Maternal Anemia as a Predictor of Childhood Anemia: Evidence from Gambian Health Data
Highlights
- There is strong evidence of the intergenerational incidence of anemia between mothers and children in The Gambia.
- We found that the intergenerational correlation coefficient for the incidence of hemoglobin is 0.17, while that for the incidence of anemia is 0.14.
- The intergenerational correlation coefficient for anemia incidence is significantly higher among families in the bottom three wealth quintiles than among those in the top two wealth quintiles.
- The significant intergenerational correlation of anemia incidence underscores the need for targeted health and dietary interventions at the household level.
Abstract
1. Introduction
2. Methods
2.1. Study and Setting
2.2. Patient
2.3. Data
2.4. Variables
2.5. Statistical Analysis
3. Results
3.1. Population Description
3.2. Multivariate Regression Results
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Accelerating Anaemia Reduction: A Comprehensive Framework for Action; World Health Organization: Geneva, Switzerland, 2023; Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Oyungu, E.; Roose, A.W.; Ombitsa, A.R.; Yang, Z.; Vreeman, R.C.; McHenry, M.S. Anemia and Iron-Deficiency Anemia in Children Born to Mothers with HIV in Western Kenya. Glob. Pediatr. Health 2021, 8, 2333794X21991035. [Google Scholar] [CrossRef]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef]
- McCann, J.C.; Ames, B.N. An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. Am. J. Clin. Nutr. 2007, 85, 931–945. [Google Scholar] [CrossRef]
- Blakstad, M.M.; Nevins, J.E.H.; Venkatramanan, S.; Przybyszewski, E.M.; Haas, J.D. Iron status is associated with worker productivity, independent of physical effort in Indian tea estate workers. Appl. Physiol. Nutr. Metab. 2020, 45, 1360–1367. [Google Scholar] [CrossRef]
- Balarajan, Y.; Ramakrishnan, U.; Ozaltin, E.; Shankar, A.H.; Subramanian, S.V. Anaemia in low-income and middle-income countries. Lancet 2011, 378, 2123–2135. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, V.R.; Gardner, G.W.; Ohira, Y.; Gunawardena, K.A.; Senewiratne, B. Iron-deficiency anemia and its effect on worker productivity and activity patterns. BMJ 1979, 2, 1546–1549. [Google Scholar] [CrossRef] [PubMed]
- Gardner, W.M.; Razo, C.; McHugh, T.A.; Hagins, H.; Vilchis-Tella, V.M.; Hennessy, C.; Taylor, H.J.; Perumal, N.; Fuller, K.; Cercy, K.M.; et al. Prevalence, years lived with disability, and trends in anaemia burden by severity and cause, 1990–2021: Findings from the global burden of disease study 2021. Lancet Haematol. 2023, 10, e713–e734. [Google Scholar] [CrossRef]
- Seifu, B.L.; Tesema, G.A. Individual-and community-level factors associated with anemia among children aged 6–23 months in sub-Saharan africa: Evidence from 32 sub-saharan African countries. Arch. Public Health 2022, 80, 183. [Google Scholar] [CrossRef] [PubMed]
- Tesema, G.A.; Worku, M.G.; Tessema, Z.T.; Teshale, A.B.; Alem, A.Z.; Yeshaw, Y.; Alamneh, T.S.; Liyew, A.M. Prevalence and determinants of severity levels of anemia among children aged 6–59 months in sub-Saharan africa: A multilevel ordinal logistic regression analysis. PLoS ONE 2021, 16, e0249978. [Google Scholar] [CrossRef]
- Correa-Agudelo, E.; Kim, H.-Y.; Musuka, G.N.; Mukandavire, Z.; Miller, F.D.; Tanser, F.; Cuadros, D.F. The epidemiological landscape of anemia in women of reproductive age in sub-saharan africa. Sci. Rep. 2021, 11, 11955. [Google Scholar] [CrossRef]
