Diesel Particulate Matter (DPM)-Induced Metabolic Disruption in Mice Is Mitigated by Sodium Copper Chlorophyllin (SCC)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diesel Particulate Matter Exposure
2.3. Tissue Permeabilization
2.4. Mitochondrial Respirometry
2.5. ATP Quantification
2.6. Glutathione/Glutathione Disulfide Redox Potential Analysis
2.7. Serum Inflammation Assessments
2.8. Adipose Tissue Histology and Characterization
2.9. Statistical Analysis
3. Results
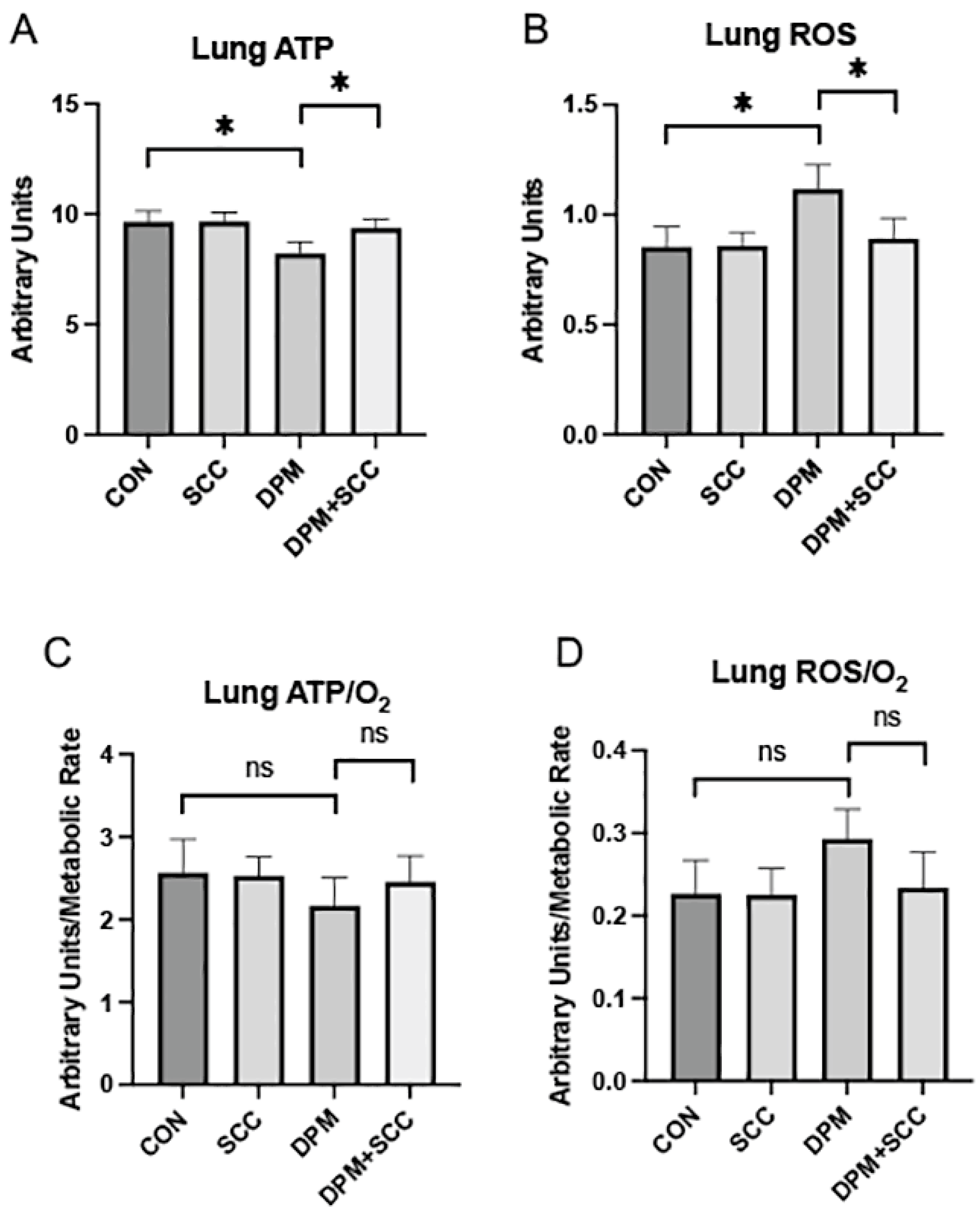
3.1. Lung Energy and Oxidative Metrics
3.2. Skeletal Muscle Bioenergetics and Oxidative Stress
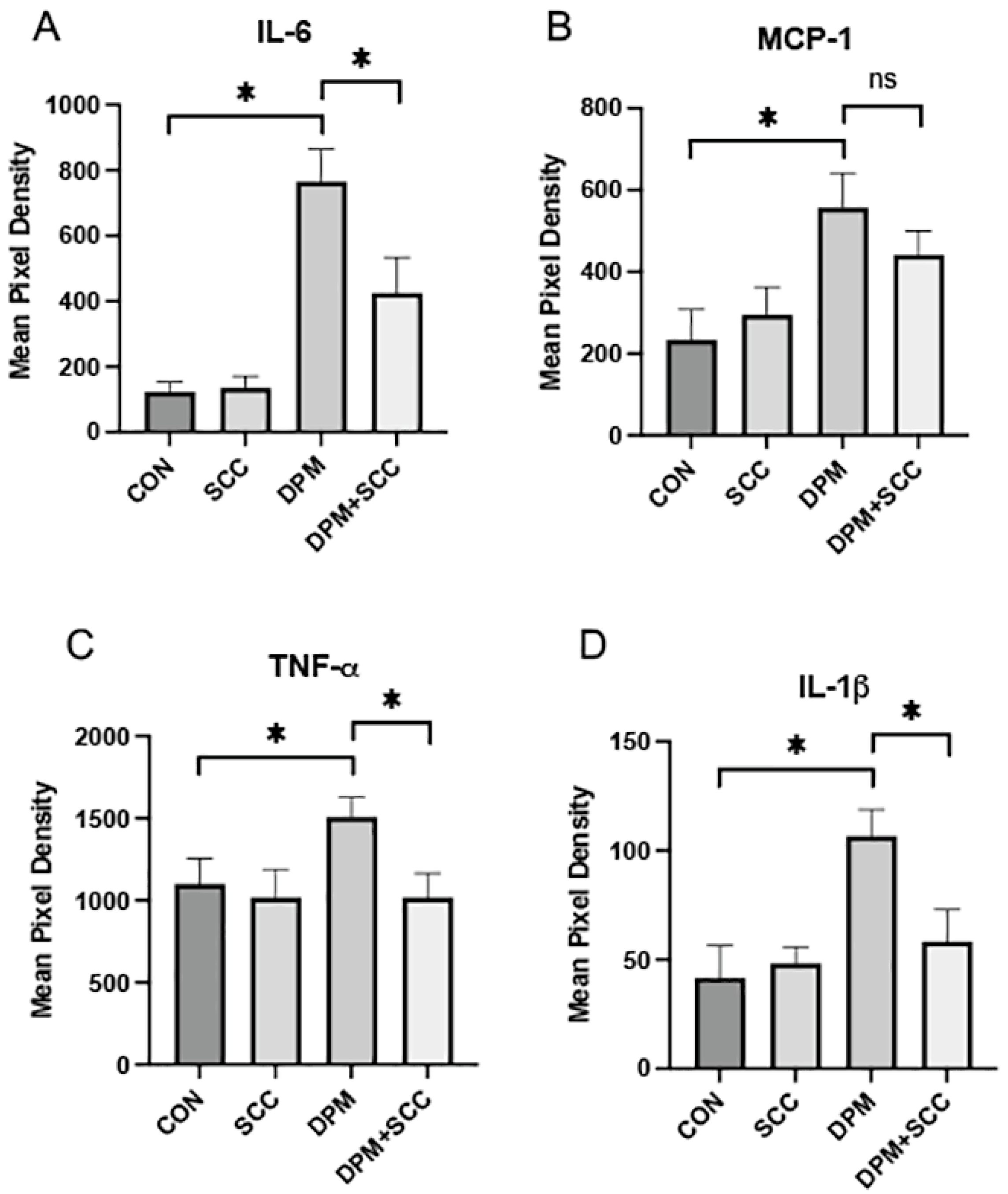
3.3. Systemic Inflammatory Cytokines
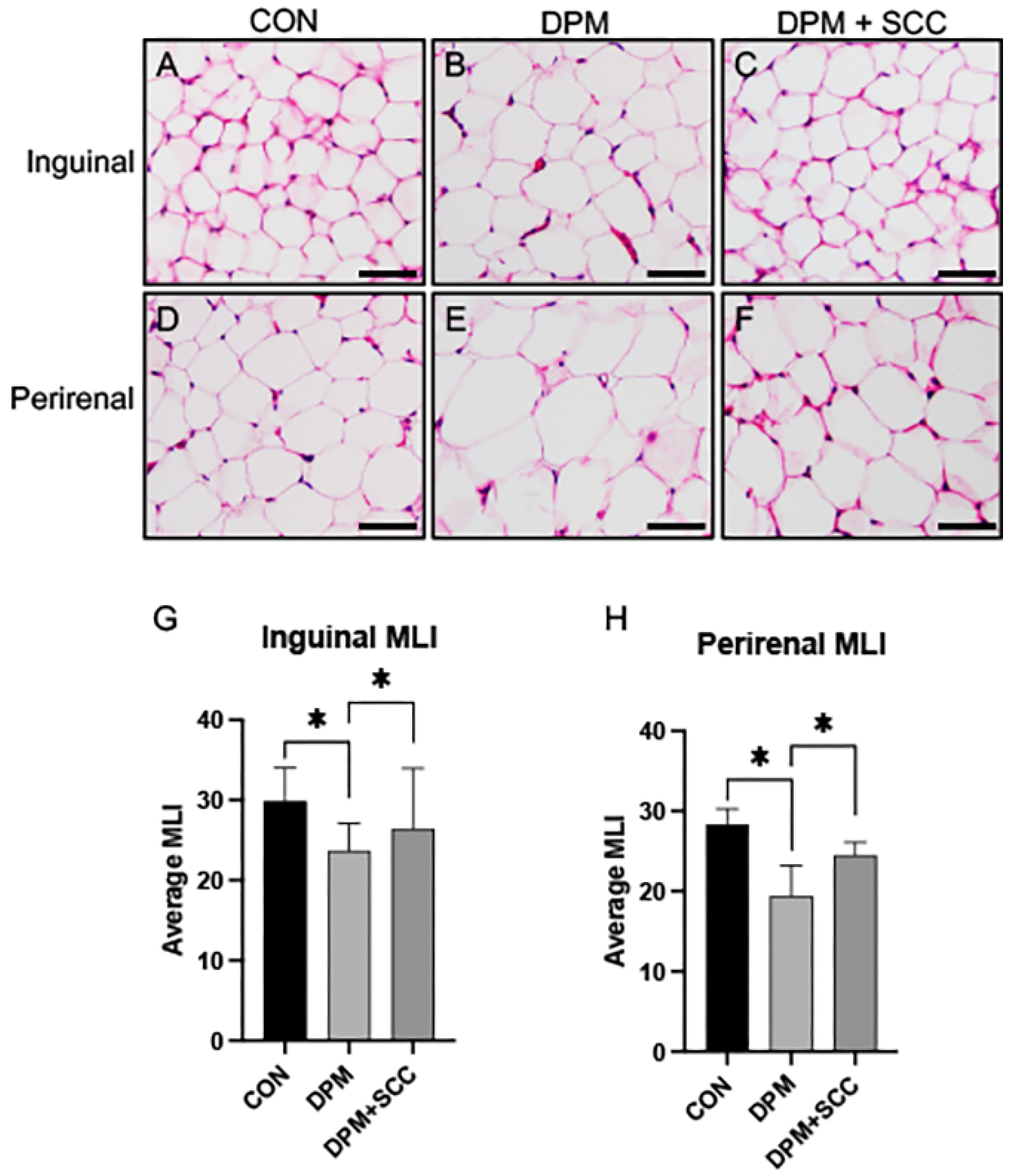
3.4. Adipose Tissue Morphometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lazzarin, M.C.; Dos Santos, J.F.; Quintana, H.T.; Pidone, F.A.M.; de Oliveira, F. Duchenne muscular dystrophy progression induced by downhill running is accompanied by increased endomysial fibrosis and oxidative damage DNA in muscle of mdx mice. J. Mol. Histol. 2023, 54, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Savary, C.C.; Bellamri, N.; Morzadec, C.; Langouët, S.; Lecureur, V.; Vernhet, L. Long term exposure to environmental concentrations of diesel exhaust particles does not impact the phenotype of human bronchial epithelial cells. Toxicol. In Vitro 2018, 52, 154–160. [Google Scholar] [CrossRef]
- Salvi, S.; Blomberg, A.; Rudell, B.; Kelly, F.; Sandström, T.; Holgate, S.T.; Frew, A. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am. J. Respir. Crit. Care Med. 1999, 159, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.I.; Song, M.K.; Kim, H.I.; Han, K.M.; Lee, K. Diesel Exhaust Particulates Induce Neutrophilic Lung Inflammation by Modulating Endoplasmic Reticulum Stress-Mediated CXCL1/KC Expression in Alveolar Macrophages. Molecules 2020, 25, 6046. [Google Scholar] [CrossRef]
