In Vivo Evidence on the Emerging Potential of Non-Digestible Oligosaccharides as Therapeutic Agents in Bacterial and Viral Infections
Abstract
1. Introduction
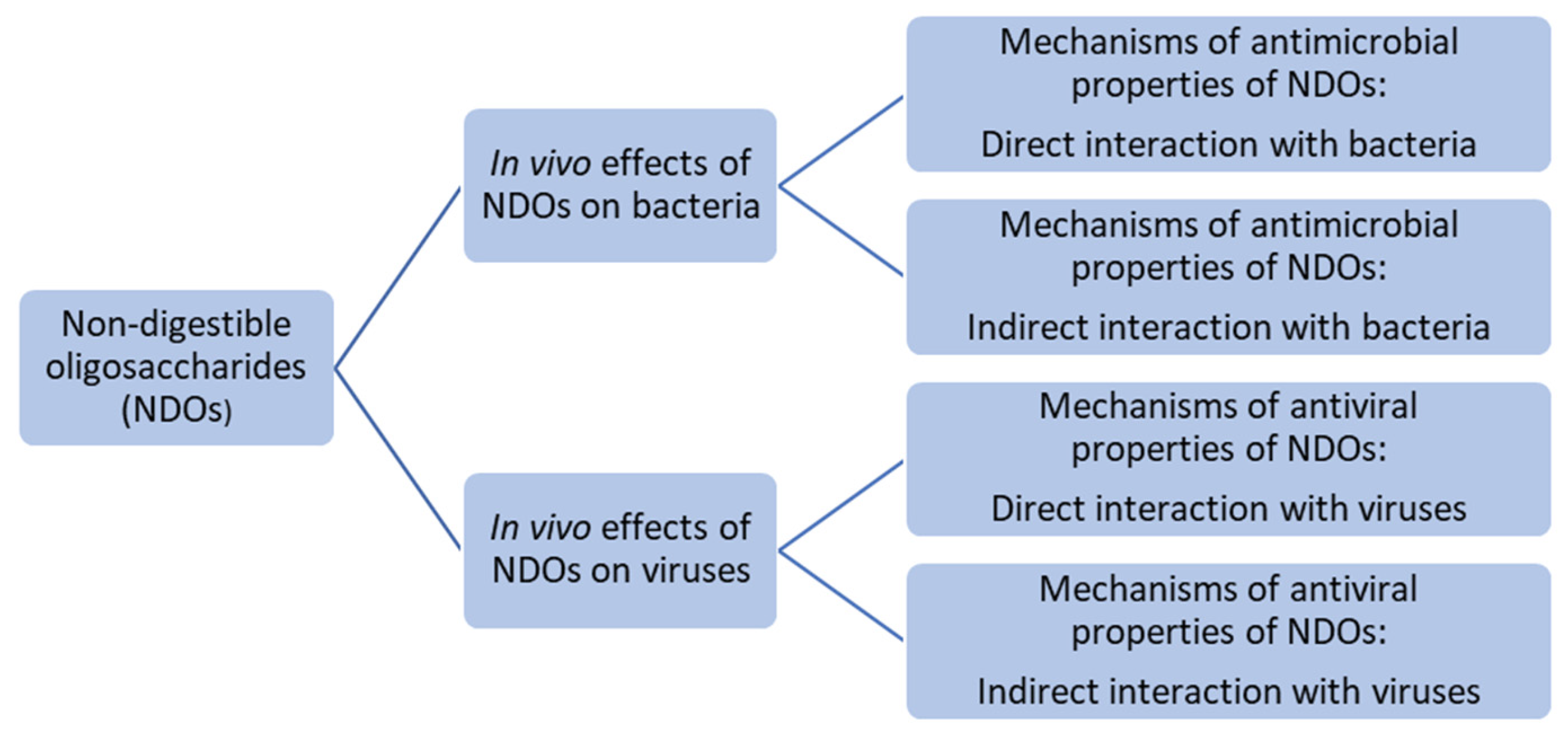
2. Antimicrobial Effect of NDOs
2.1. In Vivo Evidence for Antimicrobial and Anti-Infective Effects of NDOs
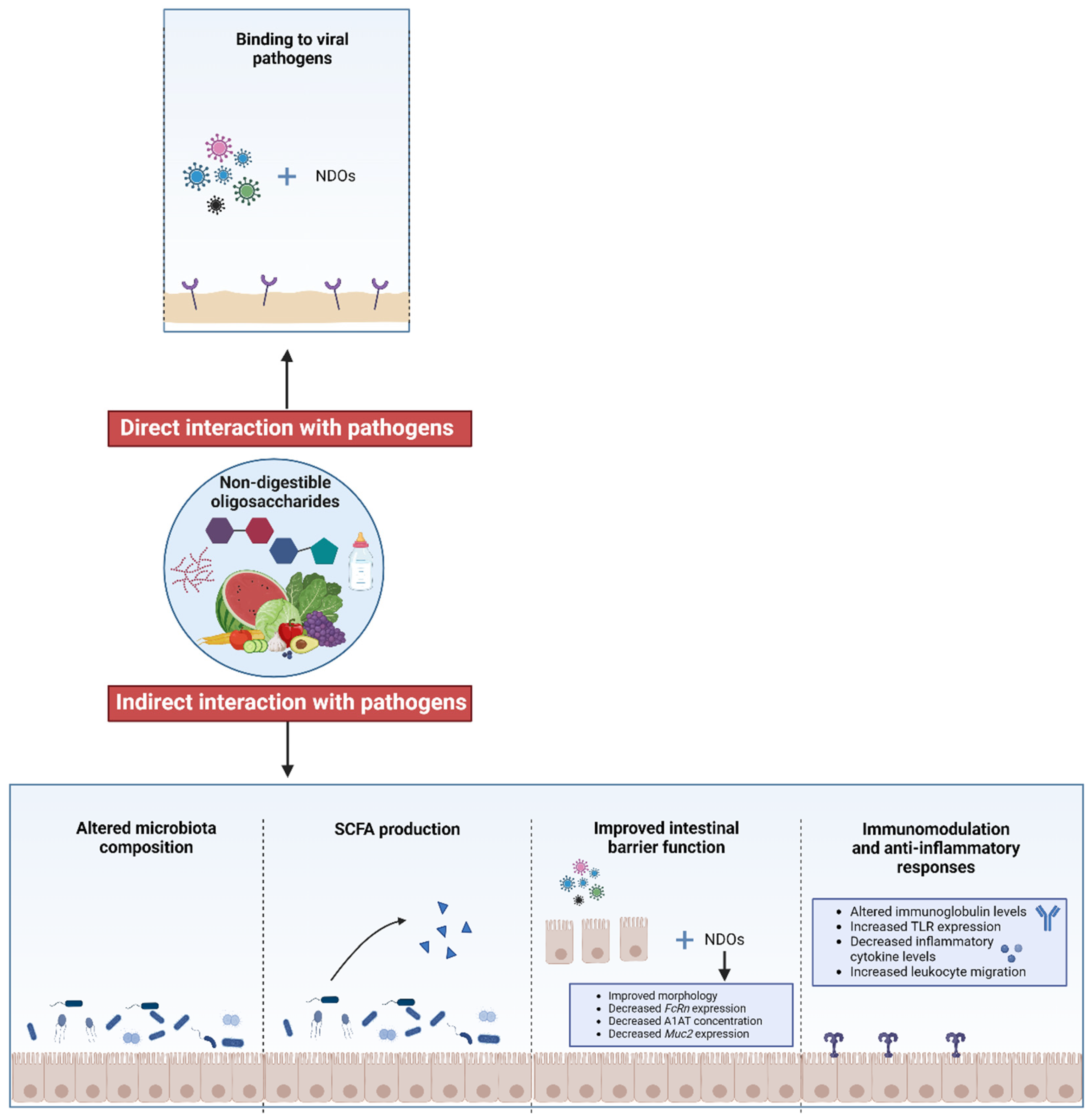
2.2. Mechanisms of Antimicrobial Properties of NDOs: Direct Interaction with Pathogens
2.2.1. NDOs Decrease Bacterial Biofilm Formation and Activity
2.2.2. NDOs Affect Interactions Between Host Cells and Bacteria: Inhibition of Recognition and Adhesion
2.2.3. NDOs Increase the Permeability of Bacterial Cell Membranes and the Efficacy of Antimicrobial Drugs
2.3. Mechanisms of Antimicrobial Properties of NDOs: Indirect Interaction with Pathogens
Effects of NDOs on Microbiota Composition, SCFA Production, Barrier, and Immune Function During Bacterial Infections
| Treatment Target | NDOs (Amount, Application, etc.) | Model Description (Pathogen, Procedure, etc.) | Effects | References |
|---|---|---|---|---|
| Infants | HMOs (2′FL + LNnT; 1.0 g/L + 0.5 g/L) | Naturally acquired infections |
| [115,116,117,118,119,120] |
| Inulin-type oligosaccharides (scFOS + lcFOS; 4 g/L + 4 g/L; 50:50 ratio ± 10% each) |
| [122] | ||
| GOS/PDX formula (GOS + PDX: 4 g/L + 4 g/L; 50:50 ratio) |
| [128] | ||
| Adults | Oral 10 g or 20 g/day 3′SL | A positive screening test for H. pylori infection |
| [41] |
| BALB/c mice | 100 μL PBS containing 2 mg neutral HMOs | C. jejuni 287ip orally |
| [39] |
| 5% pAOS extracted from citrus | P. aeruginosa strain PAO1 via airway administration |
| [49] | |
| 2 g GOS/kg BW | E. coli O157 (ATCC35150) via intragastric administration |
| [126] | |
| Oral 200, 1000, and 2000 mg/kg 3′SL or 6′SL of BW | P. aeruginosa K via intranasal inoculation |
| [50] | |
| C57BL/6 mice | 1 mg (10 μL of 100 mg/mL) purified HMOs or LNT | S. agalactiae via vaginal colonization |
| [38] |
| 5% XOS | Salmonella Typhimurium orally |
| [125] | |
| SLC: ICR mice | 2.5 mg GOS/100 μL sterile PBS by transmural injection | S. Typhimurium SL1344nalr by transmural injection |
| [44] |
| Guinea pigs | 100 g/kg GOS or XOS | L. monocytogenes orally |
| [46] |
| Rabbits | Intratracheal 20 nM LNnT or LSTc/0.2 mL saline | Pneumococcal pneumonia intratracheally |
| [42] |
| Calves | Intranasal 1.5 g GOS/10 mL saline | Naturally acquired infections |
| [43] |
| Calf milk replacer with 1% or 2% GOS |
| [48] | ||
| Calf milk replacer with 0.25% FOS |
| [52] | ||
| Piglets | 500 mg COS/kg BW | Enterotoxigenic E. coli orally administered for 3 consecutive days of the experiment |
| [123] |
| 0.4 mg/kg MOS-selenium supplemented diet | Enterotoxigenic E. coli orally administered once per week |
| [132] | |
| Chickens | 2 g XOS/kg BW | S. Enteritidis orally |
| [124] |
| 1 g MOS/kg BW |
| |||
| 1% functional GOS [1.8% w/w of commercial GOS (Oligomate™ 55NP) that contained 55–56% GOS and 44–45% monosaccharides] | A mixture of S. Typhimurium FNR-HA—kanamycin-resistant (ATCC 14028s) and S. Enteritidis FNR-HA—chloramphenicol-resistant and rifampicin-resistant (ATCC 31194) orally |
| [127] | |
| Rhesus monkeys |
| Experimentally inoculated with a mixture of 7 H. pylori strains (cagA and vacA) isolated from patients |
| [40] |
| Grass carp | 0, 200, 400, 600, 800, and 1000 mg/kg MOS | Injections of A. hydrophila |
| [130] |
3. Antiviral Effects of NDOs
3.1. In Vivo Evidence for Antiviral Effects of NDOs
3.2. Mechanisms of Antiviral Properties of NDOs: Direct Interaction with Pathogens Through Binding Affinity
3.3. Mechanisms of Antiviral Properties of NDOs: Indirect Interaction with Pathogens
3.3.1. Effects of NDOs on Intestinal Barrier Function and Intestinal Maturation in Viral Infections
3.3.2. Effects of NDOs on Immune Parameters During Viral Infections
3.3.3. Effects of NDOs on Microbiota Composition During Viral Infections
3.3.4. Effects of NDOs on SCFA Production During Viral Infections
| Treatment Target | NDOs (Amount, Application, etc.) | Model Description (Pathogen, Application, etc.) | Effects | References |
|---|---|---|---|---|
| Neonatal piglets | 2′-FL, LNnT, 6′-SL, 3′-SL, and free sialic acid (4 g/L), scGOS/lcFOS (3.6 g + 0.4 g) per liter | RV (OSU: Ohio State University) |
| [26] |
| Suckling rats | 2′-FL (0.2 g/100 g BW), scGOS/lcFOS (9:1) 0.8 g/100 g of BW and 2′-FL combined with scGOS/lcFOS (0.2 + 0.8 g/100 g BW) | RV (simian SA-11) orally inoculated at day 5 of life |
| [23] |
| Neonatal rats | 2′-FL (0.2 g/100 g BW), scGOS/lcFOS (9:1) 0.8 g/100 g of BW and 2′-FL combined with scGOS/lcFOS (0.2 + 0.8 g/100 g BW) | RV (simian SA-11) orally inoculated at day 5 of life |
| [22] |
| Chickens | 3′-SL (1 mL of 500 mM 3′-SL per day) | AI (H9N2) |
| [143] |
| Piglets | 2 mg/mL of LNnT, acidic HMO mixture (40% 6′-SL/10% 3′-SL/50% SA) Directly injected into ileal loops for 6 h | RV (OSU: Ohio State University) |
| [170] |
| C57BL/6 mouse | ScGOS/lcFOS/pAOS (9:1:10) 2% (w/w) of the total carbohydrate in the diet | RSV strain A2 (VR-1302; ATCC) and (FI)-RSV vaccine |
| [150] |
| Suckling rats | scGOS/lcFOS/pAOS (7.6:8.5:15) 0.8 g/100 g of BW | RV (simian SA-11) orally inoculated at day 7 of life |
| [28] |
| Suckling rats | scGOS/lcFOS (9:1) 0.8 g/100 g of BW | RV (simian SA-11) intragastrically inoculated at day 7 of life |
| [29] |
| Nursery pigs | MOS 0.2% (Bio-Mos, Alltech Inc., Nicholasville, KY, USA) | PRRSV (Purdue isolate P-129) intranasally inoculated at week 5 of life |
| [25] |
| Mice | 2′-FL, 3′-FL and 3′-FL + 2′-FL (first study: 750 mg/kg, second study: 150 mg/kg), 100 µL/oral gavage | Intranasal infection with H1N1 (A/Puerto Rico/8/34) |
| [144] |
| Tiger shrimp (Penaeus mondon) | MOS + peptidoglycan (0.1, 0.2 and 0.4%) | WSV-infected water for one hour |
| [21] |
| Tuebingen zebrafish | MOSs (0.2, 0.4, 0.6, and 0.8%) | SVCV bath immersion for 12 days |
| [27] |
4. Conclusions and Future Prospective
Author Contributions
Funding
Conflicts of Interest
References