- Zegeye, B.; Anyiam, F.E.; Ahinkorah, B.O.; Ameyaw, E.K.; Budu, E.; Seidu, A.-A.; Yaya, S. Prevalence of anemia and its associated factors among married women in 19 sub-saharan African countries. Arch. Public Health 2021, 79, 214. [Google Scholar] [CrossRef] [PubMed]
- Marcus, H.; Schauer, C.; Zlotkin, S. Effect of anemia on work productivity in both labor- and Nonlabor-intensive occupations: A systematic narrative synthesis. Food Nutr. Bull. 2021, 42, 289–308. [Google Scholar] [CrossRef]
- Green, R.; Datta Mitra, A. Megaloblastic Anemias: Nutritional and Other Causes. Med. Clin. N. Am. 2017, 101, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Venkatramanan, S.; Armata, I.E.; Strupp, B.J.; Finkelstein, J.L. Vitamin B-12 and Cognition in Children. Adv. Nutr. 2016, 7, 879–888. [Google Scholar] [CrossRef]
- Gebreegziabher, T.; Sidibe, S. Prevalence and contributing factors of anaemia among children aged 6–24 months and 25–59 months in Mali. J. Nutr. Sci. 2023, 12, e112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gambia Bureau of Statistics (GBoS) and ICF. The Gambia Demographic and Health Survey 2019–2020; GBoS and ICF: Banjul, The Gambia; Rockville, MD, USA, 2021; Available online: https://dhsprogram.com/pubs/pdf/FR369/FR369.pdf (accessed on 20 January 2024).
- The Gambia Bureau of Statistics—GBOS and ICF International. The Gambia Demographic and Health Survey 2013; GBOS and ICF International: Banjul, The Gambia, 2014; Available online: https://dhsprogram.com/pubs/pdf/FR289/FR289.pdf (accessed on 20 January 2024).
- Petry, N.; Jallow, B.; Sawo, Y.; Darboe, M.K.; Barrow, S.; Sarr, A.; Ceesay, P.O.; Fofana, M.N.; Prentice, A.M.; Wegmüller, R.; et al. Micronutrient deficiencies, nutritional status and the determinants of anemia in children 0–59 months of age and non-pregnant women of reproductive age in the Gambia. Nutrients 2019, 11, 2275. [Google Scholar] [CrossRef]
- Asmare, A.A.; Agmas, Y.A. Trends and Determinants of Anemia Among Under-Five Children in Gambia, Evidence from 2013–2019/2020 Gambian Demographic and Health Survey; Multilevel Binary Logistic Regression and Multivariate Decomposition Analysis. J. Food Nutr. 2022, 8, 1–20. [Google Scholar]
- World Bank. Poverty & Equity Brief, The Gambia; World Bank: Washington, DC, USA, 2023; Available online: https://databankfiles.worldbank.org/public/ddpext_download/poverty/987B9C90-CB9F-4D93-AE8C-750588BF00QA/current/Global_POVEQ_GMB.pdf (accessed on 15 December 2024).
- Ali, Z.; Scheelbeek, P.F.D.; Dalzell, S.; Hadida, G.; Segnon, A.C.; M’boob, S.; Prentice, A.M.; Green, R. Socio-economic and food system drivers of Nutrition and health transitions in the Gambia from 1990 to 2017. Glob. Food Secur. 2023, 37, 100695. [Google Scholar] [CrossRef]
- Touray, S. Poverty Trends in The Gambia: A Tale of Two Crises; World Bank Blogs: Washington, DC, USA, 2023; Available online: https://blogs.worldbank.org/en/africacan/poverty-trends-gambia-tale-two-crises#:~:text=Data%20from%20the%202020%2F21,from%20the%202015%20poverty%20levels (accessed on 15 December 2024).
- The Gambia Bureau of Statistics & The World Food Programme. The Gambia National Food Security Survey Report December 2023; OCHA: New York, NY, USA, 2023; Available online: https://reliefweb.int/report/gambia/gambia-national-food-security-survey-report-december-2023 (accessed on 15 December 2024).
