Concurrent Lead and Cadmium Exposure Among Diabetics: A Case-Control Study of Socio-Demographic and Consumption Behaviors
Highlights
- Lead exposure and diabetes risk: Blood lead (Pb) levels were significantly associated with an increased risk of type 2 diabetes (T2D) in a Pb-contaminated area of southern Thailand.
- Sociodemographic risk factors identified: Key risk factors for higher Pb exposure included lower education levels, certain occupations, low income, smoking, alcohol consumption, and high fat as well as sugar consumption.
- Implications for glycemic control: Elevated blood Pb levels were linked to poorer glycemic control.
Abstract
1. Introduction
2. Materials and Methods
2.1. Aims and Research Questions
2.2. Participants
2.3. Sociodemographic and Exposure Data
2.4. Blood and Urine Sampling
2.5. Lead and Cadmium Analysis
2.6. Body Composition Measurement
2.7. Statistical Analysis
3. Results
3.1. Socio-Demographic and Anthropometric Differences in T2D and Controls
3.2. Elevated Blood Lead Levels and Their Association with T2D
3.3. Biochemical Characteristics and Body Composition Differences in T2D Patients
4. Discussion
4.1. Cd Exposure and T2D Risk
4.2. Perspectives for Clinical Practice
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Dendup, T.; Feng, X.; Clingan, S.; Astell-Burt, T. Environmental Risk Factors for Developing Type 2 Diabetes Mellitus: A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Yang, H.; Cui, Z. Biochemical mechanism of phytoremediation process of lead and cadmium pollution with Mucor circinelloides and Trichoderma asperellum. Ecotoxicol. Environ. Saf. 2018, 157, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.H.; Jin, M.H.; Kang, J.H.; Lee, S.I.; Lee, S.; Kim, S.H.; Oh, S.Y. Relationship between heavy metal exposure and type 2 diabetes: A large-scale retrospective cohort study using occupational health examinations. BMJ Open 2021, 11, e039541. [Google Scholar] [CrossRef]
- Ghorbani Nejad, B.; Raeisi, T.; Janmohammadi, P.; Mehravar, F.; Zarei, M.; Dehghani, A.; Bahrampour, N.; Darijani, M.H.; Ahmadipour, F.; Mohajeri, M.; et al. Mercury Exposure and Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis. Int. J. Clin. Pract. 2022, 2022, 7640227. [Google Scholar] [CrossRef]
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef]
- Obeng-Gyasi, E. Sources of lead exposure in various countries. Rev. Environ. Health 2019, 34, 25–34. [Google Scholar] [CrossRef]
