Tissue-Specific Ablation of Liver Fatty Acid-Binding Protein Induces a Metabolically Healthy Obese Phenotype in Female Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of LFABP Floxed Mice
2.2. Generation of Conditional LFABP Null Mice
2.3. DNA Extraction for Genotyping
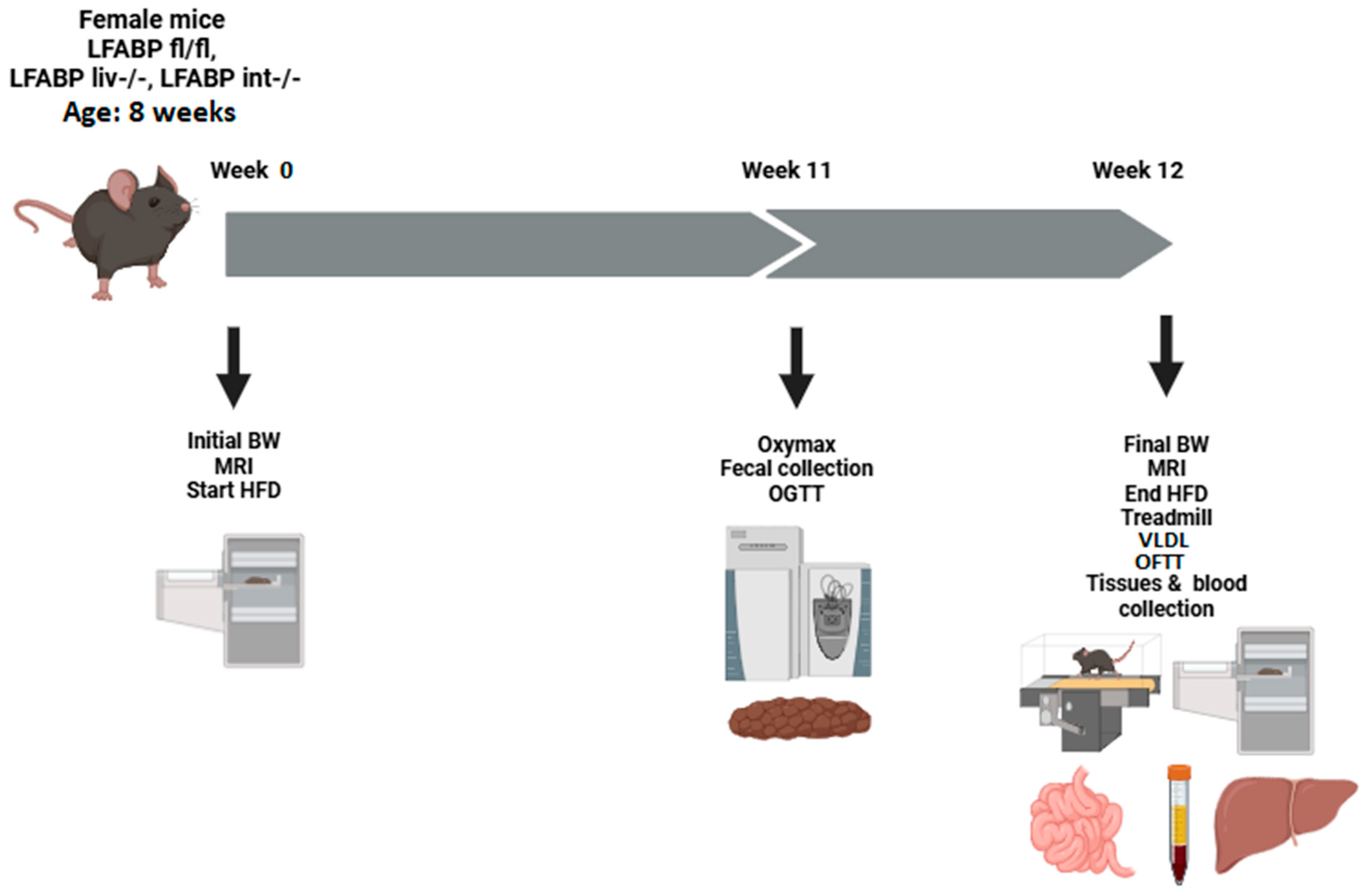
2.4. Diet and Experimental Design
2.5. Body Weight and Body Composition
2.6. Indirect Calorimetry, Activity, and Food Intake
2.7. Intestinal Transit Time
2.8. Total Fecal Excretion
2.9. Treadmill Exercise Protocol
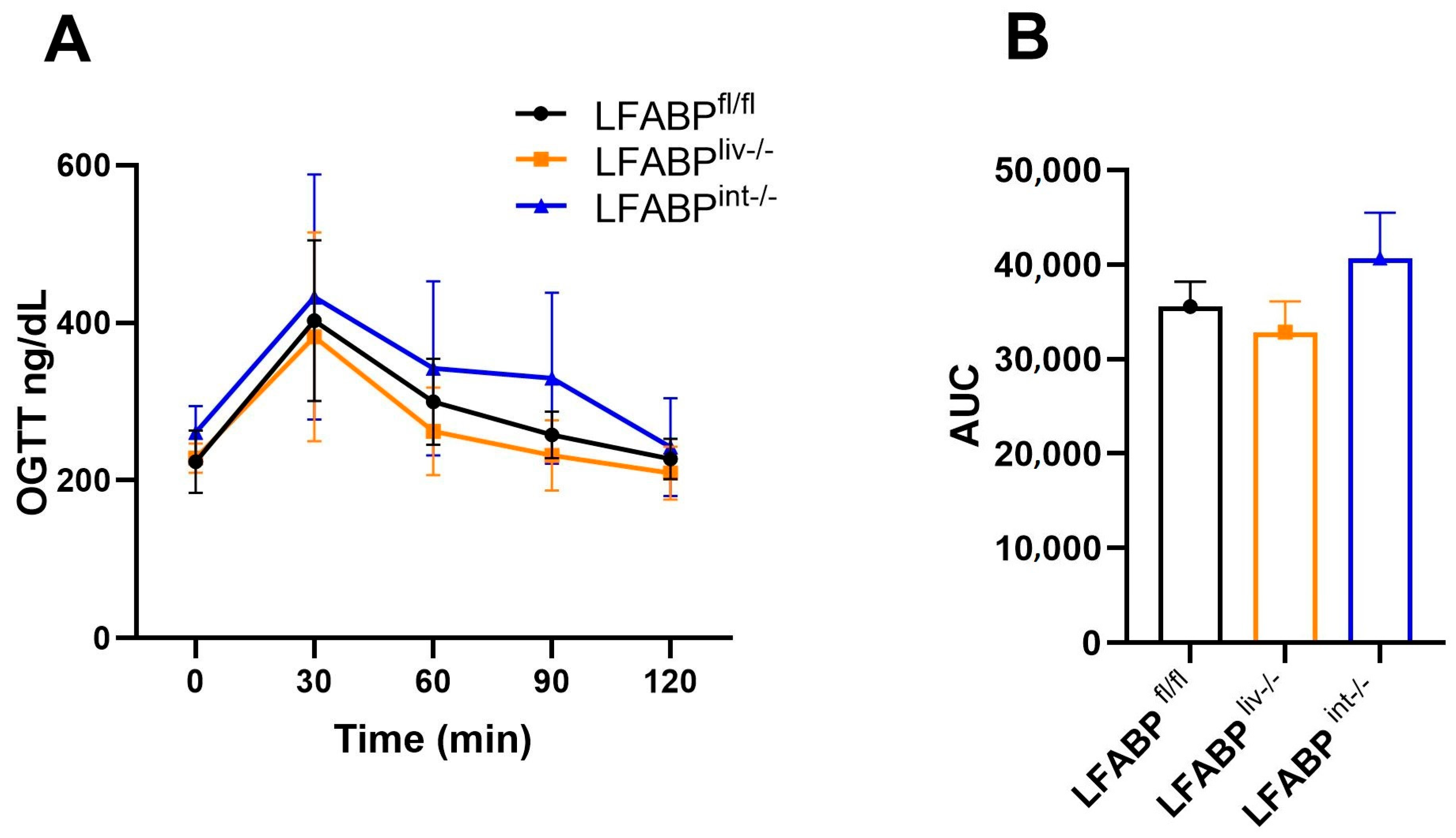
2.10. Oral Glucose Tolerance Tests (OGTT)
2.11. Tissue Preparation
2.12. Blood Preparation and Analysis
2.13. RNA Extraction and Real-Time PCR
2.14. Lipid Extraction and Metabolites Analysis
2.15. VLDL-TG Secretion Measurement
2.16. Oral Fat Tolerance Test (OFTT)
2.17. FA Oxidation Measurements
2.18. Tissue FA Uptake Assay
2.19. Statistical Analysis
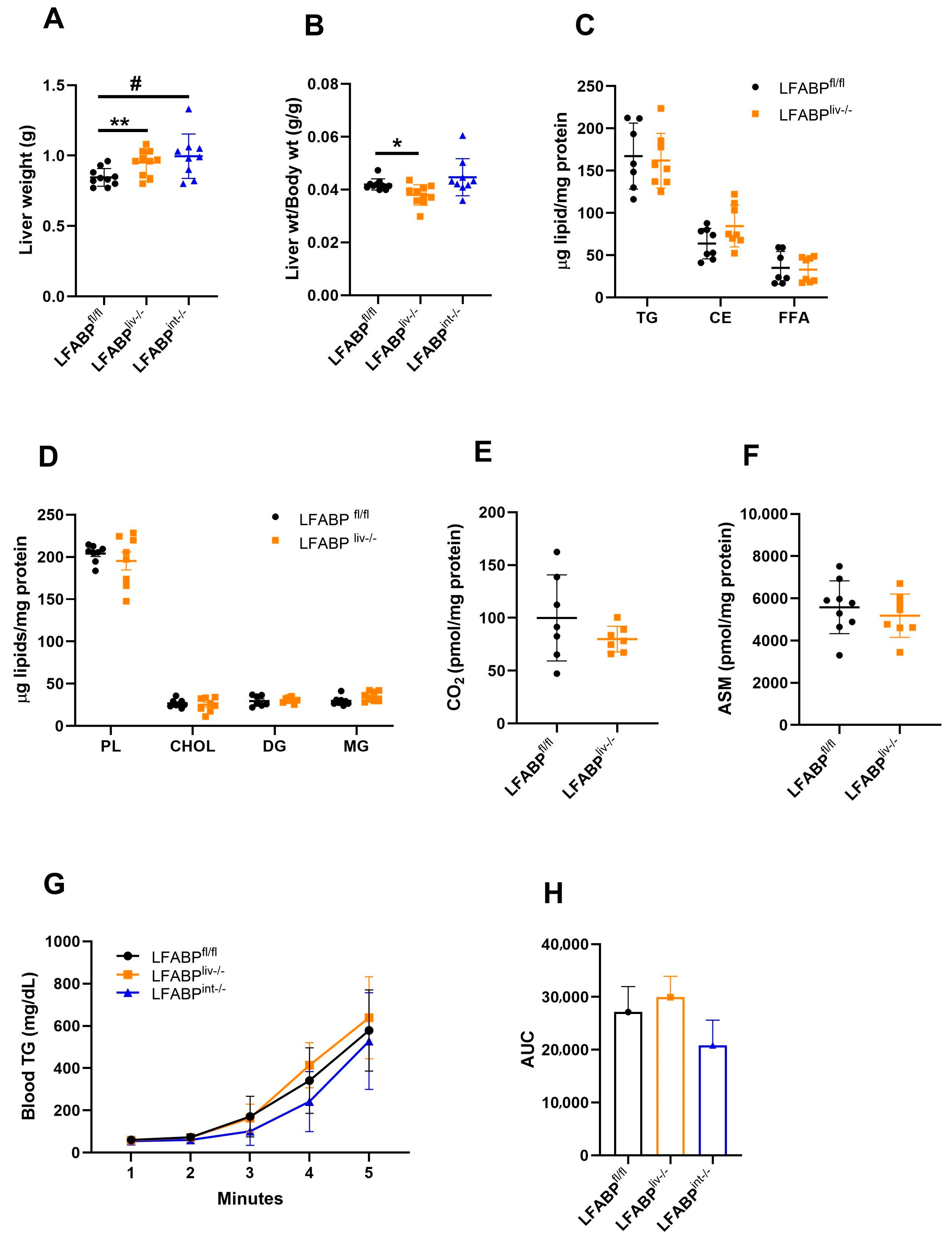
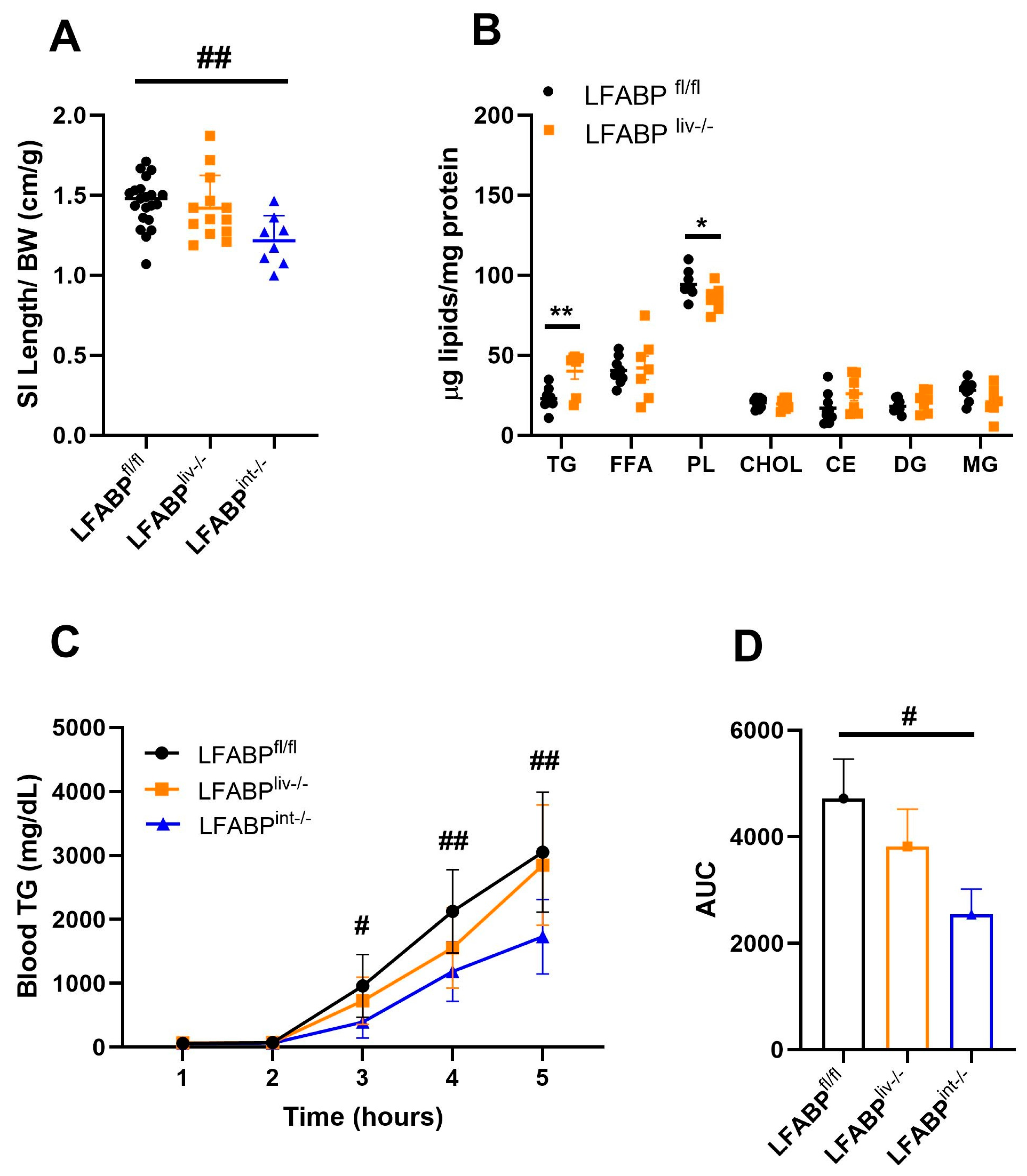
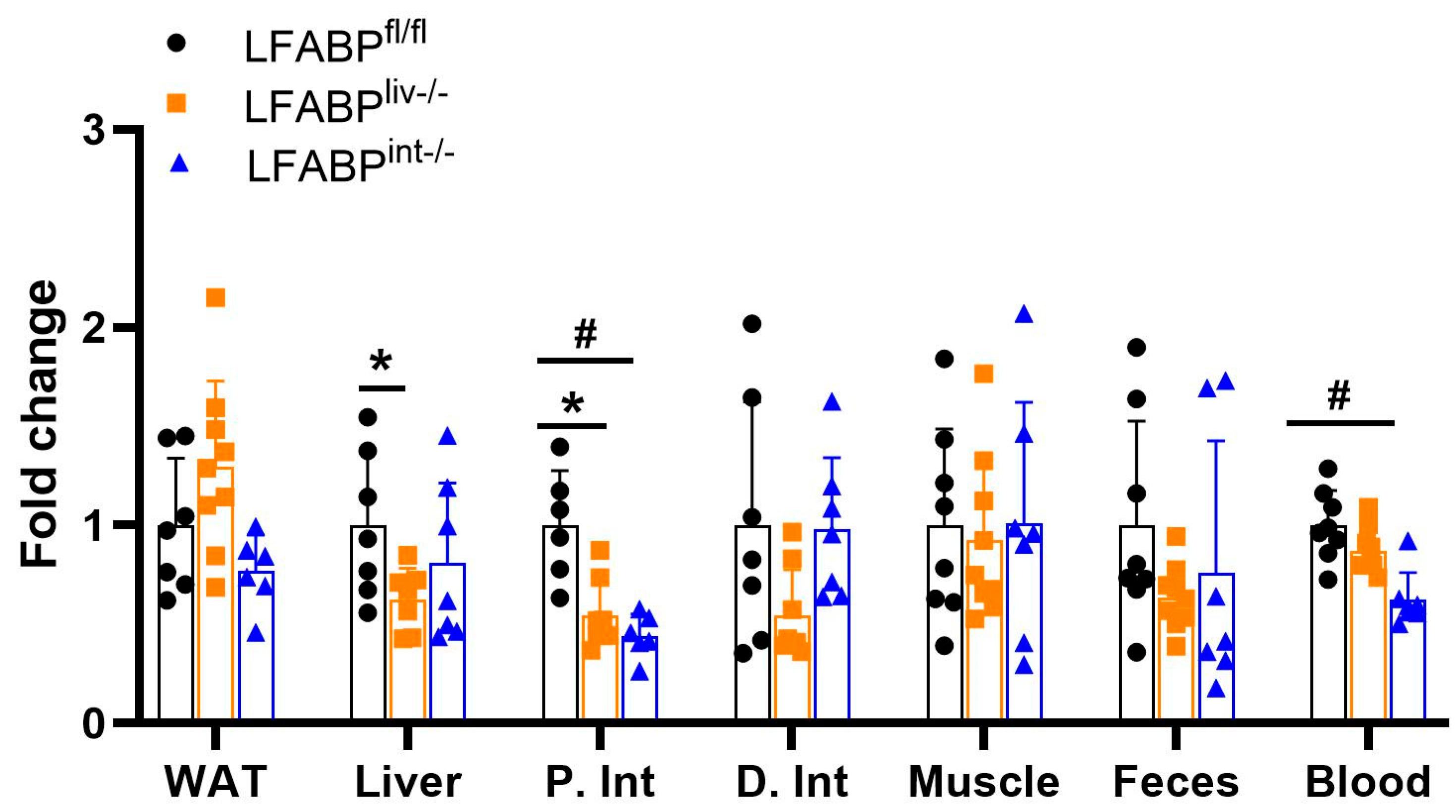
3. Results
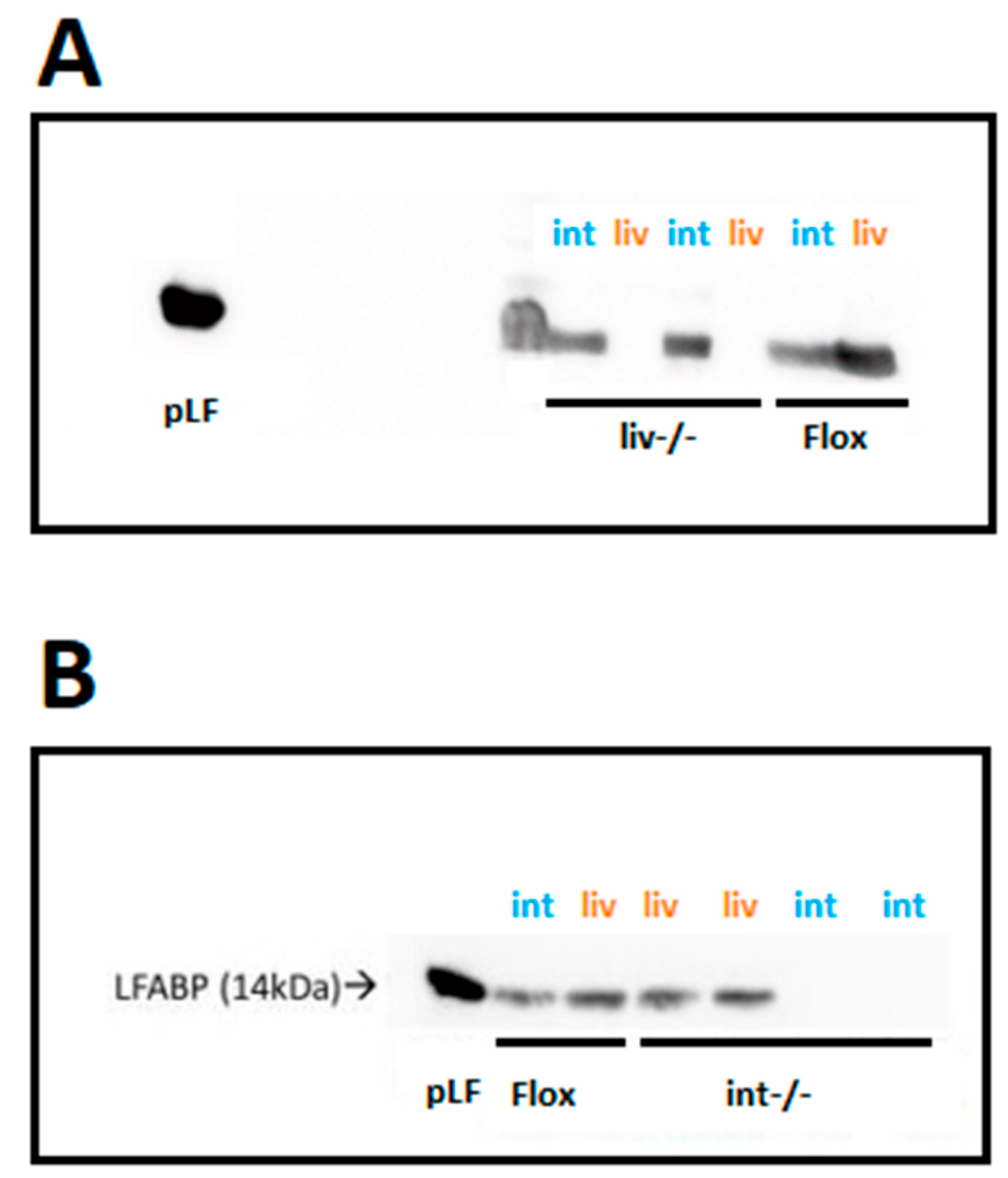
3.1. Ablation of LFABP Was Specific to the Liver in the LFABPliv-/- Mice and Specific to the Intestine in the LFABPint-/- Mice
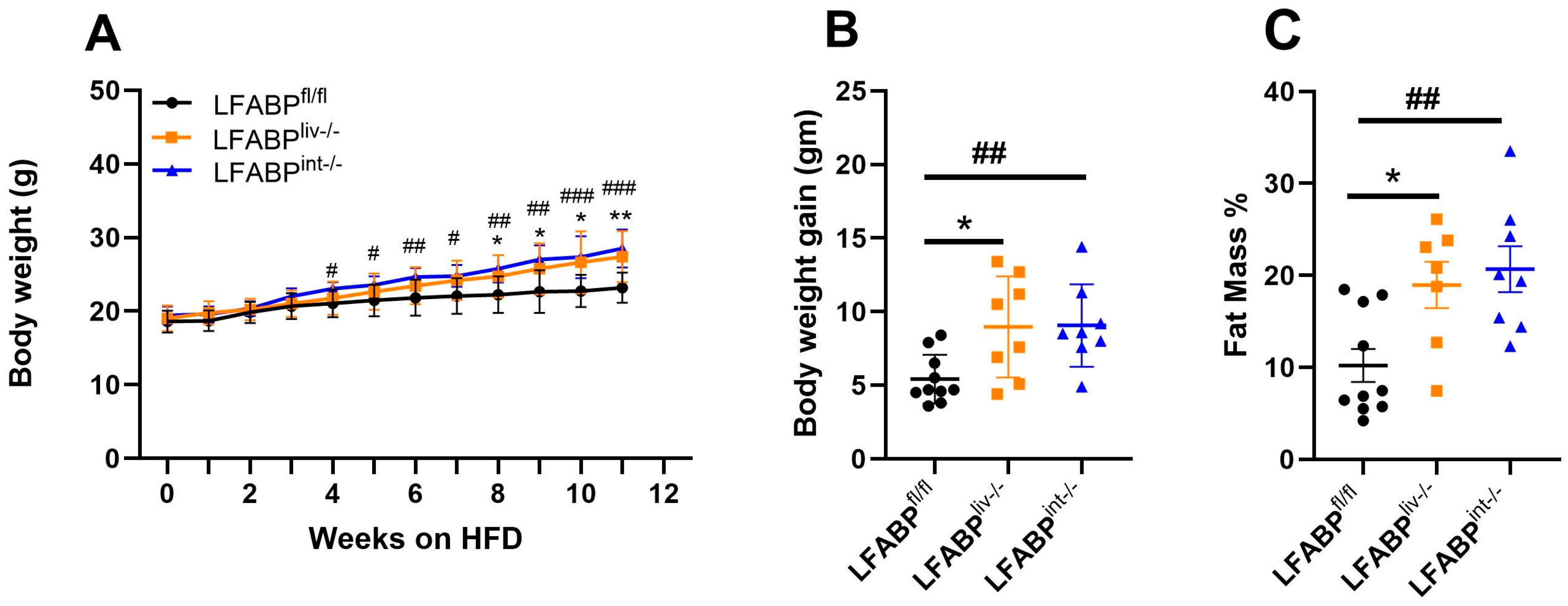
3.2. cKO LFABP Mice Have Greater Body Weight and FM Compared with the Control Floxed Mice
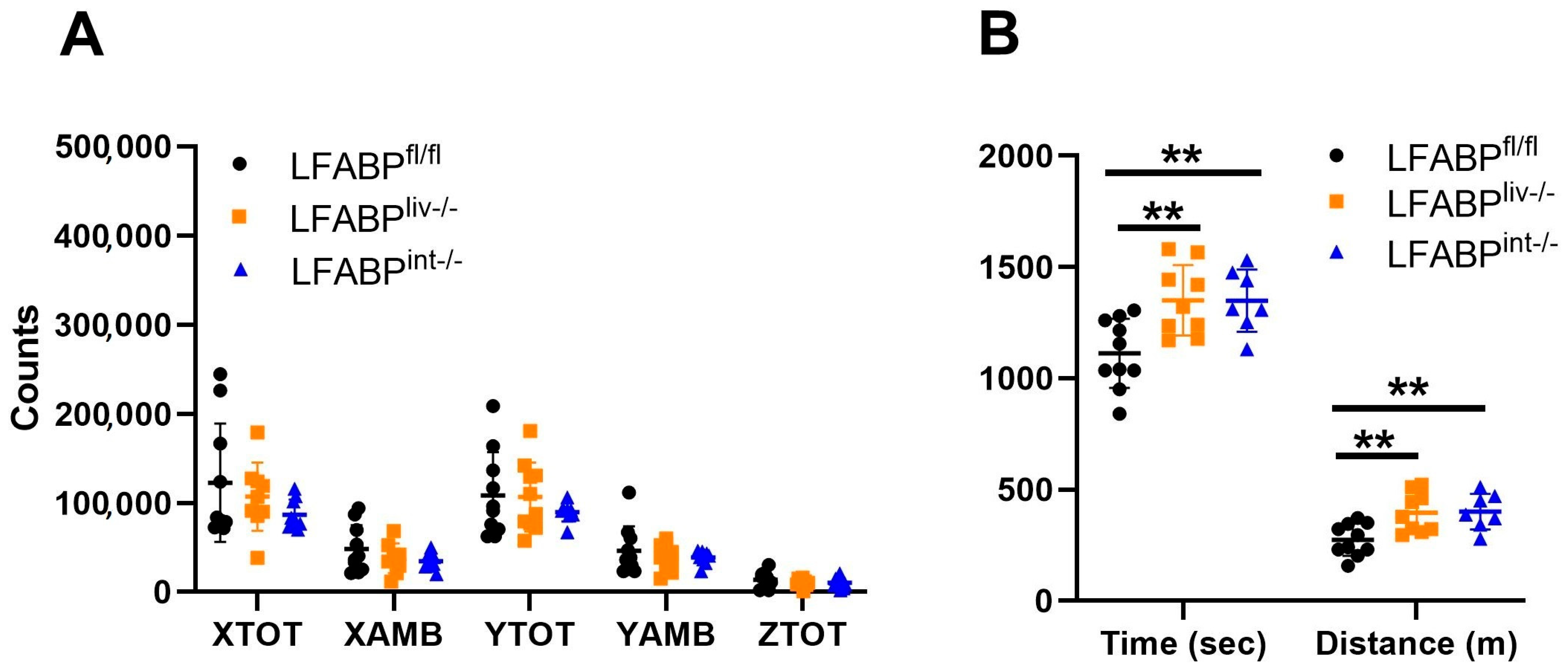
3.3. The Ablation of LFABP from Either the Liver or Intestine Does Not Alter Net Energy Absorption, Intestinal Transit Times, or Energy Expenditure
3.4. LFABPliv-/- and LFABPint-/- Mice Retain Their Exercise Capacity upon HFD Feeding Relative to LFABPfl/fl Control Mice
3.5. Liver- and Intestine-LFABP cKO Mice Do Not Display Alterations in Plasma Markers of Energy Balance
3.6. Hepatic Lipid Handling in LFABP cKO Mice
3.7. Intestinal Lipid Handling in LFABP cKO Mice
3.8. Tissue Uptake of Oral FA Is Modulated by LFABP Conditional Ablation
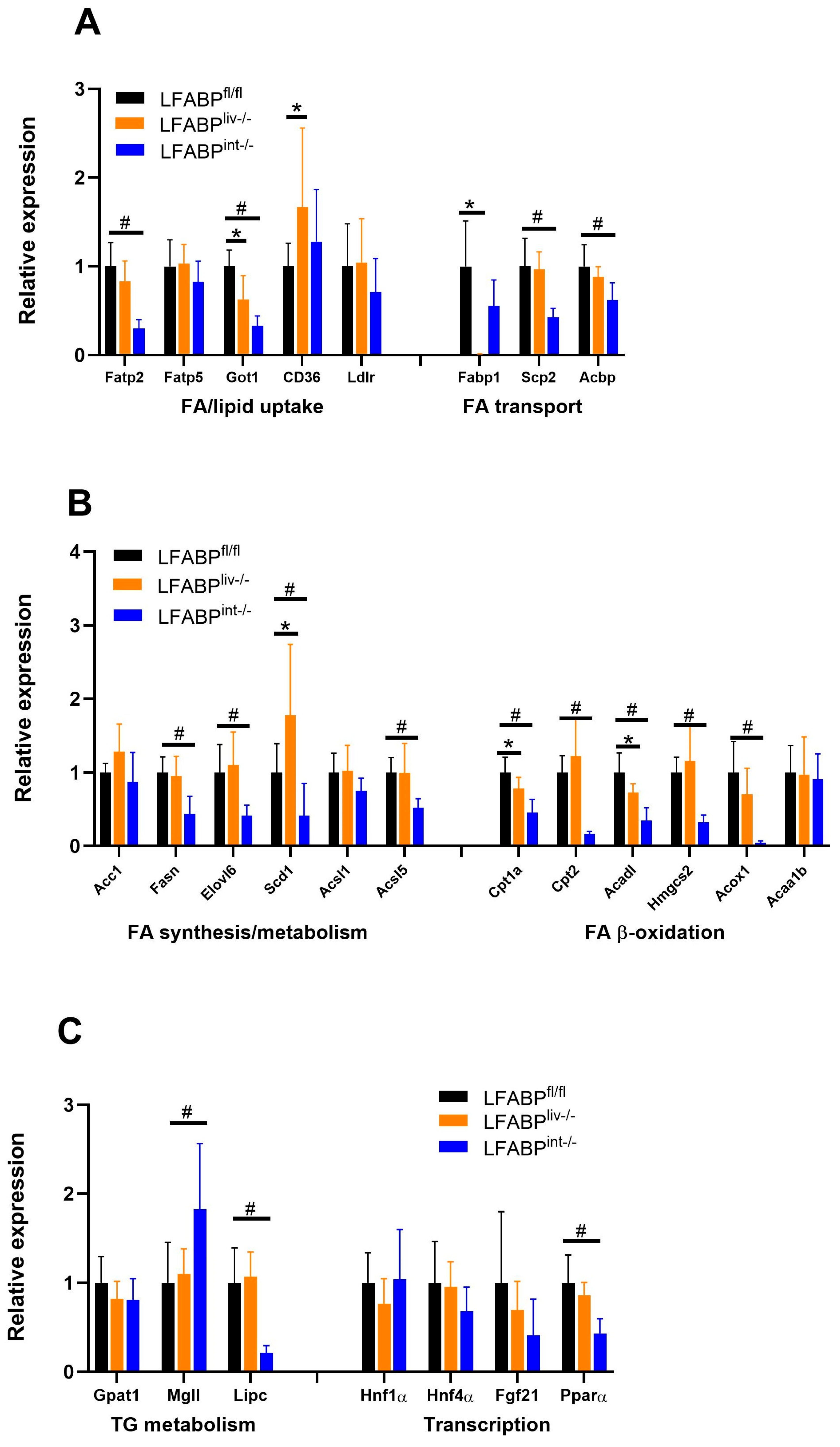
3.9. The Expression of Lipid Metabolism Genes in Liver and Intestine of LFABP cKO Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Lottenberg, A.M.; Afonso Mda, S.; Lavrador, M.S.; Machado, R.M.; Nakandakare, E.R. The role of dietary fatty acids in the pathology of metabolic syndrome. J. Nutr. Biochem. 2012, 23, 1027–1040. [Google Scholar] [CrossRef]