- Jackson, N.; Czaplewski, L.; Piddock, L.J.V. Discovery and development of new antibacterial drugs: Learning from experience? J. Antimicrob. Chemother. 2018, 73, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Claus-Desbonnet, H.; Nikly, E.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Ivanova, S.; Pierre, G.; Benbassat, N.; Katsarov, P.; Michaud, P.; Lukova, P.; et al. Polysaccharides and Their Derivatives as Potential Antiviral Molecules. Viruses 2022, 14, 426. [Google Scholar] [CrossRef]
- Slavin, J.; Carlson, J. Carbohydrates. Adv. Nutr. 2014, 5, 760–761. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Wiciński, M.; Sawicka, E.; Gębalski, J.; Kubiak, K.; Malinowski, B. Human Milk Oligosaccharides: Health Benefits, Potential Applications in Infant Formulas, and Pharmacology. Nutrients 2020, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, R.; Faas, M.M.; de Vos, P. Non-digestible carbohydrates in infant formula as substitution for human milk oligosaccharide functions: Effects on microbiota and gut maturation. Crit. Rev. Food Sci. Nutr. 2019, 59, 1486–1497. [Google Scholar] [CrossRef]
- Hobden, M.R.; Guérin-Deremaux, L.; Rowland, I.; Gibson, G.R.; Kennedy, O.B. Potential anti-obesogenic properties of non-digestible carbohydrates: Specific focus on resistant dextrin. Proc. Nutr. Soc. 2015, 74, 258–267. [Google Scholar] [CrossRef]
- Coppa, G.V.; Bruni, S.; Morelli, L.; Soldi, S.; Gabrielli, O. The First Prebiotics in Humans: Human Milk Oligosaccharides. J. Clin. Gastroenterol. 2004, 38, S80–S83. [Google Scholar] [CrossRef] [PubMed]
- Duman, H.; Bechelany, M.; Karav, S. Human Milk Oligosaccharides: Decoding Their Structural Variability, Health Benefits, and the Evolution of Infant Nutrition. Nutrients 2025, 17, 118. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Mancilha, I.M. Non-digestible oligosaccharides: A review. Carbohydr. Polym. 2007, 68, 587–597. [Google Scholar] [CrossRef]
- Muthu, M.; Gopal, J.; Chun, S.; Devadoss, A.J.P.; Hasan, N.; Sivanesan, I. Crustacean waste-derived chitosan: Antioxidant properties and future perspective. Antioxidants 2021, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.L.; Yang, Q.; Wang, Z.B. Extraction and deproteinization of mannan oligosaccharides. Z. Naturforschung C 2010, 65, 387–390. [Google Scholar] [CrossRef]
- Vera, C.; Illanes, A.; Guerrero, C. Enzymatic production of prebiotic oligosaccharides. Curr. Opin. Food Sci. 2021, 37, 160–170. [Google Scholar] [CrossRef]
- Leong, S.Y.; Duque, S.M.; Abduh, S.B.M.; Oey, I. 6-Carbohydrates. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Barba, F.J., Saraiva, J.M.A., Cravotto, G., Lorenzo, J.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 171–206. [Google Scholar]
- Wang, Y.; Guo, Q.; Goff, H.D.; LaPointe, G.l. Oligosaccharides: Structure, function and application. Encycl. Food Chem. 2019, 202–207. [Google Scholar]
- Chen, X. Human milk oligosaccharides (HMOS): Structure, function, and enzyme-catalyzed synthesis. Adv. Carbohydr. Chem. Biochem. 2015, 72, 113–190. [Google Scholar]
- Jost, T.; Lacroix, C.; Braegger, C.; Chassard, C. Impact of human milk bacteria and oligosaccharides on neonatal gut microbiota establishment and gut health. Nutr. Rev. 2015, 73, 426–437. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- Kong, C.; de Jong, A.; de Haan, B.J.; Kok, J.; de Vos, P. Human milk oligosaccharides and non-digestible carbohydrates reduce pathogen adhesion to intestinal epithelial cells by decoy effects or by attenuating bacterial virulence. Food Res. Int. 2022, 151, 110867. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C. Short-chain fatty-acid-producing bacteria: Key components of the human gut microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Apines-Amar, M.J.S.; Andrino, K.G.S.; Amar, E.C.; Cadiz, R.E.; Corre, V.L., Jr. Improved resistance against White Spot Virus (WSV) infection in tiger shrimp, Penaeus monodon by combined supplementation of peptidoglycan and mannan oligosaccharide (MOS). Extrem. Life Biospeology Astrobiol. 2014, 6, 1–9. [Google Scholar]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; van’t Land, B.; Tims, S.; Stahl, B.; Knol, J.; Garssen, J.; Franch, À.; Castell, M. Oligosaccharides modulate rotavirus-associated dysbiosis and TLR gene expression in neonatal rats. Cells 2019, 8, 876. [Google Scholar] [CrossRef] [PubMed]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; van’t Land, B.; Stahl, B.; Garssen, J.; Rodríguez-Lagunas, M.J.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Supplementation with 2′-FL and scGOS/lcFOS Ameliorates Rotavirus-Induced Diarrhea in Suckling Rats. Front. Cell Infect. Microbiol. 2018, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Bruzzese, E.; Volpicelli, M.; Squeglia, V.; Bruzzese, D.; Salvini, F.; Bisceglia, M.; Lionetti, P.; Cinquetti, M.; Iacono, G.; Amarri, S.; et al. A formula containing galacto- and fructo-oligosaccharides prevents intestinal and extra-intestinal infections: An observational study. Clin. Nutr. 2009, 28, 156–161. [Google Scholar] [CrossRef]
- Che, T.M.; Johnson, R.W.; Kelley, K.W.; Van Alstine, W.G.; Dawson, K.A.; Moran, C.A.; Pettigrew, J.E. Mannan oligosaccharide improves immune responses and growth efficiency of nursery pigs experimentally infected with porcine reproductive and respiratory syndrome virus. J. Anim. Sci. 2011, 89, 2592–2602. [Google Scholar] [CrossRef]
- Comstock, S.S.; Li, M.; Wang, M.; Monaco, M.H.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Donovan, S.M. Dietary human milk oligosaccharides but not prebiotic oligosaccharides increase circulating natural killer cell and mesenteric lymph node memory T cell populations in noninfected and rotavirus-infected neonatal piglets. J. Nutr. 2017, 147, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Xie, Y.; Li, Y.; Xie, M.; Li, M.; Zhou, W.; Chen, J.; Zhang, Z.; Yang, Y.; Ran, C. Dietary supplementation of yeast mannan enhances antiviral immunity of zebrafish (Danio rerio). Aquaculture 2023, 563, 739003. [Google Scholar] [CrossRef]