- Hellevik, O. Linear versus logistic regression when the dependent variable is a dichotomy. Qual. Quant. 2007, 43, 59–74. [Google Scholar] [CrossRef]
- Elmardi, K.A.; Adam, I.; Malik, E.M.; Ibrahim, A.A.; Elhassan, A.H.; Kafy, H.T.; Nawai, L.M.; Abdin, M.S.; Kremers, S. Anemia prevalence and determinants in under 5 years children: Findings of a cross-sectional population-based study in Sudan. BMC Pediatr. 2020, 20, 538. [Google Scholar] [CrossRef]
- Ntenda, P.A.; Nkoka, O.; Bass, P.; Senghore, T. Maternal anemia is a potential risk factor for anemia in children aged 6–59 months in Southern Africa: A Multilevel Analysis. BMC Public Health 2018, 18, 650. [Google Scholar] [CrossRef] [PubMed]
- Ngesa, O.; Mwambi, H. Prevalence and risk factors of anaemia among children aged between 6 months and 14 years in Kenya. PLoS ONE 2014, 9, e113756. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Peng, Y.; Li, J.; Lv, Y.; Zhang, Y.; Wang, P.; Yang, T.; Liu, Z. Prevalence of anemia and its risk factors among children 6–36 months old in burma. Am. J. Trop. Med. Hyg. 2012, 87, 306–311. [Google Scholar] [CrossRef]
- Gautam, S.K.; Halliday, T.J.; Mazumder, B. Cycles of Malnutrition: Intergenerational Health Transmission in India. IZA Discussion Paper No. 17684: 2025. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5134012 (accessed on 3 February 2025).
- Lemoine, A.; Tounian, P. Childhood anemia and iron deficiency in sub-saharan africa—Risk factors and prevention: A Review. Arch. Pédiatr. 2020, 27, 490–496. [Google Scholar] [CrossRef]