- Inoue, Y.; Qin, B.; Poti, J.; Sokol, R.; Gordon-Larsen, P. Epidemiology of Obesity in Adults: Latest Trends. Curr. Obes. Rep. 2018, 7, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Wycherley, T.P.; Moran, L.J.; Clifton, P.M.; Noakes, M.; Brinkworth, G.D. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 96, 1281–1298. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.W.; Qu, J.; Black, D.D.; Tso, P. Regulation of intestinal lipid metabolism: Current concepts and relevance to disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 169–183. [Google Scholar] [CrossRef]
- Canbay, A.; Bechmann, L.; Gerken, G. Lipid metabolism in the liver. Z. Gastroenterol. 2007, 45, 35–41. [Google Scholar] [CrossRef]
- Berlanga, A.; Guiu-Jurado, E.; Porras, J.A.; Auguet, T. Molecular pathways in non-alcoholic fatty liver disease. Clin. Exp. Gastroenterol. 2014, 7, 221–239. [Google Scholar] [CrossRef] [PubMed]
- Ockner, R.K.; Manning, J.A.; Poppenhausen, R.B.; Ho, W.K.L. A Binding Protein for Fatty Acids in Cytosol of Intestinal Mucosa, Liver, Myocardium, and Other Tissues. Science 1972, 177, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Sweetser, D.A.; Birkenmeier, E.H.; Hoppe, P.C.; McKeel, D.W.; Gordon, J.I. Mechanisms underlying generation of gradients in gene expression within the intestine: An analysis using transgenic mice containing fatty acid binding protein-human growth hormone fusion genes. Genes Dev. 1988, 2, 1318–1332. [Google Scholar] [CrossRef]
- Iseki, S.; Kondo, H. An immunocytochemical study on the occurrence of liver fatty-acid-binding protein in the digestive organs of rats: Specific localization in the D cells and brush cells. Acta Anat. 1990, 138, 15–23. [Google Scholar] [CrossRef]
- Newberry, E.P.; Xie, Y.; Lodeiro, C.; Solis, R.; Moritz, W.; Kennedy, S.; Barron, L.; Onufer, E.; Alpini, G.; Zhou, T.; et al. Hepatocyte and stellate cell deletion of liver fatty acid binding protein reveals distinct roles in fibrogenic injury. FASEB J. 2019, 33, 4610–4625. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Tang, Y.; Davis, V.; Hsu, F.F.; Kennedy, S.M.; Song, H.; Turk, J.; Brunt, E.M.; Newberry, E.P.; Davidson, N.O. Liver fatty acid binding protein (L-Fabp) modulates murine stellate cell activation and diet-induced nonalcoholic fatty liver disease. Hepatology 2013, 57, 2202–2212. [Google Scholar] [CrossRef] [PubMed]
- Richieri, G.V.; Ogata, R.T.; Kleinfeld, A.M. Equilibrium constants for the binding of fatty acids with fatty acid-binding proteins from adipocyte, intestine, heart, and liver measured with the fluorescent probe ADIFAB. J. Biol. Chem. 1994, 269, 23918–23930. [Google Scholar] [CrossRef] [PubMed]