- Rigo-Adrover, M.; Pérez-Berezo, T.; Ramos-Romero, S.; van Limpt, K.; Knipping, K.; Garssen, J.; Knol, J.; Franch, À.; Castell, M.; Pérez-Cano, F.J. A fermented milk concentrate and a combination of short-chain galacto-oligosaccharides/long-chain fructo-oligosaccharides/pectin-derived acidic oligosaccharides protect suckling rats from rotavirus gastroenteritis. Br. J. Nutr. 2017, 117, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Rigo-Adrover, M.; Saldaña-Ruíz, S.; Van Limpt, K.; Knipping, K.; Garssen, J.; Knol, J.; Franch, A.; Castell, M.; Pérez-Cano, F.J. A combination of scGOS/lcFOS with Bifidobacterium breve M-16V protects suckling rats from rotavirus gastroenteritis. Eur. J. Nutr. 2017, 56, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Asadpoor, M.; Peeters, C.; Henricks, P.A.J.; Varasteh, S.; Pieters, R.J.; Folkerts, G.; Braber, S. Anti-Pathogenic Functions of Non-Digestible Oligosaccharides In Vitro. Nutrients 2020, 12, 1789. [Google Scholar] [CrossRef]
- Elwakiel, M.; Hageman, J.A.; Wang, W.; Szeto, I.M.; van Goudoever, J.B.; Hettinga, K.A.; Schols, H.A. Human Milk Oligosaccharides in Colostrum and Mature Milk of Chinese Mothers: Lewis Positive Secretor Subgroups. J. Agric. Food Chem. 2018, 66, 7036–7043. [Google Scholar] [CrossRef]
- Zhang, B.; Li, L.Q.; Liu, F.; Wu, J.Y. Human milk oligosaccharides and infant gut microbiota: Molecular structures, utilization strategies and immune function. Carbohydr. Polym. 2022, 276, 118738. [Google Scholar] [CrossRef] [PubMed]
- Moubareck, C.A. Human Milk Microbiota and Oligosaccharides: A Glimpse into Benefits, Diversity, and Correlations. Nutrients 2021, 13, 1123. [Google Scholar] [CrossRef] [PubMed]
- Sankar, M.J.; Sinha, B.; Chowdhury, R.; Bhandari, N.; Taneja, S.; Martines, J.; Bahl, R. Optimal breastfeeding practices and infant and child mortality: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 3–13. [Google Scholar] [CrossRef]
- Christensen, N.; Bruun, S.; Sondergaard, J.; Christesen, H.T.; Fisker, N.; Zachariassen, G.; Sangild, P.T.; Husby, S. Breastfeeding and Infections in Early Childhood: A Cohort Study. Pediatrics 2020, 146. [Google Scholar] [CrossRef] [PubMed]
- Dinleyici, M.; Barbieur, J.; Dinleyici, E.C.; Vandenplas, Y. Functional effects of human milk oligosaccharides (HMOs). Gut Microbes 2023, 15, 2186115. [Google Scholar] [CrossRef]
- Craft, K.M.; Townsend, S.D. Mother Knows Best: Deciphering the Antibacterial Properties of Human Milk Oligosaccharides. Acc. Chem. Res. 2019, 52, 760–768. [Google Scholar] [CrossRef]
- Mejia, M.E.; Ottinger, S.; Vrbanac, A.; Babu, P.; Zulk, J.J.; Moorshead, D.; Bode, L.; Nizet, V.; Patras, K.A. Human Milk Oligosaccharides Reduce Murine Group B Streptococcus Vaginal Colonization with Minimal Impact on the Vaginal Microbiota. mSphere 2022, 7, e0088521. [Google Scholar] [CrossRef]
- Ruiz-Palacios, G.M.; Cervantes, L.E.; Ramos, P.; Chavez-Munguia, B.; Newburg, D.S. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 2003, 278, 14112–14120. [Google Scholar] [CrossRef]
- Mysore, J.V.; Wigginton, T.; Simon, P.M.; Zopf, D.; Heman-Ackah, L.M.; Dubois, A. Treatment of Helicobacter pylori infection in rhesus monkeys using a novel antiadhesion compound. Gastroenterology 1999, 117, 1316–1325. [Google Scholar] [CrossRef]
- Parente, F.; Cucino, C.; Anderloni, A.; Grandinetti, G.; Bianchi Porro, G. Treatment of Helicobacter pylori infection using a novel antiadhesion compound (3′sialyllactose sodium salt). A double blind, placebo-controlled clinical study. Helicobacter 2003, 8, 252–256. [Google Scholar] [CrossRef]
- Idänpään-Heikkilä, I.; Simon, P.M.; Zopf, D.; Vullo, T.; Cahill, P.; Sokol, K. Oligosaccharides Interfere with the Establishment and Progression of Experimental Pneumococcal Pneumonia. J. Infect. Dis. 1997, 176, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; van Putten, J.P.M.; Gilbert, M.S.; Gerrits, W.J.J.; Folkerts, G.; Braber, S. Galacto-oligosaccharides as an anti-bacterial and anti-invasive agent in lung infections. Biomaterials 2022, 283, 121461. [Google Scholar] [CrossRef]
- Searle, L.E.J.; Cooley, W.A.; Jones, G.; Nunez, A.; Crudgington, B.; Weyer, U.; Dugdale, A.H.; Tzortzis, G.; Collins, J.W.; Woodward, M.J.; et al. Purified galactooligosaccharide, derived from a mixture produced by the enzymic activity of Bifidobacterium bifidum, reduces Salmonella enterica serovar Typhimurium adhesion and invasion in vitro and in vivo. J. Med. Microbiol. 2010, 59 Pt 12, 1428–1439. [Google Scholar] [CrossRef]
- Sangwan, V.; Tomar, S.K.; Ali, B.; Singh, R.R.B.; Singh, A.K. Galactooligosaccharides reduce infection caused by Listeria monocytogenes and modulate IgG and IgA levels in mice. Int. Dairy J. 2015, 41, 58–63. [Google Scholar] [CrossRef]
- Ebersbach, T.; Jorgensen, J.B.; Heegaard, P.M.; Lahtinen, S.J.; Ouwehand, A.C.; Poulsen, M.; Frokiaer, H.; Licht, T.R. Certain dietary carbohydrates promote Listeria infection in a guinea pig model, while others prevent it. Int. J. Food Microbiol. 2010, 140, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, S.; Guardiola, F.A.; Leclercq, E.; Esteban, M.A.; Castex, M.; Sotoudeh, E.; Lee, S.M. Effects of dietary supplementation with Pediococcus acidilactici MA18/5M, galactooligosaccharide and their synbiotic on growth, innate immunity and disease resistance of rockfish (Sebastes schlegeli). Aquaculture 2018, 482, 36–44. [Google Scholar] [CrossRef]
- Cai, Y.; Gilbert, M.S.; Gerrits, W.J.J.; Folkerts, G.; Braber, S. Galacto-oligosaccharides alleviate lung inflammation by inhibiting NLRP3 inflammasome activation in vivo and in vitro. J. Adv. Res. 2022, 39, 305–318. [Google Scholar] [CrossRef]
- Bernard, H.; Desseyn, J.L.; Bartke, N.; Kleinjans, L.; Stahl, B.; Belzer, C.; Knol, J.; Gottrand, F.; Husson, M.O. Dietary pectin-derived acidic oligosaccharides improve the pulmonary bacterial clearance of Pseudomonas aeruginosa lung infection in mice by modulating intestinal microbiota and immunity. J. Infect. Dis. 2015, 211, 156–165. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.J.; Kim, J.W. Bacterial Clearance Is Enhanced by alpha2,3- and alpha2,6-Sialyllactose via Receptor-Mediated Endocytosis and Phagocytosis. Infect. Immun. 2019, 87, e00694-18. [Google Scholar] [CrossRef]
- Letellier, A.; Messier, S.; Lessard, L.; Quessy, S. Assessment of various treatments to reduce carriage of Salmonella in swine. Can. J. Vet. Res. 2000, 64, 27–31. [Google Scholar]
