The Sunshine Paradox: Unraveling Risk Factors for Low Vitamin D Status Among Non-Pregnant Women in Lebanon
Abstract
1. Introduction
2. Materials and Methods
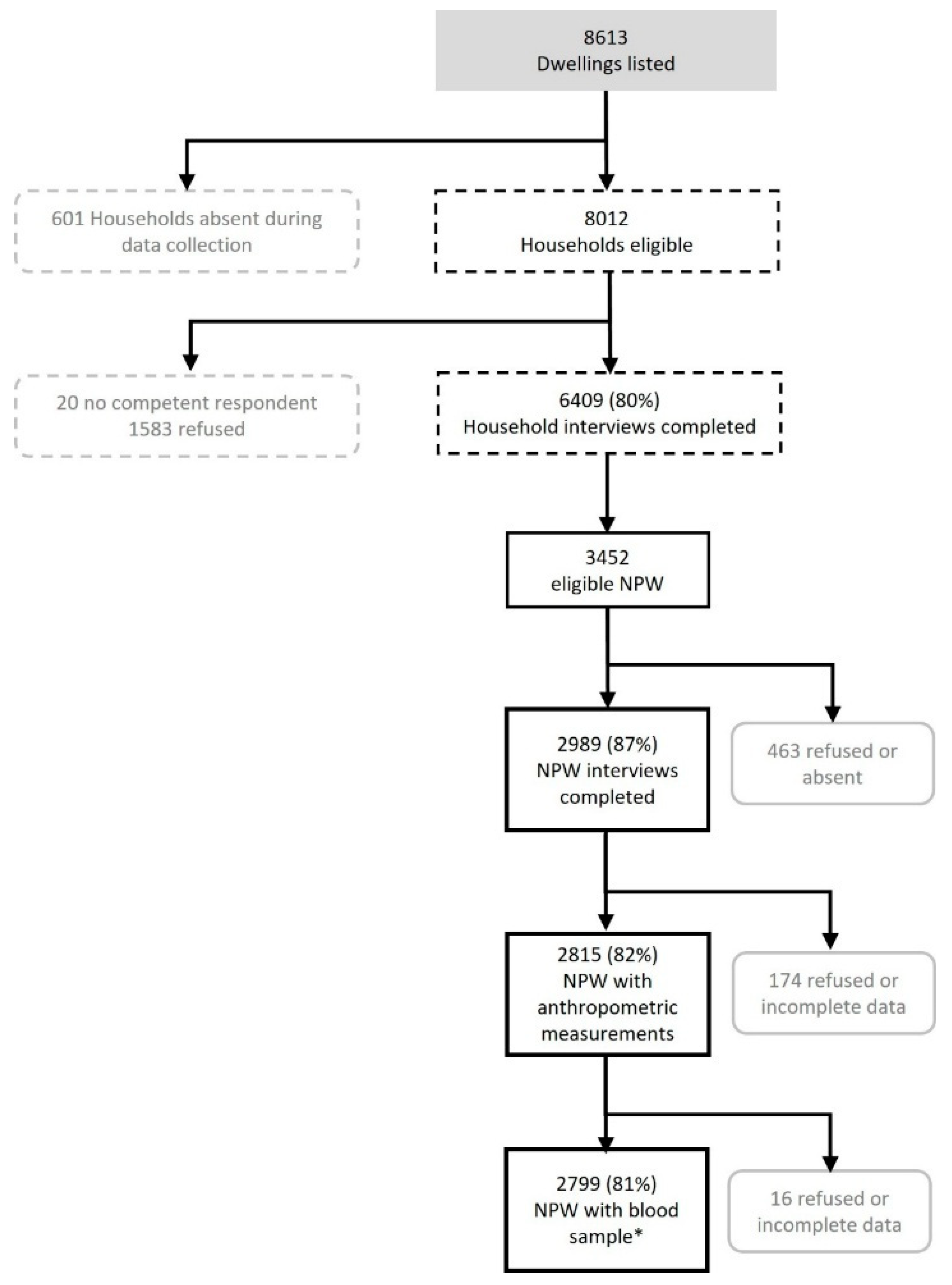
2.1. Survey Design and Participants
2.2. Data Collection
2.2.1. Questionnaires
2.2.2. Anthropometric and Blood Pressure Measurements
2.2.3. Biological Fluids Collection and Analyses
2.3. Indicators and Thresholds
2.4. Data Management and Statistical Analyses
2.5. Ethical Considerations
3. Results
3.1. Vitamin D Deficiency and Hypovitaminosis D Risk Factors
3.2. Hijab Wearing and Vitamin D Supplementation: Key Predictors of Vitamin D Status
4. Discussion
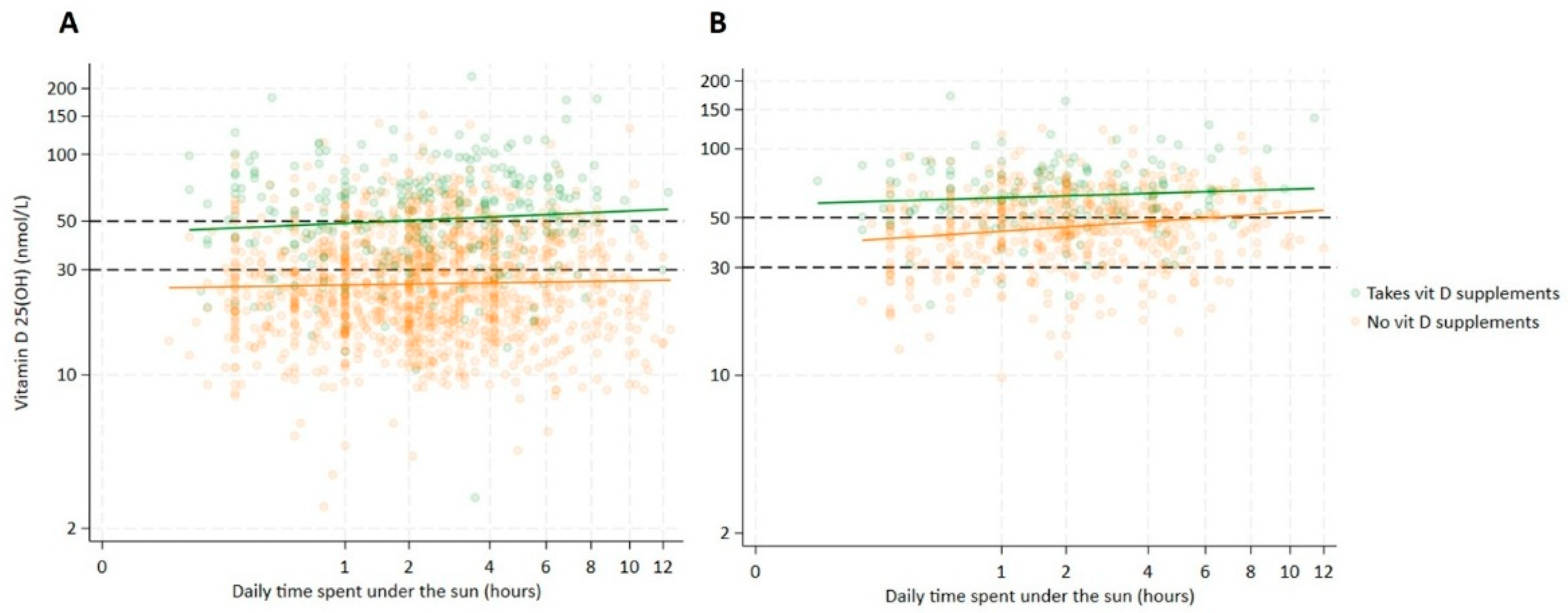
4.1. Synthesis-Related Risk Factors
4.2. Intake-Related Risk Factors
4.3. Weight-Related Risk Factors
4.4. Biochemical Risk Factors
4.5. Demographic Risk Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 25(OH)D | 25-hydroxyvitamin D |
| AGP | α1-acid glycoprotein |
| aPR | adjusted prevalence ratio |
| AUB | American University of Beirut |
| AUBMC | American University of Beirut Medical Center |
| BMI | body mass index |
| CBC | complete blood count |
| CI | confidence interval |
| CRP | C-reactive protein |
| EA | enumeration area |
| EDTA | ethylenediaminetetraacetic acid |
| ELISA | enzyme-linked immunosorbent assay |
| FANTA | Food and Nutrition Technical Assistance |
| HbA1c | glycated hemoglobin |
| HDL | high-density lipoprotein |
| HFIAS | Household Food Insecurity Access Scale |
| IOM | Institute of Medicine |
| IS | informal settlement |
| kg | kilogram |
| L | liter |
| m | meter |
| LIMA | Lebanon Integrated Micronutrient, Anthropometry, and Child Development Survey |
| mL | milliliter |
| ng | nanogram |
| nmol | nanomole |
| RBP | retinol-binding protein |
| SF | serum ferritin |
| SGA | small children for gestational age |
| SST | serum-separating tubes |
| sTfR | soluble transferrin receptor |
References
- Holick, M.F. Vitamin D: A D-Lightful Health Perspective. Nutr. Rev. 2008, 66, S182–S194. [Google Scholar] [CrossRef] [PubMed]
- Basit, S. Vitamin D in Health and Disease: A Literature Review. Br. J. Biomed. Sci. 2013, 70, 161–172. [Google Scholar] [CrossRef]
- Anjum, I.; Jaffery, S.S.; Fayyaz, M.; Samoo, Z.; Anjum, S. The Role of Vitamin D in Brain Health: A Mini Literature Review. Cureus 2018, 10, e2960. [Google Scholar] [CrossRef]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and Regional Prevalence of Vitamin D Deficiency in Population-Based Studies from 2000 to 2022: A Pooled Analysis of 7.9 Million Participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, T.; Itkonen, S.T.; Lundqvist, A.; Erkkola, M.; Koskela, T.; Lakkala, K.; Dowling, K.G.; Hull, G.L.J.; Kröger, H.; Karppinen, J.; et al. The Positive Impact of General Vitamin D Food Fortification Policy on Vitamin D Status in a Representative Adult Finnish Population: Evidence from an 11-y Follow-up Based on Standardized 25-Hydroxyvitamin D Data1, 2, 3. Am. J. Clin. Nutr. 2017, 105, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Tripkovic, L.; Wilson, L.R.; Hart, K.; Johnsen, S.; de Lusignan, S.