- Richieri, G.V.; Ogata, R.T.; Zimmerman, A.W.; Veerkamp, J.H.; Kleinfeld, A.M. Fatty acid binding proteins from different tissues show distinct patterns of fatty acid interactions. Biochemistry 2000, 39, 7197–7204. [Google Scholar] [CrossRef] [PubMed]
- Wolfrum, C.; Borchers, T.; Sacchettini, J.C.; Spener, F. Binding of fatty acids and peroxisome proliferators to orthologous fatty acid binding proteins from human, murine, and bovine liver. Biochemistry 2000, 39, 14363. [Google Scholar] [CrossRef][Green Version]
- Huang, H.; McIntosh, A.L.; Martin, G.G.; Landrock, D.; Chung, S.; Landrock, K.K.; Dangott, L.J.; Li, S.; Kier, A.B.; Schroeder, F. FABP1: A Novel Hepatic Endocannabinoid and Cannabinoid Binding Protein. Biochemistry 2016, 55, 5243–5255. [Google Scholar] [CrossRef] [PubMed]
- Thumser, A.E.; Voysey, J.E.; Wilton, D.C. The binding of lysophospholipids to rat liver fatty acid-binding protein and albumin. Biochem. J. 1994, 301 Pt 3, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Thumser, A.E.; Wilton, D.C. The binding of cholesterol and bile salts to recombinant rat liver fatty acid-binding protein. Biochem. J. 1996, 320 Pt 3, 729–733. [Google Scholar] [CrossRef]
- Lagakos, W.S.; Guan, X.; Ho, S.Y.; Sawicki, L.R.; Corsico, B.; Kodukula, S.; Murota, K.; Stark, R.E.; Storch, J. Liver fatty acid-binding protein binds monoacylglycerol in vitro and in mouse liver cytosol. J. Biol. Chem. 2013, 288, 19805–19815. [Google Scholar] [CrossRef]
- Gajda, A.M.; Zhou, Y.X.; Agellon, L.B.; Fried, S.K.; Kodukula, S.; Fortson, W.; Patel, K.; Storch, J. Direct comparison of mice null for liver or intestinal fatty acid-binding proteins reveals highly divergent phenotypic responses to high fat feeding. J. Biol. Chem. 2013, 288, 30330–30344. [Google Scholar] [CrossRef]
- Wu, G.; Tawfeeq, H.R.; Lackey, A.I.; Zhou, Y.; Sifnakis, Z.; Zacharisen, S.M.; Xu, H.; Doran, J.M.; Sampath, H.; Zhao, L.; et al. Gut Microbiota and Phenotypic Changes Induced by Ablation of Liver- and Intestinal-Type Fatty Acid-Binding Proteins. Nutrients 2022, 14, 1762. [Google Scholar] [CrossRef]
- Gajda, A.M.; Tawfeeq, H.R.; Lackey, A.I.; Zhou, Y.X.; Kanaan, H.; Pappas, A.; Xu, H.; Kodukula, S.; Storch, J. The proximal intestinal Fatty Acid-Binding Proteins liver FABP (LFABP) and intestinal FABP (IFABP) differentially modulate whole body energy homeostasis but are not centrally involved in net dietary lipid absorption: Studies of the LFABP/IFABP double knockout mouse. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2023, 1868, 159238. [Google Scholar] [CrossRef]