- Cai, Y.; Gilbert, M.S.; Gerrits, W.J.J.; Folkerts, G.; Braber, S. Anti-Inflammatory Properties of Fructo-Oligosaccharides in a Calf Lung Infection Model and in Mannheimia haemolytica-Infected Airway Epithelial Cells. Nutrients 2021, 13, 3514. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, Q.; Everaert, N.; Liu, R.; Zheng, M.; Zhao, G.; Wen, J. Effects of inulin supplementation on intestinal barrier function and immunity in specific pathogen-free chickens with Salmonella infection. J. Anim. Sci. 2020, 98, skz396. [Google Scholar] [CrossRef]
- Baurhoo, B.; Phillip, L.; Ruiz-Feria, C.A. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poult. Sci. 2007, 86, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Baurhoo, B.; Ferket, P.R.; Zhao, X. Effects of diets containing different concentrations of mannanoligosaccharide or antibiotics on growth performance, intestinal development, cecal and litter microbial populations, and carcass parameters of broilers. Poult. Sci. 2009, 88, 2262–2272. [Google Scholar] [CrossRef]
- Spring, P.; Wenk, C.; Dawson, K.A.; Newman, K.E. The effects of dietary mannaoligosaccharides on cecal parameters and the concentrations of enteric bacteria in the ceca of salmonella-challenged broiler chicks. Poult. Sci. 2000, 79, 205–211. [Google Scholar] [CrossRef]
- Bailey, J.S.; Blankenship, L.C.; Cox, N.A. Effect of fructooligosaccharide on Salmonella colonization of the chicken intestine. Poult. Sci. 1991, 70, 2433–2438. [Google Scholar] [CrossRef] [PubMed]
- Tsay, T.B.; Yang, M.C.; Chang, W.H.; Chen, P.H.; Chen, L.W. Lactobacillus salivarius reverse antibiotic-induced lung defense impairment in a ventilator model. J. Transl. Med. 2018, 16, 225. [Google Scholar] [CrossRef]
- Zhang, M.; Qiao, X.; Han, W.; Jiang, T.; Liu, F.; Zhao, X. Alginate-chitosan oligosaccharide-ZnO composite hydrogel for accelerating wound healing. Carbohydr. Polym. 2021, 266, 118100. [Google Scholar] [CrossRef]
- Chhibber, T.; Gondil, V.S.; Sinha, V.R. Development of Chitosan-Based Hydrogel Containing Antibiofilm Agents for the Treatment of Staphylococcus aureus-Infected Burn Wound in Mice. AAPS PharmSciTech 2020, 21, 43. [Google Scholar] [CrossRef]
- Henrick, B.M.; Rodriguez, L.; Lakshmikanth, T.; Pou, C.; Henckel, E.; Arzoomand, A.; Olin, A.; Wang, J.; Mikes, J.; Tan, Z.; et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 2021, 184, 3884–3898.e11. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, H.; Zheng, J.; Jiang, N.; Sun, G.; Bao, X.; Lin, A.; Liu, H. Chitosan oligosaccharides attenuate loperamide-induced constipation through regulation of gut microbiota in mice. Carbohydr. Polym. 2021, 253, 117218. [Google Scholar] [CrossRef]
- Masi, A.C.; Embleton, N.D.; Lamb, C.A.; Young, G.; Granger, C.L.; Najera, J.; Smith, D.P.; Hoffman, K.L.; Petrosino, J.F.; Bode, L.; et al. Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis. Gut 2021, 70, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Folkerts, J.; Folkerts, G.; Maurer, M.; Braber, S. Microbiota-dependent and -independent effects of dietary fibre on human health. Br. J. Pharmacol. 2020, 177, 1363–1381. [Google Scholar] [CrossRef]
- Ackerman, D.L.; Craft, K.M.; Doster, R.S.; Weitkamp, J.H.; Aronoff, D.M.; Gaddy, J.A.; Townsend, S.D. Antimicrobial and Antibiofilm Activity of Human Milk Oligosaccharides against Streptococcus agalactiae, Staphylococcus aureus, and Acinetobacter baumannii. ACS Infect. Dis. 2018, 4, 315–324. [Google Scholar] [CrossRef]
- Ackerman, D.L.; Doster, R.S.; Weitkamp, J.H.; Aronoff, D.M.; Gaddy, J.A.; Townsend, S.D. Human Milk Oligosaccharides Exhibit Antimicrobial and Antibiofilm Properties against Group B Streptococcus. ACS Infect. Dis. 2017, 3, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Buhlin, K.; Dufrene, Y.F.; Gomelsky, M.; Moroni, A.; Ramstedt, M.; Rumbaugh, K.P.; Schulte, T.; Sun, L.; Akerlund, B.; et al. Biofilm formation—What we can learn from recent developments. J. Intern. Med. 2018, 284, 332–345. [Google Scholar] [CrossRef]
- Asadpoor, M.; Ithakisiou, G.N.; van Putten, J.P.M.; Pieters, R.J.; Folkerts, G.; Braber, S. Antimicrobial Activities of Alginate and Chitosan Oligosaccharides Against Staphylococcus aureus and Group B Streptococcus. Front. Microbiol. 2021, 12, 700605. [Google Scholar] [CrossRef]
- Ortega-Gonzalez, M.; Sanchez de Medina, F.; Molina-Santiago, C.; Lopez-Posadas, R.; Pacheco, D.; Krell, T.; Martinez-Augustin, O.; Abdelali, D. Fructooligosacharides reduce Pseudomonas aeruginosa PAO1 pathogenicity through distinct mechanisms. PLoS ONE 2014, 9, e85772. [Google Scholar] [CrossRef]
- Tyrikos-Ergas, T.; Gim, S.; Huang, J.Y.; Pinzon Martin, S.; Varon Silva, D.; Seeberger, P.H.; Delbianco, M. Synthetic phosphoethanolamine-modified oligosaccharides reveal the importance of glycan length and substitution in biofilm-inspired assemblies. Nat. Commun. 2022, 13, 3954. [Google Scholar] [CrossRef]
- Vareille, M.; Kieninger, E.; Edwards, M.R.; Regamey, N. The airway epithelium: Soldier in the fight against respiratory viruses. Clin. Microbiol. Rev. 2011, 24, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Sharon, N.; Lis, H. Lectins as cell recognition molecules. Science 1989, 246, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Leusmann, S.; Menova, P.; Shanin, E.; Titz, A.; Rademacher, C. Glycomimetics for the inhibition and modulation of lectins. Chem. Soc. Rev. 2023, 52, 3663–3740. [Google Scholar] [CrossRef]
- Boukerb, A.M.; Rousset, A.; Galanos, N.; Mear, J.B.; Thepaut, M.; Grandjean, T.; Gillon, E.; Cecioni, S.; Abderrahmen, C.; Faure, K.; et al. Antiadhesive properties of glycoclusters against Pseudomonas aeruginosa lung infection. J. Med. Chem. 2014, 57, 10275–10289. [Google Scholar] [CrossRef] [PubMed]
- Imberty, A.; Mitchell, E.P.; Wimmerova, M. Structural basis of high-affinity glycan recognition by bacterial and fungal lectins. Curr. Opin. Struct. Biol. 2005, 15, 525–534. [Google Scholar] [CrossRef]
