Interplay Between Diet, Branched-Chain Amino Acids, and Myokines in Children: Vegetarian Versus Traditional Eating Habits
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Anthropometric and Nutritional Measurements
2.3. Biochemical Analyses
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R. Branched-chain amino acids and muscle protein synthesis in humans: Myth or reality? J. Int. Soc. Sports Nutr. 2017, 14, 30. [Google Scholar] [CrossRef]
- Kamei, Y.; Hatazawa, Y.; Uchitomi, R.; Yoshimura, R.; Miura, S. Regulation of skeletal muscle function by amino acids. Nutrients 2020, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Devignes, C.S.; Carmeliet, G.; Stegen, S. Amino acid metabolism in skeletal cells. Bone Rep. 2022, 17, 101620. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Bains, K. Protein, lysine and vitamin D: Critical role in muscle and bone health. Crit. Rev. Food Sci. 2022, 62, 2548–2559. [Google Scholar] [CrossRef]
- Jeong, D.; Park, K.; Lee, J.; Choi, J.; Du, H.; Jeong, H.; Li, L.; Sakai, K.; Kang, S. Effect of resistance exercise and essential amino acid intake on muscle quality, myokine, and inflammation factor in young adult males. Nutrients 2024, 16, 1688. [Google Scholar] [CrossRef]
- Nasso, R.; D’Errico, A.; Motti, M.L.; Masullo, M.; Arcone, R. Dietary protein and physical exercise for the treatment of sarcopenia. Clin. Pract. 2024, 14, 1451–1467. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–organ crosstalk: The emerging roles of myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Das, D.K.; Graham, Z.A.; Cardozo, C.P. Myokines in skeletal muscle physiology and metabolism: Recent advances and future perspectives. Acta Physiol. 2020, 228, e13367. [Google Scholar] [CrossRef]
- Liu, L.; Li, M.; Qin, Y.; Liu, L.; Xiao, Y. Serum follistatin-like 1 in children with obesity and metabolic-associated fatty liver disease. BMC Endocr. Disord. 2024, 24, 165. [Google Scholar] [CrossRef]
- Walsh, F.S.; Celeste, A.J. Myostatin: A modulator of skeletal-muscle stem cells. Biochem. Soc. Trans. 2005, 33, 1513–1517. [Google Scholar] [CrossRef]
