A 6-Month mHealth Low-Carbohydrate Dietary Intervention Ameliorates Glycaemic and Cardiometabolic Risk Profile in People with Type 2 Diabetes
Highlights
- An mHealth Low Carbohydrate Diet (LCD) application (Defeat Diabetes app) led to significant improvements in glycaemic control and cardiometabolic risk profile, as well as liver function in people with T2D after 6 months of intervention.
- A reduction in dietary carbohydrates was associated with an improvement in HbA1c by 1.0%.
- The results suggest that the Defeat Diabetes app can provide people with the education, resources, and support to help them reduce their carbohydrate intake and improve HbA1c and other health markers after 6 months of intervention.
- LCD digital apps should be considered as an additional tool that healthcare providers can offer their patients seeking lifestyle modifications to help manage T2D at minimal cost to the healthcare system.
Abstract
1. Introduction
2. Materials and Methods
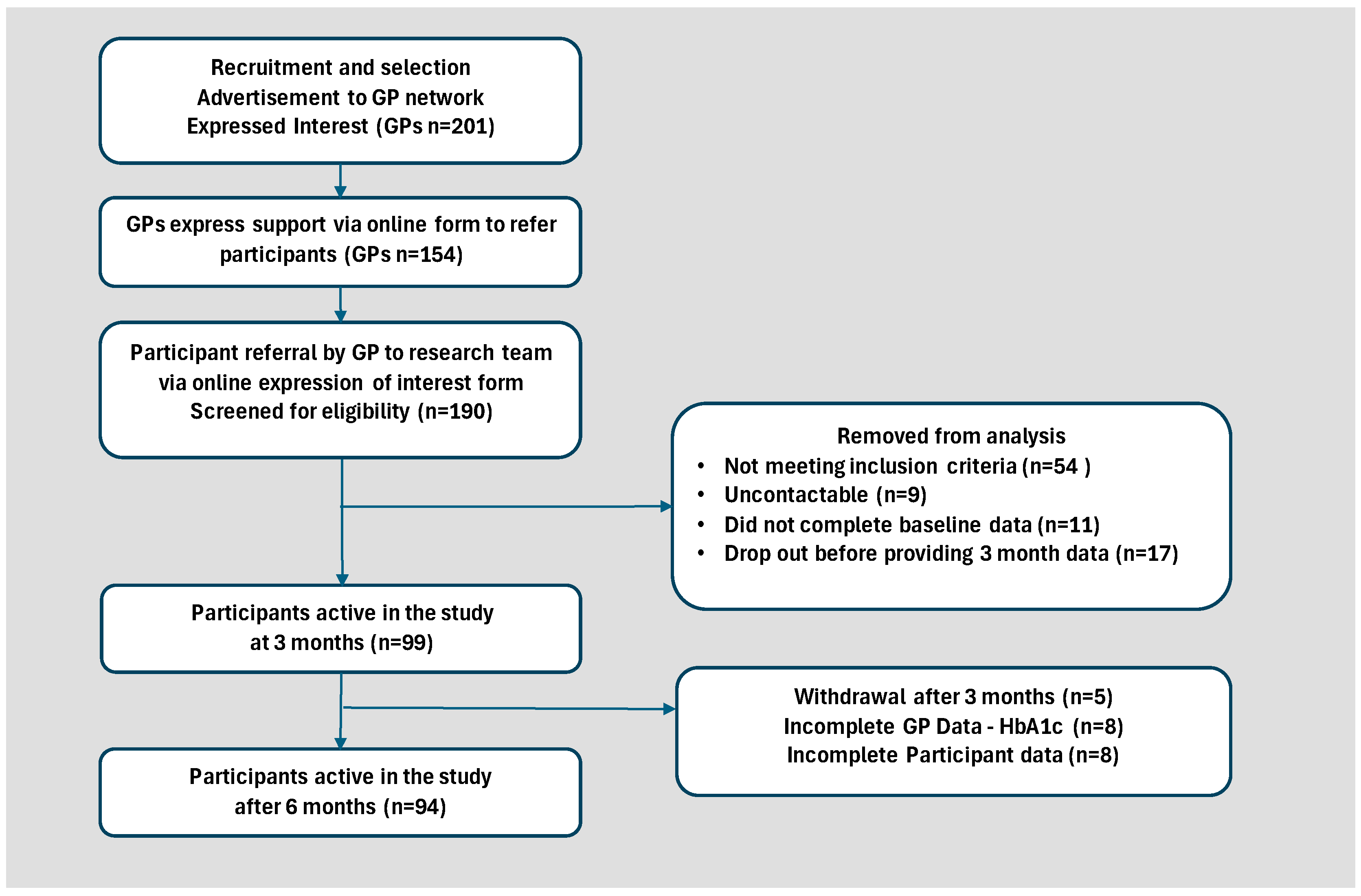
2.1. Study Design and Participants
2.2. Study Intervention
3. Outcomes
3.1. Primary Outcome
3.2. Secondary Outcomes
3.3. Participant Baseline Characteristics
3.4. Dietary Intake and Adherence to the Intervention
3.5. Impact of Physical Activity
3.6. Statistical Analyses
4. Results
4.1. Participant Baseline Characteristics
4.2. Changes in Dietary Intake
4.3. Changes in Physical Activity Levels
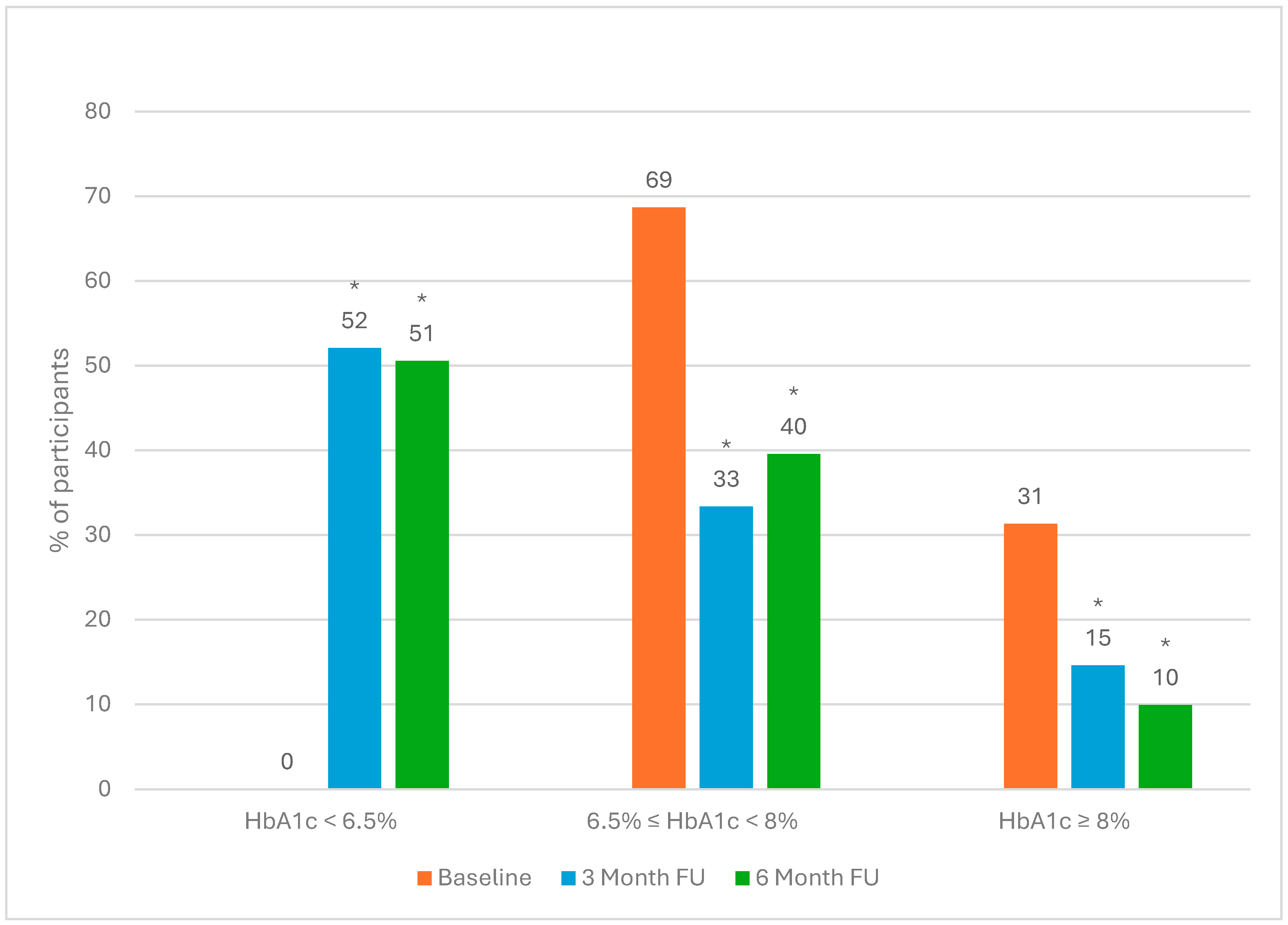