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Elliott, R.; et al. Daily Supplementation with 15 Μg Vitamin D2 Compared with Vitamin D3 to Increase Wintertime 25-Hydroxyvitamin D Status in Healthy South Asian and White European Women: A 12-Wk Randomized, Placebo-Controlled Food-Fortification Trial. Am. J. Clin. Nutr. 2017, 106, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Kiely, M.; Cashman, K.D. Summary Outcomes of the ODIN Project on Food Fortification for Vitamin D Deficiency Prevention. Int. J. Environ. Res. Public Health 2018, 15, 2342. [Google Scholar] [CrossRef]
- Zhao, L.; Tyson, N.; Liu, J.; Hébert, J.; Steck, S. Trends in Dietary Supplement Use Among US Adults Between 2009 and 2018. Curr. Dev. Nutr. 2021, 5, 701. [Google Scholar] [CrossRef]
- Kantor, E.D.; Rehm, C.D.; Du, M.; White, E.; Giovannucci, E.L. Trends in Dietary Supplement Use Among US Adults From 1999–2012. JAMA 2016, 316, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.E.; Tooze, J.A.; Gahche, J.J.; Eicher-Miller, H.A.; Guenther, P.M.; Dwyer, J.T.; Potischman, N.; Bhadra, A.; Carroll, R.J.; Bailey, R.L. Trends in Overall and Micronutrient-Containing Dietary Supplement Use in US Adults and Children, NHANES 2007–2018. J. Nutr. 2022, 152, 2789–2801. [Google Scholar] [CrossRef]
- Wan, M.; Horsfall, L.J.; Basatemur, E.; Patel, J.P.; Shroff, R.; Rait, G. Vitamin D Prescribing in Children in UK Primary Care Practices: A Population-Based Cohort Study. BMJ Open 2019, 9, e031870. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.M.; Ruppert, K.; Udupa, N.; Bassir, F.; Darakananda, K.; Solomon, D.H.; Lian, Y.; Cauley, J.A.; Karlamangla, A.S.; Greendale, G.A.; et al. Temporal Increases in 25-Hydroxyvitamin D in Midlife Women: Longitudinal Results from the Study of Women’s Health Across the Nation. Clin. Endocrinol. 2019, 91, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Raizada, N.; Goyal, A.; Singla, R.; Sreenivas, V.; Gupta, N.; Khadgawat, R. Changing Trend in Vitamin D Status from 2008 to 2016: An Experience from a Tertiary Care Institute in North India. Indian J. Endocrinol. Metab. 2020, 24, 150–154. [Google Scholar]