- Zegeye, B.; Ahinkorah, B.O.; Ameyaw, E.K.; Seidu, A.A.; Keetile, M.; Yaya, S. Determining Prevalence of Anemia and Its Associated Factors in Cameroon: A Multilevel Analysis. BioMed Res. Int. 2021, 2021, 9912549. [Google Scholar] [CrossRef] [PubMed]
- Shitu, K.; Terefe, B. Anaemia and its determinants among reproductive age women (15–49 years) in the Gambia: A multi-level analysis of 2019–2020 Gambian demographic and Health Survey Data. Arch. Public Health 2022, 80, 228. [Google Scholar] [CrossRef]
- Kumar, S.; Nahlen, B. Intergenerational persistence of health: Evidence from India. Econ. Lett. 2023, 224, 111023. [Google Scholar] [CrossRef]
- Scholz, B.D.; Gross, R.; Schultink, W.; Sastroamidjojo, S. Anemia is associated with reduced productivity of women workers even in less-physically-strenuous tasks. Br. J. Nutr. 1997, 77, 47–57. [Google Scholar] [CrossRef]
- Haas, J.D.; Brownlie, T. Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship. J. Nutr. 2001, 131, 676S–690S. [Google Scholar] [CrossRef]
- Horton, S.; Ross, J. The economics of iron deficiency. Food Policy 2003, 28, 51–75. [Google Scholar] [CrossRef]
- Larson, L.M.; Kubes, J.N.; Ramírez-Luzuriaga, M.J.; Khishen, S.; HShankar, A.; Prado, E.L. Effects of increased hemoglobin on child growth, development, and disease: A systematic review and meta-analysis. Ann. N. Y. Acad. Sci. 2019, 1450, 83–104. [Google Scholar] [CrossRef] [PubMed]
- De-Regil, L.M.; Peña-Rosas, J.P.; Fernández-Gaxiola, A.C.; Rayco-Solon, P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst. Rev. 2015, 2015, CD007950. [Google Scholar] [CrossRef] [PubMed]