4.4. Changes in Clinical Outcomes
4.5. HbA1c Reduction Grouped by Weight Loss Category After 6 Months of Intervention
4.6. Adherence to the Defeat Diabetes mHealth App and Its Impact on Glycaemic Control and Weight Loss
4.7. Medication Use Following the Intervention
4.8. Adverse Events
4.9. Participant Withdrawals Between 3- and 6-Month Follow-Up Periods
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolivas, D.; Fraser, L.; Schweitzer, R.; Brukner, P.; Moschonis, G. mHealth low-carbohydrate dietary intervention ameliorates glycaemic profile, blood pressure and weight status in people with type 2 diabetes (UNDER REVISION). NPJ Metab. Health Dis. 2025; accepted. [Google Scholar]
- Lawlor-Smith, L.; Stranks, S.N. Managing Type 2 Diabetes with Therapeutic Carbohydrate Reduction (TCR). Available online: https://www.diabetesaustralia.com.au/health-professional-guidelines/ (accessed on 3 February 2025).
- Royal Australian College of General Practitioners. Management of Type 2 Diabetes: A Handbook for General Practice; The Royal Australian College of General Practitioners: Melbourne, VIC, Australia, 2024. [Google Scholar]
- Diabetes Australia. Annual Cycle of Care. Available online: https://www.diabetesaustralia.com.au/managing-diabetes/annual-cycle-of-care/ (accessed on 3 February 2025).
- Commonwealth of Australia as Represented by the Department of Health and Aged Care. AusCVDRisk. Available online: https://www.cvdcheck.org.au/ (accessed on 3 February 2025).
