Vitamin D in Irritable Bowel Syndrome: Exploring Its Role in Symptom Relief and Pathophysiology
Abstract
1. Introduction
2. Vitamin D Uptake, Metabolism, Physiology, and the Role of Vitamin D Receptor
3. Data from Meta-Analyses on the Role of Vitamin D on IBS
3.1. Effect of Vitamin D Supplementation on Severity of Symptoms
3.2. Effect of Vitamin D Supplementation on Quality of Life (QoL)
3.3. Effect of Vitamin D Supplementation on Other Outcomes
4. Role of Vitamin D on IBS Pathophysiology
4.1. Vitamin D in Modulating Gut Microbiome Composition and Its Potential Impact on IBS
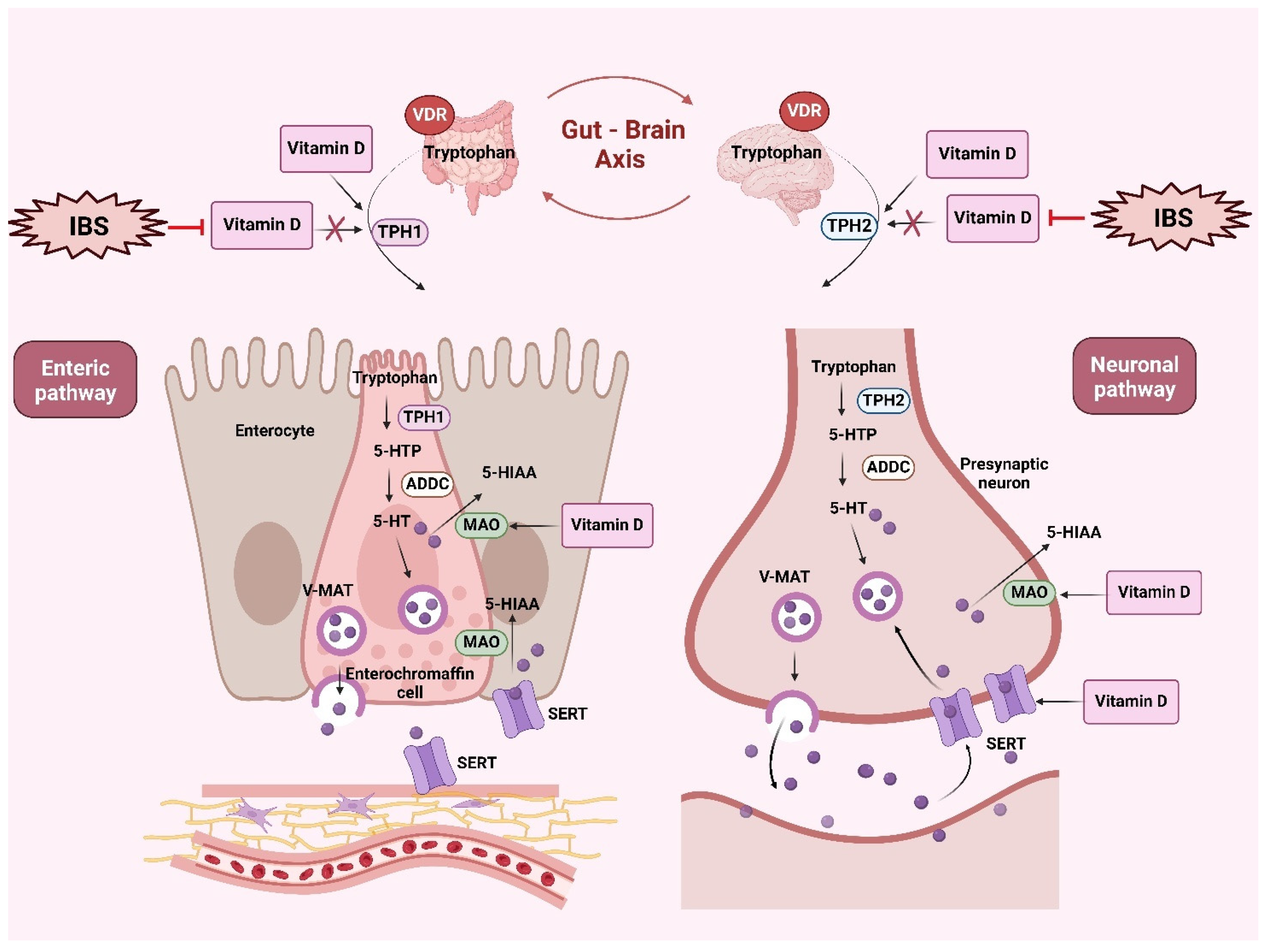
4.2. Serotonergic Signaling in IBS and the Role of Vitamin D
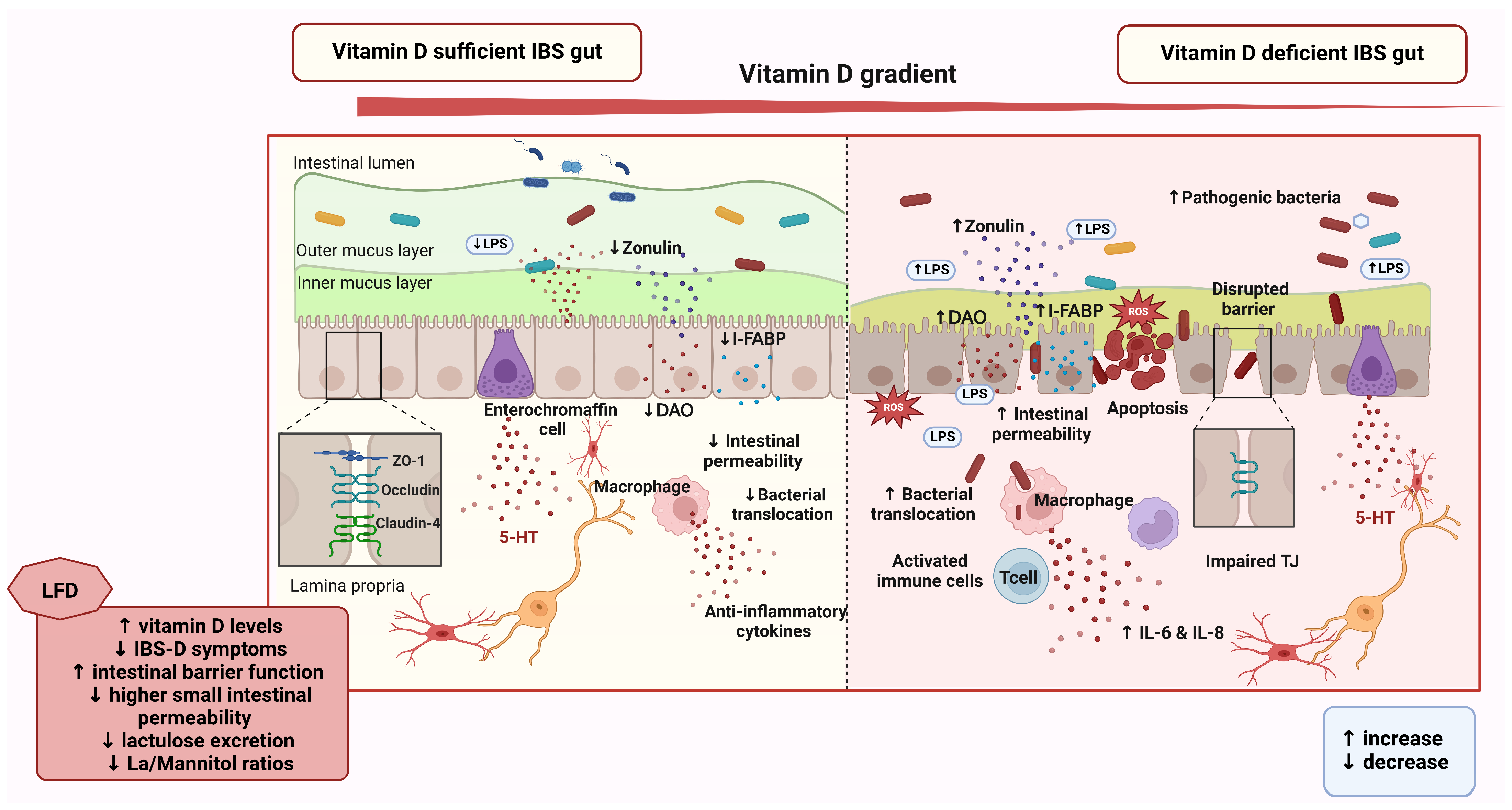
4.3. Vitamin D and Its Role in Gut Health and the Gut–Brain Axis in IBS
5. Overcoming Methodological Challenges
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Öhman, L.; Simrén, M. Pathogenesis of IBS: Role of inflammation, immunity and neuroimmune interactions. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 163–173. [Google Scholar] [CrossRef]
- Lovell, R.M.; Ford, A.C. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721.e4. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef] [PubMed]
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef]
- Ford, A.C.; Lacy, B.E.; Talley, N.J. Irritable Bowel Syndrome. J. Med. N. Engl. J. Med. 2017, 376, 2566–2578. [Google Scholar] [CrossRef]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef]
- Holtmann, G.J.; Ford, A.C.; Talley, N.J. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 2016, 1, 133–146. [Google Scholar] [CrossRef]
- Ionescu, V.A.; Gheorghe, G.; Georgescu, T.F.; Bacalbasa, N.; Gheorghe, F.; Diaconu, C.C. The Latest Data Concerning the Etiology and Pathogenesis of Irritable Bowel Syndrome. J. Clin. Med. 2024, 13, 5124. [Google Scholar] [CrossRef]
- Qin, H.Y.; Cheng, C.W.; Tang, X.D.; Bian, Z.X. Impact of psychological stress on irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 14126–14131. [Google Scholar] [CrossRef]
- Black, C.J.; Yiannakou, Y.; Houghton, L.A.; Ford, A.C. Epidemiological, Clinical, and Psychological Characteristics of Individuals with Self-reported Irritable Bowel Syndrome Based on the Rome IV vs Rome III Criteria. Clin. Gastroenterol. Hepatol. 2020, 18, 392–398.e392. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Patel, N.K. Rome Criteria and a Diagnostic Approach to Irritable Bowel Syndrome. J. Clin. Med. 2017, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Addante, R.; Naliboff, B.; Shih, W.; Presson, A.P.; Tillisch, K.; Mayer, E.A.; Chang, L. Predictors of Health-related Quality of Life in Irritable Bowel Syndrome Patients Compared with Healthy Individuals. J. Clin. Gastroenterol. 2019, 53, e142–e149. [Google Scholar] [CrossRef] [PubMed]
