Multidisciplinary Integrative Medicine Approach for Cancer Patients: A Multicenter Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and CIM Approach
2.2. Rationale for the CIM Approach Selection
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Clinicopathological Profile and Treatment Landscape of Oncology Patients Receiving Complementary Medicine
3.2. Oncological Treatment Duration, Toxicity, and Responses in Patients Receiving CIM
3.3. Implementation of CIM Approach in the Oncological Practice
3.4. Clinical Benefit, Toxicity, and Costs of Cim Approach
3.5. Factors Influencing Response to Cim and Survival
3.6. Associations Between Response to Act, Cancer Type, and Cim
3.7. Factors Predicting OS in Cancer Patients Treated with CIM
3.8. Comparative Efficacy of CIM in Oncology: Cancer-Specific Applications and Multidimensional Clinical Benefits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Complementary, Alternative, or Integrative Health: What’s in a Name? Available online: https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name (accessed on 8 August 2024).
- Berretta, M.; Morra, A.; Taibi, R.; Monari, F.; Maurea, N.; Ippolito, M.; Tirelli, U.; Fiorica, F.; Montella, L.; Facchini, G.; et al. Improved Survival and Quality of Life Through an Integrative, Multidisciplinary Oncological Approach: Pathophysiological Analysis of Four Clinical Cancer Cases and Review of the Literature. Front. Pharmacol. 2022, 13, 867907. [Google Scholar] [CrossRef] [PubMed]
- Berretta, M.; Della Pepa, C.; Tralongo, P.; Fulvi, A.; Martellotta, F.; Lleshi, A.; Nasti, G.; Fisichella, R.; Romano, C.; De Divitiis, C.; et al. Use of Complementary and Alternative Medicine (CAM) in Cancer Patients: An Italian Multicenter Survey. Oncotarget 2017, 8, 24401–24414. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, P.; Law, S.; Tian, H.; Leung, W.; Xu, C. Preventive Effect of Curcumin Against Chemotherapy-Induced Side-Effects. Front. Pharmacol. 2018, 9, 1374. [Google Scholar] [CrossRef]
- Wells, J.C.; Sidhu, A.; Ding, K.; Smoragiewicz, M.; Heng, D.Y.C.; Shepherd, F.A.; Ellis, P.M.; Bradbury, P.A.; Jonker, D.J.; Siu, L.L.; et al. Complementary Medicine Use Amongst Patients with Metastatic Cancer Enrolled in Phase III Clinical Trials. Oncologist 2022, 27, e286–e293. [Google Scholar] [CrossRef]
- Kessel, K.A.; Lettner, S.; Kessel, C.; Bier, H.; Biedermann, T.; Friess, H.; Herrschbach, P.; Gschwend, J.E.; Meyer, B.; Peschel, C.; et al. Use of Complementary and Alternative Medicine (CAM) as Part of the Oncological Treatment: Survey about Patients’ Attitude towards CAM in a University-Based Oncology Center in Germany. PLoS ONE 2016, 11, e0165801. [Google Scholar] [CrossRef]
- Berretta, M.; Rinaldi, L.; Taibi, R.; Tralongo, P.; Fulvi, A.; Montesarchio, V.; Madeddu, G.; Magistri, P.; Bimonte, S.; Trovò, M.; et al. Physician Attitudes and Perceptions of Complementary and Alternative Medicine (CAM): A Multicentre Italian Study. Front. Oncol. 2020, 10, 594. [Google Scholar] [CrossRef]
- Trillet-Lenoir, V. REPORT on Strengthening Europe in the Fight Against Cancer—Towards a Comprehensive and Coordinated Strategy|A9-0001/2022|European Parliament. Available online: https://www.europarl.europa.eu/doceo/document/A-9-2022-0001_EN.html (accessed on 8 August 2024).