- Roth, D.E.; Abrams, S.A.; Aloia, J.; Bergeron, G.; Bourassa, M.W.; Brown, K.H.; Calvo, M.S.; Cashman, K.D.; Combs, G.; De-Regil, L.M.; et al. Global Prevalence and Disease Burden of Vitamin D Deficiency: A Roadmap for Action in Low- and Middle-Income Countries. Ann. N. Y. Acad. Sci. 2018, 1430, 44–79. [Google Scholar] [CrossRef]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; El-Hajj Fuleihan, G.; Bouillon, R. Current Vitamin D Status in European and Middle East Countries and Strategies to Prevent Vitamin D Deficiency: A Position Statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, P23–P54. [Google Scholar] [CrossRef]
- Bassil, D.; Rahme, M.; Hoteit, M.; Fuleihan, G.E.-H. Hypovitaminosis D in the Middle East and North Africa. Dermato-Endocrinology 2013, 5, 274–298. [Google Scholar] [CrossRef]
- Saad, R.K.; Akiki, V.C.; Rahme, M.; Ajjour, S.; Assaad, M.; El-Hajj Fuleihan, G.A. Time Trends and Predictors of Hypovitaminosis D across the Life Course: 2009–2016. Metabolism 2020, 105, 154138. [Google Scholar] [CrossRef]
- Arabi, A.; Chamoun, N.; Nasrallah, M.P.; Tamim, H.M. Vitamin D Deficiency in Lebanese Adults: Prevalence and Predictors from a Cross-Sectional Community-Based Study. Int. J. Endocrinol. 2021, 2021, 3170129. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Tamblyn, J.A.; Finn-Sell, S.; Chan, S.-Y.; Westwood, M.; Gupta, J.; Kilby, M.D.; Gross, S.R.; Hewison, M. Vitamin D, the Placenta and Early Pregnancy: Effects on Trophoblast Function. J. Endocrinol. 2018, 236, R93–R103. [Google Scholar] [CrossRef]
- Baker, A.M.; Haeri, S.; Camargo, C.A.; Espinola, J.A.; Stuebe, A.M. A Nested Case-Control Study of Midgestation Vitamin D Deficiency and Risk of Severe Preeclampsia. J. Clin. Endocrinol. Metab. 2010, 95, 5105–5109. [Google Scholar] [CrossRef] [PubMed]
- Aghajafari, F.; Nagulesapillai, T.; Ronksley, P.E.; Tough, S.C.; O’Beirne, M.; Rabi, D.M. Association between Maternal Serum 25-Hydroxyvitamin D Level and Pregnancy and Neonatal Outcomes: Systematic Review and Meta-Analysis of Observational Studies. BMJ Br. Med. J. 2013, 346, f1169. [Google Scholar] [CrossRef] [PubMed]
- Miliku, K.; Vinkhuyzen, A.; Blanken, L.M.E.; McGrath, J.J.; Eyles, D.W.; Burne, T.H.; Hofman, A.; Tiemeier, H.; Steegers, E.A.P.; Gaillard, R.; et al. Maternal Vitamin D Concentrations during Pregnancy, Fetal Growth Patterns, and Risks of Adverse Birth Outcomes12. Am. J. Clin. Nutr. 2016, 103, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- de Mendonça, E.L.S.S.; de Lima Macêna, M.; Bueno, N.B.; de Oliveira, A.C.M.; Mello, C.S. Premature Birth, Low Birth Weight, Small for Gestational Age and Chronic Non-Communicable Diseases in Adult Life: A Systematic Review with Meta-Analysis. Early Hum. Dev. 2020, 149, 105154. [Google Scholar] [CrossRef]
- Ministry of Public Health; UNICEF-Lebanon; American University of Beirut; Mercy USA; World Food Programme; Harvard T.H. Chan School of Public Health; GroundWork. Integrated Micronutrient, Anthropometry and Child Development Survey 2023; UNICEF: Beirut, Lebanon, 2024.