- Brinchmann, B.C.; Holme, J.A.; Frerker, N.; Rambøl, M.H.; Karlsen, T.; Brinchmann, J.E.; Kubátová, A.; Kukowski, K.; Skuland, T.; Øvrevik, J. Effects of organic chemicals from diesel exhaust particles on adipocytes differentiated from human mesenchymal stem cells. Basic. Clin. Pharmacol. Toxicol. 2023, 132, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Okamura, T.; Nakajima, H.; Kitagawa, N.; Majima, S.; Okada, H.; Senmaru, T.; Ushigome, E.; Nakanishi, N.; Hamaguchi, M.; et al. Metabolic outcomes and changes in innate immunity induced by diesel exhaust particles airway exposure and high-fat high-sucrose diet. Life Sci. 2023, 326, 121794. [Google Scholar] [CrossRef]
- Tomaru, M.; Takano, H.; Inoue, K.; Yanagisawa, R.; Osakabe, N.; Yasuda, A.; Shimada, A.; Kato, Y.; Uematsu, H. Pulmonary exposure to diesel exhaust particles enhances fatty change of the liver in obese diabetic mice. Int. J. Mol. Med. 2007, 19, 17–22. [Google Scholar] [CrossRef]
- Ding, S.; Yuan, C.; Si, B.; Wang, M.; Da, S.; Bai, L.; Wu, W. Combined effects of ambient particulate matter exposure and a high-fat diet on oxidative stress and steatohepatitis in mice. PLoS ONE 2019, 14, e0214680. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Y.; Hu, X.; Liao, X.; Zhang, Y. Chlorophyll Supplementation in Early Life Prevents Diet-Induced Obesity and Modulates Gut Microbiota in Mice. Mol. Nutr. Food Res. 2019, 63, e1801219. [Google Scholar]
