X.; Lidholm, B.; Yu, C. Elevated intracellular copper contributes a unique role to kidney fibrosis by lysyl oxidase mediated matrix crosslinking. Cell Death Dis. 2020, 11, 211. [Google Scholar] [CrossRef]
- Chang, K.L.; Cheng, H.L.; Huang, L.W.; Hsieh, B.S.; Hu, Y.C.; Chih, T.T.; Shyu, H.W.; Su, S.J. Combined effects of terazosin and genistein on a metastatic, hormone-independent human prostate cancer cell line. Cancer Lett. 2009, 276, 14–20. [Google Scholar] [CrossRef]
- Ebara, M.; Fukuda, H.; Hatano, R.; Saisho, H.; Nagato, Y.; Suzuki, K.; Nakajima, K.; Yukawa, M.; Kondo, F.; Nakayama, A.; et al. Relationship between copper, zinc and metallothionein in hepatocellular carcinoma and its surrounding liver parenchyma. J. Hepatol. 2000, 33, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.F.; Ahmad, A.; Zubair, H.; Khan, H.Y.; Wang, Z.; Sarkar, F.H.; Hadi, S.M. Soy isoflavone genistein induces cell death in breast cancer cells through mobilization of endogenous copper ions and generation of reactive oxygen species. Mol. Nutr. Food Res. 2011, 55, 553–559. [Google Scholar] [CrossRef]
- Peng, F.; Lu, X.; Janisse, J.; Muzik, O.; Shields, A.F. PET of human prostate cancer xenografts in mice with increased uptake of 64CuCl2. J. Nucl. Med. 2006, 47, 1649–1652. [Google Scholar]
- Zheng, L.F.; Wei, Q.Y.; Cai, Y.J.; Fang, J.G.; Zhou, B.; Yang, L.; Liu, Z.L. DNA damage induced by resveratrol and its synthetic analogues in the presence of Cu (II) ions: Mechanism and structure-activity relationship. Free Radic. Biol. Med. 2006, 41, 1807–1816. [Google Scholar] [CrossRef]
- Daniel, K.G.; Chen, D.; Orlu, S.; Cui, Q.C.; Miller, F.R.; Dou, Q.P. Clioquinol and pyrrolidine dithiocarbamate complex with copper to form proteasome inhibitors and apoptosis inducers in human breast cancer cells. Breast Cancer Res. 2005, 7, 897–908. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y.; Jia, G.; Wang, T.; Yuan, H.; Ye, C.; Zhao, F.; et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006, 163, 109–120. [Google Scholar] [CrossRef]
- Pahl, M.V.; Culver, B.D.; Vaziri, N.D. Boron and the Kidney. J. Ren. Nutr. 2005, 15, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, H.; Jing, W.; Ke, X.; Ke-Li, Y.; Peng-Peng, S.; Cui, L.; Wei-Wei, Q.; Zhixin, L.; Hua-Zhen, L.; Hui, S.; et al. Boron Affects the Development of the Kidney Through Modulation of Apoptosis, Antioxidant Capacity, and Nrf2 Pathway in the African Ostrich Chicks. Biol. Trace Elem. Res. 2018, 186, 226–237. [Google Scholar] [CrossRef]
- Tavil Sabuncuoglu, B.; Aribal Kocaturk, P.; Yaman, O.; Ozelci Kavas, G.; Tekelioglu, M. Effects of Subacute Boric Acid Administration on Rat Kidney Tissue. Clin. Toxicol. 2006, 44, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Hwang, S.; Kim, T.H.; Kang, S.W.; Oh, K.H.; Ahn, C.; Kim, Y.H. The ratio of urinary sodium and potassium and chronic kidney disease progression. Medicine 2018, 97, e12820. [Google Scholar] [CrossRef] [PubMed]
- Aburto, N.J.; Ziolkovska, A.; Hooper, L.; Elliott, P.; Cappuccio, F.P.; Meerpohl, J.J. Effect of lower sodium intake on health: Systematic review and meta-analyses. BMJ 2013, 346, 1326. [Google Scholar] [CrossRef]
- McDonough, A.A.; Nguyen, M.T.X. How does potassium supplementation lower blood pressure? Am. J. Rental Physiol. 2012, 302, 1224–1225. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Gluba-Brzózka, A. Oxidative Stress in ESRD Patients on Dialysis and the Risk of Cardiovascular Diseases. Antioxidants 2020, 9, 1079. [Google Scholar] [CrossRef]
- Ceballos-Picot, I.; Witko-Sarsat, V.; Merad-Boudia, M.; Nguyen, A.T.; Thévenin, M.; Jaudon, M.C.; Zingraff, J.; Verger, C.; Jingers, P.; Descamps-Latscha, B. Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic. Biol. Med. 1996, 21, 845–853. [Google Scholar] [CrossRef]
- Zachara, B.A. Chapter Five—Selenium and Selenium-Dependent Antioxidants in Chronic Kidney Disease. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 131–151. Available online: https://www.sciencedirect.com/science/article/pii/S0065242314000377 (accessed on 26 April 2024).