- Satarug, S.; Gobe, G.C.; Vesey, D.A.; Phelps, K.R. Cadmium and Lead Exposure, Nephrotoxicity, and Mortality. Toxics 2020, 8, 86. [Google Scholar] [CrossRef]
- Parizi, M.G.; Sedaghat, Z.; Mazloomi, M.; Fararouei, M. Serum level of lead and cadmium is linked to facial cosmetics use among Iranian young women. Environ. Sci. Pollut. Res. Int. 2021, 28, 13913–13918. [Google Scholar] [CrossRef]
- Haswell-Elkins, M.; Satarug, S.; O’Rourke, P.; Moore, M.; Ng, J.; McGrath, V.; Walmby, M. Striking association between urinary cadmium level and albuminuria among Torres Strait Islander people with diabetes. Environ. Res. 2008, 106, 379–383. [Google Scholar] [CrossRef]
- Swaddiwudhipong, W.; Mahasakpan, P.; Funkhiew, T.; Limpatanachote, P. Changes in cadmium exposure among persons living in cadmium-contaminated areas in northwestern Thailand: A five-year follow-up. J. Med. Assoc. Thai 2010, 93, 1217–1222. [Google Scholar] [PubMed]
- Swaddiwudhipong, W.; Limpatanachote, P.; Nishijo, M.; Honda, R.; Mahasakpan, P.; Krintratun, S. Cadmium-exposed population in Mae Sot district, Tak province: 3. Associations between urinary cadmium and renal dysfunction, hypertension, diabetes, and urinary stones. J. Med. Assoc. Thai 2010, 93, 231–238. [Google Scholar] [PubMed]
- Filippini, T.; Wise, L.A.; Vinceti, M. Cadmium exposure and risk of diabetes and prediabetes: A systematic review and dose-response meta-analysis. Environ. Int. 2022, 158, 106920. [Google Scholar] [CrossRef]
- Chan, J.C.; Cho, N.H.; Tajima, N.; Shaw, J. Diabetes in the Western Pacific Region--past, present and future. Diabetes Res. Clin. Pract. 2014, 103, 244–255. [Google Scholar] [CrossRef]
- Aekplakorn, W.; Chariyalertsak, S.; Kessomboon, P.; Assanangkornchai, S.; Taneepanichskul, S.; Putwatana, P. Prevalence of Diabetes and Relationship with Socioeconomic Status in the Thai Population: National Health Examination Survey, 2004–2014. J. Diabetes Res. 2018, 2018, 1654530. [Google Scholar] [CrossRef]
- Thanapop, C.; Geater, A.F.; Robson, M.G.; Phakthongsuk, P.; Viroonudomphol, D. Exposure to lead of boatyard workers in southern Thailand. J. Occup. Health 2007, 49, 345–352. [Google Scholar] [CrossRef]
- Yimthiang, S.; Waeyang, D.; Kuraeiad, S. Screening for Elevated Blood Lead Levels and Related Risk Factors among Thai Children Residing in a Fishing Community. Toxics 2019, 7, 54. [Google Scholar] [CrossRef]