- Weichert, S.; Jennewein, S.; Hufner, E.; Weiss, C.; Borkowski, J.; Putze, J.; Schroten, H. Bioengineered 2′-fucosyllactose and 3-fucosyllactose inhibit the adhesion of Pseudomonas aeruginosa and enteric pathogens to human intestinal and respiratory cell lines. Nutr. Res. 2013, 33, 831–838. [Google Scholar] [CrossRef]
- Mortaz, E.; Nomani, M.; Adcock, I.; Folkerts, G.; Garssen, J. Galactooligosaccharides and 2′-fucosyllactose can directly suppress growth of specific pathogenic microbes and affect phagocytosis of neutrophils. Nutrition 2022, 96, 111601. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, Q.; Dai, X.; Wei, X.; Yu, Y.; Chen, X.; Li, C.; Cao, Z.; Zhang, X. A Biomimetic Non-Antibiotic Approach to Eradicate Drug-Resistant Infections. Adv. Mater. 2019, 31, e1806024. [Google Scholar] [CrossRef]
- Sattin, S.; Bernardi, A. Glycoconjugates and Glycomimetics as Microbial Anti-Adhesives. Trends Biotechnol. 2016, 34, 483–495. [Google Scholar] [CrossRef]
- Firon, N.; Ashkenazi, S.; Mirelman, D.; Ofek, I.; Sharon, N. Aromatic alpha-glycosides of mannose are powerful inhibitors of the adherence of type 1 fimbriated Escherichia coli to yeast and intestinal epithelial cells. Infect. Immun. 1987, 55, 472–476. [Google Scholar] [CrossRef]
- Wellens, A.; Garofalo, C.; Nguyen, H.; Van Gerven, N.; Slattegard, R.; Hernalsteens, J.P.; Wyns, L.; Oscarson, S.; De Greve, H.; Hultgren, S.; et al. Intervening with urinary tract infections using anti-adhesives based on the crystal structure of the FimH-oligomannose-3 complex. PLoS ONE 2008, 3, e2040. [Google Scholar] [CrossRef]
- Faustino, M.; Silva, S.; Costa, E.M.; Pereira, A.M.; Pereira, J.O.; Oliveira, A.S.; Ferreira, C.M.H.; Pereira, C.F.; Durao, J.; Pintado, M.E.; et al. Effect of Mannan Oligosaccharides Extracts in Uropathogenic Escherichia coli Adhesion in Human Bladder Cells. Pathogens 2023, 12, 885. [Google Scholar] [CrossRef] [PubMed]
- Douellou, T.; Montel, M.C.; Thevenot Sergentet, D. Invited review: Anti-adhesive properties of bovine oligosaccharides and bovine milk fat globule membrane-associated glycoconjugates against bacterial food enteropathogens. J. Dairy Sci. 2017, 100, 3348–3359. [Google Scholar] [CrossRef] [PubMed]
- Facinelli, B.; Marini, E.; Magi, G.; Zampini, L.; Santoro, L.; Catassi, C.; Monachesi, C.; Gabrielli, O.; Coppa, G.V. Breast milk oligosaccharides: Effects of 2′-fucosyllactose and 6′-sialyllactose on the adhesion of Escherichia coli and Salmonella fyris to Caco-2 cells. J. Matern. Fetal Neonatal Med. 2019, 32, 2950–2952. [Google Scholar] [CrossRef]
- Piotrowski, M.; Wultanska, D.; Obuch-Woszczatynski, P.; Pituch, H. Fructooligosaccharides and mannose affect Clostridium difficile adhesion and biofilm formation in a concentration-dependent manner. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1975–1984. [Google Scholar] [CrossRef]
- Zhu, H.; Cai, Y.; Slimmen, L.J.M.; de Bruijn, A.; van Rossum, A.M.C.; Folkerts, G.; Braber, S.; Unger, W.W.J. Galacto-Oligosaccharides as an Anti-Infective and Anti-Microbial Agent for Macrolide-Resistant and -Sensitive Mycoplasma pneumoniae. Pathogens 2023, 12, 659. [Google Scholar] [CrossRef]
- Manthey, C.F.; Autran, C.A.; Eckmann, L.; Bode, L. Human milk oligosaccharides protect against enteropathogenic Escherichia coli attachment in vitro and EPEC colonization in suckling mice. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 165–168. [Google Scholar] [CrossRef]
- Spicer, S.K.; Gaddy, J.A.; Townsend, S.D. Recent advances on human milk oligosaccharide antimicrobial activity. Curr. Opin. Chem. Biol. 2022, 71, 102202. [Google Scholar] [CrossRef]
- Di, R.; Vakkalanka, M.S.; Onumpai, C.; Chau, H.K.; White, A.; Rastall, R.A.; Yam, K.; Hotchkiss, A.T., Jr. Pectic oligosaccharide structure-function relationships: Prebiotics, inhibitors of Escherichia coli O157:H7 adhesion and reduction of Shiga toxin cytotoxicity in HT29 cells. Food Chem. 2017, 227, 245–254. [Google Scholar] [CrossRef]
- Asadpoor, M.; Varasteh, S.; Pieters, R.; Folkerts, G.; Braber, S. Differential effects of oligosaccharides on the effectiveness of ampicillin against Escherichia coli in vitro. PharmaNutrition 2021, 16, 100264. [Google Scholar] [CrossRef]
- Sharon, N. Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim. Biophys. Acta 2006, 1760, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Imberty, A.; Chabre, Y.M.; Roy, R. Glycomimetics and glycodendrimers as high affinity microbial anti-adhesins. Chemistry 2008, 14, 7490–7499. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, A.; Jimenez-Barbero, J.; Casnati, A.; De Castro, C.; Darbre, T.; Fieschi, F.; Finne, J.; Funken, H.; Jaeger, K.E.; Lahmann, M.; et al. Multivalent glycoconjugates as anti-pathogenic agents. Chem. Soc. Rev. 2013, 42, 4709–4727. [Google Scholar] [CrossRef]
- Cecioni, S.; Imberty, A.; Vidal, S. Glycomimetics versus multivalent glycoconjugates for the design of high affinity lectin ligands. Chem. Rev. 2015, 115, 525–561. [Google Scholar] [CrossRef]
- Cai, Y.; Folkerts, G.; Braber, S. Non-Digestible Oligosaccharides: A Novel Treatment for Respiratory Infections? Nutrients 2022, 14, 5033. [Google Scholar] [CrossRef]
- Duca, M.; Haksar, D.; van Neer, J.; Thies-Weesie, D.M.E.; Martinez-Alarcon, D.; de Cock, H.; Varrot, A.; Pieters, R.J. Multivalent Fucosides Targeting beta-Propeller Lectins from Lung Pathogens with Promising Anti-Adhesive Properties. ACS Chem. Biol. 2022, 17, 3515–3526. [Google Scholar] [CrossRef]
- Tin, S.; Lim, C.S.; Sakharkar, M.K.; Sakharkar, K.R. Synergistic combinations of chitosans and antibiotics in Staphylococcus aureus. Lett. Drug Des. Discov. 2010, 7, 31–35. [Google Scholar] [CrossRef]
- Craft, K.M.; Gaddy, J.A.; Townsend, S.D. Human Milk Oligosaccharides (HMOs) Sensitize Group B Streptococcus to Clindamycin, Erythromycin, Gentamicin, and Minocycline on a Strain Specific Basis. ACS Chem. Biol. 2018, 13, 2020–2026. [Google Scholar] [CrossRef]
- Das, P.; Panda, S.K.; Agarwal, B.; Behera, S.; Ali, S.M.; Pulse, M.E.; Solomkin, J.S.; Opal, S.M.; Bhandari, V.; Acharya, S. Novel Chitohexaose Analog Protects Young and Aged mice from CLP Induced Polymicrobial Sepsis. Sci. Rep. 2019, 9, 2904. [Google Scholar] [CrossRef]