- Prospero, L.; Riezzo, G.; Linsalata, M.; Orlando, A.; D’Attoma, B.; Di Masi, M.; Martulli, M.; Russo, F. Somatization in patients with predominant diarrhoea irritable bowel syndrome: The role of the intestinal barrier function and integrity. BMC Gastroenterol. 2021, 21, 235. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Black, C.J. Irritable bowel syndrome and mental health comorbidity—Approach to multidisciplinary management. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 582–596. [Google Scholar] [CrossRef]
- Sugaya, N. Work-related problems and the psychosocial characteristics of individuals with irritable bowel syndrome: An updated literature review. Biopsychosoc. Med. 2024, 18, 12. [Google Scholar] [CrossRef]
- Khan, S.; Chang, L. Diagnosis and management of IBS. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 565–581. [Google Scholar] [CrossRef]
- Billings, W.; Mathur, K.; Craven, H.J.; Xu, H.; Shin, A. Potential Benefit with Complementary and Alternative Medicine in Irritable Bowel Syndrome: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 1538–1553.e1514. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. J. Med. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Haider, F.; Ghafoor, H.; Hassan, O.F.; Farooqui, K.; Bel Khair, A.O.M.; Shoaib, F. Vitamin D and Cardiovascular Diseases: An Update. Cureus 2023, 15, e49734. [Google Scholar] [CrossRef] [PubMed]
- Seraphin, G.; Rieger, S.; Hewison, M.; Capobianco, E.; Lisse, T.S. The impact of vitamin D on cancer: A mini review. J. Steroid Biochem. Mol. Biol. 2023, 231, 106308. [Google Scholar] [CrossRef] [PubMed]
- Argano, C.; Mirarchi, L.; Amodeo, S.; Orlando, V.; Torres, A.; Corrao, S. The Role of Vitamin D and Its Molecular Bases in Insulin Resistance, Diabetes, Metabolic Syndrome, and Cardiovascular Disease: State of the Art. Int. J. Mol. Sci. 2023, 24, 15485. [Google Scholar] [CrossRef] [PubMed]
- Aggeletopoulou, I.; Thomopoulos, K.; Mouzaki, A. Vitamin D-VDR Novel Anti-Inflammatory Molecules-New Insights into Their Effects on Liver Diseases. Int. J. Mol. Sci. 2022, 23, 8465. [Google Scholar] [CrossRef]