- von Grafenstein, L.; Kumar, A.; Kumar, S.; Vollmer, S. Medium-run impacts of iron-fortified school lunch on anaemia, cognition, and learning outcomes in India. Oxf. Bull. Econ. Stat. 2023, 85, 1262–1294. [Google Scholar] [CrossRef]
- Krämer, M.; Kumar, S.; Vollmer, S. Improving child health and cognition: Evidence from a school-based nutrition intervention in India. Rev. Econ. Stat. 2021, 103, 818–834. [Google Scholar] [CrossRef]
- Krämer, M.; Kumar, S.; Vollmer, S. Anemia, diet, and cognitive development: Impact of health information on diet quality and child nutrition in rural India. J. Econ. Behav. Organ. 2021, 190, 495–523. [Google Scholar] [CrossRef]
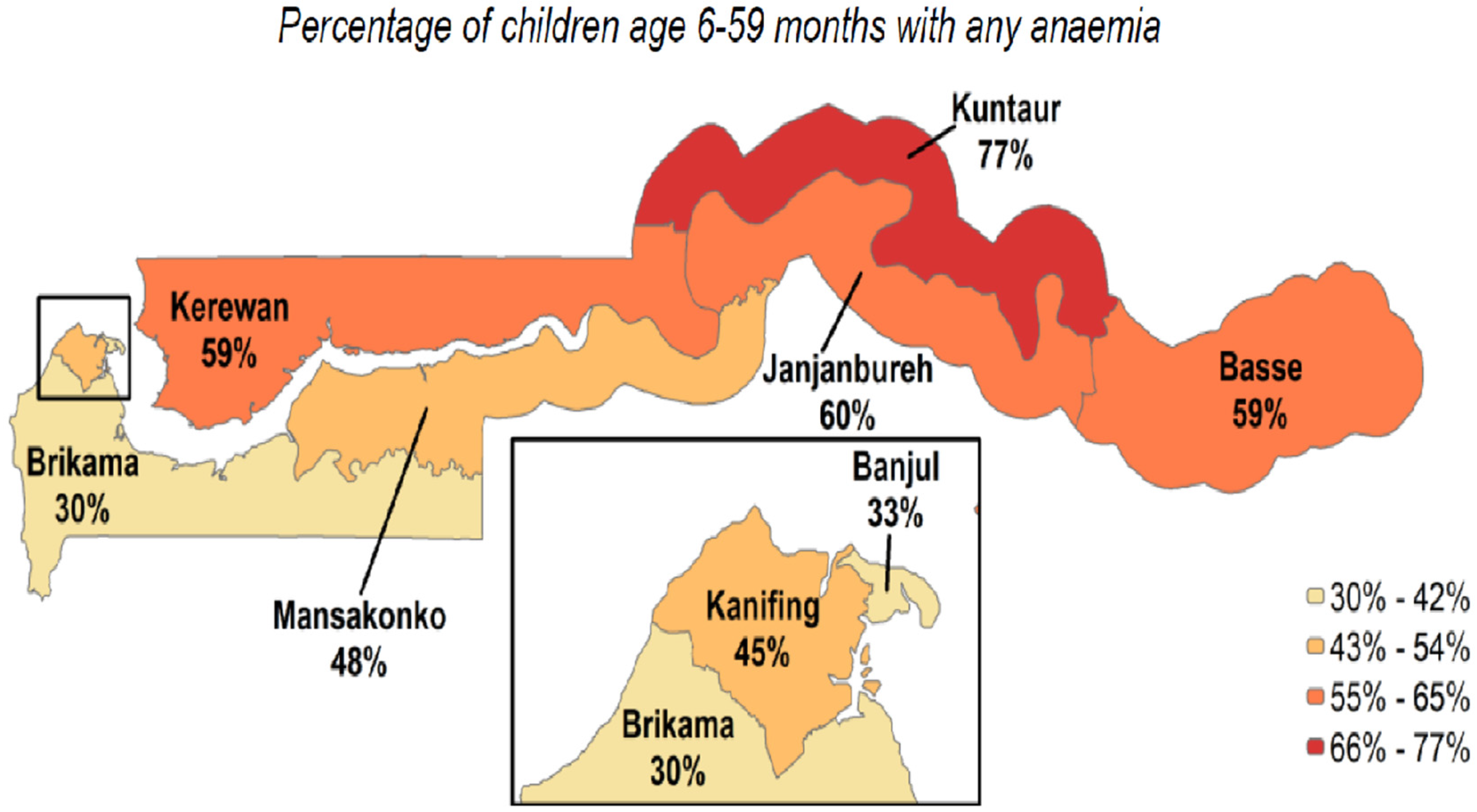
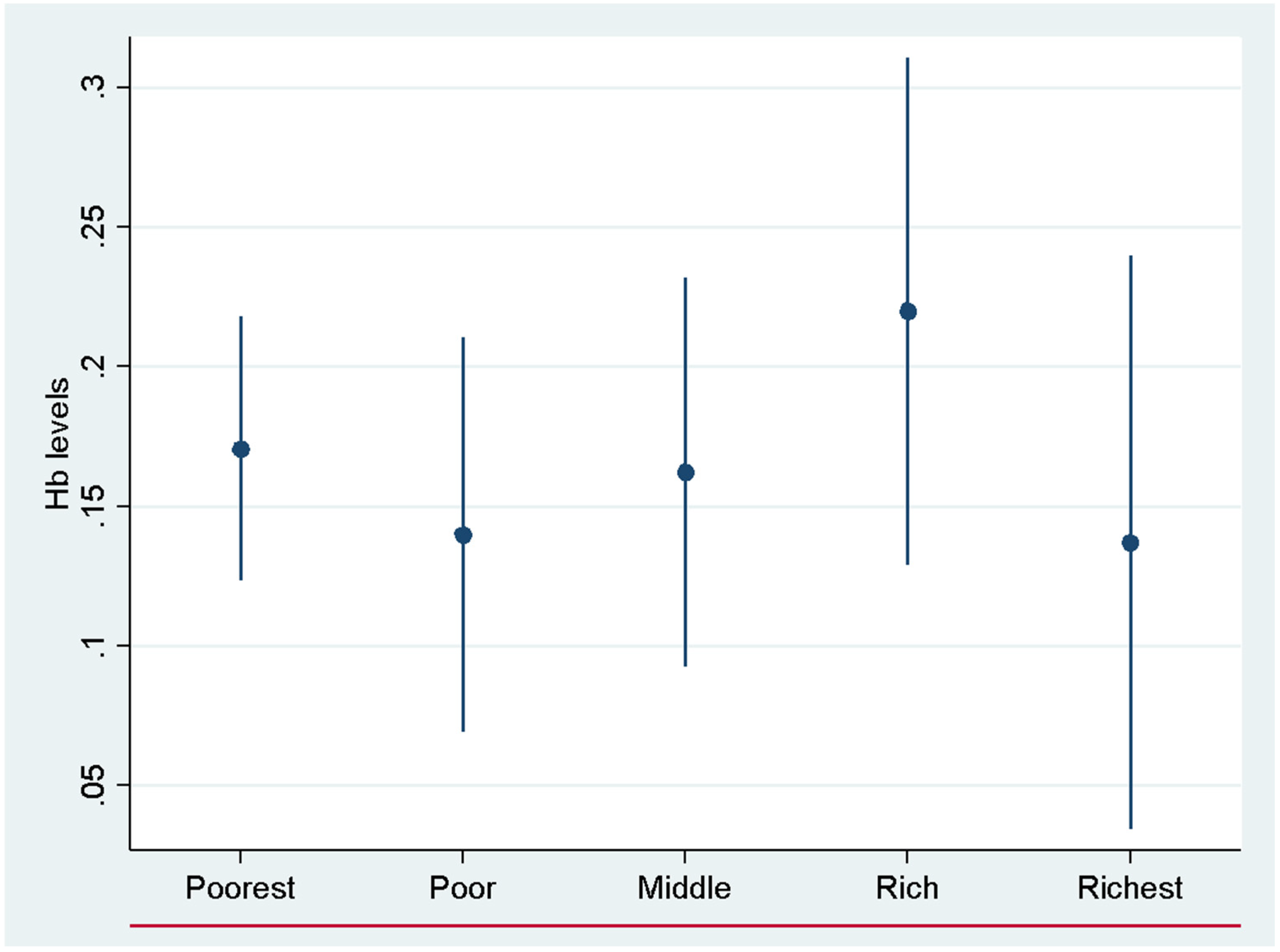

| Variables | Mean | Std Dev | Min Value | Max Value |
|---|---|---|---|---|
| Health Outcomes | ||||
| Child Hb | 10.57 | 14.36 | 27 | 144 |
| Mother Hb | 11.64 | 15.26 | 33 | 185 |
| Child is anemic (%) | 0.53 | 0.5 | 0 | 1 |
| Mother is anemic (%) | 0.52 | 0.5 | 0 | 1 |
| Child Demographics | ||||
| Birth order | 3.71 | 2.34 | 1 | 10 |
| Child age (months) | 27.52 | 17.5 | 0 | 59 |
| Female (%) | 0.48 | 0.5 | 0 | 1 |
| Household Demographics | ||||
| Mother’s education | 3.57 | 4.46 | 0 | 20 |
| Religion (Muslim) | 0.98 | 0.11 | 0 | 1 |
| Socioeconomic Status | ||||
| Poorest | 0.35 | 0.48 | 0 | 1 |
| Poor | 0.22 | 0.41 | 0 | 1 |
| Middle | 0.19 | 0.39 | 0 | 1 |
| Rich | 0.13 | 0.34 | 0 | 1 |
| Richest | 0.1 | 0.3 | 0 | 1 |
| Rural (%) | 0.39 | 0.49 | 0 | 1 |
| No. of local government regions | 8 | |||
| Child’s Hb Level | Child Is Anemic | |||
|---|---|---|---|---|
| (1) | (2) | (3) | (4) | |