- von Elm, E. STROBE initiative. The strengthening of the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344. [Google Scholar] [CrossRef]
- Trzcinka-Ochocka, M.; Brodzka, R.; Janasik, B. Useful and Fast Method for Blood Lead and Cadmium Determination Using ICP-MS and GF-AAS; Validation Parameters. J. Clin. Lab. Anal. 2016, 30, 130–139. [Google Scholar] [CrossRef]
- Thai Health Promotion Foundation. Screening Form for Food Consumption Behavior (Sweet, Fat, Salty). Available online: https://dol.thaihealth.or.th/Media/Index/3a123c3a-39b0-ee11-8100-00155d1aab77 (accessed on 1 February 2021).
- Carlsson, S.; Andersson, T.; Talbäck, M.; Feychting, M. Incidence and prevalence of type 2 diabetes by occupation: Results from all Swedish employees. Diabetologia 2020, 63, 95–103. [Google Scholar] [CrossRef]
- Guth, K.; Bourgeois, M.; Johnson, G.; Harbison, R. Assessment of lead exposure controls on bridge painting projects using worker blood lead levels. Regul. Toxicol. Pharmacol. 2020, 115, 104698. [Google Scholar] [CrossRef] [PubMed]
- Montes-Santiago, J. The lead-poisoned genius: Saturnism in famous artists across five centuries. Prog. Brain Res. 2013, 203, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, T.R.; Carey, R.N.; Peters, S.; Glass, D.C.; Benke, G.; Reid, A.; Fritschi, L. The Australian Work Exposures Study: Occupational Exposure to Lead and Lead Compounds. Ann. Occup. Hyg. 2016, 60, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Noh, J.; Choi, J.W.; Park, E.C. Association of Education and Smoking Status on Risk of Diabetes Mellitus: A Population-Based Nationwide Cross-Sectional Study. Int. J. Environ. Res. Public Health 2017, 14, 655. [Google Scholar] [CrossRef]
- de Oliveira, C.M.; Viater Tureck, L.; Alvares, D.; Liu, C.; Horimoto, A.; Balcells, M.; de Oliveira Alvim, R.; Krieger, J.E.; Pereira, A.C. Relationship between marital status and incidence of type 2 diabetes mellitus in a Brazilian rural population: The Baependi Heart Study. PLoS ONE 2020, 15, e0236869. [Google Scholar] [CrossRef]
- Jiao, J.; Liu, G.; Shin, H.J.; Hu, F.B.; Rimm, E.B.; Rexrode, K.M.; Manson, J.E.; Zong, G.; Sun, Q. Dietary fats and mortality among patients with type 2 diabetes: Analysis in two population based cohort studies. BMJ 2019, 366, l4009. [Google Scholar] [CrossRef]