- Triantos, C.; Aggeletopoulou, I.; Thomopoulos, K.; Mouzaki, A. Vitamin D-Liver Disease Association: Biological Basis and Mechanisms of Action. Hepatology 2021, 74, 1065–1073. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Marangos, M.; Assimakopoulos, S.F.; Mouzaki, A.; Thomopoulos, K.; Triantos, C. Vitamin D and Microbiome: Molecular Interaction in Inflammatory Bowel Disease Pathogenesis. Am. J. Pathol. 2023, 193, 656–668. [Google Scholar] [CrossRef]
- Triantos, C.; Aggeletopoulou, I.; Mantzaris, G.J.; Mouzaki, A. Molecular basis of vitamin D action in inflammatory bowel disease. Autoimmun. Rev. 2022, 21, 103136. [Google Scholar] [CrossRef]
- Daryabor, G.; Gholijani, N.; Kahmini, F.R. A review of the critical role of vitamin D axis on the immune system. Exp Mol. Pathol. 2023, 132–133, 104866. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, Z.; Musch, M.W.; Ning, G.; Sun, J.; Hart, J.; Bissonnette, M.; Li, Y.C. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G208–G216. [Google Scholar] [CrossRef]
- Yan, C.; Hu, C.; Chen, X.; Jia, X.; Zhu, Z.; Ye, D.; Wu, Y.; Guo, R.; Jiang, M. Vitamin D improves irritable bowel syndrome symptoms: A meta-analysis. Heliyon 2023, 9, e16437. [Google Scholar] [CrossRef]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Bilezikian, J.P. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocr. Rev. 2024, 45, 625–654. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Vitamin D—Effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef] [PubMed]
- Battault, S.; Whiting, S.J.; Peltier, S.L.; Sadrin, S.; Gerber, G.; Maixent, J.M. Vitamin D metabolism, functions and needs: From science to health claims. Eur. J. Nutr. 2013, 52, 429–441. [Google Scholar] [CrossRef]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef]
- Saponaro, F.; Saba, A.; Zucchi, R. An update on vitamin D metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef]
- Cashman, K.D.; Kinsella, M.; McNulty, B.A.; Walton, J.; Gibney, M.J.; Flynn, A.; Kiely, M. Dietary vitamin D2—A potentially underestimated contributor to vitamin D nutritional status of adults? Br. J. Nutr. 2014, 112, 193–202. [Google Scholar] [CrossRef]
- Maurya, V.K.; Aggarwal, M. Factors influencing the absorption of vitamin D in GIT: An overview. J. Food Sci. Technol. 2017, 54, 3753–3765. [Google Scholar] [CrossRef]
- Wei, X.; Pandohee, J.; Xu, B. Recent developments and emerging trends in dietary vitamin D sources and biological conversion. Crit Rev. Food Sci. Nutr. 2023, 64, 10121–10137. [Google Scholar] [CrossRef]