- Baig, M.H.; Ahmad, K.; Moon, J.S.; Park, S.Y.; Lim, J.H.; Chun, H.J.; Qadri, A.F.; Hwang, Y.C.; Jan, A.T.; Ahmad, S.S.; et al. Myostatin and its regulation: A comprehensive review of myostatin inhibiting strategies. Front. Physiol. 2022, 13, 876078. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, C.; Hui, G.Z.; Amanda, W.Z.; Lau, H.Y.; Lokireddy, S.; Xiaojia, G.; Mouly, V.; Butter-Browne, G.; Gluckman, P.D.; Sharma, M.; et al. Human myostatin negatively regulates human myoblast growth and differentiation. Am. J. Physiol. Cell Physiol. 2011, 301, C196–C203. [Google Scholar] [CrossRef] [PubMed]
- Kappes, E.C.; Kattamuri, C.; Czepnik, M.; Yarawsky, A.E.; Brule, E.; Wang, Y.; Ongaro, L.; Herr, A.B.; Walton, K.L.; Bernard, D.J.; et al. Follistatin forms a stable complex with inhibin A that does not interfere with activin A antagonism. Endocrinology 2023, 164, 1–114. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.N.; Little, H.C.; Clark, D.J.; Zhang, H.; Wong, G.W. Protein modifications critical for myonectin/erythroferrone secretion and oligomer assembly. Biochemistry 2020, 59, 2684–2697. [Google Scholar] [CrossRef]
- Seldin, M.M.; Peterson, J.M.; Byerly, M.S.; Wei, Z.; Wong, G.W. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J. Biol. Chem. 2012, 287, 11968–11980. [Google Scholar] [CrossRef]
- Hamasaki, H. Effects of exercise on circulating muscle-related cytokines in adults with type 2 diabetes and/or obesity. Curr. Diabetes Rev. 2023, 19, e121222211873. [Google Scholar] [CrossRef]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, C. Muscle, bone, and fat crosstalk: The biological role of myokines, osteokines, and adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef]
- Bowser, M.; Herberg, S.; Arounleut, P.; Shi, X.; Fulzele, S.; Hill, W.D.; Isales, C.M.; Hamrick, M.W. Effects of the activin A-myostatin-follistatin system on aging bone and muscle progenitor cells. Exp. Gerontol. 2013, 48, 290–297. [Google Scholar] [CrossRef]
- Alexy, U. Diet and growth of vegetarian and vegan children. BMJ Nutr. Prev. Health 2023, 6, e000697. [Google Scholar] [CrossRef]
- Senesi, P.; Luzi, L.; Terruzzi, I. Adipokines, myokines, and cardiokines: The role of nutritional interventions. Int. J. Mol. Sci. 2020, 21, 8372. [Google Scholar] [CrossRef] [PubMed]