- Chen, Y.; He, D.; Yang, T.; Zhou, H.; Xiang, S.; Shen, L.; Wen, J.; Chen, S.; Peng, S.; Gan, Y. Relationship between body composition indicators and risk of type 2 diabetes mellitus in Chinese adults. BMC Public Health 2020, 20, 452. [Google Scholar] [CrossRef]
- Ye, J. Mechanisms of insulin resistance in obesity. Front. Med. 2013, 7, 14–24. [Google Scholar] [CrossRef]
- Borné, Y.; Fagerberg, B.; Persson, M.; Sallsten, G.; Forsgard, N.; Hedblad, B.; Barregard, L.; Engström, G. Cadmium exposure and incidence of diabetes mellitus--results from the Malmö Diet and Cancer study. PLoS ONE 2014, 9, e112277. [Google Scholar] [CrossRef]
- Swaddiwudhipong, W.; Limpatanachote, P.; Mahasakpan, P.; Krintratun, S.; Punta, B.; Funkhiew, T. Progress in cadmium-related health effects in persons with high environmental exposure in northwestern Thailand: A five-year follow-up. Environ. Res. 2012, 112, 194–198. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, J.B.; Hafida, S.; Stemmer, P.; Adhami, A.; Leff, T. Lead (Pb) exposure promotes diabetes in obese rodents. J. Trace Elem. Med. Biol. 2017, 39, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Leff, T.; Stemmer, P.; Tyrrell, J.; Jog, R. Diabetes and Exposure to Environmental Lead (Pb). Toxics 2018, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Yimthiang, S.; Pouyfung, P.; Khamphaya, T.; Kuraeiad, S.; Wongrith, P.; Vesey, D.A.; Gobe, G.C.; Satarug, S. Effects of Environmental Exposure to Cadmium and Lead on the Risks of Diabetes and Kidney Dysfunction. Int. J. Environ. Res. Public Health 2022, 19, 2259. [Google Scholar] [CrossRef]
- Ashraf, M.W. Levels of heavy metals in popular cigarette brands and exposure to these metals via smoking. Sci. World J. 2012, 2012, 729430. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Cabral-Pinto, M.M.S.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Tüzen, M. Determination of heavy metals in fish samples of the middle Black Sea (Turkey) by graphite furnace atomic absorption spectrometry. Food Chem. 2003, 80, 119–123. [Google Scholar] [CrossRef]
- Lv, Y.; Yao, Y.; Ye, J.; Guo, X.; Dou, J.; Shen, L.; Zhang, A.; Xue, Z.; Yu, Y.; Jin, L. Association of Blood Pressure with Fasting Blood Glucose Levels in Northeast China: A Cross-Sectional Study. Sci. Rep. 2018, 8, 7917. [Google Scholar] [CrossRef]
