The “Jekyll Side” of the S100B Protein: Its Trophic Action in the Diet
Abstract
1. Introduction
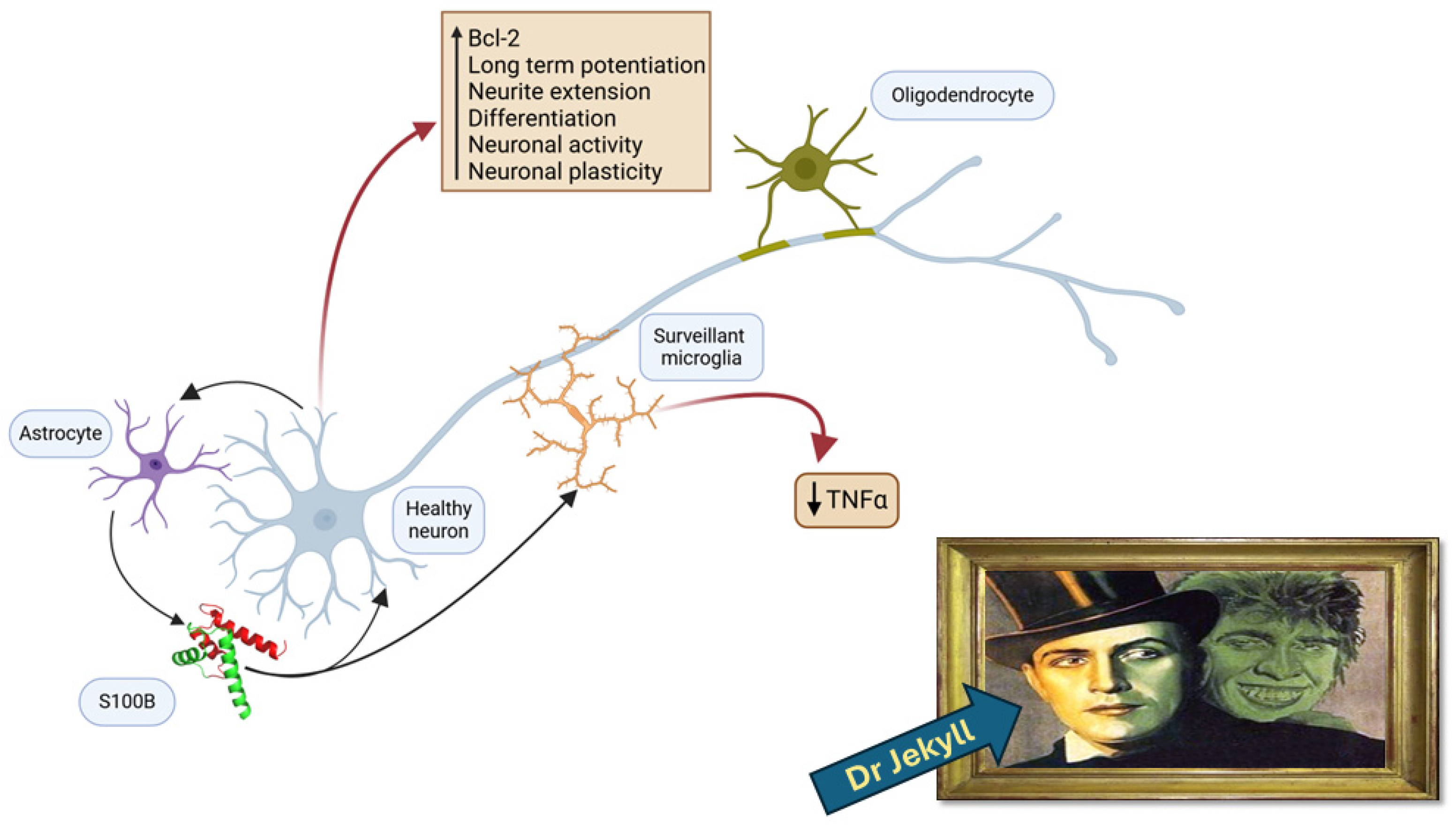
2. The S100B Protein as a Protective and Trophic Factor
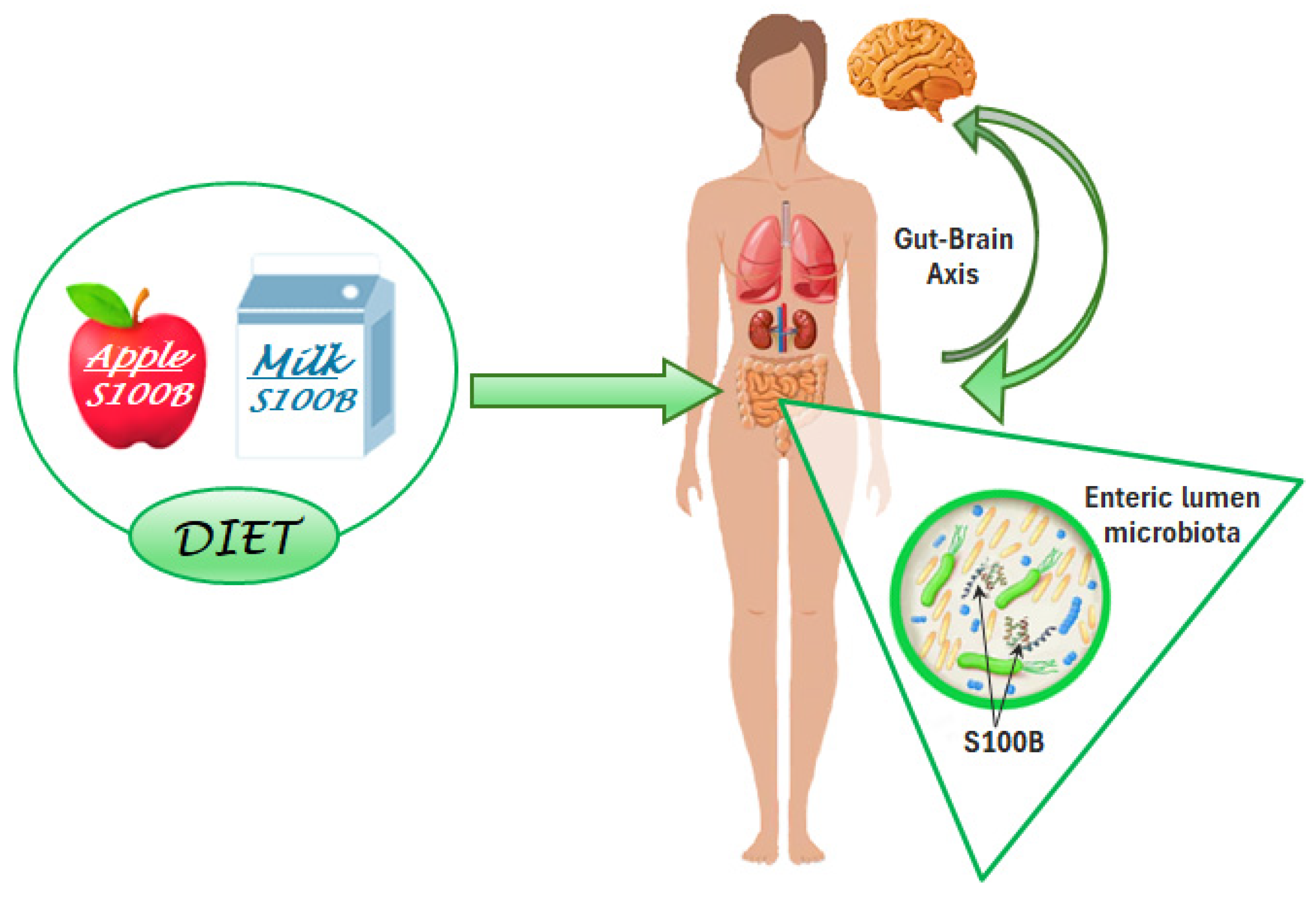
3. The S100B Protein as a Nutrient in Different Aliments
3.1. Milk
3.2. Edible Plants
3.3. Gut Microbiota Interactions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Michetti, F.; Clementi, M.E.; Di Liddo, R.; Valeriani, F.; Ria, F.; Rende, M.; Di Sante, G.; Romano Spica, V. The S100B Protein: A Multifaceted Pathogenic Factor More Than a Biomarker. Int. J. Mol. Sci. 2023, 24, 9605. [Google Scholar] [CrossRef] [PubMed]
- Michetti, F.; Cocchia, D. S-100-like immunoreactivity in a planarian. An immunochemical and immunocytochemical study. Cell Tissue Res. 1982, 223, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Endo, T. Immunohistochemical demonstration of S-100 protein in the brain neurosecretory cells of invertebrates (insects and earthworms). Neurosci. Lett. 1988, 90, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Michetti, F.; Grilli Caiola, M.; Botti, F.; Bertini, G.; Cocchia, D. Immunochemical and immunohistochemical detection of S-100-like immunoreactivity in spinach tissues. J. Histochem. Cytochem. 1992, 40, 839–843. [Google Scholar] [CrossRef]
- Romano Spica, V.; Volpini, V.; Valeriani, F.; Carotenuto, G.; Arcieri, M.; Platania, S.; Castrignanò, T.; Clementi, M.E.; Michetti, F. In Silico Predicting the Presence of the S100B Motif in Edible Plants and Detecting Its Immunoreactive Materials: Perspectives for Functional Foods, Dietary Supplements and Phytotherapies. Int. J. Mol. Sci. 2024, 25, 9813. [Google Scholar] [CrossRef]
- Gazzolo, D.; Monego, G.; Corvino, V.; Bruschettini, M.; Bruschettini, P.; Zelano, G.; Michetti, F. Human. milk contains S100B protein. Biochim. Biophys. Acta 2003, 1619, 209–212. [Google Scholar] [CrossRef]
- Aşkan, Ö.Ö.; Karpuzoğlu, F.H.; Kayı, A.B.; Özden, T.A.; Gökçay, G.; Keskindemirci, G. Unique content of breastmilk: Neurotrophic growth factors in breastmilk at 2 years and beyo. Eur. J. Pediatr. 2024, 183, 4729–4734. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Kaur, J.; Singh, H.; Naqvi, S. Intracellular DAMPs in Neurodegeneration and Their Role in Clinical Therapeutics. Mol. Neurobiol. 2023, 60, 3600–3616. [Google Scholar] [CrossRef]