- Ewy, M.W.; Patel, A.; Abdelmagid, M.G.; Elfadil, O.M.; Bonnes, S.L.; Salonen, B.R.; Hurt, R.T.; Mundi, M.S. Plant-based diet: Is it as good as an animal-based diet when it comes to protein? Curr. Nutr. Rep. 2022, 11, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Peretti, N.; Darmaun, D.; Chouraqui, J.P.; Bocquet, A.; Briend, A.; Feillet, F.; Frelut, M.L.; Guimber, D.; Hankard, R.; Lapillonne, A.; et al. Vegetarian diet in children and adolescents: A health benefit? Arch. Pediatr. 2020, 27, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Cofnas, N. Is vegetarianism healthy for children? Crit. Rev. Food Sci. Nutr. 2019, 59, 2052–2060. [Google Scholar] [CrossRef]
- Dietrich, S.; Trefflich, I.; Ueland, P.M.; Menzel, J.; Penczynski, K.J.; Abraham, K.; Weikert, C. Amino acid intake and plasma concentrations and their interplay with gut microbiota in vegans and omnivores in Germany. Eur. J. Nutr. 2022, 61, 2103–2114. [Google Scholar] [CrossRef]
- Hovinen, T.; Korkalo, L.; Freese, R.; Skaffari, E.; Isohanni, P.; Niemi, M.; Nevalainen, J.; Gylling, H.; Zamboni, N.; Erkkola, M.; et al. Vegan diet in young children remodels metabolism and challenges the statuses of essential nutrients. EMBO Mol. Med. 2021, 13, e13492. [Google Scholar] [CrossRef]
- Mariotti, F.; Gardner, C.D. Dietary protein and amino acids in vegetarian diets: A review. Nutrients 2019, 11, 2661. [Google Scholar] [CrossRef]
- Neufingerl, N.; Eilander, A. Nutrient intake and status in adults consuming plant-based diets compared to meat-eaters: A systematic review. Nutrients 2022, 14, 29. [Google Scholar] [CrossRef]
- Ambroszkiewicz, J.; Gajewska, J.; Mazur, J.; Kuśmierska, K.; Klemarczyk, W.; Rowicka, G.; Strucińska, M.; Chełchowska, M. Dietary and circulating amino acid concentrations in relations with bone metabolism markers in children following vegetarian and omnivorous diets. Nutrients 2023, 15, 1376. [Google Scholar] [CrossRef]
- Płudowski, P.; Kos-Kudła, B.; Walczak, M.; Fal, A.; Zozulińska-Ziółkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewiński, A.; et al. Guidelines for Preventing and Treating Vitamin D Deficiency: A 2023 Update in Poland. Nutrients 2023, 15, 695. [Google Scholar] [CrossRef]
- Wajszczyk, B.; Chwojnowska, Z.; Nasiadko, D.; Rybaczuk, M. Dieta 5.0 Software for Individual and Group Nutrition Assessment and Diet Planning; National Food and Nutrition Institute: Warsaw, Poland, 2015. [Google Scholar]