- Lebanese Palestinian Dialogue Committee; Central Administration of Statistics; Palestinian Central Bureau of Statistics. The Population and Housing Census in Palestinian Camps and Gatherings—2017, Key Findings Report (Population, Buildings and Housing Units); Lebanese Palestinian Dialogue Committee: Beirut, Lebanon, 2018. [Google Scholar]
- Filmer, D.; Pritchett, L. The Effect of Household Wealth on Educational Attainment: Demographic and Health Survey Evidence; Policy Research Working Paper; World Bank Publications: Washington, DC, USA, 1998. (In English) [Google Scholar]
- Coates, J.; Swindale, A.; Bilinsky, P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Food Access: Indicator Guide: Version 3; Food and Nutrition Technical Assistance (FANTA III): Washington, DC, USA, 2007. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Minimum Dietary Diversity for Women: A Guide for Measurement; FAO: Rome, Italy, 2016; Volume 82, p. 499. [Google Scholar]
- Erhardt, J.G.; Estes, J.E.; Pfeiffer, C.M.; Biesalski, H.K.; Craft, N.E. Combined Measurement of Ferritin, Soluble Transferrin Receptor, Retinol Binding Protein, and C-Reactive Protein by an Inexpensive, Sensitive, and Simple Sandwich Enzyme-Linked Immunosorbent Assay Technique. J. Nutr. 2004, 134, 3127–3132. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- WHO. Guideline on Haemoglobin Cutoffs to Defne Anaemia in Individuals and Populations; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- WHO. Serum Ferritin Concentrations for the Assessment of Iron Status and Iron Deficiency in Populations; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Thurnham, D.I.; McCabe, G.P.; Northrop-Clewes, C.A.; Nestel, P. Effects of Subclinical Infection on Plasma Retinol Concentrations and Assessment of Prevalence of Vitamin a Deficiency: Meta-Analysis. Lancet 2003, 362, 2052–2058. [Google Scholar] [CrossRef]
- WHO. Serum and Red Blood Cell Folate Concentrations for Assessing Folate Status in Populations; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- de Benoist, B. Conclusions of a WHO Technical Consultation on Folate and Vitamin B12 Deficiencies. Food Nutr. Bull. 2008, 29, S238–S244. [Google Scholar] [CrossRef] [PubMed]
- IZiNCG. Secretariat Assessing Population Zinc Status with Serum Zinc Concentration; IZiNCG: Davis, CA, USA, 2012. [Google Scholar]
- WHO. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Grundy, S.M.; Brewer, H.B.; Cleeman, J.I.; Smith, S.C.; Lenfant, C. Definition of Metabolic Syndrome. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef]
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report. Pediatrics 2011, 128, S213. [Google Scholar] [CrossRef] [PubMed]
- WHO. Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee. World Health Organ. Tech. Rep. Ser. 1995, 854, 1–452. [Google Scholar]
- WHO. Prevention of Cardiovascular Disease: Guidelines for Assessment and Management of Total Cardiovascular Risk; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- WHO. Serum Retinol Concentrations for Determining the Prevalence of Vitamin A Deficiency in Populations. In Vitamin and Mineral Nutrition Information System; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Ministry of Health; UNICEF; GroundWork. Oman National Nutrition Survey 2017; UNICEF: Muscat, Oman, 2018. [Google Scholar]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D Deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Hussein, I.H.; Mansour, A.A.; Nwayyir, H.A.; Almomin, A.M.S.A.; Alibrahim, N.T.Y.; Alidrisi, H.A.; Al-Waeli, D.K.J.; Zaboon, I.A.; Hussein, R.N.; Mohammed, A.G.; et al. Real-Life Data on Total Vitamin D3 (25-Hydroxyvitamin D) Concentrations in Basrah, Iraq. Biomed. Pharmacol. J. 2021, 14, 2191–2198. [Google Scholar] [CrossRef]
- Alpdemir, M.; Alpdemir, M.F. Vitamin D Deficiency Status in Turkey: A Meta-Analysis. Int. J. Med. Biochem. 2019, 2, 118–131. [Google Scholar] [CrossRef]
- Botros, R.; Al, S.H.; Mansour, H.; Guirgis, M. High Prevalence of Severe Vitamin D Deficiency in Egyptian Females. In Proceedings of the Endocrine Abstracts, 21st European Congress of Endocrinology, Lyon, France, 18–21 May 2019; European Society of Endocrinology: Lyon, France, 2019; p. P473. [Google Scholar]
- Ministry of Health; UNICEF; WFP; Jordan Health Aid Society International; Department of Statistics; Biolab; GroundWork. Jordan National Micronutrient and Nutritional Survey 2019; UNICEF: Amman, Jordan, 2021. [Google Scholar]