- Huttunen, H.J.; Kuja-Panula, J.; Sorci, G.; Agneletti, A.L.; Donato, R.; Rauvala, H. Co-regulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J. Biol. Chem. 2000, 275, 40096–40105. [Google Scholar] [CrossRef]
- Carapeto, A.P.; Marcuello, C.; Faísca, P.F.N.; Rodrigues, M.S. Morphological and Biophysical Study of S100A9 Protein Fibrils by Atomic Force Microscopy Imaging and Nanomechanical Analysis. Biomolecules 2024, 14, 1091. [Google Scholar] [CrossRef]
- Gambichler, T.; Brown, V.; Steuke, A.K.; Schmitz, L.; Stockfleth, E.; Susok, L. Baseline laboratory parameters predicting clinical outcome in melanoma patients treated with ipilimumab: A single-centre analysis. J. Eur. Acad. Dermatol. Venereol. 2017, 32, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, M.; Enk, A.H.; Hassel, J.C. S100 as Serum Tumor Marker in Advanced Uveal Melanoma. Biomolecules 2023, 13, 529. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 1–53. [Google Scholar] [CrossRef]
- Orsini, M.; Di Liddo, R.; Valeriani, F.; Mancin, M.; D’Incà, R.; Castagnetti, A.; Aceti, A.; Parnigotto, P.P.; Romano Spica, V.; Michetti, F. In Silico Evaluation of Putative S100B Interacting Proteins in Healthy and IBD Gut Microbiota. Cells 2020, 9, 1697. [Google Scholar] [CrossRef]
- Romano Spica, V.; Valeriani, F.; Orsini, M.; Clementi, M.E.; Seguella, L.; Gianfranceschi, G.; Di Liddo, R.; Di Sante, G.; Ubaldi, F.; Ria, F.; et al. S100B Affects Gut Microbiota Biodiversity. Int. J. Mol. Sci. 2023, 24, 2248. [Google Scholar] [CrossRef] [PubMed]
- Levine, L.; Moore, B.W. Structural relatedness of a vertebrate brain acidic protein as measured immunochemically. Neurosci. Res. Prog. Bull 1965, 3, 18–23. [Google Scholar]
- Moore, B.W.; Perez, V.J.; Gehring, M. Assay and regional distribution of a soluble protein characteristic of the nervous system. J. Neurochem. 1968, 15, 265–272. [Google Scholar] [CrossRef]
- Kessler, D.; Levine, L.; Fasman, G.D. Conformational and immunological properties of a bovine brain acidic protein (S-100) Biochemistry. Easton 1968, 7, 758–764. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Herschman, H.R.; Levine, L. Appearance of a brain specific antigen (th S-100 protein) during human foetal development. J. Neurochem. 1970, 17, 247–251. [Google Scholar] [CrossRef]