- Qu, Y.; Xu, J.; Zhou, H.; Dong, R.; Kang, M.; Zhao, J. Chitin Oligosaccharide (COS) Reduces Antibiotics Dose and Prevents Antibiotics-Caused Side Effects in Adolescent Idiopathic Scoliosis (AIS) Patients with Spinal Fusion Surgery. Mar. Drugs 2017, 15, 70. [Google Scholar] [CrossRef]
- Ghai, I. A barrier to entry: Examining the bacterial outer membrane and antibiotic resistance. Appl. Sci. 2023, 13, 4238. [Google Scholar] [CrossRef]
- Elie, C.R.; David, G.; Schmitzer, A.R. Strong antibacterial properties of anion transporters: A result of depolarization and weakening of the bacterial membrane. J. Med. Chem. 2015, 58, 2358–2366. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Mondal, A.; Yadav, V.; Sarkar, A.; Banerjee, R.; Sanpui, P.; Jaiswal, A. Mechanistic Insight into the Antibacterial Activity of Chitosan Exfoliated MoS2 Nanosheets: Membrane Damage, Metabolic Inactivation, and Oxidative Stress. ACS Appl. Bio. Mater. 2019, 2, 2738–2755. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hwang, H.-m.; Aker, W.G.; Wang, P.; Lin, Y.; Jiang, X.; He, X. Synergistic combination of marine oligosaccharides and azithromycin against Pseudomonas aeruginosa. Microbiol. Res. 2014, 169, 759–767. [Google Scholar] [CrossRef]
- Khan, S.; Tondervik, A.; Sletta, H.; Klinkenberg, G.; Emanuel, C.; Onsoyen, E.; Myrvold, R.; Howe, R.A.; Walsh, T.R.; Hill, K.E.; et al. Overcoming drug resistance with alginate oligosaccharides able to potentiate the action of selected antibiotics. Antimicrob. Agents Chemother. 2012, 56, 5134–5141. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Zhang, N.; Jin, M.; Wang, K.; Zhang, Z.; Shah, N.P.; Wei, H. Functional oligosaccharide fermentation in the gut: Improving intestinal health and its determinant factors—A review. Carbohydr. Polym. 2022, 284, 119043. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Akritidou, T.; Akkermans, S.; Smet, C.; Gaspari, S.; Sharma, C.; Matthews, E.; Van Impe, J.F. Gut microbiota of the small intestine as an antimicrobial barrier against foodborne pathogens: Impact of diet on the survival of S. typhimurium and L. monocytogenes during in vitro digestion. Food Res. Int. 2023, 173, 113292. [Google Scholar] [CrossRef]
- Siracusa, F.; Schaltenberg, N.; Kumar, Y.; Lesker, T.R.; Steglich, B.; Liwinski, T.; Cortesi, F.; Frommann, L.; Diercks, B.P.; Bonisch, F.; et al. Short-term dietary changes can result in mucosal and systemic immune depression. Nat. Immunol. 2023, 24, 1473–1486. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.B.; Huang, H.; Ning, Y.; Xiao, J. Probiotics in the New Era of Human Milk Oligosaccharides (HMOs): HMO Utilization and Beneficial Effects of Bifidobacterium longum subsp. infantis M-63 on Infant Health. Microorganisms 2024, 12, 1014. [Google Scholar] [CrossRef] [PubMed]
- Li, K.L.; Wang, B.Z.; Li, Z.P.; Li, Y.L.; Liang, J.J. Alterations of intestinal flora and the effects of probiotics in children with recurrent respiratory tract infection. World J. Pediatr. 2019, 15, 255–261. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Zolnowska, M.; Berni Canani, R.; Ludman, S.; Tengelyi, Z.; Moreno-Alvarez, A.; Goh, A.E.N.; Gosoniu, M.L.; Kirwan, B.A.; Tadi, M.; et al. Effects of an Extensively Hydrolyzed Formula Supplemented with Two Human Milk Oligosaccharides on Growth, Tolerability, Safety and Infection Risk in Infants with Cow’s Milk Protein Allergy: A Randomized, Multi-Center Trial. Nutrients 2022, 14, 530. [Google Scholar] [CrossRef] [PubMed]
- Puccio, G.; Alliet, P.; Cajozzo, C.; Janssens, E.; Corsello, G.; Sprenger, N.; Wernimont, S.; Egli, D.; Gosoniu, L.; Steenhout, P. Effects of Infant Formula with Human Milk Oligosaccharides on Growth and Morbidity: A Randomized Multicenter Trial. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 624–631. [Google Scholar] [CrossRef]
- van Stigt, A.H.; Oude Rengerink, K.; Bloemenkamp, K.W.M.; de Waal, W.; Prevaes, S.; Le, T.M.; van Wijk, F.; Nederend, M.; Hellinga, A.H.; Lammers, C.S.; et al. Analysing the protection from respiratory tract infections and allergic diseases early in life by human milk components: The PRIMA birth cohort. BMC Infect. Dis. 2022, 22, 152. [Google Scholar] [CrossRef]
- Martin, F.P.; Tytgat, H.L.P.; Krogh Pedersen, H.; Moine, D.; Eklund, A.C.; Berger, B.; Sprenger, N. Host-microbial co-metabolites modulated by human milk oligosaccharides relate to reduced risk of respiratory tract infections. Front. Nutr. 2022, 9, 935711. [Google Scholar] [CrossRef]
- Dogra, S.K.; Martin, F.P.; Donnicola, D.; Julita, M.; Berger, B.; Sprenger, N. Human Milk Oligosaccharide-Stimulated Bifidobacterium Species Contribute to Prevent Later Respiratory Tract Infections. Microorganisms 2021, 9, 1939. [Google Scholar] [CrossRef]
- Berger, B.; Porta, N.; Foata, F.; Grathwohl, D.; Delley, M.; Moine, D.; Charpagne, A.; Siegwald, L.; Descombes, P.; Alliet, P.; et al. Linking Human Milk Oligosaccharides, Infant Fecal Community Types, and Later Risk To Require Antibiotics. mBio 2020, 11, e03196-19. [Google Scholar] [CrossRef]
- Lamberti, L.M.; Zakarija-Grkovic, I.; Fischer Walker, C.L.; Theodoratou, E.; Nair, H.; Campbell, H.; Black, R.E. Breastfeeding for reducing the risk of pneumonia morbidity and mortality in children under two: A systematic literature review and meta-analysis. BMC Public Health 2013, 13 (Suppl. S3), S18. [Google Scholar] [CrossRef]
- Neumer, F.; Urraca, O.; Alonso, J.; Palencia, J.; Varea, V.; Theis, S.; Rodriguez-Palmero, M.; Moreno-Munoz, J.A.; Guarner, F.; Veereman, G.; et al. Long-Term Safety and Efficacy of Prebiotic Enriched Infant Formula—A Randomized Controlled Trial. Nutrients 2021, 13, 1276. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Meng, T.; He, W.; Huang, H.; Liu, C.; Fu, X.; He, J.; Yin, Y.; Xiao, D. Dietary Chito-oligosaccharides Improve Intestinal Immunity via Regulating Microbiota and Th17/Treg Balance-Related Immune Signaling in Piglets Challenged by Enterotoxigenic E. coli. J. Agric. Food Chem. 2021, 69, 15195–15207. [Google Scholar] [CrossRef]
- Pourabedin, M.; Chen, Q.; Yang, M.; Zhao, X. Mannan- and xylooligosaccharides modulate caecal microbiota and expression of inflammatory-related cytokines and reduce caecal Salmonella Enteritidis colonisation in young chickens. FEMS Microbiol. Ecol. 2017, 93, fiw226. [Google Scholar] [CrossRef]