- Christakos, S.; Ajibade, D.V.; Dhawan, P.; Fechner, A.J.; Mady, L.J. Vitamin D: Metabolism. Rheum Dis. Clin. N. Am. 2012, 38, 1–11. [Google Scholar] [CrossRef]
- Latic, N.; Erben, R.G. FGF23 and Vitamin D Metabolism. JBMR Plus. 2021, 5, e10558. [Google Scholar] [CrossRef] [PubMed]
- Bergwitz, C.; Jüppner, H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu. Rev. Med. 2010, 61, 91–104. [Google Scholar] [CrossRef]
- Carlberg, C. Genome-wide (over)view on the actions of vitamin D. Front. Physiol. 2014, 5, 167. [Google Scholar] [CrossRef]
- Sirajudeen, S.; Shah, I.; Al Menhali, A. A Narrative Role of Vitamin D and Its Receptor: With Current Evidence on the Gastric Tissues. Int. J. Mol. Sci. 2019, 20, 3832. [Google Scholar] [CrossRef]
- Voltan, G.; Cannito, M. Vitamin D: An Overview of Gene Regulation, Ranging from Metabolism to Genomic Effects. Genes 2023, 14, 1691. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.W.; Okamura, W.H.; Hammond, M.W.; Bishop, J.E.; Dormanen, M.C.; Bouillon, R.; van Baelen, H.; Ridall, A.L.; Daane, E.; Khoury, R.; et al. Comparison of 6-s-cis- and 6-s-trans-locked analogs of 1alpha,25-dihydroxyvitamin D3 indicates that the 6-s-cis conformation is preferred for rapid nongenomic biological responses and that neither 6-s-cis- nor 6-s-trans-locked analogs are preferred for genomic biological responses. Mol. Endocrinol. 1997, 11, 1518–1531. [Google Scholar] [CrossRef] [PubMed]
- Hii, C.S.; Ferrante, A. The Non-Genomic Actions of Vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef]
- Abuelazm, M.; Muhammad, S.; Gamal, M.; Labieb, F.; Amin, M.A.; Abdelazeem, B.; Brašić, J.R. The Effect of Vitamin D Supplementation on the Severity of Symptoms and the Quality of Life in Irritable Bowel Syndrome Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 2618. [Google Scholar] [CrossRef]
- Bin, Y.; Kang, L.; Lili, Y. Vitamin D status in irritable bowel syndrome and the impact of supplementation on symptoms: A systematic review and meta-analysis. Nutr. Hosp. 2022, 39, 1144–1152. [Google Scholar] [CrossRef]
- Chong, R.I.H.; Yaow, C.Y.L.; Loh, C.Y.L.; Teoh, S.E.; Masuda, Y.; Ng, W.K. Vitamin D supplementation for irritable bowel syndrome: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2022, 37, 993–1003. [Google Scholar] [CrossRef]
- Huang, H.; Lu, L.; Chen, Y.; Zeng, Y.; Xu, C. The efficacy of vitamin D supplementation for irritable bowel syndrome: A systematic review with meta-analysis. Nutr. J. 2022, 21, 24. [Google Scholar] [CrossRef]