- Mao, J.J.; Greenlee, H.; Bao, T.; Ismaila, N.; Bruera, E. Integrative Medicine for Pain Management in Oncology: Society for Integrative Oncology-ASCO Guideline Summary and Q&A. JCO Oncol. Pract. 2023, 19, 45–48. [Google Scholar] [CrossRef]
- Greenlee, H.; DuPont-Reyes, M.J.; Balneaves, L.G.; Carlson, L.E.; Cohen, M.R.; Deng, G.; Johnson, J.A.; Mumber, M.; Seely, D.; Zick, S.M.; et al. Clinical Practice Guidelines on the Evidence-based Use of Integrative Therapies during and after Breast Cancer Treatment. CA Cancer J. Clin. 2017, 67, 194–232. [Google Scholar] [CrossRef]
- Kempen, J.H. Appropriate Use and Reporting of Uncontrolled Case Series in the Medical Literature. Am. J. Ophthalmol. 2011, 151, 7–10.e1. [Google Scholar] [CrossRef]
- Berretta, M.; Quagliariello, V.; Maurea, N.; Di Francia, R.; Sharifi, S.; Facchini, G.; Rinaldi, L.; Piezzo, M.; Manuela, C.; Nunnari, G.; et al. Multiple Effects of Ascorbic Acid against Chronic Diseases: Updated Evidence from Preclinical and Clinical Studies. Antioxidants 2020, 9, 1182. [Google Scholar] [CrossRef]
- Berretta, M.; Quagliariello, V.; Bignucolo, A.; Facchini, S.; Maurea, N.; Di Francia, R.; Fiorica, F.; Sharifi, S.; Bressan, S.; Richter, S.N.; et al. The Multiple Effects of Vitamin D against Chronic Diseases: From Reduction of Lipid Peroxidation to Updated Evidence from Clinical Studies. Antioxidants 2022, 11, 1090. [Google Scholar] [CrossRef] [PubMed]
- Arora, J.; Wang, J.; Weaver, V.; Zhang, Y.; Cantorna, M.T. Novel Insight into the Role of the Vitamin D Receptor in the Development and Function of the Immune System. J. Steroid Biochem. Mol. Biol. 2022, 219, 106084. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yang, J.; Liu, J.; Wang, Y.; Mu, J.; Zeng, Q.; Deng, S.; Zhou, H. Signaling Pathways in Cancer-Associated Fibroblasts and Targeted Therapy for Cancer. Signal Transduct. Target. Ther. 2021, 6, 218. [Google Scholar] [CrossRef] [PubMed]
- Ottaiano, A.; Facchini, S.; Santorsola, M.; Nasti, G.; Facchini, G.; Montella, L.; Maurea, N.; Cascella, M.; Iervolino, D.; Facchini, B.A.; et al. Circulating Vitamin D Level and Its Impact on Mortality and Recurrence in Stage III Colorectal Cancer Patients: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 3012. [Google Scholar] [CrossRef]
- Ottaiano, A.; Iacovino, M.L.; Santorsola, M.; Facchini, S.; Iervolino, D.; Perri, F.; Nasti, G.; Quagliariello, V.; Maurea, N.; Ronchi, A.; et al. Circulating Vitamin D Level before Initiating Chemotherapy Impacts on the Time-to-Outcome in Metastatic Colorectal Cancer Patients: Systematic Review and Meta-Analysis. J. Transl. Med. 2024, 22, 119. [Google Scholar] [CrossRef]
- Ottaiano, A.; Facchini, B.A.; Iacovino, M.; Santorsola, M.; Facchini, S.; Di Mauro, G.; Toscano, E.; Montopoli, M.; Di Mauro, A.; Quagliariello, V.; et al. Impact of Vitamin D Levels on Progression-Free Survival and Response to Neoadjuvant Chemotherapy in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 4206. [Google Scholar] [CrossRef]
- Legesse Bedada, T.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for Cancer Alternative Prevention and Treatment. Biomed. Pharmacother. 2020, 129, 110409. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Lekshmi Priya, K.S.; Maheswary, D.; Ravi, S.S.S.; Leela, K.V.; Lathakumari, R.H.; Malavika, G. The Impact of Probiotics on Oral Cancer: Mechanistic Insights and Therapeutic Strategies. Oral Oncol. Rep. 2025, 13, 100715. [Google Scholar] [CrossRef]
- Kingkaew, E. Somboon Tanasupawat Probiotics Based Anticancer Immunity in Skin Cancer. Available online: http://www.eurekaselect.com/chapter/19234 (accessed on 31 January 2025).
- Mechanism of Probiotic Action in Anticancer Immunity. Available online: http://www.eurekaselect.com/chapter/19233 (accessed on 4 February 2025).