- Ambroszkiewicz, J.; Klemarczyk, W.; Mazur, J.; Gajewska, J.; Rowicka, G.; Strucińska, M.; Chełchowska, M. Serum hepcidin and soluble transferrin receptor in the assessment of iron metabolism in children on a vegetarian diet. Biol. Trace Elem. Res. 2017, 180, 182–190. [Google Scholar] [CrossRef]
- Marsh, K.A.; Munn, E.A.; Baines, S.K. Protein and vegetarian diets. Med. J. Aust. 2013, 199, S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Kaji, K.; Nishimura, N.; Enomoto, M.; Fujimoto, Y.; Murata, K.; Takaya, H.; Kawaratani, H.; Moriya, K.; Namisaki, T.; et al. Angiotensin receptor blockers potentiate the protective effect of branched-chain amino acids on skeletal muscle atrophy in cirrhotic rats. Mol. Nutr. Food Res. 2021, 65, 2100526. [Google Scholar] [CrossRef] [PubMed]
- Konstantis, G.; Pourzitaki, C.; Chourdakis, M.; Kitsikidou, E.; Germanidis, G. Efficacy of branched chain amino acids supplementation in liver cirrhosis: A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 1171–1190. [Google Scholar] [CrossRef] [PubMed]
- Xiu, S.; Chhetri, J.K.; Sun, L.; Mu, Z.; Wang, L. Association of serum prealbumin with risk of osteoporosis in older adults with type 2 diabetes mellitus: A cross-sectional study. Ther. Adv. Chronic Dis. 2019, 10, 204062. [Google Scholar] [CrossRef]
- Caso, G.; Scalfi, L.; Marra, M.; Covino, A.; Muscaritoli, M.; McNurlan, M.A.; Garlick, P.J.; Contaldo, F. Albumin synthesis is diminished in men consuming a predominantly vegetarian diet. J. Nutr. 2000, 130, 528–533. [Google Scholar] [CrossRef][Green Version]
- Ambroszkiewicz, J.; Gajewska, J.; Mazur, J.; Klemarczyk, W.; Rowicka, G.; Ołtarzewski, M.; Strucińska, M.; Chełchowska, M. Does a vegetarian diet affect the levels of myokine and adipokine in prepubertal children? J. Clin. Med. 2021, 10, 3995. [Google Scholar] [CrossRef]
- Ambroszkiewicz, J.; Gajewska, J.; Szamotulska, K.; Rowicka, G.; Klemarczyk, W.; Strucińska, M.; Chełchowska, M. Comparative analysis of myokines and bone metabolism markers in prepubertal vegetarian and omnivorous children. Nutrients 2024, 16, 2009. [Google Scholar] [CrossRef]