- Møller, P.; Daneshvar, B.; Loft, S.; Wallin, H.; Poulsen, H.E.; Autrup, H.; Ravn-Haren, G.; Dragsted, L.O. Oxidative DNA damage in vitamin C-supplemented guinea pigs after intratracheal instillation of diesel exhaust particles. Toxicol. Appl. Pharmacol. 2003, 189, 39–44. [Google Scholar] [CrossRef]
- Flury, M.; Mathison, J.B.; Harsh, J.B. In situ mobilization of colloids and transport of cesium in Hanford sediments. Environ. Sci. Technol. 2002, 36, 5335–5341. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; You, Y.; Hua, M.; Wu, P.; Liu, Y.; Chen, Z.; Zhang, L.; Wei, H.; Li, Y.; Luo, M.; et al. Chlorophyllin Modulates Gut Microbiota and Inhibits Intestinal Inflammation to Ameliorate Hepatic Fibrosis in Mice. Front. Physiol. 2018, 9, 1671. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O.; Pfeiffer, A.F. Metabolic effects of dietary fiber consumption and prevention of diabetes. J. Nutr. 2008, 138, 439–442. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, X.; Pandol, S.J.; Han, Y.P.; Zheng, X. Green Plant Pigment, Chlorophyllin, Ameliorates Non-alcoholic Fatty Liver Diseases (NAFLDs) Through Modulating Gut Microbiome in Mice. Front. Physiol. 2021, 12, 739174. [Google Scholar] [CrossRef]
- Warren, C.E.; Campbell, K.M.; Kirkham, M.N.; Saito, E.R.; Remund, N.P.; Cayabyab, K.B.; Kim, I.J.; Heimuli, M.S.; Reynolds, P.R.; Arroyo, J.A.; et al. The Effect of Diesel Exhaust Particles on Adipose Tissue Mitochondrial Function and Inflammatory Status. Int. J. Mol. Sci. 2024, 25, 4322. [Google Scholar] [CrossRef]
- Ahmed, T.M.; Bergvall, C.; Åberg, M.; Westerholm, R. Determination of oxygenated and native polycyclic aromatic hydrocarbons in urban dust and diesel particulate matter standard reference materials using pressurized liquid extraction and LC-GC/MS. Anal. Bioanal. Chem. 2015, 407, 427–438. [Google Scholar] [CrossRef]