- Cara, K.C.; Taylor, S.F. The effects of vitamin D intake and status on symptom severity and quality-of-life in adults with irritable bowel syndrome (IBS): A systematic review and meta-analysis. Crit Rev. Food Sci. Nutr. 2024, 1–14. [Google Scholar] [CrossRef]
- Veraza, D.I.; Calderon, G.; Jansson-Knodell, C.; Aljaras, R.; Foster, E.D.; Xu, H.; Biruete, A.; Shin, A. A systematic review and meta-analysis of diet and nutrient intake in adults with irritable bowel syndrome. Neurogastroenterol. Motil. 2024, 36, e14698. [Google Scholar] [CrossRef] [PubMed]
- Abbasnezhad, A.; Amani, R.; Hajiani, E.; Alavinejad, P.; Cheraghian, B.; Ghadiri, A. Effect of vitamin D on gastrointestinal symptoms and health-related quality of life in irritable bowel syndrome patients: A randomized double-blind clinical trial. Neurogastroenterol. Motil. 2016, 28, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Abuelazm, M.; Abdelazeem, B. Vitamin D supplementation for irritable bowel syndrome: Concerns about the meta-analysis. J. Gastroenterol. Hepatol. 2022, 37, 1402–1403. [Google Scholar] [CrossRef]
- Głąbska, D.; Kołota, A. Vitamin D Supplementation and Mental Health in Inflammatory Bowel Diseases and Irritable Bowel Syndrome Patients: A Systematic Review. Nutrients 2021, 13, 3662. [Google Scholar] [CrossRef]
- Tangestani, H.; Boroujeni, H.K. Vitamin D and The Gut Microbiota: A Narrative Literature Review. Clin. Nutr. Res. 2021, 10, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.A.; Miller, C.W.; El-Abbassi, A.M.; Cutchins, D.C.; Cutchins, C.; Grant, W.B.; Peiris, A.N. Antimicrobial implications of vitamin D. Dermatoendocrinol 2011, 3, 220–229. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.G. Vitamin D Receptor Influences Intestinal Barriers in Health and Disease. Cells 2022, 11, 1129. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Regulation of Immune Function. Curr. Osteoporos. Rep. 2022, 20, 186–193. [Google Scholar] [CrossRef]
- Fenercioglu, A.K. The Anti-Inflammatory Roles of Vitamin D for Improving Human Health. Curr. Issues Mol. Biol. 2024, 46, 13514–13525. [Google Scholar] [CrossRef]
- Ogbu, D.; Xia, E.; Sun, J. Gut instincts: Vitamin D/vitamin D receptor and microbiome in neurodevelopment disorders. Open Biol. 2020, 10, 200063. [Google Scholar] [CrossRef]