- Wenger, B.; Friedman, H. S-100 Protein in Embryonic Chick Retinae. Nature 1970, 228, 1214. [Google Scholar] [CrossRef]
- Cicero, T.J.; Cowan, W.M.; Moore, B.W. Changes in the concentrations of the two brain specific proteins, s-100 and 14-3-2, during the development of the avian optic tectum. Brain Res. 1970, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Herschman, H.R.; Levine, L.; De Vellis, J. Appearance of a brain-specific antigen (S-100 protein) in the developing rat brain. J. Neurochem. 1971, 18, 629–633. [Google Scholar] [CrossRef]
- Cicero, T.J.; Provine, R.R. The levels of the brain-specific proteins, S-100 and 14-3-2, in the developing chick spinal cord 1972. Brain Res. 1972, 44, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Van Hartesveldt, C.; Moore, B.; Hartman, B.K. Transient midline raphe glial structure in the developing rat. J. Comp. Neurol. 1986, 253, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ortega, K.; Canul-Euan, A.A.; Solis-Paredes, J.M.; Borboa-Olivares, H.; Reyes-Muñoz, E.; Estrada-Gutierrez, G.; Camacho-Arroyo, I.F. S100B actions on glial and neuronal cells in the developing brain: An overview. Front. Neurosci. 2024, 18, 1425525. [Google Scholar] [CrossRef]
- Kligman, D.; Marshak, D.R. Purification and characterization of a neurite extension factor from bovine brain. Proc. Natl. Acad. Sci. USA 1985, 82, 7136–7139. [Google Scholar] [CrossRef]
- Kligman, D.; Hsieh, L.J. Neurite extension factor induces rapid morphological differentiation of mouse neuroblastoma cells in defined medium. Dev. Brain Res. 1987, 33, 296–300. [Google Scholar] [CrossRef]
- Winningham-Major, F.; Staecker, J.L.; Barger, S.W.; Coats, S.; Van Eldik, L.J. Neurite extension and neuronal survival activities of recombinant S100/3 proteins that differ in the content and position of cysteine residues. J. Cell Biol. 1989, 109, 3063–3071. [Google Scholar] [CrossRef]
- Selinfreund, R.H.; Barger, S.W.; Pledger, W.J.; Van Eldik, L.J. Neurotrophic protein S100 beta stimulates glial cell proliferation. Proc. Natl. Acad. Sci. USA 1991, 88, 3554–3558. [Google Scholar] [CrossRef] [PubMed]