- Pang, J.; Wang, S.; Wang, Z.; Wu, Y.; Zhang, X.; Pi, Y.; Han, D.; Zhang, S.; Wang, J. Xylo-oligosaccharide alleviates Salmonella induced inflammation by stimulating Bifidobacterium animalis and inhibiting Salmonella colonization. FASEB J. 2021, 35, e21977. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Wang, Y.; Peng, B.; Liu, J.; Zhang, B.; Lv, H.; Wang, S. Protection of Galacto-Oligosaccharide against E. coli O157 Colonization through Enhancing Gut Barrier Function and Modulating Gut Microbiota. Foods 2020, 9, 1710. [Google Scholar] [CrossRef]
- Hughes, R.A.; Ali, R.A.; Mendoza, M.A.; Hassan, H.M.; Koci, M.D. Impact of Dietary Galacto-Oligosaccharide (GOS) on Chicken’s Gut Microbiota, Mucosal Gene Expression, and Salmonella Colonization. Front. Vet. Sci. 2017, 4, 192. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, G.; Buccigrossi, V.; Borgia, E.; Piacentini, D.; Visentin, F.; Cantarutti, L.; Baiardi, P.; Felisi, M.; Spagnuolo, M.I.; Zanconato, S.; et al. Galacto-Oligosaccharide/Polidextrose Enriched Formula Protects against Respiratory Infections in Infants at High Risk of Atopy: A Randomized Clinical Trial. Nutrients 2018, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, H.; Teng, Y.; Qin, N.; Ren, X.; Xia, X. A high-sucrose diet causes microbiota composition shift and promotes the susceptibility of mice to Salmonella Typhimurium infection. Food Funct. 2023, 14, 2836–2846. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Feng, L.; Jiang, W.D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Li, S.W.; Zhong, C.B.; et al. Dietary mannan oligosaccharides strengthens intestinal immune barrier function via multipath cooperation during Aeromonas Hydrophila infection in grass carp (Ctenopharyngodon idella). Front. Immunol. 2022, 13, 1010221. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, X.; Liu, Y.; Zheng, Y.; Li, H.; Zhang, M.; He, Y.; Cheng, H.; Xu, J.; Chen, X.; et al. Dietary Mannan Oligosaccharides Enhance the Non-Specific Immunity, Intestinal Health, and Resistance Capacity of Juvenile Blunt Snout Bream (Megalobrama amblycephala) Against Aeromonas hydrophila. Front. Immunol. 2022, 13, 863657. [Google Scholar] [CrossRef]
- Zha, A.; Tu, R.; Qi, M.; Wang, J.; Tan, B.; Liao, P.; Wu, C.; Yin, Y. Mannan oligosaccharides selenium ameliorates intestinal mucosal barrier, and regulate intestinal microbiota to prevent Enterotoxigenic Escherichia coli-induced diarrhea in weaned piglets. Ecotoxicol. Environ. Saf. 2023, 264, 115448. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.; Tang, H.; Zhang, Y.; Huang, X.; Xu, M. Potential of gut-derived short-chain fatty acids to control enteric pathogens. Front. Microbiol. 2022, 13, 976406. [Google Scholar] [CrossRef]
- Pace, F.; Rudolph, S.E.; Chen, Y.; Bao, B.; Kaplan, D.L.; Watnick, P.I. The Short-Chain Fatty Acids Propionate and Butyrate Augment Adherent-Invasive Escherichia coli Virulence but Repress Inflammation in a Human Intestinal Enteroid Model of Infection. Microbiol. Spectr. 2021, 9, e0136921. [Google Scholar] [CrossRef]
- Ney, L.M.; Wipplinger, M.; Grossmann, M.; Engert, N.; Wegner, V.D.; Mosig, A.S. Short chain fatty acids: Key regulators of the local and systemic immune response in inflammatory diseases and infections. Open Biol. 2023, 13, 230014. [Google Scholar] [CrossRef]
- Mavrogeni, M.E.; Asadpoor, M.; Henricks, P.A.J.; Keshavarzian, A.; Folkerts, G.; Braber, S. Direct Action of Non-Digestible Oligosaccharides against a Leaky Gut. Nutrients 2022, 14, 4699. [Google Scholar] [CrossRef]
- Pujari, R.; Banerjee, G. Impact of prebiotics on immune response: From the bench to the clinic. Immunol. Cell Biol. 2021, 99, 255–273. [Google Scholar] [CrossRef]
- Hayen, S.M.; Knulst, A.C.; Garssen, J.; Otten, H.G.; Willemsen, L.E. Fructo-oligosaccharides modify human DC maturation and peanut-induced autologous T-cell response of allergic patients in vitro. Front. Immunol. 2021, 11, 600125. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Niharika, J.; Kondepudi, K.K.; Bishnoi, M.; Tingirikari, J.M.R. Recent understanding of human milk oligosaccharides in establishing infant gut microbiome and roles in immune system. Food Res. Int. 2022, 151, 110884. [Google Scholar] [CrossRef] [PubMed]
- Weichert, S.; Koromyslova, A.; Singh, B.K.; Hansman, S.; Jennewein, S.; Schroten, H.; Hansman, G.S. Structural basis for norovirus inhibition by human milk oligosaccharides. J. Virol. 2016, 90, 4843–4848. [Google Scholar] [CrossRef]
- Newburg, D.S.; Walker, W.A. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr. Res. 2007, 61, 2–8. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Rotavirus surveillance--worldwide, 2001–2008. MMWR Morb. Mortal. Wkly. Rep. 2008, 57, 1255–1257. [Google Scholar]
- Pandey, R.P.; Kim, D.H.; Woo, J.; Song, J.; Jang, S.H.; Kim, J.B.; Cheong, K.M.; Oh, J.S.; Sohng, J.K. Broad-spectrum neutralization of avian influenza viruses by sialylated human milk oligosaccharides: In vivo assessment of 3′-sialyllactose against H9N2 in chickens. Sci. Rep. 2018, 8, 2563. [Google Scholar] [CrossRef]
- Moon, S.; Lee, K.; Park, M.; Moon, J.; Park, S.H.; Kim, S.; Hwang, J.; Yoon, J.W.; Jeon, S.M.; Kim, J.S.; et al. 3-Fucosyllactose-mediated modulation of immune response against virus infection. Int. J. Antimicrob. Agents 2024, 64, 107187. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.C.; Jin, C.X.; Liu, K.X.; Yang, Z.C. Microbiota-derived short chain fatty acids: Their role and mechanisms in viral infections. Biomed. Pharmacother. 2023, 160, 114414. [Google Scholar] [CrossRef]
- Vandenplas, Y.; De Greef, E.; Veereman, G. Prebiotics in infant formula. Gut Microbes 2014, 5, 681–687. [Google Scholar] [CrossRef]
- Chutipongtanate, S.; Morrow, A.L.; Newburg, D.S. Human Milk Oligosaccharides: Potential Applications in COVID-19. Biomedicines 2022, 10, 346. [Google Scholar] [CrossRef]
- Jang, D.; Lee, D.; Shin, Y.C.; Lee, J.S.; Jung, J.; Ryoo, S. Low molecular weight chitooligosaccharide inhibits infection of SARS-CoV-2 in vitro. J. Appl. Microbiol. 2022, 133, 1089–1098. [Google Scholar] [CrossRef]