- Aziz, M.A.; Majeed, G.H.; Diab, K.S.; Al-Tamimi, R.J. The association of oxidant-antioxidant status in patients with chronic renal failure. Ren. Fail. 2016, 38, 20–26. [Google Scholar] [CrossRef]
- Wu, C.Y.; Wong, C.S.; Chung, C.J.; Wu, M.Y.; Huang, Y.L.; Ao, P.L.; Lin, Y.F.; Lin, Y.C.; Shiue, H.S.; Su, C.T.; et al. The association between plasma selenium and chronic kidney disease related to lead, cadmium and arsenic exposure in a Taiwanese population. J. Hazard. Mater. 2019, 375, 224–232. [Google Scholar] [CrossRef]
- Balasubramanian, S.K.; Jittiwat, J.; Manikandan, J.; Ong, C.N.; Yu, L.E.; Ong, W.Y. Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats. Biomaterials 2010, 31, 2034–2042. [Google Scholar] [PubMed]
- Niżnik, Ł.; Noga, M.; Kobylarz, D.; Frydrych, A.; Krośniak, A.; Kapka-Skrzypczak, L.; Jurowski, K. Gold Nanoparticles (AuNPs)—Toxicity, Safety and Green Synthesis: A Critical Review. Int. J. Mol. Sci. 2024, 25, 4057. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Cho, M.; Jeong, J.; Choi, M.; Han, B.S.; Shin, H.S.; Hong, J.; Hyun Chung, B.; Jeong, J.; Cho, M.H. Size-dependent tissue kinetics of PEG-coated gold nanoparticles. Toxicol. Appl. Pharmacol. 2010, 245, 116–123. [Google Scholar]
- Correa Segura, F.; Macías Macías, F.I.; Velázquez Delgado, K.A.; Ramos-Godinez, M.P.; Ruiz-Ramírez, A.; Flores, P.; Huerta-Garcia, E.; Lopez-Marure, R. Food-grade titanium dioxide (E171) and zinc oxide nanoparticles induce mitochondrial permeability and cardiac damage after oral exposure in rats. Nanotoxicology 2024, 18, 122–133. [Google Scholar] [CrossRef]
- Canup, B.; Rogers, P.; Paredes, A.; Manheng, W.; Lyn-Cook, B.; Fahmi, T. Investigation of sex-based differences in the immunotoxicity of silver nanoparticles. Nanotoxicology 2024, 18, 134–159. [Google Scholar] [PubMed]
- He, R.; Ding, X.; Zhang, T.; Mei, L.; Zhu, S.; Wang, C.; Liao, Y.; Wang, D.; Wang, H.; Guo, J.; et al. Study on myocardial toxicity induced by lead halide perovskites nanoparticles. Nanotoxicology 2023, 17, 449–470. [Google Scholar]
- Nagaraju, S.A.; Rao, P.J.; Priyadarshini, P. Assessment of acute and subacute toxicity, pharmacokinetics, and biodistribution of eugenol nanoparticles after oral exposure in Wistar rats. Nanotoxicology 2024, 18, 87–105. [Google Scholar] [CrossRef]
- Falkiewicz, K.; Fryca, I.; Ciura, K.; Mikolajczyk, A.; Jagiello, K.; Puzyn, T. A bibliometric analysis of the recent achievements in pulmonary safety of nanoparticles. Nanotoxicology 2023, 17, 547–561. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Alhadlaq, H.A.; Alrokayan, S.A. Assessment of the lung toxicity of copper oxide nanoparticles: Current status. Nanomedicine 2015, 10, 2365–2377. [Google Scholar]




| Groups | Final Body Weight ± SD (g) | Kidney Weight ± SD (g) | Ratio ± SD (%) | p |
|---|---|---|---|---|
| Standard | 233.3 ± 17.3 | 1.636 ± 0.197 | 0.7 ± 0.06 | |
| Macrogenistein | 214.5 ± 7.1 | 1.750 ± 0.104 | 0.82 a ±0.05 | p ≤ 0.001 |
| Microgenistein | 225.9 ± 13.9 | 1.708 ± 0.082 | 0.76 c ± 0.04 | p ≤ 0.05 |
| Nanogenistein | 221.1 ± 10.4 | 1.745 ± 0.145 | 0.79 b ± 0.07 | p ≤ 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrajnowska, D.; Szterk, A.; Ofiara, K.; Kowalczyk, P.; Bobrowska-Korczak, B. The Genistein Supply and Elemental Composition of Rat Kidneys in an Induced Breast Cancer Model. Nutrients 2025, 17, 1184. https://doi.org/10.3390/nu17071184
Skrajnowska D, Szterk A, Ofiara K, Kowalczyk P, Bobrowska-Korczak B. The Genistein Supply and Elemental Composition of Rat Kidneys in an Induced Breast Cancer Model. Nutrients. 2025; 17(7):1184. https://doi.org/10.3390/nu17071184
Chicago/Turabian StyleSkrajnowska, Dorota, Arkadiusz Szterk, Karol Ofiara, Paweł Kowalczyk, and Barbara Bobrowska-Korczak. 2025. "The Genistein Supply and Elemental Composition of Rat Kidneys in an Induced Breast Cancer Model" Nutrients 17, no. 7: 1184. https://doi.org/10.3390/nu17071184
APA StyleSkrajnowska, D., Szterk, A., Ofiara, K., Kowalczyk, P., & Bobrowska-Korczak, B. (2025). The Genistein Supply and Elemental Composition of Rat Kidneys in an Induced Breast Cancer Model. Nutrients, 17(7), 1184. https://doi.org/10.3390/nu17071184