- Barger, S.W.; Wolchok, S.R.; Van Eldik, L.J. Disulfide-linked S100 beta dimers and signal transduction. Biochim. Biophys. Acta 1992, 1160, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Selinfreund, R.H.; Barger, S.W.; Welsh, M.J.; Van Eldik, L.J. Antisense inhibition of glial S100 beta production results in alterations in cell morphology, cytoskeletal organization, and cell proliferation. J. Cell Biol. 1990, 111 Pt 1, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Z.; Wang, Z.; Shao, G.; Li, X. Epigenetic regulation in adult neural stem cells. Front. Cell Dev. Biol. 2024, 12, 1331074. [Google Scholar] [CrossRef] [PubMed]
- Kleindienst, A.; McGinn, M.J.; Harvey, H.B.; Colello, R.J.; Hamm, R.J.; Bullock, M.R. Enhanced hippocampal neurogenesis by intraventricular S100B infusion is associated with improved cognitive recovery after traumatic brain injury. J. Neurotrauma 2005, 22, 645–655. [Google Scholar] [CrossRef]
- Kleindienst, A.; Grünbeck, F.; Buslei, R.; Emtmann, I.; Buchfelder, M. Intraperitoneal treatment with S100B enhances hippocampal neurogenesis in juvenile mice and after experimental brain injury. Acta Neurochir. 2013, 155, 1351–1360. [Google Scholar] [CrossRef]
- Baecker, J.; Wartchow, K.; Sehm, T.; Ghoochani, A.; Buchfelder, M.; Kleindienst, A. Treatment with the Neurotrophic Protein S100B Increases Synaptogenesis after Traumatic Brain Injury. J. Neurotrauma 2020, 37, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.; Wartchow, K.M.; Buchfelder, M.; Souza, D.O.; Gonçalves, C.A.; Kleindienst, A. Longterm Increased S100B Enhances Hippocampal Progenitor Cell Proliferation in a Transgenic Mouse Model. Int. J. Mol. Sci. 2022, 23, 9600. [Google Scholar] [CrossRef]
- Cristóvão Joana, S.; Morris Vanessa, K.; Cardoso, I.; Leal Sónia, S.; Martínez, J.; Botelho Hugo, M. The neuronal S100B protein is a calcium-tuned suppressor of amyloid-β aggregation. Sci. Adv. 2018, 4, eaaq1702. [Google Scholar] [CrossRef]
- Cristovao, J.S.; Figueira, A.J.; Carapeto, A.P.; Rodrigues, M.S.; Cardoso, I.; Gomes, C.M. The S100B Alarmin Is a Dual-Function Chaperone Suppressing Amyloid-beta Oligomerization through Combined Zinc Chelation and Inhibition of Protein Aggregation ACS Chem. Neuro sci. 2020, 11, 2753–2760. [Google Scholar]