- Newberry, E.P.; Xie, Y.; Kennedy, S.M.; Luo, J.; Davidson, N.O. Protection against Western diet-induced obesity and hepatic steatosis in liver fatty acid-binding protein knockout mice. Hepatology 2006, 44, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Newberry, E.P.; Kennedy, S.M.; Xie, Y.; Sternard, B.T.; Luo, J.; Davidson, N.O. Diet-induced obesity and hepatic steatosis in L-Fabp/mice is abrogated with SF, but not PUFA, feeding and attenuated after cholesterol supplementation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2008, 294, G307–G314. [Google Scholar] [CrossRef] [PubMed]
- Douglass, J.D.; Malik, N.; Chon, S.H.; Wells, K.; Zhou, Y.X.; Choi, A.S.; Joseph, L.B.; Storch, J. Intestinal mucosal triacylglycerol accumulation secondary to decreased lipid secretion in obese and high fat fed mice. Front. Physiol. 2012, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gajda, A.M.; Zhou, Y.X.; Panetta, C.; Sifnakis, Z.; Fatima, A.; Henderson, G.C.; Storch, J. Muscle metabolic reprogramming underlies the resistance of liver fatty acid-binding protein (LFABP)-null mice to high-fat feeding-induced decline in exercise capacity. J. Biol. Chem. 2019, 294, 15358–15372. [Google Scholar] [CrossRef]
- Primeau, V.; Coderre, L.; Karelis, A.D.; Brochu, M.; Lavoie, M.E.; Messier, V.; Sladek, R.; Rabasa-Lhoret, R. Characterizing the profile of obese patients who are metabolically healthy. Int. J. Obes. 2011, 35, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.G.; Glade, M.J.; Meguid, M.M. Metabolically healthy obese individuals: Key protective factors. Nutrition 2016, 32, 14–20. [Google Scholar] [CrossRef]
- Farabi, S.S.; Smith, G.I.; Yoshino, J.; Klein, S. Metabolically Healthy Obesity is not a Myth. JCEM Case Rep. 2023, 1, luad015. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Mittendorfer, B.; Klein, S. Metabolically healthy obesity: Facts and fantasies. J. Clin. Investig. 2019, 129, 3978–3989. [Google Scholar] [CrossRef]
- Bass, N.M.; Manning, J.A.; Ockner, R.K.; Gordon, J.I.; Seetharam, S.; Alpers, D.H. Regulation of the biosynthesis of two distinct fatty acid-binding proteins in rat liver and intestine. Influences of sex difference and of clofibrate. J. Biol. Chem. 1985, 260, 1432–1436. [Google Scholar] [CrossRef] [PubMed]
- Ockner, R.K.; Burnett, D.A.; Lysenko, N.; Manning, J.A. Sex differences in long chain fatty acid utilization and fatty acid binding protein concentration in rat liver. J. Clin. Investig. 1979, 64, 172–181. [Google Scholar] [CrossRef]