- Stokes, C.S.; Lammert, F.; Volmer, D.A. Analytical Methods for Quantification of Vitamin D and Implications for Research and Clinical Practice. Anticancer Res. 2018, 38, 1137. [Google Scholar]
- Daniel, D. Bikle Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar]
- Libon, F.; Cavalier, E.; Nikkels, A.F. Skin Color Is Relevant to Vitamin D Synthesis. Dermatology 2013, 227, 250–254. [Google Scholar] [CrossRef]
- Richard, A.; Rohrmann, S.; Quack Lötscher, K.C. Prevalence of Vitamin D Deficiency and Its Associations with Skin Color in Pregnant Women in the First Trimester in a Sample from Switzerland. Nutrients 2017, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Zanoletti, A.; Kareem, K.Y.; Adelodun, B.; Kumar, P.; Ajibade, F.O.; Silva, L.F.O.; Phillips, A.J.; Kartheeswaran, T.; Bontempi, E.; et al. Skin Protection from Solar Ultraviolet Radiation Using Natural Compounds: A Review. Environ. Chem. Lett. 2024, 22, 273–295. [Google Scholar] [CrossRef]
- Farrar, M.D.; Kift, R.; Felton, S.J.; Berry, J.L.; Durkin, M.T.; Allan, D.; Vail, A.; Webb, A.R.; Rhodes, L.E. Recommended Summer Sunlight Exposure Amounts Fail to Produce Sufficient Vitamin D Status in UK Adults of South Asian Origin123. Am. J. Clin. Nutr. 2011, 94, 1219–1224. [Google Scholar] [CrossRef]
- Mishal, A.A. Effects of Different Dress Styles on Vitamin D Levels in Healthy Young Jordanian Women. Osteoporos. Int. 2001, 12, 931–935. [Google Scholar] [CrossRef]
- Batieha, A.; Khader, Y.; Jaddou, H.; Hyassat, D.; Batieha, Z.; Khateeb, M.; Belbisi, A.; Ajlouni, K. Vitamin D Status in Jordan: Dress Style and Gender Discrepancies. Ann. Nutr. Metab. 2011, 58, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Gannagé-Yared, M.-H.; Maalouf, G.; Khalife, S.; Challita, S.; Yaghi, Y.; Ziade, N.; Chalfoun, A.; Norquist, J.; Chandler, J. Prevalence and Predictors of Vitamin D Inadequacy amongst Lebanese Osteoporotic Women. Br. J. Nutr. 2008, 101, 487–491. [Google Scholar] [CrossRef]
- Silva, M.C.; Furlanetto, T.W. Intestinal Absorption of Vitamin D: A Systematic Review. Nutr. Rev. 2018, 76, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.L.; Rees, J.R.; Peacock, J.L.; Mott, L.A.; Amos, C.I.; Bostick, R.M.; Figueiredo, J.C.; Ahnen, D.J.; Bresalier, R.S.; Burke, C.A.; et al. Genetic Variants in CYP2R1, CYP24A1, and VDR Modify the Efficacy of Vitamin D3 Supplementation for Increasing Serum 25-Hydroxyvitamin D Levels in a Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2014, 99, E2133–E2137. [Google Scholar] [CrossRef]
- Tobias, D.K.; Luttmann-Gibson, H.; Mora, S.; Danik, J.; Bubes, V.; Copeland, T.; LeBoff, M.S.; Cook, N.R.; Lee, I.-M.; Buring, J.E.; et al. Association of Body Weight with Response to Vitamin D Supplementation and Metabolism. JAMA Netw. Open 2023, 6, e2250681. [Google Scholar] [CrossRef]
- Haghighat, N.; Sohrabi, Z.; Bagheri, R.; Akbarzadeh, M.; Esmaeilnezhad, Z.; Ashtary-Larky, D.; Barati-Boldaji, R.; Zare, M.; Amini, M.; Hosseini, S.V.; et al. A Systematic Review and Meta-Analysis of Vitamin D Status of Patients with Severe Obesity in Various Regions Worldwide. Obes. Facts 2023, 16, 519–539. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemy, Z.; Lotfi, K.; Heidari, Z.; Saneei, P. Serum Vitamin D Levels in Relation to Abdominal Obesity in Children and Adolescents: A Systematic Review and Dose-Response Meta-Analysis. Front. Nutr. 2022, 9, 806459. [Google Scholar] [CrossRef]
- Pereira-Santos, M.; Costa, P.R.F.; Assis, A.M.O.; Santos, C.A.S.T.; Santos, D.B. Obesity and Vitamin D Deficiency: A Systematic Review and Meta-Analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef]
- Walsh, J.S.; Bowles, S.; Evans, A.L. Vitamin D in Obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased Bioavailability of Vitamin D in Obesity123. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Pannu, P.K.; Zhao, Y.; Soares, M.J. Reductions in Body Weight and Percent Fat Mass Increase the Vitamin D Status of Obese Subjects: A Systematic Review and Metaregression Analysis. Nutr. Res. 2016, 36, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.; Xiao, L.; Imayama, I.; Duggan, C.R.; Bain, C.; Foster-Schubert, K.E.; Kong, A.; Campbell, K.L.; Wang, C.-Y.; Neuhouser, M.L.; et al. Effects of Weight Loss on Serum Vitamin D in Postmenopausal Women12345. Am. J. Clin. Nutr. 2011, 94, 95–103. [Google Scholar] [CrossRef]