- Cristóvão, J.S.; Moreira, G.G.; Rodrigues, F.E.P.; Carapeto, A.P.; Rodrigues, M.S.; Cardoso, I. Cu(2+)-binding to S100B triggers polymerization of disulfide cross-linked tetramers with enhanced chaperone activity against amyloid-β aggregation. Chem. Commun. 2021, 57, 379–382. [Google Scholar] [CrossRef]
- Moreira, G.G.; Cantrelle, F.X.; Quezada, A.; Carvalho, F.S.; Cristóvão, J.S.; Sengupta, U. Dynamic interactions and Ca2+-binding modulate the holdase-type chaperone activity of S100B preventing tau aggregation and seeding. Nat. Commun. 2021, 12, 6292. [Google Scholar] [CrossRef]
- Figueira, A.J.; Moreira, G.G.; Saavedra, J.; Cardoso, I.; Gomes, C.M. Tetramerization of the S100B chaperone spawns a Ca2+ independent regulatory surface that enhances anti-aggregation activity and client specificity. J. Mol. Biol. 2022, 434, 167791. [Google Scholar] [CrossRef] [PubMed]
- Coelho, R.; De Benedictis, C.A.; Sauer, A.K.; Figueira, A.J.; Faustino, H.; Grabrucker, A.M.; Gomes, C.M. Secondary Modification of S100B Influences Anti Amyloid-β Aggregation Activity and Alzheimer’s Disease Pathology. Int. J. Mol. Sci. 2024, 25, 1787. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy for Infant and Young Child Feeding; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Petersohn, I.; Hellinga, A.H.; Van Lee, L.; Keukens, N.; Bont, L.; Hettinga, K.A.; Feskens, E.J.E.M. Maternal diet and human milk composition: An updated systematic review. Front. Nutr. Jan. 2024, 23, 1320560. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xia, W.; Zhang, Z.; Wu, K. S100B protein, brain-derived neurotrophic factor, and glial cell line-derived neurotrophic factor in human milk. PLoS ONE 2011, 6, e21663. [Google Scholar] [CrossRef]
- Galvano, F.; Frigiola, A.; Gagliardi, L.; Ciotti, S.; Bognanno, M.; Iacopino, A.M.; Nigro, F.; Tina, G.L.; Cavallaro, D.; Mussap, M.; et al. S100B milk concentration in mammalian species. Front. Biosci. 2009, 1, 542–546. [Google Scholar] [CrossRef]