- Narayanan, S.; de Mores, A.R.; Cohen, L.; Anwar, M.M.; Lazar, F.; Hicklen, R.; Lopez, G.; Yang, P.; Bruera, E. Medicinal Mushroom Supplements in Cancer: A Systematic Review of Clinical Studies. Curr. Oncol. Rep. 2023, 25, 569–587. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, Q.; Rong, C.; Wang, S.; Zhao, Z.; Liu, Y.; Xu, J. Immunomodulatory Effects of Edible and Medicinal Mushrooms and Their Bioactive Immunoregulatory Products. J. Fungi 2020, 6, 269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Liu, H.; Yuan, Y.; Wang, Q.; Wang, L.; Wu, J. Lentinan Progress in Inflammatory Diseases and Tumor Diseases. Eur. J. Med. Res. 2024, 29, 8. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Chang, Y.; Zhang, L. Coriolus Versicolor Polysaccharopeptide as an Immunotherapeutic in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J. Current Uses of Mushrooms in Cancer Treatment and Their Anticancer Mechanisms. Int. J. Mol. Sci. 2022, 23, 10502. [Google Scholar] [CrossRef]
- Chay, W.Y.; Tham, C.K.; Toh, H.C.; Lim, H.Y.; Tan, C.K.; Lim, C.; Wang, W.-W.; Choo, S.-P. Coriolus Versicolor (Yunzhi) Use as Therapy in Advanced Hepatocellular Carcinoma Patients with Poor Liver Function or Who Are Unfit for Standard Therapy. J. Altern. Complement. Med. 2017, 23, 648–652. [Google Scholar] [CrossRef]
- Neuwirthová, J.; Urbánková, P.; Smilek, P. Immunostimulatory and Anticancer Effect of Reishi and Coriol Extracts at the Level of Clinical Studies and Their Implementation in Practice. Klin. Onkol. 2020, 33, 426–434. [Google Scholar] [CrossRef]
- Kim, H.S.; Hong, J.T.; Kim, Y.; Han, S.-B. Stimulatory Effect of β-Glucans on Immune Cells. Immune Netw. 2011, 11, 191–195. [Google Scholar] [CrossRef]
- Roda, E.; Luca, F.D.; Iorio, C.D.; Ratto, D.; Siciliani, S.; Ferrari, B.; Cobelli, F.; Borsci, G.; Priori, E.C.; Chinosi, S.; et al. Novel Medicinal Mushroom Blend as a Promising Supplement in Integrative Oncology: A Multi-Tiered Study Using 4T1 Triple-Negative Mouse Breast Cancer Model. Int. J. Mol. Sci. 2020, 21, 3479. [Google Scholar] [CrossRef]
- Chan, G.C.-F.; Chan, W.K.; Sze, D.M.-Y. The Effects of Beta-Glucan on Human Immune and Cancer Cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef]
- Lam, K.-L.; Chi-Keung Cheung, P. Non-Digestible Long Chain Beta-Glucans as Novel Prebiotics. Bioact. Carbohydr. Diet. Fibre 2013, 2, 45–64. [Google Scholar] [CrossRef]