- Tsuji, K.; Maeda, T.; Kawane, T.; Matsunuma, A.; Horiuchi, N. Leptin Stimulates Fibroblast Growth Factor 23 Expression in Bone and Suppresses Renal 1α,25-dihydroxyvitamin D3 Synthesis in Leptin-deficient Ob/Ob Mice. J. Bone Miner. Res. 2010, 25, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Ribas de Farias Costa, P.; Miranda Pereira, E.; Russoni de Lima Lago, I.; Marlucia Oliveira, A. Does Vitamin D Deficiency Increase the Risk of Obesity in Adults and the Elderly? A Systematic Review of Prospective Cohort Studies. Public Health 2021, 190, 123–131. [Google Scholar] [CrossRef]
- Marwaha, R.K.; Garg, M.K.; Mahalle, N.; Bhadra, K.; Tandon, N. Role of Parathyroid Hormone in Determination of Fat Mass in Patients with Vitamin D Deficiency. Indian J. Endocrinol. Metab. 2017, 21, 848–853. [Google Scholar] [CrossRef]
- Abbas, M.A. Physiological Functions of Vitamin D in Adipose Tissue. J. Steroid Biochem. Mol. Biol. 2017, 165, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, Z.S.; Kafeshani, M.; Tavasoli, P.; Zadeh, A.H.; Entezari, M.H. Effect of Vitamin D Supplementation on Weight Loss, Glycemic Indices, and Lipid Profile in Obese and Overweight Women: A Clinical Trial Study. Int. J. Prev. Med. 2018, 9, 63. [Google Scholar] [PubMed]
- Song, C.; Sun, H.; Wang, B.; Song, C.; Lu, H. Association Between Vitamin D Status and Undernutrition Indices in Children: A Systematic Review and Meta-Analysis of Observational Studies. Front. Pediatr. 2021, 9, 665749. [Google Scholar] [CrossRef]
- Orces, C. The Association between Body Mass Index and Vitamin D Supplement Use among Adults in the United States. Cureus 2019, 11, e5721. [Google Scholar] [CrossRef]
- Radkhah, N.; Zarezadeh, M.; Jamilian, P.; Ostadrahimi, A. The Effect of Vitamin D Supplementation on Lipid Profiles: An Umbrella Review of Meta-Analyses. Adv. Nutr. 2023, 14, 1479–1498. [Google Scholar] [CrossRef]
- Nakhl, S.; Sleilaty, G.; El Samad, S.; Saliba, Y.; Chahine, R.; Farès, N. Association between Vitamin D Deficiency and Lipid and Non-Lipid Markers of Cardiovascular Diseases in the Middle East Region. Eur. J. Clin. Nutr. 2019, 73, 850–858. [Google Scholar] [CrossRef]
- Higashi, Y. Endothelial Function in Dyslipidemia: Roles of LDL-Cholesterol, HDL-Cholesterol and Triglycerides. Cells 2023, 12, 1293. [Google Scholar] [CrossRef] [PubMed]
- Querfeld, U.W.E.; Hoffmann, M.M.; Klaus, G.D.; Eifinger, F.; Ackerschott, M.; Michalk, D.; Kern, P.A. Antagonistic Effects of Vitamin D and Parathyroid Hormone on Lipoprotein Lipase in Cultured Adipocytes. J. Am. Soc. Nephrol. 1999, 10, 2158–2164. [Google Scholar] [CrossRef]
- Gholamzad, A.; Khakpour, N.; Kabipour, T.; Gholamzad, M. Association between Serum Vitamin D Levels and Lipid Profiles: A Cross-Sectional Analysis. Sci. Rep. 2023, 13, 21058. [Google Scholar] [CrossRef]
- Lu, Z.; Jiao, Y.; Li, J. Higher Genetically Predicted Triglycerides, LDL, and HDL Increase the Vitamin D Deficiency: A Mendelian Randomization Study. Front. Nutr. 2022, 9, 862942. [Google Scholar] [CrossRef]
- Han, Y.-Y.; Hsu, S.H.-J.; Su, T.-C. Association between Vitamin D Deficiency and High Serum Levels of Small Dense LDL in Middle-Aged Adults. Biomedicines 2021, 9, 464. [Google Scholar] [CrossRef]
- Smith, E.M.; Tangpricha, V. Vitamin D and Anemia: Insights into an Emerging Association. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 432–438. [Google Scholar] [CrossRef]
- Ben Alon, D.; Chaimovitz, C.; Dvilansky, A.; Lugassy, G.; Douvdevani, A.; Shany, S.; Nathan, I. Novel Role of 1,25(OH)2D3 in Induction of Erythroid Progenitor Cell Proliferation. Exp. Hematol. 2002, 30, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Karabayir, N.; Teber, B.G.; Dursun, H.K.; Pehlivan, L.S. Is There an Association Between Vitamin B12 Level and Vitamin D Status in Children? J. Pediatr. Hematol. Oncol. 2022, 44, e677–e681. [Google Scholar] [CrossRef]
- Rahman, A.; Al-Taiar, A.; Shaban, L.; Al-Sabah, R.; Mojiminiyi, O. Plasma 25-Hydroxyvitamin D Is Positively Associated with Folate and Vitamin B12 Levels in Adolescents. Nutr. Res. 2020, 79, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.; Cooper, S.C.; Ghosh, S.; Hewison, M. The Role of Vitamin D in Inflammatory Bowel Disease: Mechanism to Management. Nutrients 2019, 11, 1019. [Google Scholar] [CrossRef] [PubMed]
- Bauman, W.A.; Shaw, S.; Jayatilleke, E.; Spungen, A.M.; Herbert, V. Increased Intake of Calcium Reverses Vitamin B12 Malabsorption Induced by Metformin. Diabetes Care 2000, 23, 1227–1231. [Google Scholar] [CrossRef]
- Shane, B.; Stokstad, E.L.R. Vitamin B12-Folate Interrelationships. Annu. Rev. Nutr. 1985, 5, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Zhang, J.; Huo, C.; Zhang, M.; Xiao, J.; Peng, J.; Wang, G.; Wang, C.; Li, Y. The Association of Vitamin D Deficiency, Age and Depression in US Adults: A Cross-Sectional Analysis. BMC Psychiatry 2023, 23, 534. [Google Scholar] [CrossRef]