- Barton, D.B.; Betteridge, B.C.; Earley, T.D.; Curtis, C.S.; Robinson, A.B.; Reynolds, P.R. Primary alveolar macrophages exposed to diesel particulate matter increase RAGE expression and activate RAGE signaling. Cell Tissue Res. 2014, 358, 229–238. [Google Scholar] [CrossRef]
- Walton, C.M.; Saito, E.R.; Warren, C.E.; Larsen, J.G.; Remund, N.P.; Reynolds, P.R.; Hansen, J.M.; Bikman, B.T. Yerba Maté (Ilex paraguariensis) Supplement Exerts Beneficial, Tissue-Specific Effects on Mitochondrial Efficiency and Redox Status in Healthy Adult Mice. Nutrients 2023, 15, 4454. [Google Scholar] [CrossRef]
- Hsia, C.C.; Hyde, D.M.; Ochs, M.; Weibel, E.R. An official research policy statement of the American Thoracic Society/European Respiratory Society: Standards for quantitative assessment of lung structure. Am. J. Respir. Crit. Care Med. 2010, 181, 394–418. [Google Scholar] [CrossRef]
- Martinez-Santibanez, G.; Singer, K.; Cho, K.W.; DelProposto, J.L.; Mergian, T.; Lumeng, C.N. Obesity-induced remodeling of the adipose tissue elastin network is independent of the metalloelastase MMP-12. Adipocyte 2015, 4, 264–272. [Google Scholar] [CrossRef]
- Mendez, R.; Zheng, Z.; Fan, Z.; Rajagopalan, S.; Sun, Q.; Zhang, K. Exposure to fine airborne particulate matter induces macrophage infiltration, unfolded protein response, and lipid deposition in white adipose tissue. Am. J. Transl. Res. 2013, 5, 224–234. [Google Scholar] [PubMed]