- Sharma, E.; Bairwa, R.; Lal, P.; Pattanayak, S.; Chakrapani, K.; Poorvasandhya, R.; Kumar, A.; Altaf, M.A.; Tiwari, R.K.; Lal, M.K.; et al. Edible Mushrooms Trending in Food: Nutrigenomics, Bibliometric, from Bench to Valuable Applications. Heliyon 2024, 10, e36963. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The Importance of Glutathione in Human Disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Kurtovic, S.; Modén, O.; Shokeer, A.; Mannervik, B. Structural Determinants of Glutathione Transferases with Azathioprine Activity Identified by DNA Shuffling of Alpha Class Members. J. Mol. Biol. 2008, 375, 1365–1379. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Chen, C.; Xu, Y.; Shen, J.; Guo, H.; Li, H.; Li, X.; Kang, D.; Shao, Y.; Zhu, Z.; et al. Is the Combinational Administration of Doxorubicin and Glutathione a Reasonable Proposal? Acta Pharmacol. Sin. 2019, 40, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Salas-Vega, S.; Iliopoulos, O.; Mossialos, E. Assessment of Overall Survival, Quality of Life, and Safety Benefits Associated with New Cancer Medicines. JAMA Oncol. 2017, 3, 382–390. [Google Scholar] [CrossRef]
- Lopez, G.; McQuade, J.; Cohen, L.; Williams, J.T.; Spelman, A.R.; Fellman, B.; Li, Y.; Bruera, E.; Lee, R.T. Integrative Oncology Physician Consultations at a Comprehensive Cancer Center: Analysis of Demographic, Clinical and Patient Reported Outcomes. J. Cancer 2017, 8, 395–402. [Google Scholar] [CrossRef]
- Popović, V.; Živković, J.; Davidović, S.; Stevanović, M.; Stojković, D. Mycotherapy of Cancer: An Update on Cytotoxic and Antitumor Activities of Mushrooms, Bioactive Principles and Molecular Mechanisms of Their Action. Curr. Top. Med. Chem. 2013, 13, 2791–2806. [Google Scholar] [CrossRef]
- De Luca, F.; Roda, E.; Rossi, P.; Bottone, M.G. Medicinal Mushrooms in Metastatic Breast Cancer: What Is Their Therapeutic Potential as Adjuvant in Clinical Settings? Curr. Issues Mol. Biol. 2024, 46, 7577–7591. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Jin, X.; Ruiz Beguerie, J.; Sze, D.M.-Y.; Chan, G.C.F. Ganoderma lucidum (Reishi Mushroom) for Cancer Treatment. Cochrane Database Syst. Rev. 2016, 4, CD007731. [Google Scholar] [CrossRef]
- Rodriguez-Arrastia, M.; Martinez-Ortigosa, A.; Rueda-Ruzafa, L.; Folch Ayora, A.; Ropero-Padilla, C. Probiotic Supplements on Oncology Patients’ Treatment-Related Side Effects: A Systematic Review of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 4265. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.-Q.; Li, L. The Potential Role of Probiotics in Cancer Prevention and Treatment. Nutr. Cancer 2016, 68, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Luo, X.; Yang, D.; Li, Y.; Gong, T.; Li, B.; Cheng, J.; Chen, R.; Guo, X.; Yuan, W. Effects of Probiotic Supplementation on Related Side Effects after Chemoradiotherapy in Cancer Patients. Front. Oncol. 2022, 12, 1032145. [Google Scholar] [CrossRef]
- Ohigashi, S.; Hoshino, Y.; Ohde, S.; Onodera, H. Functional Outcome, Quality of Life, and Efficacy of Probiotics in Postoperative Patients with Colorectal Cancer. Surg. Today 2011, 41, 1200–1206. [Google Scholar] [CrossRef]