| Mother’s Hb level | 0.181 *** | 0.165 *** | ||
| (0.0155) | (0.015) | |||
| Mother is anemic | 0.147 *** | 0.135 *** | ||
| (0.0167) | (0.016) | |||
| Child controls | Yes | Yes | Yes | Yes |
| Household controls | Yes | Yes | Yes | Yes |
| Region-fixed effects | No | Yes | No | Yes |
| Observations | 3249 | 3249 | 3249 | 3249 |
| Child Is Anemic | ||||
|---|---|---|---|---|
| Children’s Age | Mother’s Education | |||
| <12 months | ≥12 months | <5 years | ≥5 years | |
| (1) | (2) | (3) | (4) | |
| Mother is anemic | 0.16 *** | 0.13 *** | 0.138 *** | 0.129 *** |
| (0.05) | (0.017) | (0.021) | (0.027) | |
| Child controls | Yes | Yes | Yes | Yes |
| Household controls | Yes | Yes | Yes | Yes |
| Region-fixed effects | No | Yes | No | Yes |
| Observations | 373 | 2874 | 1994 | 1255 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sowe, A.; Wood, E.; Gautam, S.K. Maternal Anemia as a Predictor of Childhood Anemia: Evidence from Gambian Health Data. Nutrients 2025, 17, 879. https://doi.org/10.3390/nu17050879
Sowe A, Wood E, Gautam SK. Maternal Anemia as a Predictor of Childhood Anemia: Evidence from Gambian Health Data. Nutrients. 2025; 17(5):879. https://doi.org/10.3390/nu17050879
Chicago/Turabian StyleSowe, Alhagie, Elizabeth Wood, and Santosh Kumar Gautam. 2025. "Maternal Anemia as a Predictor of Childhood Anemia: Evidence from Gambian Health Data" Nutrients 17, no. 5: 879. https://doi.org/10.3390/nu17050879
APA StyleSowe, A., Wood, E., & Gautam, S. K. (2025). Maternal Anemia as a Predictor of Childhood Anemia: Evidence from Gambian Health Data. Nutrients, 17(5), 879. https://doi.org/10.3390/nu17050879