- Orces, C.; Lorenzo, C.; Guarneros, J.E. The Prevalence and Determinants of Vitamin D Inadequacy among U.S. Older Adults: National Health and Nutrition Examination Survey 2007–2014. Cureus 2019, 11, e5300. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Baylin, A.; Levy, P.D. Vitamin D Deficiency and Insufficiency among US Adults: Prevalence, Predictors and Clinical Implications. Br. J. Nutr. 2018, 119, 928–936. [Google Scholar] [CrossRef]

| Indicator | Condition | Cutoffs |
|---|---|---|
| Blood biomarkers | ||
| 25(OH)D [30] | Hypovitaminosis D Vitamin D insufficiency Vitamin D deficiency | <50 nmol/L (20 ng/mL) 30 nmol/L (12 ng/mL)–50 nmol/L (20 ng/mL) <30 nmol/L (12 ng/mL) |
| Hemoglobin 1 [31] | Anemia | <120 g/L |
| Retinol-binding protein 2, 3 | Vitamin A deficiency | <0.62 µmol/L |
| Plasma ferritin 3 [32] | Iron deficiency | <15 µg/L |
| α1-acid-glycoprotein [33] | Convalescent inflammation | >1 g/L |
| C-reactive protein [33] | Early or acute inflammation | >5 mg/L |
| Folate [34] | Folate deficiency | <4.4 ng/mL (<10 nmol/L) |
| Vitamin B12 [35] | Vitamin B12 deficiency | <203 mg/L (150 pmol/L) |
| Zinc [36] | Zinc deficiency | Morning sample in a fasting state: <70 μg/dL Morning sample in a non-fasting state: <66 μg/dL Afternoon sample in a non-fasting state: <59 μg/dL |
| HbA1c [37] | Elevated HbA1c Pre-diabetes Diabetes | ≥5.7% (39 mmol/mol) 5.7–6.4% (39–47 mmol/mol) ≥6.5% (48 mmol/mol) |
| Triglycerides [38,39] | Hypertriglyceridemia | ≥18 years: Fasting: ≥150 mg/dL (1.69 mmol/L) Non-fasting: ≥200 mg/dL (2.26 mmol/L) <18 years: Fasting: ≥90 mg/dL (1.02 mmol/L) Non-fasting: ≥130 mg/dL (1.47 mmol/L) |
| Total cholesterol [38,39] | Hypercholesterolemia | ≥18 years: ≥240 mg/dL (6.2 mmol/L) <18 years: ≥200 mg/dL (5.17 mmol/L) |
| HDL-cholesterol [38,39] | Low HDL-cholesterol | ≥18 years: <50 mg/dL (1.3 mmol/L) <18 years: <40 mg/dL (1.03 mmol/L) |
| Anthropometry and blood pressure | ||
| Body Mass Index (BMI) 4 [40] | Underweight Normal weight Overweight Obesity | <18.5 kg/m2 18.5 to 24.9 kg/m2 25 to 29.9 kg/m2 ≥30 kg/m2 |
| Blood pressure [41] | Hypertension | Systolic blood pressure ≥ 140 mmHg Diastolic blood pressure ≥ 90 mmHg |
| Characteristics | N a | % b | 95% CI c |
|---|---|---|---|
| Total | 2803 | 100.0 | |
| Age group (in years) | |||
| 15–19 | 456 | 16.4 | [14.2, 18.9] |
| 20–29 | 751 | 25.3 | [22.5, 28.3] |
| 30–39 | 753 | 26.6 | [23.2, 30.3] |
| 40–49 | 843 | 31.8 | [28.7, 35.0] |
| Skin color | |||
| Very white/white | 1051 | 37.1 | [34.0, 40.2] |
| Olive | 1529 | 54.2 | [51.7, 56.7] |
| Dark/very dark | 213 | 8.5 | [6.7, 10.6] |
| Unable to observe | 10 | 0.3 | [0.1, 0.6] |
| Nationality | |||
| Lebanese | 2002 | 71.6 | [66.8, 76.0] |
| Syrian | 666 | 25.0 | [20.8, 29.8] |
| Palestinian | 97 | 2.0 | [1.2, 3.3] |
| Other | 29 | 1.1 | [0.6,1.9] |
| Not known | 9 | 0.2 | [0.1, 0.6] |
| Wealth quintile | |||
| Lowest | 677 | 23.2 | [19.6, 27.2] |
| Second | 525 | 20.3 | [16.8, 24.3] |
| Middle | 560 | 20.0 | [17.2, 23.2] |
| Fourth | 526 | 17.9 | [15.1, 21.1] |
| Highest | 515 | 18.6 | [15.0, 22.7] |
| Educational level | |||
| Basic secondary or less | 1488 | 55.4 | [51.6, 59.1] |
| Complete secondary or more | 1306 | 44.3 | [40.6, 48.1] |
| Not known | 9 | 0.2 | [0.1, 0.6] |
| Household food insecure access | |||
| Secure | 897 | 34.4 | [29.8, 39.3] |
| Mild | 272 | 9.0 | [7.0, 11.4] |
| Moderate | 859 | 28.6 | [25.3, 32.2] |
| Severe | 775 | 28.0 | [24.0, 32.4] |
| Household sanitation | |||
| Inadequate | 113 | 4.1 | [2.8, 6.0] |
| Adequate | 2636 | 93.4 | [91.2, 95.1] |
| Not known | 54 | 2.5 | [1.5,4.1] |
| Wearing hijab | |||
| Yes | 1920 | 58.3 | [51.9, 64.4] |
| No | 874 | 41.4 | [35.3, 47.8] |
| Not known | 9 | 0.2 | [0.1, 0.6] |
| Variables | Vitamin D Deficiency | Hypovitaminosis D | ||
|---|---|---|---|---|
| Known Risk Factors | aPR a [95% CI] | p-Value b | aPR a [95% CI] | p-Value b |
| Skin color | ||||
| Very white/white | reference | reference | ||
| Olive | 1.14 [1.03, 1.26] | 0.010 | 1.05 [1.00, 1.11] | 0.062 |
| Dark/very dark | 1.28 [1.09, 1.51] | 0.003 | 1.10 [1.00, 1.21] | 0.040 |
| Wearing hijab | ||||
| No | reference | reference | ||
| Yes | 3.76 [3.05, 4.63] | <0.001 | 1.47 [1.36, 1.58] | <0.001 |
| Daily sun exposure | ||||
| <1 h | reference | reference | ||
| 1–2 h | 0.84 [0.74, 0.96] | 0.012 | 0.90 [0.84, 0.96] | 0.002 |
| 2–3 h | 0.84 [0.74, 0.95] | 0.006 | 0.91 [0.85, 0.98] | 0.008 |