- Xu, X.; Yavar, Z.; Verdin, M.; Ying, Z.; Mihai, G.; Kampfrath, T.; Wang, A.; Zhong, M.; Lippmann, M.; Chen, L.C.; et al. Effect of early particulate air pollution exposure on obesity in mice: Role of p47phox. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2518–2527. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, C.; Xu, Z.; Tzan, K.; Zhong, M.; Wang, A.; Lippmann, M.; Chen, L.C.; Rajagopalan, S.; Sun, Q. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol. Sci. 2011, 124, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Mukherjee, B.; Brook, R.D.; Gatts, G.A.; Yang, F.; Sun, Q.; Brook, J.R.; Fan, Z.; Rajagopalan, S. Air-Pollution and Cardiometabolic Diseases (AIRCMD): A prospective study investigating the impact of air pollution exposure and propensity for type II diabetes. Sci. Total Environ. 2013, 448, 72–78. [Google Scholar] [CrossRef]
- Matthiessen, C.; Lucht, S.; Hennig, F.; Ohlwein, S.; Jakobs, H.; Jöckel, K.H.; Moebus, S.; Hoffmann, B. Long-term exposure to airborne particulate matter and NO(2) and prevalent and incident metabolic syndrome—Results from the Heinz Nixdorf Recall Study. Environ. Int. 2018, 116, 74–82. [Google Scholar] [CrossRef]
- Hwang, M.J.; Kim, J.H.; Koo, Y.S.; Yun, H.Y.; Cheong, H.K. Impacts of ambient air pollution on glucose metabolism in Korean adults: A Korea National Health and Nutrition Examination Survey study. Environ. Health 2020, 19, 70. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, Y.; Chang, Q.; Ji, C.; Zhao, Y.; Zhang, H. Exposure to ambient air pollution and metabolic kidney diseases: Evidence from the Northeast China Biobank. Nephrol. Dial. Transplant. 2023, 38, 2222–2231. [Google Scholar] [CrossRef]