- Ockner, R.K.; Lysenko, N.; Manning, J.A.; Monroe, S.E.; Burnett, D.A. Sex steroid modulation of fatty acid utilization and fatty acid binding protein concentration in rat liver. J. Clin. Investig. 1980, 65, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Diolintzi, A. The Role of Liver Fatty Acid-Binding Protein (LFABP; FABP1) in Adipose Tissues Cellularity and Function: Implications of Interorgan Signaling. Ph.D. Thesis, Rutgers University, New Brunswick, NJ, USA, 2023. [Google Scholar]
- Lagakos, W.S.; Gajda, A.M.; Agellon, L.; Binas, B.; Choi, V.; Mandap, B.; Russnak, T.; Zhou, Y.X.; Storch, J. Different functions of intestinal and liver-type fatty acid-binding proteins in intestine and in whole body energy homeostasis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 300, G803–G814. [Google Scholar] [CrossRef]
- Madison, B.B.; Dunbar, L.; Qiao, X.T.; Braunstein, K.; Braunstein, E.; Gumucio, D.L. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J. Biol. Chem. 2002, 277, 33275–33283. [Google Scholar] [CrossRef]
- McLean, J.A.; Tobin, G. Animal and Human Calorimetry; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Nagakura, Y.; Naitoh, Y.; Kamato, T.; Yamano, M.; Miyata, K. Compounds possessing 5-HT3 receptor antagonistic activity inhibit intestinal propulsion in mice. Eur. J. Pharmacol. 1996, 311, 67–72. [Google Scholar] [CrossRef]
- Ma, X.; Hamadeh, M.J.; Christie, B.R.; Foster, J.A.; Tarnopolsky, M.A. Impact of treadmill running and sex on hippocampal neurogenesis in the mouse model of amyotrophic lateral sclerosis. PLoS ONE 2012, 7, e36048. [Google Scholar] [CrossRef]
- Tanner, C.B.; Madsen, S.R.; Hallowell, D.M.; Goring, D.M.; Moore, T.M.; Hardman, S.E.; Heninger, M.R.; Atwood, D.R.; Thomson, D.M. Mitochondrial and performance adaptations to exercise training in mice lacking skeletal muscle LKB1. Am. J. Physiol.-Endocrinol. Metab. 2013, 305, E1018–E1029. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Brewster, M.; Quittner, H. Separation of plasma lipids by thin-layer chromatography. Ann. Clin. Lab. Sci. (1971) 1973, 3, 79–84. [Google Scholar] [PubMed]
- Huynh, F.K.; Green, M.F.; Koves, T.R.; Hirschey, M.D. Measurement of fatty acid oxidation rates in animal tissues and cell lines. Methods Enzymol. 2014, 542, 391–405. [Google Scholar] [CrossRef]
- Coburn, C.T.; Knapp, F.F., Jr.; Febbraio, M.; Beets, A.L.; Silverstein, R.L.; Abumrad, N.A. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J. Biol. Chem. 2000, 275, 32523–32529. [Google Scholar] [CrossRef]
- Hagberg, C.E.; Falkevall, A.; Wang, X.; Larsson, E.; Huusko, J.; Nilsson, I.; van Meeteren, L.A.; Samen, E.; Lu, L.; Vanwildemeersch, M.; et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature 2010, 464, 917–921. [Google Scholar] [CrossRef]