- Magnussen, C.; Ojeda, F.M.; Leong, D.P.; Alegre-Diaz, J.; Amouyel, P.; Aviles-Santa, L.; De Bacquer, D.; Ballantyne, C.M.; Bernabé-Ortiz, A.; Bobak, M.; et al. Global Effect of Modifiable Risk Factors on Cardiovascular Disease and Mortality. N. Engl. J. Med. 2023, 389, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 11. Chronic Kidney Disease and Risk Management: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S175–S184. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Sookoian, S. From NAFLD to MASLD: Updated naming and diagnosis criteria for fatty liver disease. J. Lipid Res. 2024, 65, 100485. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.H. Elevated Liver Function Tests in Type 2 Diabetes. Clin. Diabetes 2005, 23, 115–119. [Google Scholar] [CrossRef]
- Ballestri, S.; Zona, S.; Targher, G.; Romagnoli, D.; Baldelli, E.; Nascimbeni, F.; Roverato, A.; Guaraldi, G.; Lonardo, A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016, 31, 936–944. [Google Scholar] [CrossRef]
- Cho, N.H.; Jang, H.C.; Choi, S.H.; Kim, H.R.; Lee, H.K.; Chan, J.C.N.; Lim, S. Abnormal Liver Function Test Predicts Type 2 Diabetes. Diabetes Care 2007, 30, 2566–2568. [Google Scholar] [CrossRef]
- Al-Jameil, N.; Khan, F.A.; Arjumand, S.; Khan, M.F.; Tabassum, H. Associated liver enzymes with hyperlipidemic profile in type 2 diabetes patients. Int. J. Clin. Exp. Pathol. 2014, 7, 4345–4349. [Google Scholar]
- Rock, C.L.; Flatt, S.W.; Pakiz, B.; Taylor, K.S.; Leone, A.F.; Brelje, K.; Heath, D.D.; Quintana, E.L.; Sherwood, N.E. Weight Loss, Glycemic Control, and Cardiovascular Disease Risk Factors in Response to Differential Diet Composition in a Weight Loss Program in Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2014, 37, 1573–1580. [Google Scholar] [CrossRef]
- Tay, J.; Luscombe-Marsh, N.D.; Thompson, C.H.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S.; Brinkworth, G.D. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: A randomized trial. Am. J. Clin. Nutr. 2015, 102, 780–790. [Google Scholar] [CrossRef]
- Snorgaard, O.; Poulsen, G.M.; Andersen, H.K.; Astrup, A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2017, 5, e000354. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, J.Z.; Day, A.; Brinkworth, G.D.; Sato, J.; Yamada, S.; Jönsson, T.; Beardsley, J.; Johnson, J.A.; Thabane, L.; Johnston, B.C. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: Systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 2021, 372, m4743. [Google Scholar] [CrossRef] [PubMed]
- Riddle, M.C.; Cefalu, W.T.; Evans, P.H.; Gerstein, H.C.; Nauck, M.A.; Oh, W.K.; Rothberg, A.E.; le Roux, C.W.; Rubino, F.; Schauer, P.; et al. Consensus Report: Definition and Interpretation of Remission in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2022, 107, 1–9. [Google Scholar] [CrossRef]
- Soltani, S.; Jayedi, A.; Abdollahi, S.; Vasmehjani, A.A.; Meshkini, F.; Shab-Bidar, S. Effect of carbohydrate restriction on body weight in overweight and obese adults: A systematic review and dose–response meta-analysis of 110 randomized controlled trials. Front. Nutr. 2023, 10, 1287987. [Google Scholar] [CrossRef]
- Unwin, D.; Delon, C.; Unwin, J.; Tobin, S.; Taylor, R. What predicts drug-free type 2 diabetes remission? Insights from an 8-year general practice service evaluation of a lower carbohydrate diet with weight loss. BMJ Nutr. Prev. Health 2023, 6, 46–55. [Google Scholar] [CrossRef]
- Athinarayanan, S.J.; Adams, R.N.; Hallberg, S.J.; McKenzie, A.L.; Bhanpuri, N.H.; Campbell, W.W.; Volek, J.S.; Phinney, S.D.; McCarter, J.P. Long-Term Effects of a Novel Continuous Remote Care Intervention Including Nutritional Ketosis for the Management of Type 2 Diabetes: A 2-Year Non-randomized Clinical Trial. Front. Endocrinol. 2019, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Charlot, A.; Zoll, J. Beneficial Effects of the Ketogenic Diet in Metabolic Syndrome: A Systematic Review. Diabetology 2022, 3, 292–309. [Google Scholar] [CrossRef]
- Gavidia, K.; Kalayjian, T. Treating Diabetes Utilizing a Low Carbohydrate Ketogenic Diet and Intermittent Fasting Without Significant Weight Loss: A Case Report. Front. Nutr. 2021, 8, 687081. [Google Scholar] [CrossRef]
- Hawkins, M.A.; Zinn, C.; Delon, C. The application of carbohydrate-reduction in general practice: A medical audit. J. Metab. Health 2023, 6, 11. [Google Scholar] [CrossRef]
- O’Neill, B.J. Effect of low-carbohydrate diets on cardiometabolic risk, insulin resistance, and metabolic syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 301–307. [Google Scholar] [CrossRef]
- Rafiullah, M.; Musambil, M.; David, S.K. Effect of a very low-carbohydrate ketogenic diet vs recommended diets in patients with type 2 diabetes: A meta-analysis. Nutr. Rev. 2022, 80, 488–502. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, X.; Chen, L.; Chen, T.; Yu, J.; Wu, C.; Zheng, J. Ketogenic Diets and Cardio-Metabolic Diseases. Front. Endocrinol. 2021, 12, 753039. [Google Scholar] [CrossRef]
- Oyabu, C.; Hashimoto, Y.; Fukuda, T.; Tanaka, M.; Asano, M.; Yamazaki, M.; Fukui, M. Impact of low-carbohydrate diet on renal function: A meta-analysis of over 1000 individuals from nine randomised controlled trials. Br. J. Nutr. 2016, 116, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Unwin, D.; Unwin, J.; Crocombe, D.; Delon, C.; Guess, N.; Wong, C. Renal function in patients following a low carbohydrate diet for type 2 diabetes: A review of the literature and analysis of routine clinical data from a primary care service over 7 years. Curr. Opin. Endocrinol. Diabetes Obes 2021, 28, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.M.; Guzman, G.; Correa De Mello, L.L.; Trein, B.; Spina, L.; Bussade, I.; Marques Prata, J.; Sajoux, I.; Countinho, W. Efficacy of a 2-Month Very Low-Calorie Ketogenic Diet (VLCKD) Compared to a Standard Low-Calorie Diet in Reducing Visceral and Liver Fat Accumulation in Patients with Obesity. Front. Endocrinol. 2020, 11, 607. [Google Scholar] [CrossRef]