- Nigro, F.; Gagliardi, L.; Ciotti, S.; Galvano, F.; Pietri, A.; Tina, G.L.; Cavallaro, D.; La Fauci, L.; Iacopino, L.; Bognanno, M.; et al. S100B Protein concentration in milk-formulas for preterm and term infants. Correlation with industrial preparation procedures. Mol. Nutr. Food Res. 2008, 52, 609–613. [Google Scholar] [CrossRef]
- Peila, C.; Coscia, A.; Bertino, E.; Li Volti, G.; Galvano, F.; Visser, G.H.A.; Gazzolo, D.; Holder, J. Pasteurization affects S100B concentrations in human milk. Matern. Fetal Neonatal Med. 2018, 31, 513–517. [Google Scholar] [CrossRef]
- Ghaffar, T.; Volpini, V.; Platania, S.; Glogowski, P.A.; Gianfranceschi, G.; Vassioukovitch, O.; Valeriani, F.; Michetti, F.; Romano Spica, V. A novel role for S100B in diet and gut-microbiota regulation. In Proceedings of the Gut Microbiota for Health World Summit 2025, Washington, DC, USA, 15–16 March 2025; Available online: https://agau.gastro.org/cw/course-details?entryId=17324988#nav-home (accessed on 16 January 2024).
- Charpentier, T.H.; Wilder, P.T.; Liriano, M.A.; Varney, K.M.; Pozharski, E.; MacKerell, A.D.; Coop, A.; Toth, E.A.; Weber, D.J. Divalent metal ion complexes of S100B in the absence and presence of pentamidine. J. Mol. Biol. 2008, 382, 56–73. [Google Scholar] [CrossRef]
- Ferri, G.L.; Probert, L.; Cocchia, D.; Michetti, F.; Marangos, P.J.; Polak, J.M. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature 1982, 297, 409–410. [Google Scholar] [CrossRef]
| Food Source | S100B Concentration |
|---|---|
| Farm animal milk | 0.03–180 µg/L |
| Cheese–Dairy | 0.01–0.4 µg/Kg |
| Fruit–Vegetables | 0.04–180 µg/Kg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michetti, F.; Romano Spica, V. The “Jekyll Side” of the S100B Protein: Its Trophic Action in the Diet. Nutrients 2025, 17, 881. https://doi.org/10.3390/nu17050881
Michetti F, Romano Spica V. The “Jekyll Side” of the S100B Protein: Its Trophic Action in the Diet. Nutrients. 2025; 17(5):881. https://doi.org/10.3390/nu17050881
Chicago/Turabian StyleMichetti, Fabrizio, and Vincenzo Romano Spica. 2025. "The “Jekyll Side” of the S100B Protein: Its Trophic Action in the Diet" Nutrients 17, no. 5: 881. https://doi.org/10.3390/nu17050881
APA StyleMichetti, F., & Romano Spica, V. (2025). The “Jekyll Side” of the S100B Protein: Its Trophic Action in the Diet. Nutrients, 17(5), 881. https://doi.org/10.3390/nu17050881