- Richardson, M.A.; Sanders, T.; Palmer, J.L.; Greisinger, A.; Singletary, S.E. Complementary/Alternative Medicine Use in a Comprehensive Cancer Center and the Implications for Oncology. J. Clin. Oncol. 2000, 18, 2505–2514. [Google Scholar] [CrossRef]
- Drug Interactions Checker—Medscape Drug Reference Database. Available online: https://reference.medscape.com/drug-interactionchecker?faf=1&ecd=ppc_google_rem-traf_mscp_interactions_md-hdle-cohort_us&gad_source=1&gclid=CjwKCAiA34S7BhAtEiwACZzv4SB-wHL8zhym8YVPRnL-vSGtIni5ElNDMVEdPtonSdVCeG3PjWTJuRoCjp8QAvD_BwE (accessed on 4 February 2025).
- Drug Interaction Checker ← Quickly Check Your Meds. Available online: https://www.drugs.com/drug_interactions.html (accessed on 4 February 2025).
- Johnson, S.B.; Park, H.S.; Gross, C.P.; Yu, J.B. Complementary Medicine, Refusal of Conventional Cancer Therapy, and Survival Among Patients with Curable Cancers. JAMA Oncol. 2018, 4, 1375–1381. [Google Scholar] [CrossRef]
- Buckner, C.A.; Lafrenie, R.M.; Dénommée, J.A.; Caswell, J.M.; Want, D.A. Complementary and Alternative Medicine Use in Patients before and after a Cancer Diagnosis. Curr. Oncol. 2018, 25, e275–e281. [Google Scholar] [CrossRef]
| Variable | No. (%) |
|---|---|
| Age | |
| Median: 62 y; range (28–83 y) | |
| <70 y | 41 (75.9) |
| ≥70 y | 13 (24.1) |
| Gender | |
| Female | 31 (57.4) |
| Male | 23 (42.6) |
| Type of cancer | |
| Breast | 14 (25.9) |
| CNS | 11 (20.4) |
| Colorectal | 9 (16.6) |
| Urogynecological | 6 (11.1) |
| GI non colorectal | 4 (7.4) |
| Hematologic | 4 (7.4) |
| Multiple cancers | 2 (3.7) |
| Lung | 2 (3.7) |
| Other | 2 (3.7) |
| Stage | |
| Advanced | 45 (83.3) |
| Early | 9 (16.7) |
| Grading | |
| G1 | 17 (31.5) |
| G2/3 | 37 (68.5) |
| Metastatic sites | |
| Not applicable | 11 (20.3) |
| Lymph nodes | 6 (11.1) |
| Liver | 4 (7.4) |
| Bone | 3 (5.5) |
| Peritoneum/Pleura | 3 (5.5) |
| Lung | 1 (1.8) |
| Multiple sites | 18 (33.3) |
| Other * | 8 (14.8) |
| PS ECOG | |
| 0–1 | 42 (77.7) |
| 2–3 | 12 (22.2) |
| Type of oncological treatment | |
| Chemotherapy | 20 (37.7) |
| Hormone therapy | 8 (14.8) |
| Target therapy | 5 (9.2) |
| Anti-angiogenic drugs | 4 (7.4) |
| Immunotherapy | 1 (1.8) |
| Radiotherapy | 1 (1.8) |
| None | 2 (3.7) |
| Combinations | 13 (24.1) |
| Variable | No. (%) |
|---|---|
| Lifestyle | |
| Healthy | 26 (48.1) |
| Sedentary | 17 (31.5) |
| Active | 7 (13.0) |
| Smoking | 4 (7.4) |
| Comorbidities | |
| None | 33 (61.1) |
| Cardiovascular | 8 (14.8) |
| Metabolic | 5 (9.3) |
| Liver | 3 (5.6) |
| Anxiety–depression syndrome | 3 (5.6) |
| Multiple morbidities | 2 (3.7) |
| Variable | No. (%) |