- Jurimae, J.; Remmel, L.; Tamm, A.L.; Purge, P.; Maasalu, K.; Tillmann, V. Follistatin is associated with bone mineral density in lean adolescent girls with increased physical activity. Children 2023, 10, 1226. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, T.; Mokou, M.; Li, L.; Li, P.; Song, J.; Liu, H.; Zhu, Z.; Liu, D.; Yang, M.; et al. Follistatin-like 1 as a novel adipo-myokine related to insulin resistance and physical activity. J. Clin. Endocrinol. Metab. 2020, 105, 629. [Google Scholar] [CrossRef]
- Gilson, H.; Schakman, O.; Kalista, S.; Lause, P.; Tsuchida, K.; Thissen, J.P. Follistatin induces muscle hypertrophy through satellite cell proliferation and inhibition of both myostatin and activin. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E157–E164. [Google Scholar] [CrossRef] [PubMed]
- Günther, A.L.B.; Remer, T.; Kroke, A.; Buyken, A.E. Early protein intake and later obesity risk: Which protein sources at which time points throughout infancy and childhood are important for body mass index and body fat percentage at 7 years of age? Am. J. Clin. Nutr. 2007, 86, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Levin, S.; Barnard, N. Cardio-metabolic benefits of plant-based diets. Nutrients 2017, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Gerić, M.; Jakaša, I.; Peremin, I.; Domijan, A.M.; Vučić Lovrenčić, M.; Kežić, S.; Bituh, M.; Moraes de Andrade, V. Inflammatory, oxidative and DNA damage status in vegetarians: Is the future of human diet green? Crit. Rev. Food Sci. Nutr. 2023, 63, 3189–3221. [Google Scholar] [CrossRef]
- Thorisdottir, B.; Gunnarsdottir, I.; Palsson, G.I.; Halldorsson, T.I.; Thorsdottir, I. Animal protein intake at 12 months is associated with growth factors at the age of six. Acta Paediatr. 2014, 103, 512–517. [Google Scholar] [CrossRef]
- Pimpin, L.; Jebb, S.A.; Johnson, L.; Llewellyn, C.; Ambrosini, G.L. Sources and patterns of protein intake and risk of overweight or obesity in young UK twins. Br. J. Nutr. 2018, 120, 820–829. [Google Scholar] [CrossRef]
- Gauze-Gnagne, C.; Raynard, F.; Djohan, Y.F.; Laurent, C.; Feillet-Coudray, C.; Coudray, C.; Monde, A.; Koffi, G.; Morena, M.; Camara-Cisse, M.; et al. Impact of diets rich in olive oil, palm oil or lard on myokine expression in rats. Food Funct. 2020, 11, 9114–9128. [Google Scholar] [CrossRef]