- Lopez, D.V.; Al-Jaberi, F.A.H.; Woetmann, A.; Ødum, N.; Bonefeld, C.M.; Kongsbak-Wismann, M.; Geisler, C. Macrophages Control the Bioavailability of Vitamin D and Vitamin D-Regulated T Cell Responses. Front. Immunol. 2021, 12, 722806. [Google Scholar] [CrossRef]
- Hamza, F.N.; Daher, S. Immunomodulatory Properties of Vitamin D in the Intestinal and Respiratory Systems. Nutrients 2023, 15, 1696. [Google Scholar] [CrossRef]
- Ostaff, M.J.; Stange, E.F.; Wehkamp, J. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol. Med. 2013, 5, 1465–1483. [Google Scholar] [CrossRef]
- Akimbekov, N.S.; Digel, I.; Sherelkhan, D.K.; Lutfor, A.B.; Razzaque, M.S. Vitamin D and the Host-Gut Microbiome: A Brief Overview. Acta Histochem. Cytochem. 2020, 53, 33–42. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Triantos, C. Microbiome Shifts and Their Impact on Gut Physiology in Irritable Bowel Syndrome. Int. J. Mol. Sci. 2024, 25, 12395. [Google Scholar] [CrossRef]
- Singh, P.; Rawat, A.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci. Rep. 2020, 10, 21641. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Goulart, R.A. Irritable bowel syndrome: A review of the general aspects and the potential role of vitamin D. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 345–359. [Google Scholar] [CrossRef]
- Matthews, S.W.; Plantinga, A.; Burr, R.; Cain, K.C.; Savidge, T.; Kamp, K. Exploring the Role of Vitamin D and the Gut Microbiome: A Cross-Sectional Study of Individuals with Irritable Bowel Syndrome and Healthy Controls. Biol. Res. Nurs. 2023, 25, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Quigley, E.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.; Moayyedi, P. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: Systematic review and meta-analysis. Am. J. Gastroenterol. 2014, 109, 1350–1365, quiz 1366. [Google Scholar] [CrossRef]
- Patrick, R.P.; Ames, B.N. Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism. FASEB J. 2014, 28, 2398–2413. [Google Scholar] [CrossRef]
- Kaneko, I.; Sabir, M.S.; Dussik, C.M.; Whitfield, G.K.; Karrys, A.; Hsieh, J.C.; Haussler, M.R.; Meyer, M.B.; Pike, J.W.; Jurutka, P.W. 1,25-Dihydroxyvitamin D regulates expression of the tryptophan hydroxylase 2 and leptin genes: Implication for behavioral influences of vitamin D. FASEB J. 2015, 29, 4023–4035. [Google Scholar] [CrossRef]
- McCann, J.C.; Ames, B.N. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008, 22, 982–1001. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Chen, Y.; Du, J. Critical roles of intestinal epithelial vitamin D receptor signaling in controlling gut mucosal inflammation. J. Steroid Biochem. Mol. Biol. 2015, 148, 179–183. [Google Scholar] [CrossRef]