|---|---|
| Oncological treatment duration | |
| <6 months | 11 (20.4) |
| ≥6–<12 months | 11 (20.4) |
| ≥12 months | 32 (59.2) |
| Toxicity * | |
| ≤G2 | 35 (64.8) |
| Hematologic | 15 |
| Non-hematologic | 41 |
| Fatigue | 20 |
| Gastrointestinal | 13 |
| Neurologic | 3 |
| Cutis | 1 |
| Renal | 1 |
| ≥G3 | 2 (3.7) |
| Renal | 1 |
| Gastrointestinal | 1 |
| No toxicity | 17 (31.5) |
| Best response ** | |
| Complete response | 10 (20.4) |
| Partial response | 19 (38.7) |
| Stable disease | 17 (34.7) |
| Progressive disease | 3 (6.1) |
| Variable | No. (%) |
|---|---|
| PS ECOG at the start of CIM | |
| 0–1 | 39 (72.2) |
| 2–3 | 14 (25.9) |
| Type of complementary therapy | |
| U-CARE + Polytherapy-based complementary medicine | 25 (46.3) |
| U-CARE | 22 (40.7) |
| Polytherapy-based complementary medicine | 7 (12.9) |
| Line of therapy associated with CIM | |
| First | 20 (37.0) |
| Second | 17 (31.4) |
| Beyond second line | 12 (22.2) |
| Adjuvant | 3 (5.6) |
| No systemic therapy | 2 (3.8) |
| Relationship between the timing of CIM and ACTs | |
| Initiation at the time of cancer diagnosis | 4 (7.4) |
| Initiation at the onset of ACT | 6 (11.1) |
| Initiation during ACT | 39 (72.2) |
| Initiation following the completion of ACT | 5 (9.3) |
| Motivations for initiating CIM | |
| General support | 22 (40.7) |
| Support for toxicity | 7 (12.1) |
| Immunomodulation | 1 (1.8) |
| More than one of the above reasons | 24 (44.4) |
| Duration of CIM | |
| ≤3 months | 20 (37.0) |
| >3–≤6 months | 14 (25.9) |
| >6 months | 20 (37.0) |
| Variable | No. (%) |
|---|---|
| Type of clinical benefit | |
| Fatigue and QoL improvement | 24 (44.4) |
| Toxicity improvement | 6 (11.1) |
| QoL improvement | 6 (11.1) |
| No benefit | 6 (11.1) |
| Fatigue and toxicity improvement | 2 (3.7) |
| Improvement in tumor shrinkage | 1 (1.8) |
| Toxicity and QoL improvement | 1 (1.8) |
| Improvement in tumor shrinkage and QoL | 1 (1.8) |
| Multiple benefits | 7 (12.1) |
| Type of toxicity * | |
| No toxicity | 50 (92.6) |
| Gastrointestinal | 3 (5.5) |
| Liver | 1 (1.8) |
| Costs | |
| <EUR 100 per month | 40 (74.1) |
| >EUR 100 per month | 14 (35.9) |
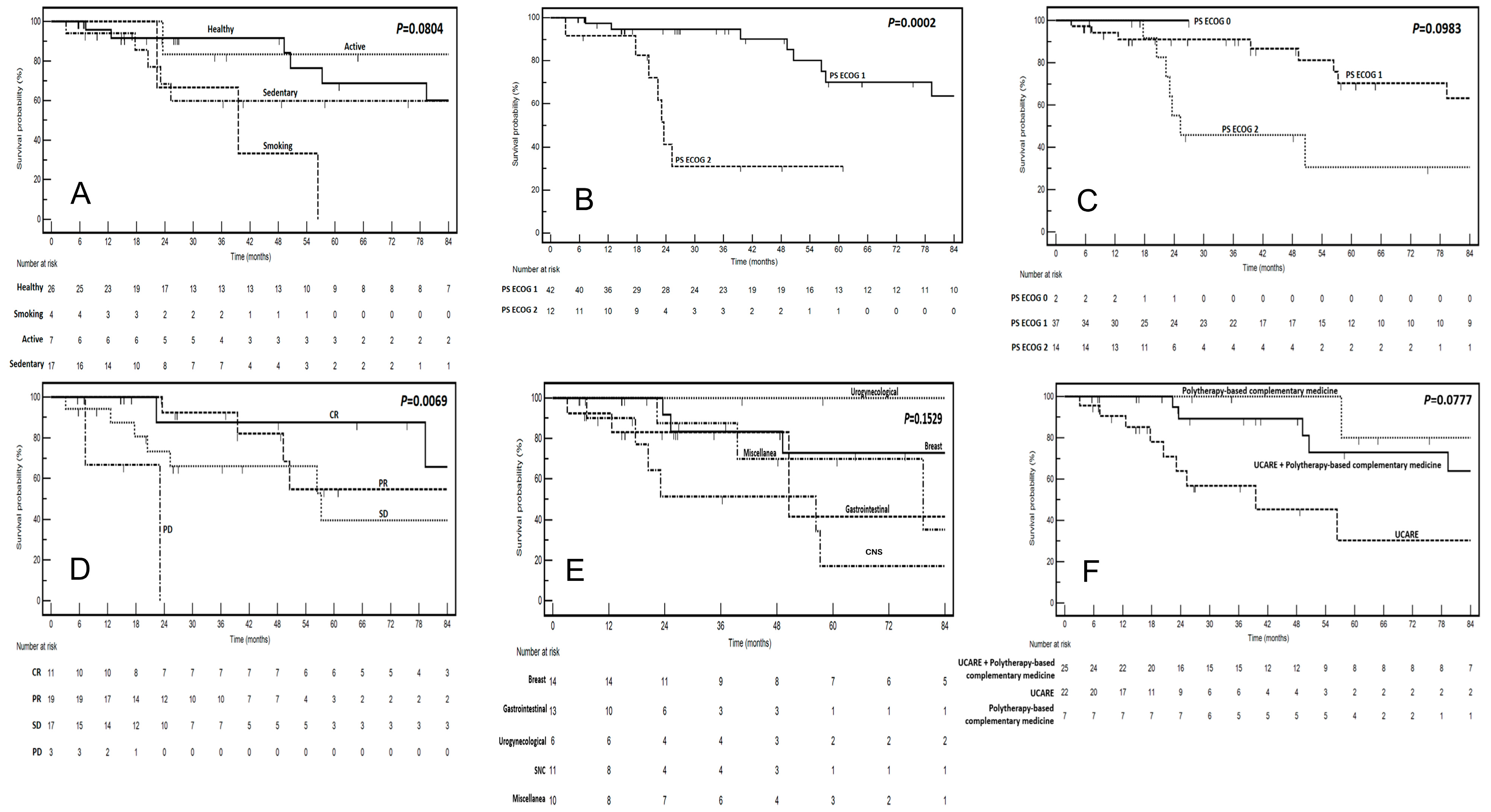
| Variable | Clinical Benefit from CIM | p (at Chi-Square test) | HR for OS | 95% CIs | p (at Log-Rank Test) | |
|---|---|---|---|---|---|---|
| Yes | No | |||||
| Age | ||||||
| <70 y | 35 | 6 | 1 | |||
| ≥70 y | 13 | 0 | 0.1472 | 0.97 | 0.31–3.01 | 0.9663 |
| Gender | ||||||
| Male | 20 | 3 | 1 | |||
| Female | 28 | 3 | 0.6998 | 0.50 | 0.17–1.42 | 0.1960 |
| Lifestyle | ||||||
| Healthy | 23 | 3 | 1 | |||
| Sedentary | 16 | 1 | 1.72 | 0.57–5.14 | ||
| Active | 6 | 1 | 0.91 | 0.24–3.33 | ||
| Smoking | 3 | 1 | 0.7225 | 4.55 | 0.51–40.3 | 0.0804 |