- Unwin, D.; Unwin, J. Low carbohydrate diet to achieve weight loss and improve HbA1c in type 2 diabetes and pre-diabetes: Experience from one general practice. Pract. Diabetes 2014, 31, 76–79. [Google Scholar] [CrossRef]
- Ryan, M.C.; Abbasi, F.; Lamendola, C.; Carter, S.; McLaughlin, T.L. Serum Alanine Aminotransferase Levels Decrease Further With Carbohydrate Than Fat Restriction in Insulin-Resistant Adults. Diabetes Care 2007, 30, 1075–1080. [Google Scholar] [CrossRef]
- Willett, W.C.; Leibel, R.L. Dietary fat is not a major determinant of body fat. Am. J. Med. 2002, 113 (Suppl. 9B), 47s–59s. [Google Scholar] [CrossRef]
- Ashwell, M.; Gibson, S. Waist-to-height ratio as an indicator of ‘early health risk’: Simpler and more predictive than using a ‘matrix’ based on BMI and waist circumference. BMJ Open 2016, 6, e010159. [Google Scholar] [CrossRef]
- Ke, J.-F.; Wang, J.-W.; Lu, J.-X.; Zhang, Z.-H.; Liu, Y.; Li, L.-X. Waist-to-height ratio has a stronger association with cardiovascular risks than waist circumference, waist-hip ratio and body mass index in type 2 diabetes. Diabetes Res. Clin. Pract. 2022, 183, 109151. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, X.; Lo, K.; Huang, Y.; Feng, Y. The effect of total cholesterol/high-density lipoprotein cholesterol ratio on mortality risk in the general population. Front. Endocrinol. 2022, 13, 1012383. [Google Scholar] [CrossRef]
- Yang, M.; Rigdon, J.; Tsai, S.A. Association of triglyceride to HDL cholesterol ratio with cardiometabolic outcomes. J. Investig. Med. 2019, 67, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, C.E.; Rodriguez Polanco, S.; Bousvarou, M.D.; Papakonstantinou, E.J.; Peña Genao, E.; Guzman, E.; Kostara, C.E. The Triglyceride/High-Density Lipoprotein Cholesterol (TG/HDL-C) Ratio as a Risk Marker for Metabolic Syndrome and Cardiovascular Disease. Diagnostics 2023, 13, 929. [Google Scholar] [CrossRef]
- Flores-Guerrero, J.L.; Been, R.A.; Shalaurova, I.; Connelly, M.A.; van Dijk, P.R.; Dullaart, R.P.F. Triglyceride/HDL cholesterol ratio and lipoprotein insulin resistance Score: Associations with subclinical atherosclerosis and incident cardiovascular disease. Clin. Chim. Acta 2024, 553, 117737. [Google Scholar] [CrossRef] [PubMed]
- Che, B.; Zhong, C.; Zhang, R.; Pu, L.; Zhao, T.; Zhang, Y.; Han, L. Triglyceride-glucose index and triglyceride to high-density lipoprotein cholesterol ratio as potential cardiovascular disease risk factors: An analysis of UK biobank data. Cardiovasc. Diabetol. 2023, 22, 34. [Google Scholar] [CrossRef]
- European Society of Cardiology. TG/HDL Ratio as Surrogate Marker for Insulin Resistance. Available online: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-8/TG-HDL-ratio-as-surrogate-marker-for-insulin-resistance (accessed on 3 February 2025).
- Lelis, D.d.F.; Calzavara, J.V.S.; Santos, R.D.; Sposito, A.C.; Griep, R.H.; Barreto, S.M.; Molina, M.d.C.B.; Schmidt, M.I.; Duncan, B.B.; Bensenor, I.; et al. Reference values for the triglyceride to high-density lipoprotein ratio and its association with cardiometabolic diseases in a mixed adult population: The ELSA-Brasil study. J. Clin. Lipidol. 2021, 15, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Moschonis, G.; Siopis, G.; Jung, J.; Eweka, E.; Willems, R.; Kwasnicka, D.; Asare, B.Y.-A.; Kodithuwakku, V.; Verhaeghe, N.; Vedanthan, R.; et al. Effectiveness, reach, uptake, and feasibility of digital health interventions for adults with type 2 diabetes: A systematic review and meta-analysis of randomised controlled trials. Lancet Digit. Health 2023, 5, e125–e143. [Google Scholar] [CrossRef]
- Kolivas, D.; Fraser, L.; Schweitzer, R.; Brukner, P.; Moschonis, G. Effectiveness of a Digitally Delivered Continuous Care Intervention (Defeat Diabetes) on Type 2 Diabetes Outcomes: A 12-Month Single-Arm, Pre-Post Intervention Study. Nutrients 2023, 15, 2153. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Defeat Diabetes. Available online: https://www.defeatdiabetes.com.au/ (accessed on 3 February 2025).
- Australian Government Department of Health. MBS Online Medicare Benefits Schedule. Available online: http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Home (accessed on 3 February 2025).
- Babic, N.; Valjevac, A.; Zaciragic, A.; Avdagic, N.; Zukic, S.; Hasic, S. The Triglyceride/HDL Ratio and Triglyceride Glucose Index as Predictors of Glycemic Control in Patients with Diabetes Mellitus Type 2. Med. Arch. 2019, 73, 163. [Google Scholar] [CrossRef] [PubMed]
- Jamar, G.; Almeida, F.R.D.; Gagliardi, A.; Sobral, M.R.; Ping, C.T.; Sperandio, E.; Romiti, M.; Arantes, R.; Dourado, V.Z. Evaluation of waist-to-height ratio as a predictor of insulin resistance in non-diabetic obese individuals. A cross-sectional study. Sao Paulo Med. J. 2017, 135, 462–468. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Obesity: Identification, Assessment and Management. Available online: https://www.nice.org.uk/guidance/cg189/chapter/Recommendations (accessed on 3 February 2025).