- Pan, K.; Jiang, S.; Du, X.; Zeng, X.; Zhang, J.; Song, L.; Zhou, J.; Kan, H.; Sun, Q.; Xie, Y.; et al. AMPK activation attenuates inflammatory response to reduce ambient PM(2.5)-induced metabolic disorders in healthy and diabetic mice. Ecotoxicol. Environ. Saf. 2019, 179, 290–300. [Google Scholar] [CrossRef]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Sandberg, M.; Patil, J.; D’Angelo, B.; Weber, S.G.; Mallard, C. NRF2-regulation in brain health and disease: Implication of cerebral inflammation. Neuropharmacology 2014, 79, 298–306. [Google Scholar] [CrossRef]
- Bivol, L.M.; Berge, R.K.; Iversen, B.M. Tetradecylthioacetic acid prevents the inflammatory response in two-kidney, one-clip hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R438–R447. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radford, J.H.; Evans, E.P.; Edwards, I.T.; Arroyo, J.A.; Bikman, B.T.; Reynolds, P.R. Diesel Particulate Matter (DPM)-Induced Metabolic Disruption in Mice Is Mitigated by Sodium Copper Chlorophyllin (SCC). Nutrients 2025, 17, 717. https://doi.org/10.3390/nu17040717
Radford JH, Evans EP, Edwards IT, Arroyo JA, Bikman BT, Reynolds PR. Diesel Particulate Matter (DPM)-Induced Metabolic Disruption in Mice Is Mitigated by Sodium Copper Chlorophyllin (SCC). Nutrients. 2025; 17(4):717. https://doi.org/10.3390/nu17040717
Chicago/Turabian StyleRadford, Jack H., Ethan P. Evans, Isaac T. Edwards, Juan A. Arroyo, Benjamin T. Bikman, and Paul R. Reynolds. 2025. "Diesel Particulate Matter (DPM)-Induced Metabolic Disruption in Mice Is Mitigated by Sodium Copper Chlorophyllin (SCC)" Nutrients 17, no. 4: 717. https://doi.org/10.3390/nu17040717
APA StyleRadford, J. H., Evans, E. P., Edwards, I. T., Arroyo, J. A., Bikman, B. T., & Reynolds, P. R. (2025). Diesel Particulate Matter (DPM)-Induced Metabolic Disruption in Mice Is Mitigated by Sodium Copper Chlorophyllin (SCC). Nutrients, 17(4), 717. https://doi.org/10.3390/nu17040717