| Type of cancer | ||||||
| Breast | 13 | 1 | 1 | |||
| CNS | 10 | 1 | 3.74 | 1.03–13.56 | ||
| Gastrointestinal | 12 | 1 | 2.45 | 0.63–9.51 | ||
| Urogynecological | 6 | 0 | 0.81 | 0.18–3.63 | ||
| Miscellanea | 7 | 3 | 0.3101 | 1.73 | 0.46–6.49 | 0.1529 |
| Type of complementary therapy | ||||||
| U-CARE | 23 | 2 | 1 | |||
| U-CARE + Polytherapy-based complementary medicine | 18 | 4 | 0.49 | 0.18–1.36 | ||
| Polytherapy-based complementary medicine | 7 | 0 | 0.3273 | 0.19 | 0.05–0.78 | 0.0777 |
| Duration of CIM | ||||||
| <3 months | 17 | 3 | 1 | |||
| >3–≤6 months | 13 | 1 | 0.75 | 0.24–2.34 | ||
| >6 months | 18 | 2 | 0.7579 | 0.29 | 0.09–0.92 | 0.1551 |
| PS ECOG at cancer diagnosis | ||||||
| 0 | - | - | ||||
| 1 | 37 | 5 | 1 | |||
| 2 | 11 | 1 | 0.7309 | 16.8 | 3.78–74.5 | 0.0002 |
| 3 | - | - | ||||
| PS ECOG at CIM start | ||||||
| 0 | 1 | 1 | ||||
| 1 | 32 | 5 | 1 | |||
| 2 | 14 | 0 | 0.0844 | 2.46 | 0.83–7.28 | 0.0983 |
| 3 | - | - | ||||
| Stage | ||||||
| Early | 8 | 1 | 1 | |||
| Advanced | 40 | 5 | 1.0000 | 1.73 | 0.52–5.76 | 0.3684 |
| Timing of CIM initiation | ||||||
| At the time of cancer diagnosis | 4 | 0 | 1 | |||
| At the onset of ACT | 6 | 0 | 0.33 | 0.04–2.45 | ||
| During ACT | 39 | 6 | 0.16 | 0.01–1.74 | ||
| Following the completion of ACT | 5 | 0 | 0.4582 | 2.02 | 0.47–8.06 | 0.1831 |
| Line of therapy associated with CIM | ||||||
| First | 18 | 2 | 1 | |||
| Second | 14 | 3 | 1.23 | 0.37–4.12 | ||
| Beyond second line | 11 | 1 | 1.37 | 0.39–4.76 | ||
| Adjuvant | 2 | 0 | 1.19 | 0.29–4.81 | ||
| No systemic therapy | 3 | 0 | 0.8303 | 1.26 | 0.14–11.0 | 0.9896 |
| Best response to oncological treatment | ||||||
| Complete response | 9 | 1 | 1 | |||
| Partial response | 17 | 2 | 1.43 | 0.44–4.57 | ||
| Stable disease | 15 | 2 | 2.18 | 0.71–6.70 | ||
| Progressive disease | 2 | 1 | 0.7186 | 11.91 | 0.29–48.37 | 0.0076 |
| Variable | Best Response to Oncological Treatments | ||||
|---|---|---|---|---|---|
| CR | PR | SD | PD | p | |
| Type of cancer | |||||
| Breast | 3 | 7 | 3 | 0 | |
| CNS | 0 | 0 | 8 | 2 | |
| Gastrointestinal | 2 | 7 | 2 | 0 | |
| Urogynecological | 0 | 1 | 3 | 1 | |
| Miscellanea | 5 | 4 | 1 | 0 | 0.0019 |
| Type of complementary therapy | |||||
| U-CARE + Polytherapy-based complementary medicine | 6 | 11 | 4 | 1 | |
| U-CARE | 1 | 6 | 12 | 2 | |
| Polytherapy-based complementary medicine | 3 | 2 | 1 | 0 | 0.041 |
| Variable | HR for OS | 95% CIs | p |
|---|---|---|---|
| Age | |||
| <70 y | 1 | ||
| ≥70 y | 1.16 | 0.33–4.12 | 0.7236 |
| Gender | |||
| Male | 1 | ||
| Female | 0.46 | 0.15–1.38 | 0.2151 |
| PS ECOG * | |||
| 0–1 | 1 | ||
| 2 | 4.86 | 1.24–19.0 | 0.0232 |
| PS ECOG ** | |||
| 0–1 | 1 | ||