- Borges, C.A.; Slater, B.; Santaliestra-Pasías, A.M.; Mouratidou, T.; Huybrechts, I.; Widhalm, K.; Gottrand, F.; Manios, Y.; Jimenez-Pavón, D.; Valtueña, J.; et al. Dietary Patterns in European and Brazilian Adolescents: Comparisons and Associations with Socioeconomic Factors. Nutrients 2018, 10, 57. [Google Scholar] [CrossRef]
- Cunha, C.M.L.; Canuto, R.; Rosa, P.B.Z.; Longarai, L.S.; Schuch, I. Association between dietary patterns and socioeconomic factors and food environment in a city in the South of Brazil. Cien Saude Colet. 2022, 27, 687–700. [Google Scholar] [CrossRef]
- Brunström, M.; Carlberg, B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: Systematic review and meta-analyses. BMJ 2016, 352, i717. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Il’yasova, D.; Ivanova, A. Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care 2003, 26, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Zhi, X.; Xu, M.; Li, B.; Zhang, Z. Gender-specific differences of interaction between cadmium exposure and obesity on prediabetes in the NHANES 2007-2012 population. Endocrine 2018, 61, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Wallia, A.; Allen, N.B.; Badon, S.; El Muayed, M. Association between urinary cadmium levels and prediabetes in the NHANES 2005-2010 population. Int. J. Hyg. Environ. Health 2014, 217, 854–860. [Google Scholar] [CrossRef]
- Oosterwijk, M.M.; Hagedoorn, I.J.M.; Maatman, R.G.H.J.; Bakker, S.J.L.; Navis, G.; Laverman, G.D. Cadmium, active smoking, and renal function deterioration in patients with type 2 diabetes. Nephrol. Dial. Transplant. 2023, 38, 876–883. [Google Scholar] [CrossRef]
- Cangelosi, G.; Mancin, S.; Pantanetti, P.; Nguyen, C.T.T.; Morales Palomares, S.; Biondini, F.; Sguanci, M.; Petrelli, F. Lifestyle Medicine Case Manager Nurses for Type Two Diabetes Patients: An Overview of a Job Description Framework—A Narrative Review. Diabetology 2024, 5, 375–388. [Google Scholar] [CrossRef]
- Cangelosi, G.; Grappasonni, I.; Pantanetti, P.; Scuri, S.; Garda, G.; Cuc Thi Thu, N.; Petrelli, F. Nurse Case Manager Lifestyle Medicine (NCMLM) in the Type Two Diabetes patient concerning post COVID-19 Pandemic management: Integrated-Scoping literature review. Ann. Ig. Med. Prev. Comunita 2022, 34, 585–602. [Google Scholar] [CrossRef]