- Perry, C.A.; van Guilder, G.P.; Butterick, T.A. Decreased myostatin in response to a controlled DASH diet is associated with improved body composition and cardiometabolic biomarkers in older adults: Results from a controlled-feeding diet interventional study. BMC Nutr. 2022, 8, 24. [Google Scholar] [CrossRef]
- Kysel, P.; Haluzikova, D.; Pleyerova, I.; Reznickova, K.; Lankova, I.; Lacinova, Z.; Havrlantova, T.; Mraz, M.; Kasperova, B.J.; Kovarova, V.; et al. Different effects of cyclical ketogenic vs. nutritionally balanced reduction diet on serum concentrations of myokines in healthy young males undergoing combined resistance/aerobic training. Nutrients 2023, 15, 1720. [Google Scholar] [CrossRef]
- Siramolpiwat, S.; Limthanetkul, N.; Pornthisarn, B.; Vilaichone, R.K.; Chonprasertsuk, S.; Bhanthumkomol, P.; Nunanan, P.; Issariyakulkarn, N. Branched-chain amino acids supplementation improves liver frailty index in frail compensated cirrhotic patients: A randomized controlled trial. BMC Gastroenterol. 2023, 23, 154. [Google Scholar] [CrossRef]
| Vegetarians (n = 42) | Omnivores (n = 22) | p Value | |
|---|---|---|---|
| Boys, n (%) | 21 (50%) | 11 (50%) | |
| Age (years) | 6.3 (5.4–8.4) | 6.0 (4.8–7.5) | 0.197 |
| BMI (kg/m2) | 15.3 ± 1.4 | 15.2 ± 1.2 | 0.561 |
| Energy (kcal/d) | 1349 (1056–1634) | 1476 (1261–1736) | 0.210 |
| Carbohydrates, % of energy | 56.1 ± 4.5 | 52.0 ± 5.2 | 0.007 |
| Fat, % of energy | 29.2 ± 4.4 | 29.8 ± 5.6 | 0.867 |
| Protein, % of energy | 12.1 ± 2.2 | 15.9 ± 4.1 | <0.001 |
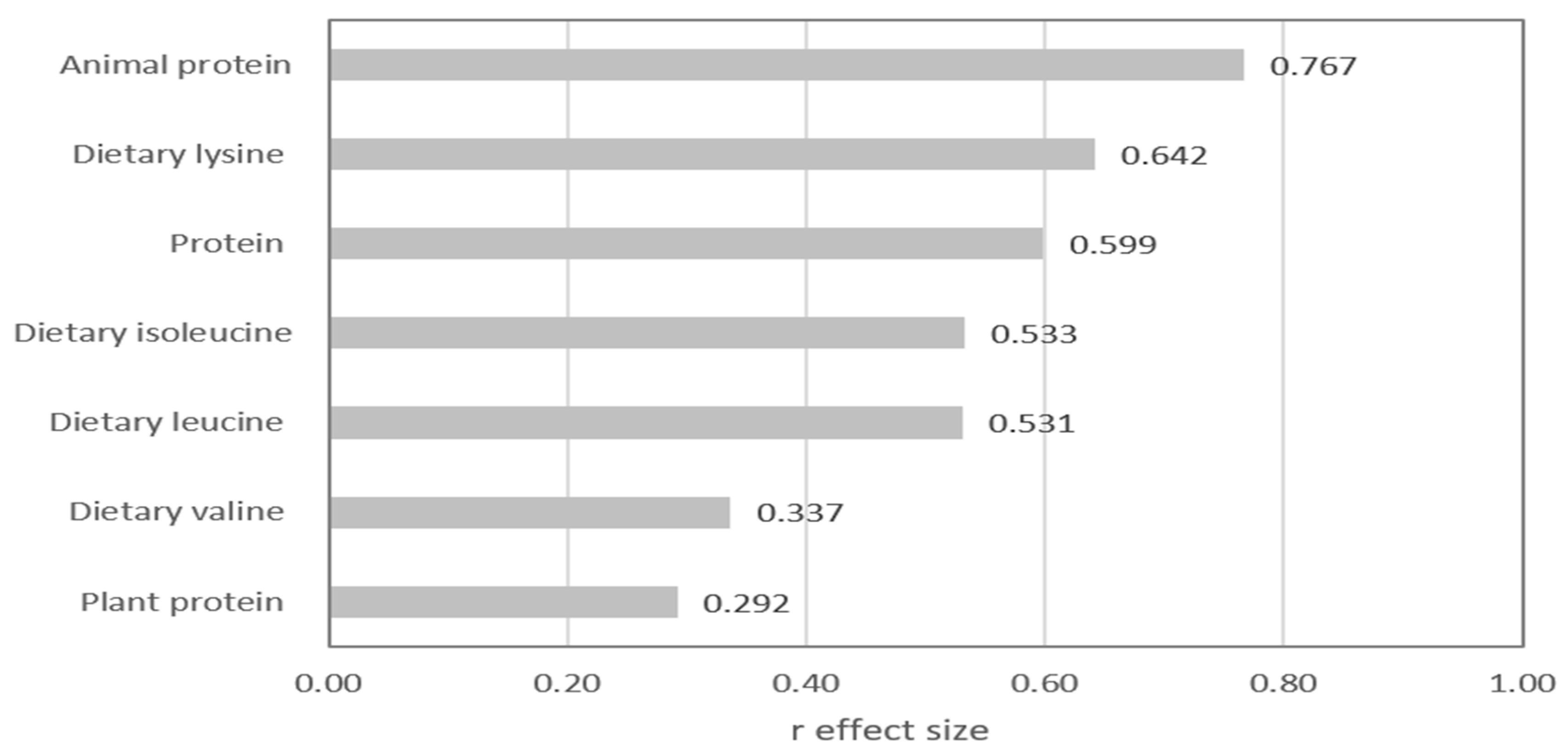
| Protein (g/d) | 34.5 (27.8–42.3) | 54.8 (44.8–67.7) | <0.001 |
| Animal protein (g/d) | 13.3 (5.2–17.6) | 36.9 (27.5–46.7) | <0.001 |
| Plant protein (g/d) | 21.9 (18.1–26.3) | 18.8 (14.7–22.2) | 0.026 |
| Dietary leucine (mg/d) | 2734 (2063–3408) | 4275 (3175–5338) | <0.001 |
| Dietary isoleucine (mg/d) | 1635 (1280–2083) | 2670 (1924–3310) | <0.001 |
| Dietary valine (mg/d) | 2024 (1581–2584) | 2670 (1924–3310) | 0.010 |
| Dietary lysine (mg/d) | 1956 (1470–2376) | 3762 (2755–4729) | <0.001 |
| Vegetarians | Omnivores | p Value | |
|---|---|---|---|
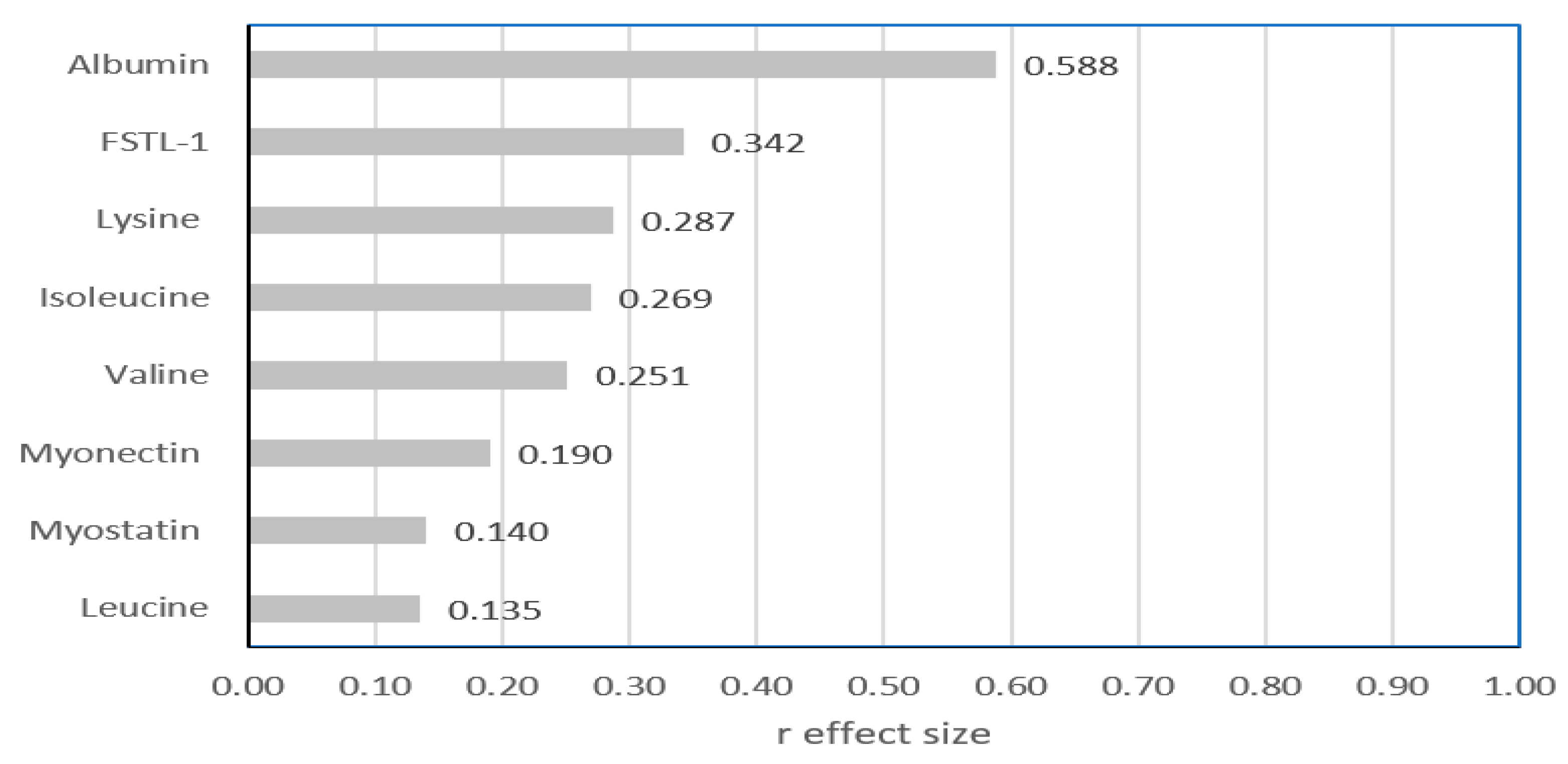
| Albumin (mg/mL) | 51.1 (45.7–56.5) | 62.8 (56.6–68.2) | <0.001 |
| Leucine (µmol/L) | 109 (94–124) | 115 (92–146) | 0.280 |
| Isoleucine (µmol/L) | 51 (43–63) | 57 (51–76) | 0.031 |
| Valine (µmol/L) | 186 (166–209) | 206 (176–267) | 0.045 |