- Xyris. FoodWorks Professional 10; Xyris Software (Australia) Pty Ltd.: Brisbane City, Australia, 2020. [Google Scholar]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.; Stewart, S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Dening, J.; Mohebbi, M.; Abbott, G.; George, E.S.; Ball, K.; Islam, S.M.S. A web-based low carbohydrate diet intervention significantly improves glycaemic control in adults with type 2 diabetes: Results of the T2Diet Study randomised controlled trial. Nutr. Diabetes 2023, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Flores-Hernández, M.N.; Martínez-Coria, H.; López-Valdés, H.E.; Arteaga-Silva, M.; Arrieta-Cruz, I.; Gutiérrez-Juárez, R. Efficacy of a High-Protein Diet to Lower Glycemic Levels in Type 2 Diabetes Mellitus: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 10959. [Google Scholar] [CrossRef]
- Morell, P.; Fiszman, S. Revisiting the role of protein-induced satiation and satiety. Food Hydrocoll. 2017, 68, 199–210. [Google Scholar] [CrossRef]
- Samra, R.A. Fat Detection: Taste, Texture, and Post Ingestive Effects. In Fats and Satiety; Montmayeur, J.P., le Coutre, J., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010; Chapter 15; Available online: https://www.ncbi.nlm.nih.gov/books/NBK53550/ (accessed on 3 February 2025).
- Offringa, L.C.; Hartle, J.C.; Rigdon, J.; Gardner, C.D. Changes in Quantity and Sources of Dietary Fiber from Adopting Healthy Low-Fat vs. Healthy Low-Carb Weight Loss Diets: Secondary Analysis of DIETFITS Weight Loss Diet Study. Nutrients 2021, 13, 3625. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Cardiovascular Disease: Risk Assessment and Reduction, Including Lipid Modification. Available online: https://www.nice.org.uk/guidance/ng238?UID=3279581122023122671124 (accessed on 3 February 2025).
- McBride, P. Triglycerides and risk for coronary artery disease. Curr. Atheroscler. Rep. 2008, 10, 386–390. [Google Scholar] [CrossRef]
- Bhanpuri, N.H.; Hallberg, S.J.; Williams, P.T.; McKenzie, A.L.; Ballard, K.D.; Campbell, W.W.; McCarter, J.P.; Phinney, S.D.; Volek, J.S. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: An open label, non-randomized, controlled study. Cardiovasc. Diabetol. 2018, 17, 56. [Google Scholar] [CrossRef]
- Glandt, M.; Ailon, N.Y.; Berger, S.; Unwin, D. Use of a very low carbohydrate diet for prediabetes and type 2 diabetes: An audit. J. Metab. Health 2024, 7, a87. [Google Scholar] [CrossRef]
- Unwin, D.J.; Cuthbertson, D.J.; Feinman, R.D.; Sprung, V.S. A pilot study to explore the role of a low-carbohydrate intervention to improve GGT levels and HbA 1c. Diabesity Pract. 2015, 4, 102. [Google Scholar]
- Ghasemi, P.; Jafari, M.; Maskouni, S.J.; Hosseini, S.A.; Amiri, R.; Hejazi, J.; Chambari, M.; Tavasolian, R.; Rahimlou, M. Impact of very low carbohydrate ketogenic diets on cardiovascular risk factors among patients with type 2 diabetes; GRADE-assessed systematic review and meta-analysis of clinical trials. Nutr. Metab. 2024, 21, 50. [Google Scholar] [CrossRef]
- Tay, J.; Thompson, C.H.; Luscombe-Marsh, N.D.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Brinkworth, G.D. Long-Term Effects of a Very Low Carbohydrate Compared with a High Carbohydrate Diet on Renal Function in Individuals with Type 2 Diabetes: A Randomized Trial. Medicine 2015, 94, e2181. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Sithamparapillai, A.; Brimble, K.S.; Banfield, L.; Morton, R.W.; Phillips, S.M. Changes in Kidney Function Do Not Differ between Healthy Adults Consuming Higher- Compared with Lower- or Normal-Protein Diets: A Systematic Review and Meta-Analysis. J. Nutr. 2018, 148, 1760–1775. [Google Scholar] [CrossRef]
- Zainordin, N.A.; Eddy Warman, N.A.; Mohamad, A.F.; Abu Yazid, F.A.; Ismail, N.H.; Chen, X.W.; Koshy, M.; Abdul Rahman, T.H.; Mohd Ismail, N.; Abdul Ghani, R. Safety and efficacy of very low carbohydrate diet in patients with diabetic kidney disease—A randomized controlled trial. PLoS ONE 2021, 16, e0258507. [Google Scholar] [CrossRef]