- Plass, D.; Beloconi, A.; Bessems, J.; Buekers, J.; Kienzler, S.; Martinez, G.S.; Purece, A.; Vounatsou, P. Estimating the environmental burden of disease resulting from exposure to chemicals in European countries–potentials and challenges revealed in selected case studies. Environ. Res. 2025, 269, 120828. [Google Scholar] [CrossRef]
- Wu, K.; Chen, Y.; Huang, W. Combined Molecular Toxicity Mechanism of Heavy Metals Mixtures. In Toxicological Assessment of Combined Chemicals in the Environment; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2025; Chapter 9; pp. 125–172. [Google Scholar] [CrossRef]
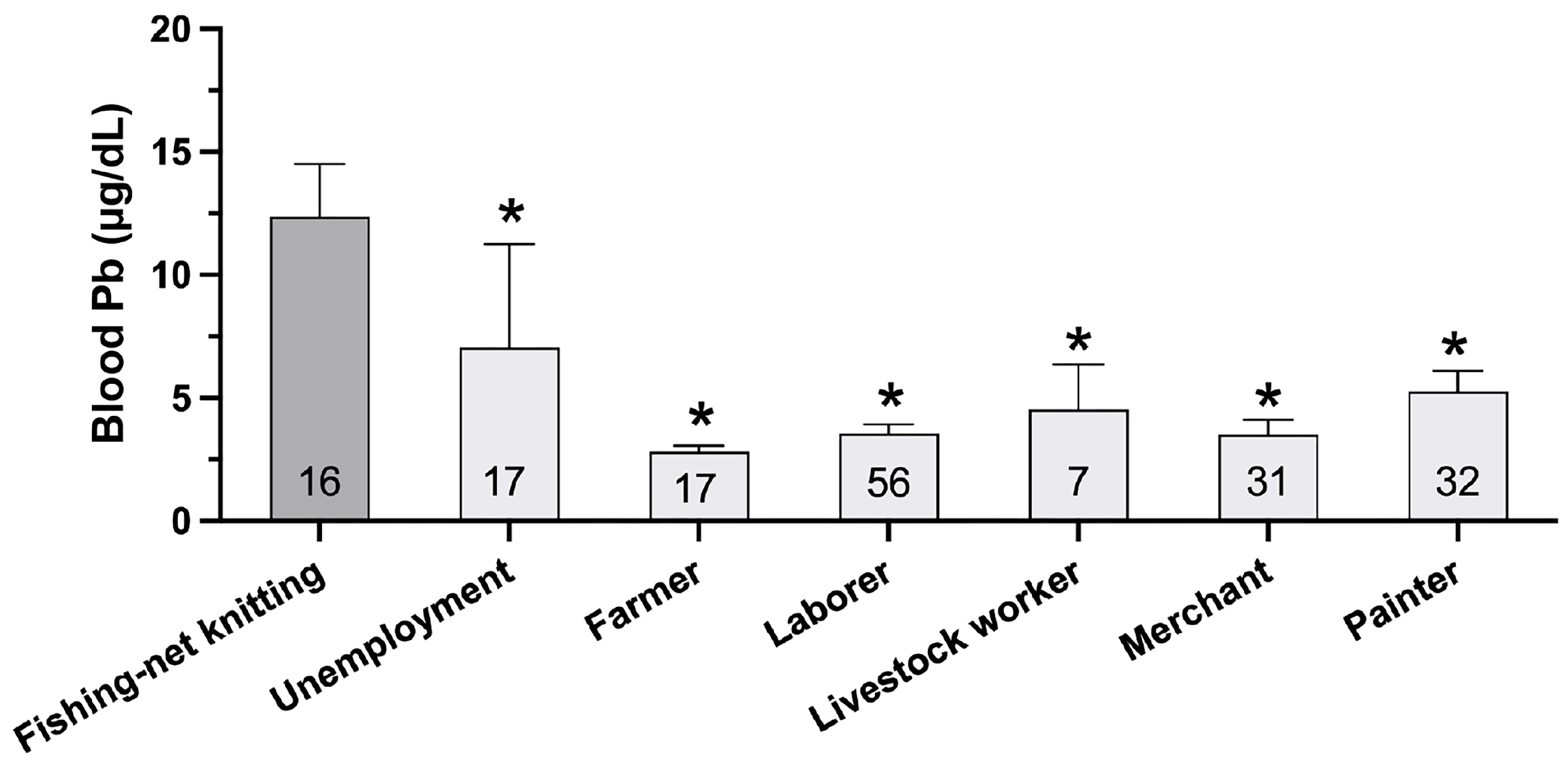
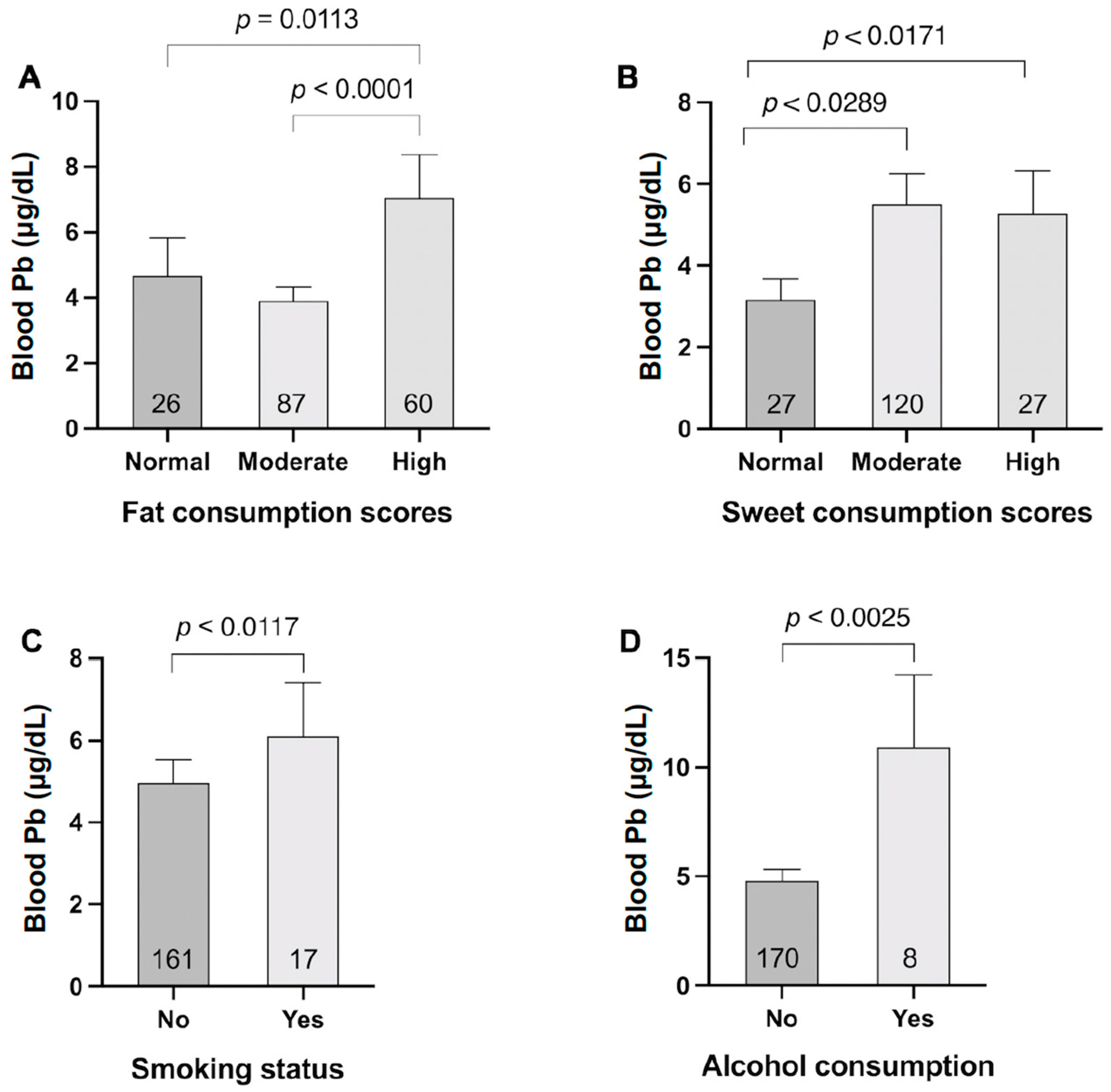
| Variables | Type 2 Diabetes | Non-Diabetic Control | p-Value | ||
|---|---|---|---|---|---|
| n = 88 | n = 90 | ||||
| Mean or n | SD or % b | Mean or n | SD or % b | ||
| Women | 71 | 80.7 | 73 | 81.1 | 0.92 |
| Age, years | 60 | 9 | 59 | 10 | 0.55 |
| BMI, kg/m2 | 26.06 | 4.9 | 24.74 | 4.3 | 0.058 |
| Waist circumference, cm | 95.15 | 13.1 | 87.57 | 10.1 | <0.001 * |
| Marital status | |||||
| Married | 50 | 56.8 | 65 | 72.2 | 0.03 * |
| Education | |||||
| Primary school | 74 | 84.1 | 54 | 60.0 | 0.001 |
| Secondary school | 12 | 13.6 | 24 | 26.7 | |
| College and university | 2 | 2.3 | 12 | 13.3 | |
| Occupations | |||||
| Unemployment | 3 | 3.4 | 14 | 15.6 | <0.001 * |
| Color painter | 28 | 31.8 | 3 | 3.3 | |
| Merchant | 17 | 19.3 | 14 | 15.6 | |
| Livestock worker | 3 | 3.4 | 4 | 4.4 | |
| Laborer | 25 | 28.4 | 31 | 34.4 | |
| Fishing-net knitting | 7 | 8.0 | 10 | 11.1 | |
| Farmer | 5 | 5.7 | 12 | 13.3 | |
| Fishery | 0 | 0.0 | 2 | 2.2 | |
| Income (Baht/month) | |||||
| <9000 | 70 | 79.5 | 63 | 70.0 | 0.142 |
| ≥9000 | 18 | 20.5 | 27 | 30.0 | |
| Smoking | 7 | 8.0 | 10 | 11.1 | 0.474 |
| Alcohol consumption | 6 | 6.8 | 2 | 2.2 | 0.141 |
| Sweet consumption score a | |||||
| Normal | 13 | 14.8 | 14 | 16.1 | 0.832 |
| Moderate | 60 | 68.2 | 61 | 56.3 | |
| High | 15 | 17.0 | 12 | 27.6 | |
| Fat consumption score a | |||||
| Normal | 12 | 13.8 | 14 | 16.1 | 0.113 |
| Moderate | 38 | 43.7 | 49 | 56.3 | |
| High | 15 | 17.0 | 12 | 27.6 | |
| Fat consumption score a | |||||
| Normal | 12 | 13.8 | 14 | 16.1 | 0.113 |
| Moderate | 38 | 43.7 | 49 | 56.3 | |
| High | 37 | 42.5 | 24 | 27.6 | |
| Salt consumption score a | |||||
| Normal | 7 | 8.0 | 9 | 10.3 | 0.401 |
| Moderate | 76 | 87.4 | 70 | 80.5 | |
| High | 4 | 4.6 | 8 | 9.2 | |
| Variables | Type 2 Diabetes | Non-Diabetic Control | p-Value | ||