| Lysine (µmol/L) | 123 (88–151) | 147 (115–174) | 0.022 |
| FSTL-1 (ng/mL) | 7.7 (6.5–9.4) | 9.7 (7.5–13.9) | 0.012 |
| MSTN (ng/mL) | 1.1 (0.7–2.2) | 1.4 (0.9–2.4) | 0.264 |
| Myonectin (ng/mL) | 7.0 (4.8–8.7) | 8.1 (6.4–8.9) | 0.129 |
| Leucine | Isoleucine | Valine | Lysine | |||||
|---|---|---|---|---|---|---|---|---|
| Rho * | p | Rho * | p | Rho * | p | Rho * | p | |
| Age | −0.044 | 0.728 | −0.024 | 0.849 | −0.020 | 0.877 | 0.099 | 0.435 |
| BMI | 0.129 | 0.325 | −0.023 | 0.860 | 0.078 | 0.551 | 0.042 | 0.753 |
| Energy | 0.094 | 0.475 | −0.157 | 0.231 | −0.109 | 0.407 | −0.296 | 0.022 |
| Protein | 0.151 | 0.257 | 0.139 | 0.297 | 0.343 | 0.008 | 0.344 | 0.008 |
| Animal protein | 0.118 | 0.376 | 0.197 | 0.138 | 0.300 | 0.022 | 0.288 | 0.028 |
| Plant protein | 0.128 | 0.337 | −0.039 | 0.773 | 0.132 | 0.322 | 0.111 | 0.408 |
| Dietary leucine | 0.037 | 0.780 | 0.059 | 0.658 | 0.251 | 0.057 | 0.262 | 0.047 |
| Dietary isoleucine | 0.035 | 0.796 | 0.062 | 0.643 | 0.256 | 0.052 | 0.254 | 0.054 |
| Dietary valine | 0.028 | 0.836 | 0.023 | 0.863 | 0.247 | 0.061 | 0.227 | 0.086 |
| Dietary lysine | 0.070 | 0.602 | 0.126 | 0.347 | 0.301 | 0.022 | 0.304 | 0.022 |
| Leucine | Isoleucine | Valine | Lysine | |||||
|---|---|---|---|---|---|---|---|---|
| Rho * | p | Rho * | p | Rho * | p | Rho * | p | |
| Age | 0.012 | 0.942 | 0.110 | 0.490 | 0.108 | 0.197 | 0.286 | 0.066 |
| BMI | 0.013 | 0.935 | −0.113 | 0.476 | −0.007 | 0.964 | −0.081 | 0.610 |
| Energy | −0.069 | 0.673 | −0.186 | 0.251 | −0.165 | 0.308 | −0.377 | 0.018 |
| Protein | 0.304 | 0.064 | 0.184 | 0.268 | 0.467 | 0.003 | 0.461 | 0.004 |
| Animal protein | 0.201 | 0.226 | 0.157 | 0.345 | 0.309 | 0.059 | 0.289 | 0.078 |
| Plant protein | 0.232 | 0.161 | 0.059 | 0.727 | 0.294 | 0.073 | 0.321 | 0.057 |
| Dietary leucine | 0.224 | 0.176 | 0.143 | 0.393 | 0.385 | 0.017 | 0.409 | 0.011 |
| Dietary isoleucine | 0.214 | 0.196 | 0.147 | 0.377 | 0.375 | 0.020 | 0.398 | 0.013 |
| Dietary valine | 0.226 | 0.172 | 0.147 | 0.377 | 0.397 | 0.014 | 0.417 | 0.009 |
| Dietary lysine | 0.170 | 0.308 | 0.128 | 0.444 | 0.348 | 0.032 | 0.361 | 0.026 |
| FSTL-1 | MSTN | Myonectin | ||||
|---|---|---|---|---|---|---|
| Rho * | p | Rho * | p | Rho * | p | |
| Age | −0.008 | 0.957 | −0.442 | <0.001 | −0.232 | 0.065 |