- Tirosh, A.; Golan, R.; Harman-Boehm, I.; Henkin, Y.; Schwarzfuchs, D.; Rudich, A.; Kovsan, J.; Fiedler, G.M.; Blüher, M.; Stumvoll, M.; et al. Renal Function Following Three Distinct Weight Loss Dietary Strategies During 2 Years of a Randomized Controlled Trial. Diabetes Care 2013, 36, 2225–2232. [Google Scholar] [CrossRef]
- Cheng, Y.; Zheng, G.; Song, Z.; Zhang, G.; Rao, X.; Zeng, T. Association between dietary protein intake and risk of chronic kidney disease: A systematic review and meta-analysis. Front. Nutr. 2024, 11, 1408424. [Google Scholar] [CrossRef]
- Carballo-Casla, A.; Avesani, C.M.; Beridze, G.; Ortolá, R.; García-Esquinas, E.; Lopez-Garcia, E.; Dai, L.; Dunk, M.M.; Stenvinkel, P.; Lindholm, B.; et al. Protein Intake and Mortality in Older Adults with Chronic Kidney Disease. JAMA Netw. Open 2024, 7, e2426577. [Google Scholar] [CrossRef]
- Nam, K.H.; An, S.Y.; Joo, Y.S.; Lee, S.; Yun, H.-R.; Jhee, J.H.; Han, S.H.; Yoo, T.-H.; Kang, S.-W.; Park, J.T. Carbohydrate-Rich Diet Is Associated with Increased Risk of Incident Chronic Kidney Disease in Non-Diabetic Subjects. J. Clin. Med. 2019, 8, 793. [Google Scholar] [CrossRef]
- Athinarayanan, S.J.; Roberts, C.G.P.; Vangala, C.; Shetty, G.K.; McKenzie, A.L.; Weimbs, T.; Volek, J.S. The case for a ketogenic diet in the management of kidney disease. BMJ Open Diabetes Res. Care 2024, 12, e004101. [Google Scholar] [CrossRef]
- Cameron, A.J.; Dunstan, D.W.; Owen, N.; Zimmet, P.Z.; Barr, E.L.M.; Tonkin, A.M.; Magliano, D.J.; Murray, S.G.; Welborn, T.A.; Shaw, J.E. Health and mortality consequences of abdominal obesity: Evidence from the AusDiab study. Med. J. Aust. 2009, 191, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Stranges, S.; Dorn, J.M.; Muti, P.; Freudenheim, J.L.; Farinaro, E.; Russell, M.; Nochajski, T.H.; Trevisan, M. Body fat distribution, relative weight, and liver enzyme levels: A population-based study. Hepatology 2004, 39, 754–763. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, B.I.; Kim, H.J.; Kim, H.; Kim, S.H.; Park, D.I.; Cho, Y.K.; Sung, I.K.; Sohn, C.I.; Jeon, W.K.; et al. Clinical features associated with improvement of fatty liver disease. Intern. Med. J. 2005, 35, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Sam, S.; Haffner, S.; Davidson, M.H.; D’Agostino, R.B.; Feinstein, S.; Kondos, G.; Perez, A.; Mazzone, T. Hypertriglyceridemic Waist Phenotype Predicts Increased Visceral Fat in Subjects with Type 2 Diabetes. Diabetes Care 2009, 32, 1916–1920. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.P.; Su, Z.J.; Jiang, J.; Sun, B.Q.; Liu, Z.Z.; Ceng, Z.X. Correlation of severity of non-alcoholic fatty liver disease with visceral adipose tissue area, body mass index, and waist circumference. J. Clin. Hepatol. 2013, 29, 445–448. [Google Scholar]
- Kelley, D.E.; McKolanis, T.M.; Hegazi, R.A.F.; Kuller, L.H.; Kalhan, S.C. Fatty liver in type 2 diabetes mellitus: Relation to regional adiposity, fatty acids, and insulin resistance. Am. J. Physiol.-Endocrinol. Metab. 2003, 285, E906–E916. [Google Scholar] [CrossRef]
- John, J.; Sakarde, A.; Chafle, J.; Amle, D.; Jose, J.; Sakhare, V.; Rathod, B.D. An Assessment of the Utility of Serum Fructosamine in the Diagnosis and Monitoring of Diabetes Mellitus. Cureus 2023, 15, e33549. [Google Scholar] [CrossRef]
- Abbasi, F.; Shiffman, D.; Tong, C.H.; Devlin, J.J.; McPhaul, M.J. Insulin Resistance Probability Scores for Apparently Healthy Individuals. J. Endocr. Soc. 2018, 2, 1050–1057. [Google Scholar] [CrossRef]
- Del Rio Szupszynski, K.P.; De Ávila, A.C. The Transtheoretical Model of Behavior Change: Prochaska and DiClemente’s Model; Springer International Publishing: Cham, Switzerland, 2021; pp. 205–216. [Google Scholar]
- U.K. Prospective Diabetes Study Group. U.K. Prospective Diabetes Study 16: Overview of 6 Years’ Therapy of Type II Diabetes: A Progressive Disease. Diabetes 1995, 44, 1249–1258. [Google Scholar] [CrossRef]
| Total Sample | Male | Female | p Value | |
|---|---|---|---|---|
| (n = 99) | (n = 44) | (n = 55) | ||
| Socio-demographics | ||||
| Age (years) (Mean (SD)) | 58.4 (11.3) | 57.6 (11.2) | 59.1 (11.4) | 0.518 |
| Education Level (%) | 0.636 | |||
| Up to Secondary | 34.3 | 31.8 | 36.4 | |
| Higher education | 65.7 | 68.2 | 63.6 | |
| Country of Birth (%) | 0.129 | |||