| 2 | 3.15 | 1.18–8.36 | 0.3001 |
| Type of cancer | |||
| Breast | 1 | ||
| CNS | 3.76 | 1.08–14.6 | 0.0816 |
| Gastrointestinal | 2.46 | 0.63–10.06 | 0.1222 |
| Urogynecological | 0.81 | 0.17–3.84 | 0.2948 |
| Miscellanea | 1.83 | 0.44–6.52 | 0.3308 |
| Type of complementary therapy | |||
| U-CARE | 1 | ||
| U-CARE + polytherapy-based complementary medicine | 0.48 | 0.17–1.35 | 0.9117 |
| Polytherapy-based complementary medicine | 0.18 | 0.04–0.77 | 0.9882 |
| Variable | Type of Complementary Therapy | |||
|---|---|---|---|---|
| PCM | U-CARE | U-CARE + PCM | p | |
| Type of cancer | ||||
| Breast | 3 | 3 | 8 | |
| CNS | 1 | 10 | 0 | |
| Gastrointestinal | 2 | 6 | 5 | |
| Urogynecological | 0 | 0 | 6 | |
| Miscellanea | 1 | 3 | 6 | 0.0041 |
| Type of clinical benefit | ||||
| Fatigue and QoL improvement | 3 | 1 | 20 | |
| Toxicity improvement | 0 | 4 | 2 | |
| QoL improvement | 1 | 5 | 0 | |
| No benefit | 1 | 5 | 0 | |
| Fatigue and toxicity improvement | 0 | 0 | 2 | |
| Improvement in tumor shrinkage | 0 | 1 | 0 | |
| Toxicity and QoL improvement | 0 | 1 | 0 | |
| Improvement in tumor shrinkage and QoL | 1 | 0 | 0 | |
| Multiple benefits | 1 | 5 | 1 | 0.0003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berretta, M.; Quagliariello, V.; Ottaiano, A.; Santorsola, M.; Di Francia, R.; Carroccio, P.; Maurea, N.; Buonomo, O.C.; Facchini, G.; Di Mauro, G.; et al. Multidisciplinary Integrative Medicine Approach for Cancer Patients: A Multicenter Retrospective Study. Nutrients 2025, 17, 1012. https://doi.org/10.3390/nu17061012
Berretta M, Quagliariello V, Ottaiano A, Santorsola M, Di Francia R, Carroccio P, Maurea N, Buonomo OC, Facchini G, Di Mauro G, et al. Multidisciplinary Integrative Medicine Approach for Cancer Patients: A Multicenter Retrospective Study. Nutrients. 2025; 17(6):1012. https://doi.org/10.3390/nu17061012
Chicago/Turabian StyleBerretta, Massimiliano, Vincenzo Quagliariello, Alessandro Ottaiano, Mariachiara Santorsola, Raffaele Di Francia, Patrizia Carroccio, Nicola Maurea, Oreste Claudio Buonomo, Gaetano Facchini, Giordana Di Mauro, and et al. 2025. "Multidisciplinary Integrative Medicine Approach for Cancer Patients: A Multicenter Retrospective Study" Nutrients 17, no. 6: 1012. https://doi.org/10.3390/nu17061012
APA StyleBerretta, M., Quagliariello, V., Ottaiano, A., Santorsola, M., Di Francia, R., Carroccio, P., Maurea, N., Buonomo, O. C., Facchini, G., Di Mauro, G., Montopoli, M., Toscano, E., Gelsomino, C., Picone, A., Franchina, T., Muscolino, P., Bignucolo, A., Vanni, G., Ciappina, G., & Montella, L. (2025). Multidisciplinary Integrative Medicine Approach for Cancer Patients: A Multicenter Retrospective Study. Nutrients, 17(6), 1012. https://doi.org/10.3390/nu17061012