- van Diepen, J.A.; Stienstra, R.; Vroegrijk, I.O.; van den Berg, S.A.; Salvatori, D.; Hooiveld, G.J.; Kersten, S.; Tack, C.J.; Netea, M.G.; Smit, J.W.; et al. Caspase-1 deficiency in mice reduces intestinal triglyceride absorption and hepatic triglyceride secretion. J. Lipid Res. 2013, 54, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Atshaves, B.P.; McIntosh, A.L.; Storey, S.M.; Landrock, K.K.; Kier, A.B.; Schroeder, F. High dietary fat exacerbates weight gain and obesity in female liver fatty acid binding protein gene-ablated mice. Lipids 2010, 45, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Uchida, A.; Whitsitt, M.C.; Eustaquio, T.; Slipchenko, M.N.; Leary, J.F.; Cheng, J.X.; Buhman, K.K. Reduced triglyceride secretion in response to an acute dietary fat challenge in obese compared to lean mice. Front. Physiol. 2012, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Morino, K.; Ugi, S.; Nakagawa, F.; Lemecha, M.; Ida, S.; Ohashi, N.; Sato, D.; Fujita, Y.; Maegawa, H. Microbiome potentiates endurance exercise through intestinal acetate production. Am. J. Physiol.-Endocrinol. Metab. 2019, 316, E956–E966. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Lauretani, F.; Milani, C.; Nouvenne, A.; Tana, C.; Del Rio, D.; Maggio, M.; Ventura, M.; Meschi, T. Aging Gut Microbiota at the Cross-Road between Nutrition, Physical Frailty, and Sarcopenia: Is There a Gut-Muscle Axis? Nutrients 2017, 9, 1303. [Google Scholar] [CrossRef]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef]
- Newberry, E.P.; Xie, Y.; Kennedy, S.; Han, X.; Buhman, K.K.; Luo, J.; Gross, R.W.; Davidson, N.O. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J. Biol. Chem. 2003, 278, 51664–51672. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Tawfeeq, H.R.; Ali, J. Assessment of Liver Enzymes Activity in Patients with Rheumatoid Arthritis in Nineveh province. Tikrit J. Pharmaceut. Sci. 2012, 8, 138–144. [Google Scholar] [CrossRef]
- Kneeman, J.M.; Misdraji, J.; Corey, K.E. Secondary causes of nonalcoholic fatty liver disease. Therap. Adv. Gastroenterol. 2012, 5, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Erol, E.; Kumar, L.S.; Cline, G.W.; Shulman, G.I.; Kelly, D.P.; Binas, B. Liver fatty acid binding protein is required for high rates of hepatic fatty acid oxidation but not for the action of PPARalpha in fasting mice. FASEB J. 2004, 18, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Neeli, I.; Siddiqi, S.A.; Siddiqi, S.; Mahan, J.; Lagakos, W.S.; Binas, B.; Gheyi, T.; Storch, J.; Mansbach, C.M., 2nd. Liver fatty acid-binding protein initiates budding of pre-chylomicron transport vesicles from intestinal endoplasmic reticulum. J. Biol. Chem. 2007, 282, 17974–17984. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, S.; Saleem, U.; Abumrad, N.A.; Davidson, N.O.; Storch, J.; Siddiqi, S.A.; Mansbach, C.M., 2nd. A novel multiprotein complex is required to generate the prechylomicron transport vesicle from intestinal ER. J. Lipid Res. 2010, 51, 1918–1928. [Google Scholar] [CrossRef]
- Guzmán, C.; Benet, M.; Pisonero-Vaquero, S.; Moya, M.; García-Mediavilla, M.V.; Martínez-Chantar, M.L.; González-Gallego, J.; Castell, J.V.; Sánchez-Campos, S.; Jover, R. The human liver fatty acid binding protein (FABP1) gene is activated by FOXA1 and PPARα; and repressed by C/EBPα: Implications in FABP1 down-regulation in nonalcoholic fatty liver disease. Biochim. Biophys. Acta 2013, 1831, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Wolfrum, C.; Borrmann, C.M.; Borchers, T.; Spener, F. Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors alpha- and gamma-mediated gene expression via liver fatty acid binding protein: A signaling path to the nucleus. Proc. Natl. Acad. Sci. USA 2001, 98, 2323–2328. [Google Scholar] [CrossRef]
- McIntosh, A.L.; Petrescu, A.D.; Hostetler, H.A.; Kier, A.B.; Schroeder, F. Liver-type fatty acid binding protein interacts with hepatocyte nuclear factor 4α. FEBS Lett. 2013, 587, 3787–3791. [Google Scholar] [CrossRef]
- Huang, H.; Starodub, O.; McIntosh, A.; Kier, A.B.; Schroeder, F. Liver fatty acid-binding protein targets fatty acids to the nucleus. Real time confocal and multiphoton fluorescence imaging in living cells. J. Biol. Chem. 2002, 277, 29139–29151. [Google Scholar] [CrossRef] [PubMed]
- Chmurzyńska, A. The multigene family of fatty acid-binding proteins (FABPs): Function, structure and polymorphism. J. Appl. Genet. 2006, 47, 39–48. [Google Scholar] [CrossRef]
- Weisiger, R.A. Cytoplasmic transport of lipids: Role of binding proteins. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1996, 115, 319–331. [Google Scholar] [CrossRef]
- Cheng, A.; Jia, W.; Kawahata, I.; Fukunaga, K. A novel fatty acid-binding protein 5 and 7 inhibitor ameliorates oligodendrocyte injury in multiple sclerosis mouse models. EBioMedicine 2021, 72, 103582. [Google Scholar] [CrossRef] [PubMed]
- Babaev, V.R.; Runner, R.P.; Fan, D.; Ding, L.; Zhang, Y.; Tao, H.; Erbay, E.; Görgün, C.Z.; Fazio, S.; Hotamisligil, G.S.; et al. Macrophage Mal1 deficiency suppresses atherosclerosis in low-density lipoprotein receptor-null mice by activating peroxisome proliferator-activated receptor-γ-regulated genes. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1283–1290. [Google Scholar] [CrossRef]