| Australia | 60.6 | 52.3 | 67.3 | |
| Overseas | 39.4 | 47.7 | 32.7 | |
| Employment Status (%) | 0.031 | |||
| Unemployed | 5.1 | 0 | 9.3 | |
| Casual/Part-time/Full-time | 63.3 | 75.0 | 53.7 * | |
| Retired | 31.6 | 25.0 | 37.0 * | |
| No. of People in Household (%) | 0.637 | |||
| One person | 18.2 | 16.3 | 20.0 | |
| Two or more people | 80.8 | 83.7 | 80.0 | |
| Years with Type 2 Diabetes (Median (IQR)) | 2.9 (6.0) | 2.0 (5.1) | 4.0 (7.0) | 0.017 |
| Time since diagnosis (%) | 0.003 | |||
| Up to six years | 68.7 | 79.5 | 60.0 | |
| Six or more years | 31.3 | 20.5 | 40.0 | |
| Co-existing medical conditions (%) | 0.387 | |||
| None | 22.2 | 18.2 | 25.5 | |
| One or more | 77.8 | 81.8 | 74.5 | |
| Diabetes Medications (%) | 0.485 | |||
| No Medication | 28.3 | 31.8 | 25.5 | |
| Medications | 71.7 | 68.2 | 74.5 | |
| Antihypertensive Medications (%) | 0.857 | |||
| No Medication | 46.5 | 45.5 | 47.3 | |
| Medications | 53.5 | 54.5 | 52.7 | |
| Cholesterol-Lowering Medications (%) | 0.821 | |||
| No Medication | 44.4 | 43.2 | 45.5 | |
| Medications | 55.6 | 56.8 | 54.5 | |
| IPAQ Activity Level (%) | 0.495 | |||
| Low | 26.3 | 20.5 | 30.9 | |
| Medium | 41.4 | 45.5 | 38.2 | |
| High | 32.3 | 34.1 | 30.9 |
| Baseline | 6-Month Follow-Up | 6-Month Change | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dietary Intake of | n | Mean | SD | n | Mean | SD | Mean Change | (95% CI) Lower | (95% CI) Upper | p Value |
| Energy (kJ/day) | ||||||||||
| Total Sample | 99 | 7969 | 2378 | 91 | 6667 | 1869 | −1301 | −1917 | −685 | <0.001 |
| Males | 44 | 8791 | 2357 | 37 | 7542 | 1857 | −1249 | −2201 | −297 | 0.01 |
| Females | 55 | 7311 | 2202 | 54 | 6068 | 1640 | −1243 | −1981 | −504 | 0.00 |
| CHO (%kJ/day) | ||||||||||
| Total Sample | 99 | 32 | 10 | 91 | 18 | 10 | −14 | −17 | −11 | <0.001 |
| Males | 44 | 29 | 12 | 37 | 17 | 9 | −12 | −17 | −7 | <0.001 |
| Females | 55 | 34 | 8 | 54 | 19 | 10 | −16 | −19 | −12 | <0.001 |
| Protein (%kJ/day) | ||||||||||
| Total Sample | 99 | 22 | 6 | 91 | 28 | 7 | 6 | 4 | 8 | <0.001 |
| Males | 44 | 23 | 7 | 37 | 29 | 7 | 6 | 2 | 9 | <0.001 |
| Females | 55 | 21 | 4 | 54 | 27 | 8 | 6 | 4 | 8 | <0.001 |
| Total fat (%kJ/day) | ||||||||||
| Total Sample | 99 | 40 | 7 | 91 | 49 | 9 | 9 | 6 | 11 | <0.001 |
| Males | 44 | 41 | 8 | 37 | 48 | 8 | 7 | 4 | 11 | <0.001 |
| Females | 55 | 40 | 7 | 54 | 49 | 9 | 10 | 6 | 13 | <0.001 |
| SAT fat (%kJ/day) | ||||||||||
| Total Sample | 99 | 14 | 4 | 91 | 18 | 5 | 4 | 3 | 5 | <0.001 |
| Males | 44 | 13 | 3 | 37 | 17 | 4 | 4 | 2 | 5 | <0.000 |
| Females | 55 | 15 | 5 | 54 | 19 | 5 | 4 | 2 | 6 | <0.001 |
| MONO fat (%fat/day) | ||||||||||
| Total Sample | 99 | 44 | 7 | 91 | 44 | 6 | 0 | −1 | 2 | 0.71 |
| Males | 44 | 46 | 6 | 37 | 46 | 6 | 0 | −3 | 3 | 0.94 |
| Females | 55 | 42 | 7 | 54 | 43 | 6 | 1 | −2 | 3 | 0.60 |
| POLY fat (%fat/day) | ||||||||||
| Total Sample | 99 | 17 | 6 | 91 | 14 | 5 | −3 | −4 | −1 | <0.001 |
| Males | 44 | 19 | 6 | 37 | 15 | 4 | −4 | −6 | −2 | <0.001 |
| Females | 55 | 16 | 5 | 54 | 14 | 5 | −2 | −4 | 0 | 0.04 |
| Fibre (g/day) | ||||||||||
| Total Sample | 99 | 21 | 8 | 91 | 19 | 8 | −3 | −5 | 0 | 0.02 |
| Males | 44 | 23 | 9 | 37 | 19 | 9 | −4 | −8 | 0 | 0.07 |
| Females | 55 | 20 | 7 | 54 | 18 | 8 | −2 | −5 | 1 | 0.17 |
| Baseline | 6-Month Follow-Up | 6-Month Change | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | Mean Change | (95% CI) Lower | (95% CI) Upper | p Value | |
| Diabetes blood markers | ||||||||||
| HbA1c % | ||||||||||
| Total Sample | 99 | 7.7 | 1.3 | 91 | 6.8 | 0.9 | −1.0 | −1.3 | −0.6 | <0.001 |
| Males | 44 | 7.9 | 1.3 | 38 | 6.8 | 0.9 | −1.2 | −1.7 | −0.7 | <0.001 |
| Females | 55 | 7.6 | 1.3 | 53 | 6.7 | 1.0 | −0.8 | −1.2 | −0.4 | <0.001 |
| Fasting plasma glucose mmol/L | ||||||||||
| Total Sample | 93 | 8.6 | 2.7 | 81 | 7.4 | 1.8 | −1.3 | −2.0 | −0.6 | <0.001 |
| Males | 41 | 8.3 | 2.2 | 30 | 7.2 | 1.6 | −1.0 | −2.0 | −0.1 | 0.04 |
| Females | 52 | 8.9 | 3.1 | 51 | 7.5 | 1.9 | −1.4 | −2.4 | −0.4 | 0.01 |
| Liver enzymes | ||||||||||
| ALT U/L | ||||||||||
| Total Sample | 97 | 39.7 | 28.8 | 86 | 30.2 | 16.4 | −9.3 | −16.3 | −2.4 | 0.01 |