- Dussik, C.M.; Hockley, M.; Grozić, A.; Kaneko, I.; Zhang, L.; Sabir, M.S.; Park, J.; Wang, J.; Nickerson, C.A.; Yale, S.H.; et al. Gene Expression Profiling and Assessment of Vitamin D and Serotonin Pathway Variations in Patients with Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2018, 24, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Sabir, M.S.; Haussler, M.R.; Mallick, S.; Kaneko, I.; Lucas, D.A.; Haussler, C.A.; Whitfield, G.K.; Jurutka, P.W. Optimal vitamin D spurs serotonin: 1,25-dihydroxyvitamin D represses serotonin reuptake transport (SERT) and degradation (MAO-A) gene expression in cultured rat serotonergic neuronal cell lines. Genes Nutr. 2018, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Pertile, R.A.; Cui, X.; Eyles, D.W. Vitamin D signaling and the differentiation of developing dopamine systems. Neuroscience 2016, 333, 193–203. [Google Scholar] [CrossRef]
- Patrick, R.P.; Ames, B.N. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: Relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 2015, 29, 2207–2222. [Google Scholar] [CrossRef]
- Khalighi Sikaroudi, M.; Mokhtare, M.; Shidfar, F.; Janani, L.; Faghihi Kashani, A.; Masoodi, M.; Agah, S.; Dehnad, A.; Shidfar, S. Effects of vitamin D3 supplementation on clinical symptoms, quality of life, serum serotonin (5-hydroxytryptamine), 5-hydroxy-indole acetic acid, and ratio of 5-HIAA/5-HT in patients with diarrhea-predominant irritable bowel syndrome: A randomized clinical trial. Excli J. 2020, 19, 652–667. [Google Scholar] [CrossRef]
- Grozić, A.; Coker, K.; Dussik, C.M. Identification of putative transcriptomic biomarkers in irritable bowel syndrome (IBS): Differential gene expression and regulation of TPH1 and SERT by vitamin D. PLoS ONE 2022, 17, e0275683. [Google Scholar] [CrossRef] [PubMed]
- Tazzyman, S.; Richards, N.; Trueman, A.R.; Evans, A.L.; Grant, V.A.; Garaiova, I.; Plummer, S.F.; Williams, E.A.; Corfe, B.M. Vitamin D associates with improved quality of life in participants with irritable bowel syndrome: Outcomes from a pilot trial. BMJ Open Gastroenterol. 2015, 2, e000052. [Google Scholar] [CrossRef] [PubMed]
- Linsalata, M.; Riezzo, G.; Orlando, A. The Relationship between Low Serum Vitamin D Levels and Altered Intestinal Barrier Function in Patients with IBS Diarrhoea Undergoing a Long-Term Low-FODMAP Diet: Novel Observations from a Clinical Trial. Nutrients 2021, 13, 1011. [Google Scholar] [CrossRef] [PubMed]