| >3 h | 0.83 [0.74, 0.93] | 0.001 | 0.90 [0.85, 0.96] | 0.001 |
| Use of sunscreen | ||||
| No | reference | reference | ||
| Yes | 0.82 [0.74, 0.91] | <0.001 | 0.89 [0.84, 0.95] | <0.001 |
| Vitamin D supplementation | ||||
| No | reference | reference | ||
| Yes | 0.30 [0.24, 0.39] | <0.001 | 0.55 [0.49, 0.62] | <0.001 |
| Multivitamin supplementation | ||||
| No | reference | reference | ||
| Yes | 0.99 [0.81, 1.22] | 0.950 | 0.93 [0.81, 1.05] | 0.231 |
| Demographic characteristics | ||||
| Age group in years | ||||
| 15–19 | 1.57 [1.37, 1.79] | <0.001 | 1.38 [1.27, 1.49] | <0.001 |
| 20–29 | 1.33 [1.17,1.52] | <0.001 | 1.28 [1.19, 1.37] | <0.001 |
| 30–39 | 1.17 [1.03, 1.33] | 0.019 | 1.12 [1.04, 1.20] | 0.003 |
| 40–49 | reference | reference | ||
| Wealth quintile | ||||
| Lowest | 1.21 [0.99, 1.47] | 0.068 | 1.13 [1.02, 1.25] | 0.016 |
| Second | 1.28 [1.05, 1.56] | 0.015 | 1.17 [1.06, 1.28] | 0.002 |
| Middle | 1.25 [1.03, 1.53] | 0.026 | 1.04 [0.94, 1.15] | 0.484 |
| Fourth | 1.15 [0.93, 1.42] | 0.190 | 1.10 [1.00, 1.22] | 0.062 |
| Highest | reference | reference | ||
| Educational level | ||||
| Basic secondary or less | reference | reference | ||
| Complete secondary or more | 0.82 [0.74, 0.92] | <0.001 | 0.96 [0.90, 1.01] | 0.127 |
| Household food security access | ||||
| Secure | reference | reference | ||
| Mild | 1.14 [0.94, 1.38] | 0.185 | 1.02 [0.92, 1.13] | 0.751 |
| Moderate | 1.00 [0.88, 1.15] | 0.974 | 1.02 [0.95, 1.09] | 0.625 |
| Severe | 1.10 [0.96, 1.26] | 0.175 | 0.98 [0.91, 1.05] | 0.571 |
| Other risk factors | ||||
| Minimum dietary diversity | ||||
| No | reference | - | - | |
| Yes | 0.98 [0.90, 1.07] | 0.614 | - | - |
| Tobacco smoking | ||||
| No | reference | reference | ||
| Yes | 0.95 [0.86, 1.05] | 0.282 | 1.00 [0.95, 1.05] | 0.955 |
| Currently breastfeeding | ||||
| No | reference | reference | ||
| Yes | 1.00 [0.80, 1.25] | 0.977 | 0.95 [0.84, 1.08] | 0.431 |
| BMI classes | ||||
| Underweight | - | - | 1.13 [1.02, 1.25] | 0.016 |
| Healthy weight | - | - | reference | |
| Overweight | - | - | 1.06 [1.00, 1.13] | 0.068 |
| Obese | - | - | 1.12 [1.04, 1.20] | 0.001 |
| Elevated serum triglycerides | ||||
| No | reference | - | - | |
| Yes | 0.87 [0.77, 0.97] | 0.016 | - | - |
| Low serum HDL-cholesterol | ||||
| No | reference | reference | ||
| Yes | 1.08 [0.98, 1.20] | 0.116 | 1.06 [1.00, 1.11] | 0.049 |
| Metabolic syndrome | ||||
| No | - | - | reference | |
| Yes | - | - | 1.02 [0.96, 1.09] | 0.544 |
| Anemia | ||||
| No | reference | reference | ||
| Yes | 1.14 [1.04, 1.26] | 0.007 | 1.02 [0.97, 1.08] | 0.441 |
| Iron deficiency | ||||
| No | reference | reference | ||
| Yes | 1.00 [0.91, 1.10] | 0.990 | 1.01 [0.96, 1.06] | 0.743 |
| Vitamin A deficiency | ||||
| No | - | - | reference | |
| Yes | - | - | 1.15 [0.98, 1.34] | 0.078 |
| Folate deficiency | ||||
| No | - | - | reference | |
| Yes | - | - | 1.10 [1.04, 1.17] | 0.001 |
| Vitamin B12 deficiency | ||||
| No | reference | reference | ||
| Yes | 1.29 [1.19, 1.41] | <0.001 | 1.11 [1.06, 1.16] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Mallah, C.; Yarparvar, A.; Galetti, V.; Obeid, O.; Boutros, M.; Safadi, G.; ZeinEddine, R.; Ezzeddine, N.E.H.; Kouzeiha, M.; Kobayter, D.; et al. The Sunshine Paradox: Unraveling Risk Factors for Low Vitamin D Status Among Non-Pregnant Women in Lebanon. Nutrients 2025, 17, 804. https://doi.org/10.3390/nu17050804
El-Mallah C, Yarparvar A, Galetti V, Obeid O, Boutros M, Safadi G, ZeinEddine R, Ezzeddine NEH, Kouzeiha M, Kobayter D, et al. The Sunshine Paradox: Unraveling Risk Factors for Low Vitamin D Status Among Non-Pregnant Women in Lebanon. Nutrients. 2025; 17(5):804. https://doi.org/10.3390/nu17050804
Chicago/Turabian StyleEl-Mallah, Carla, Amirhossein Yarparvar, Valeria Galetti, Omar Obeid, Mira Boutros, Gloria Safadi, Razan ZeinEddine, Nour El Hoda Ezzeddine, Maya Kouzeiha, Diana Kobayter, and et al. 2025. "The Sunshine Paradox: Unraveling Risk Factors for Low Vitamin D Status Among Non-Pregnant Women in Lebanon" Nutrients 17, no. 5: 804. https://doi.org/10.3390/nu17050804
APA StyleEl-Mallah, C., Yarparvar, A., Galetti, V., Obeid, O., Boutros, M., Safadi, G., ZeinEddine, R., Ezzeddine, N. E. H., Kouzeiha, M., Kobayter, D., Wirth, J. P., Abi Zeid Daou, M., Asfahani, F., Hilal, N., Hamadeh, R., Abiad, F., & Petry, N. (2025). The Sunshine Paradox: Unraveling Risk Factors for Low Vitamin D Status Among Non-Pregnant Women in Lebanon. Nutrients, 17(5), 804. https://doi.org/10.3390/nu17050804