| BMI | 0.074 | 0.604 | −0.069 | 0.601 | 0.076 | 0.565 |
| Energy intake | −0.044 | 0.761 | 0.152 | 0.246 | 0.119 | 0.364 |
| Protein intake | 0.278 | 0.054 | 0.124 | 0.352 | 0.093 | 0.485 |
| Animal protein | 0.335 | 0.010 | 0.334 | 0.019 | 0.277 | 0.035 |
| Plant protein | −0.141 | 0.334 | −0.102 | 0.445 | −0.306 | 0.019 |
| Dietary leucine | 0.253 | 0.080 | 0.141 | 0.292 | −0.149 | 0.263 |
| Dietary isoleucine | 0.267 | 0.064 | 0.164 | 0.219 | −0.128 | 0.339 |
| Dietary valine | 0.214 | 0.139 | 0.119 | 0.374 | −0.165 | 0.216 |
| Dietary lysine | 0.288 | 0.045 | 0.229 | 0.084 | 0.049 | 0.714 |
| Serum leucine | 0.104 | 0.452 | 0.118 | 0.351 | 0.098 | 0.443 |
| Serum isoleucine | 0.140 | 0.313 | 0.045 | 0.722 | 0.146 | 0.251 |
| Serum valine | 0.155 | 0.264 | 0.101 | 0.429 | 0.107 | 0.402 |
| Serum lysine | 0.135 | 0.332 | 0.045 | 0.725 | 0.035 | 0.783 |
| Serum albumin | 0.215 | 0.119 | 0.188 | 0.136 | 0.177 | 0.162 |
| FSTL-1 | - | - | 0.027 | 0.847 | 0.054 | 0.697 |
| MSTN | - | - | 0.261 | 0.038 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambroszkiewicz, J.; Chełchowska, M.; Mazur, J.; Rowicka, G.; Klemarczyk, W.; Strucińska, M.; Gajewska, J. Interplay Between Diet, Branched-Chain Amino Acids, and Myokines in Children: Vegetarian Versus Traditional Eating Habits. Nutrients 2025, 17, 834. https://doi.org/10.3390/nu17050834
Ambroszkiewicz J, Chełchowska M, Mazur J, Rowicka G, Klemarczyk W, Strucińska M, Gajewska J. Interplay Between Diet, Branched-Chain Amino Acids, and Myokines in Children: Vegetarian Versus Traditional Eating Habits. Nutrients. 2025; 17(5):834. https://doi.org/10.3390/nu17050834
Chicago/Turabian StyleAmbroszkiewicz, Jadwiga, Magdalena Chełchowska, Joanna Mazur, Grażyna Rowicka, Witold Klemarczyk, Małgorzata Strucińska, and Joanna Gajewska. 2025. "Interplay Between Diet, Branched-Chain Amino Acids, and Myokines in Children: Vegetarian Versus Traditional Eating Habits" Nutrients 17, no. 5: 834. https://doi.org/10.3390/nu17050834
APA StyleAmbroszkiewicz, J., Chełchowska, M., Mazur, J., Rowicka, G., Klemarczyk, W., Strucińska, M., & Gajewska, J. (2025). Interplay Between Diet, Branched-Chain Amino Acids, and Myokines in Children: Vegetarian Versus Traditional Eating Habits. Nutrients, 17(5), 834. https://doi.org/10.3390/nu17050834