- Yu, W.Y.; Li, Y.; Liu, D.D.; Wang, Y.L.; Li, J.J.; Du, Y.G.; Gao, G.F.; Li, Z.M.; Xu, Y.Q.; Wei, J.H. Evaluation and Mechanistic Investigation of Human Milk Oligosaccharide against SARS-CoV-2. J. Agric. Food Chem. 2023, 71, 16102–16113. [Google Scholar] [CrossRef]
- Schijf, M.A.; Kruijsen, D.; Bastiaans, J.; Coenjaerts, F.E.; Garssen, J.; van Bleek, G.M.; van’t Land, B. Specific dietary oligosaccharides increase Th1 responses in a mouse respiratory syncytial virus infection model. J. Virol. 2012, 86, 11472–11482. [Google Scholar] [CrossRef]
- Hill, B. National and international impacts of white spot disease of shrimp. Bull.-Eur. Assoc. Fish Pathol. 2002, 22, 58–65. [Google Scholar]
- Stevens, J.; Blixt, O.; Glaser, L.; Taubenberger, J.K.; Palese, P.; Paulson, J.C.; Wilson, I.A. Glycan microarray analysis of the hemagglutinins from modern and pandemic influenza viruses reveals different receptor specificities. J. Mol. Biol. 2006, 355, 1143–1155. [Google Scholar] [CrossRef]
- Sureda, E.A.; Weström, B.; Pierzynowski, S.G.; Prykhodko, O. Maturation of the Intestinal Epithelial Barrier in Neonatal Rats Coincides with Decreased FcRn Expression, Replacement of Vacuolated Enterocytes and Changed Blimp-1 Expression. PLoS ONE 2016, 11, 0164775. [Google Scholar]
- Jeurissen, S.H.; Lewis, F.; van der Klis, J.D.; Mroz, Z.; Rebel, J.M.; Ter Huurne, A.A. Parameters and techniques to determine intestinal health of poultry as constituted by immunity, integrity, and functionality. Curr. Issues Intest. Microbiol. 2002, 3, 1–14. [Google Scholar]
- Donovan, S.M.; Comstock, S.S. Human milk oligosaccharides influence neonatal mucaosal and systemic immunity. Ann. Nutr. Metab. 2017, 69 (Suppl. S2), 41–51. [Google Scholar] [CrossRef] [PubMed]
- Mazanec, M.B.; Nedrud, J.G.; Kaetzel, C.S.; Lamm, M.E. A three-tiered view of the role of IgA in mucosal defense. Immunol. Today 1993, 14, 430–435. [Google Scholar] [CrossRef]
- Desselberger, U.; Huppertz, H.-I. Immune responses to rotavirus infection and vaccination and associated correlates of protection. J. Infect. Dis. 2011, 203, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Moro, G.E.; Boehm, G. Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J. Nutr. 2007, 137, 2420–2424. [Google Scholar] [CrossRef]
- Newburg, D.S.; He, Y. Neonatal gut microbiota and human milk glycans cooperate to attenuate infection and inflammation. Clin. Obstet. Gynecol. 2015, 58, 814–826. [Google Scholar] [CrossRef]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-like receptors. Annu. Rev. Immunol. 2003, 21, 335–376. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Boehm, G.n.; Matthews, C.; Stahl, B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef]
- Ortega-Gonzalez, M.; Ocon, B.; Romero-Calvo, I.; Anzola, A.; Guadix, E.; Zarzuelo, A.; Suarez, M.D.; Sanchez de Medina, F.; Martinez-Augustin, O. Nondigestible oligosaccharides exert nonprebiotic effects on intestinal epithelial cells enhancing the immune response via activation of TLR4-NFkappaB. Mol. Nutr. Food Res. 2014, 58, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Kiewiet, M.B.G.; Groeneveld, A.; Nauta, A.; de Vos, P. Human milk oligosaccharides and its acid hydrolysate LNT2 show immunomodulatory effects via TLRs in a dose and structure-dependent way. J. Funct. Foods 2019, 59, 174–184. [Google Scholar] [CrossRef]
- Guinane, C.M.; Cotter, P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Therap. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef]
- Monedero, V.; Collado, M.C.; Rodríguez-Díaz, J. Therapeutic Opportunities in Intestinal Microbiota-Virus Interactions. Trends Biotechnol. 2018, 36, 645–648. [Google Scholar] [CrossRef]
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Pattaroni, C.; Lopez-Mejia, I.C.; Riva, E.; Pernot, J.; Ubags, N.; Fajas, L.; Nicod, L.P.; Marsland, B.J. Dietary Fiber Confers Protection against Flu by Shaping Ly6c− Patrolling Monocyte Hematopoiesis and CD8+ T Cell Metabolism. Immunity 2018, 48, 992–1005.e8. [Google Scholar] [CrossRef]
- Imai, K.; Ochiai, K.; Okamoto, T. Reactivation of latent HIV-1 infection by the periodontopathic bacterium Porphyromonas gingivalis involves histone modification. J. Immunol. 2009, 182, 3688–3695. [Google Scholar] [CrossRef] [PubMed]
- Chemudupati, M.; Kenney, A.D.; Smith, A.C.; Fillinger, R.J.; Zhang, L.; Zani, A.; Liu, S.-L.; Anderson, M.Z.; Sharma, A.; Yount, J.S. Butyrate reprograms expression of specific interferon-stimulated genes. J. Virol. 2020, 94, 10–1128. [Google Scholar] [CrossRef]
- Hester, S.N.; Chen, X.; Li, M.; Monaco, M.H.; Comstock, S.S.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Donovan, S.M. Human milk oligosaccharides inhibit rotavirus infectivity in vitro and in acutely infected piglets. Br. J. Nutr. 2013, 110, 1233–1242. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afsharnia, A.; Cai, Y.; Nauta, A.; Groeneveld, A.; Folkerts, G.; Wösten, M.M.S.M.; Braber, S. In Vivo Evidence on the Emerging Potential of Non-Digestible Oligosaccharides as Therapeutic Agents in Bacterial and Viral Infections. Nutrients 2025, 17, 1068. https://doi.org/10.3390/nu17061068
Afsharnia A, Cai Y, Nauta A, Groeneveld A, Folkerts G, Wösten MMSM, Braber S. In Vivo Evidence on the Emerging Potential of Non-Digestible Oligosaccharides as Therapeutic Agents in Bacterial and Viral Infections. Nutrients. 2025; 17(6):1068. https://doi.org/10.3390/nu17061068
Chicago/Turabian StyleAfsharnia, Amirmohammad, Yang Cai, Arjen Nauta, Andre Groeneveld, Gert Folkerts, Marc M. S. M. Wösten, and Saskia Braber. 2025. "In Vivo Evidence on the Emerging Potential of Non-Digestible Oligosaccharides as Therapeutic Agents in Bacterial and Viral Infections" Nutrients 17, no. 6: 1068. https://doi.org/10.3390/nu17061068
APA StyleAfsharnia, A., Cai, Y., Nauta, A., Groeneveld, A., Folkerts, G., Wösten, M. M. S. M., & Braber, S. (2025). In Vivo Evidence on the Emerging Potential of Non-Digestible Oligosaccharides as Therapeutic Agents in Bacterial and Viral Infections. Nutrients, 17(6), 1068. https://doi.org/10.3390/nu17061068