- Yabuki, Y.; Matsuo, K.; Kawahata, I.; Fukui, N.; Mizobata, T.; Kawata, Y.; Owada, Y.; Shioda, N.; Fukunaga, K. Fatty Acid Binding Protein 3 Enhances the Spreading and Toxicity of α-Synuclein in Mouse Brain. Int. J. Mol. Sci. 2020, 21, 2230. [Google Scholar] [CrossRef]
- Storch, J.; Corsico, B. The Multifunctional Family of Mammalian Fatty Acid-Binding Proteins. Annu. Rev. Nutr. 2023, 43, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]


| LFABPfl/fl | LFABPliv-/- | LFABPint-/- | |
|---|---|---|---|
| Glucose (ng/dL) | 240.1 ± 24.68 | 228.22 ± 18.34 | 260.86 ± 33.84 |
| Insulin (ng/mL) | 0.37 ± 0.09 | 0.40 ± 0.04 | 0.30 ± 0.05 |
| Leptin (ng/mL) | 4.74 ± 2.34 | 16.81 ± 8.20 ** | 6.87 ± 4.41 |
| Leptin index | 1.54 ± 0.60 | 2.05 ± 0.22 | 1.03 ± 0.24 |
| Adiponectin (ng/mL) | 14,406.04 ± 5931.61 | 14,946.52 ± 2494.73 | ND |
| Adiponectin index | 4322.25 ± 2787.70 | 2246.00 ± 1123.28 | ND |
| NEFA (μM) | 357.74 ± 153.58 | 402.42 ± 210.23 | ND |
| TG (mg/dL) | 19.34 ± 11.44 | 16.70 ± 11.31 | ND |
| Cholesterol (μM) | 1298.41 ± 468.77 | 1414.29 ± 349.85 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tawfeeq, H.R.; Lackey, A.I.; Zhou, Y.; Diolintzi, A.; Zacharisen, S.M.; Lau, Y.H.; Quadro, L.; Storch, J. Tissue-Specific Ablation of Liver Fatty Acid-Binding Protein Induces a Metabolically Healthy Obese Phenotype in Female Mice. Nutrients 2025, 17, 753. https://doi.org/10.3390/nu17050753
Tawfeeq HR, Lackey AI, Zhou Y, Diolintzi A, Zacharisen SM, Lau YH, Quadro L, Storch J. Tissue-Specific Ablation of Liver Fatty Acid-Binding Protein Induces a Metabolically Healthy Obese Phenotype in Female Mice. Nutrients. 2025; 17(5):753. https://doi.org/10.3390/nu17050753
Chicago/Turabian StyleTawfeeq, Hiba Radhwan, Atreju I. Lackey, Yinxiu Zhou, Anastasia Diolintzi, Sophia M. Zacharisen, Yin Hei Lau, Loredana Quadro, and Judith Storch. 2025. "Tissue-Specific Ablation of Liver Fatty Acid-Binding Protein Induces a Metabolically Healthy Obese Phenotype in Female Mice" Nutrients 17, no. 5: 753. https://doi.org/10.3390/nu17050753
APA StyleTawfeeq, H. R., Lackey, A. I., Zhou, Y., Diolintzi, A., Zacharisen, S. M., Lau, Y. H., Quadro, L., & Storch, J. (2025). Tissue-Specific Ablation of Liver Fatty Acid-Binding Protein Induces a Metabolically Healthy Obese Phenotype in Female Mice. Nutrients, 17(5), 753. https://doi.org/10.3390/nu17050753