- Uberti, F.; Trotta, F.; Cavalli, R.; Galla, R.; Caldera, F. Enhancing Vitamin D3 Efficacy: Insights from Complexation with Cyclodextrin Nanosponges and Its Impact on Gut-Brain Axes in Physiology and IBS Syndrome. Int. J. Mol. Sci. 2024, 25, 2189. [Google Scholar] [CrossRef]

| Meta-Analysis | Date | No. of Studies | No. of Participants | Comparing Arms | Effect Size | 95% CI | p Value | I2 (%) | Effect Size | 95% CI | p Value | I2 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Severity of Symptoms | Quality of Life | |||||||||||
| Abuelazm et al. [48] | 2022 | 6 | 616 | Vitamin D vs. placebo in IBS | −45.82 (MD) | [−93.62, 1.98] | 0.06 | 97.07 | 6.19 (MD) | [0.35, 12.03] | 0.04 | 97.07 |
| Bin et al. [49] | 2022 | 12 | 1331 | Vitamin D vs. placebo in IBS | −0.43 (SMD) | [−0.89, 0.03] | - | 85.6 | 0.65 (SMD) | [0.14, 1.15] | - | 85.6 |
| Chong et al. [50] | 2022 | 8 | 685 | Vitamin D vs. placebo in IBS | −0.77 (SMD) | [−1.47, −0.07] | 0.04 | 91 | 0.54 (SMD) | [−0.34, 1.41] | 0.15 | 87 |
| Huang et al. [51] | 2022 | 4 | 335 | Vitamin D vs. placebo in IBS | −55.55 (WMD) | [−70.22, −40.87] | <0.001 | 53.7 | 14.98 (WMD) | [12.06, 17.90] | <0.001 | 0.0 |
| Cara et al. [52] | 2024 | 12 | - | Vitamin D vs. placebo in IBS | −25.89 (MD) | [−55.26, 3.48] | 0.15 | 92.8 | 3.19 (MD) | [2.14, 4.24] | 0.00001 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aggeletopoulou, I.; Geramoutsos, G.; Pastras, P.; Triantos, C. Vitamin D in Irritable Bowel Syndrome: Exploring Its Role in Symptom Relief and Pathophysiology. Nutrients 2025, 17, 1028. https://doi.org/10.3390/nu17061028
Aggeletopoulou I, Geramoutsos G, Pastras P, Triantos C. Vitamin D in Irritable Bowel Syndrome: Exploring Its Role in Symptom Relief and Pathophysiology. Nutrients. 2025; 17(6):1028. https://doi.org/10.3390/nu17061028
Chicago/Turabian StyleAggeletopoulou, Ioanna, Georgios Geramoutsos, Ploutarchos Pastras, and Christos Triantos. 2025. "Vitamin D in Irritable Bowel Syndrome: Exploring Its Role in Symptom Relief and Pathophysiology" Nutrients 17, no. 6: 1028. https://doi.org/10.3390/nu17061028
APA StyleAggeletopoulou, I., Geramoutsos, G., Pastras, P., & Triantos, C. (2025). Vitamin D in Irritable Bowel Syndrome: Exploring Its Role in Symptom Relief and Pathophysiology. Nutrients, 17(6), 1028. https://doi.org/10.3390/nu17061028