| Males | 43 | 40.1 | 28.5 | 36 | 31.5 | 18.5 | −8.0 | −18.8 | 2.7 | 0.14 |
| Females | 54 | 39.4 | 29.4 | 50 | 29.2 | 14.8 | −10.2 | −19.4 | −1.0 | 0.03 |
| GGT U/L | ||||||||||
| Total Sample | 95 | 53.3 | 54.0 | 86 | 34.3 | 24.9 | −18.8 | −31.4 | −6.3 | 0.00 |
| Males | 42 | 58.3 | 59.2 | 36 | 37.2 | 24.5 | −21.4 | −42.6 | −0.3 | 0.05 |
| Females | 53 | 49.4 | 49.8 | 50 | 32.2 | 25.2 | −17.4 | −32.9 | −1.9 | 0.03 |
| Waist circumference cm | ||||||||||
| Total Sample | 96 | 113.6 | 15.3 | 86 | 108.8 | 14.6 | −4.6 | −8.9 | −0.2 | 0.04 |
| Males | 42 | 114.7 | 15.4 | 33 | 112.6 | 16.0 | −2.2 | −9.5 | 5.1 | 0.55 |
| Females | 54 | 112.8 | 15.3 | 53 | 106.5 | 13.2 | −6.3 | −11.7 | −0.9 | 0.02 |
| Cardiometabolic risk ratios | ||||||||||
| Total Cholesterol/HDL-c | ||||||||||
| Total Sample | 98 | 4.16 | 1.26 | 85 | 3.76 | 1.23 | −0.38 | −0.70 | −0.05 | 0.03 |
| Males | 44 | 4.29 | 1.30 | 37 | 3.93 | 1.26 | −0.28 | −0.78 | 0.22 | 0.26 |
| Females | 54 | 4.06 | 1.24 | 48 | 3.62 | 1.21 | −0.44 | −0.89 | 0.00 | 0.52 |
| TRIG/HDL-c (mmol/L/mmol/L) | ||||||||||
| Total Sample | 98 | 2.00 | 1.74 | 85 | 1.48 | 1.18 | −0.50 | −0.92 | −0.08 | 0.02 |
| Males | 44 | 2.45 | 2.27 | 37 | 1.72 | 1.45 | −0.70 | −1.56 | 0.16 | 0.11 |
| Females | 54 | 1.64 | 1.03 | 48 | 1.29 | 0.90 | −0.35 | −0.70 | 0.00 | 0.05 |
| Waist to Height ratio | ||||||||||
| Total Sample | 96 | 0.67 | 0.09 | 86 | 0.65 | 0.08 | −0.02 | −0.05 | 0.00 | 0.05 |
| Males | 42 | 0.65 | 0.09 | 33 | 0.65 | 0.09 | −0.01 | −0.05 | 0.04 | 0.81 |
| Females | 54 | 0.69 | 0.08 | 53 | 0.65 | 0.07 | −0.04 | −0.07 | −0.01 | 0.01 |
| Δ HbA1c (%) | |||||||
|---|---|---|---|---|---|---|---|
| ≥5% of Weight Loss | <5% of Weight Loss (Including Weight Gain) | ||||||
| n | Mean Change | SD | n | Mean Change | SD | p Value | |
| Total Sample | 39 | −1.2 | 1.1 | 50 | −0.7 | 1.3 | 0.047 |
| Males | 12 | −1.3 | 0.9 | 24 | −1.2 | 1.6 | 0.907 |
| Females | 27 | −1.2 | 1.2 | 26 | −0.3 | 0.7 | <0.001 |
| 6-Month Change | |||||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | Mean Change | (95% CI) Lower | (95% CI) Upper | p Value | |
| Δ HbA1c (%) | −0.7 | −1.2 | −0.1 | 0.017 | |||
| ≤50 g CHO per day | 30 | −1.4 | 1.4 | ||||
| >50 g CHO per day | 56 | −0.7 | 1.1 | ||||
| Weight Loss (kg) | −4.2 | −6.2 | −2.2 | <0.001 | |||
| ≤50 g CHO per day | 31 | −8.0 | 5.2 | ||||
| >50 g CHO per day | 56 | −3.8 | 4.1 | ||||
| Male | |||||||
| Δ HbA1c (%) | −0.5 | −1.6 | 0.6 | 0.387 | |||
| ≤50 g CHO per day | 10 | −1.6 | 1.7 | ||||
| >50 g CHO per day | 24 | −1.1 | 1.3 | ||||
| Weight Loss (kg) | −3.8 | −7.4 | −0.1 | 0.044 | |||
| ≤50 g CHO per day | 11 | −7.7 | 6.2 | ||||
| >50 g CHO per day | 24 | −3.9 | 4.3 | ||||
| Female | |||||||
| Δ HbA1c (%) | −0.9 | −1.5 | −0.3 | 0.005 | |||
| ≤50 g CHO per day | 20 | −1.3 | 1.3 | ||||
| >50 g CHO per day | 32 | −0.4 | 0.8 | ||||
| Weight Loss (kg) | −4.5 | −7.0 | −2.1 | <0.001 | |||
| ≤50 g CHO per day | 20 | −8.2 | 4.6 | ||||
| >50 g CHO per day | 32 | −3.7 | 4.0 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolivas, D.; Fraser, L.; Schweitzer, R.; Brukner, P.; Moschonis, G. A 6-Month mHealth Low-Carbohydrate Dietary Intervention Ameliorates Glycaemic and Cardiometabolic Risk Profile in People with Type 2 Diabetes. Nutrients 2025, 17, 937. https://doi.org/10.3390/nu17060937
Kolivas D, Fraser L, Schweitzer R, Brukner P, Moschonis G. A 6-Month mHealth Low-Carbohydrate Dietary Intervention Ameliorates Glycaemic and Cardiometabolic Risk Profile in People with Type 2 Diabetes. Nutrients. 2025; 17(6):937. https://doi.org/10.3390/nu17060937
Chicago/Turabian StyleKolivas, Despina, Liz Fraser, Ronald Schweitzer, Peter Brukner, and George Moschonis. 2025. "A 6-Month mHealth Low-Carbohydrate Dietary Intervention Ameliorates Glycaemic and Cardiometabolic Risk Profile in People with Type 2 Diabetes" Nutrients 17, no. 6: 937. https://doi.org/10.3390/nu17060937
APA StyleKolivas, D., Fraser, L., Schweitzer, R., Brukner, P., & Moschonis, G. (2025). A 6-Month mHealth Low-Carbohydrate Dietary Intervention Ameliorates Glycaemic and Cardiometabolic Risk Profile in People with Type 2 Diabetes. Nutrients, 17(6), 937. https://doi.org/10.3390/nu17060937