|---|---|---|---|---|---|
| n = 88 | n = 90 | ||||
| Mean or n | SD or % a | Mean or n | SD or % a | ||
| Fasting blood glucose, mg/dL | 168.7 | 67.58 | 94.87 | 12.40 | <0.001 * |
| ≥110 mg/dL | 78 | 88.6 | 8 | 8.9 | <0.001 * |
| SBP, mmHg | 141.3 | 17.07 | 134.9 | 16.79 | 0.006 * |
| >140 | 47 | 53.4 | 31 | 35.2 | 0.025 |
| DBS, mmHg | 83.44 | 9.78 | 84.09 | 9.107 | 0.325 |
| >90 | 21 | 23.9 | 23 | 26.4 | 0.391 |
| Toxic metal exposure | |||||
| Blood Pb (µg/dL) | 5.74 | 5.64 | 3.60 | 3.71 | 0.001 * |
| ≥3.0 µg/dL | 25 | 28.1 | 58 | 65.9 | <0.001 * |
| Blood Cd (µg/L) | 0.53 | 0.60 | 0.65 | 0.85 | 0.158 |
| ≥0.4 µg/L | 56 | 62.2 | 60 | 68.2 | 0.404 * |
| Basal metabolic rate (BMR) b | |||||
| Low | 43 | 48.9 | 31 | 37.8 | 0.34 |
| Normal | 31 | 35.2 | 36 | 43.9 | |
| High | 14 | 15.9 | 15 | 18.3 | |
| % Body fat c | |||||
| Low | 11 | 12.5 | 30 | 33.3 | 0.01 * |
| Normal | 38 | 43.2 | 39 | 43.3 | |
| High | 21 | 23.9 | 16 | 17.8 | |
| Very high | 18 | 20.5 | 5 | 5.6 | |
| Visceral Fat Rating Scale d | |||||
| Normal | 53 | 60.2 | 62 | 68.9 | 0.46 |
| High | 29 | 33.0 | 24 | 26.7 | |
| Very high | 6 | 6.8 | 4 | 4.4 | |
| Bone Mass e | |||||
| Low | 27 | 23.5 | 28 | 23.7 | 0.951 |
| Normal | 88 | 76.5 | 90 | 76.3 | |
| Water f | |||||
| Normal | 70 | 79.5 | 75 | 83.3 | 0.52 |
| Low | 18 | 20.5 | 15 | 16.7 | |
| Blood Lead Levels | p-Value | ||||
|---|---|---|---|---|---|
| Variables | ≥3 µg/dL, n = 83 | <3.0 µg/dL, n = 94 | |||
| Mean or n | SD or % b | Mean or n | SD or % b | ||
| Women | 61 | 73.5 | 82 | 87.2 | 0.021 * |
| SBP, mmHg | 138.7 | 17.32 | 137.8 | 17.00 | 0.366 |
| DBP, mmHg | 83.85 | 9.268 | 83.74 | 9.594 | 0.899 |
| MAP, mmHg | 101.3 | 11.34 | 101.5 | 10.70 | 0.425 |
| Blood Cd | 0.6956 | 0.7431 | 0.4997 | 0.7247 | 0.006 * |
| ≥0.4 µg/L | 64 | 71.1 | 52 | 55.3 | 0.002 * |
| Fasting blood glucose (mg/dL) | 148.6 | 70.31 | 115.9 | 47.18 | <0.001 * |
| ≥110 | 51 | 61.4 | 35 | 37.2 | 0.001 * |
| Education | |||||
| Primary school | 72 | 86.8 | 55 | 58.5 | <0.001 * |
| Secondary school | 9 | 10.8 | 27 | 28.3 | |
| University | 2 | 2.4 | 12 | 12.8 | |
| Occupations | |||||
| Unemployment | 5 | 6.0 | 12 | 12.8 | <0.001* |
| Painter | 23 | 27.7 | 8 | 8.5 | |
| Merchant | 9 | 10.8 | 22 | 23.4 | |
| Livestock worker | 2 | 2.4 | 5 | 5.3 | |
| Laborer | 22 | 26.5 | 34 | 36.2 | |
| Fishing-net knitting | 14 | 16.9 | 2 | 2.1 | |
| Farmer | 6 | 7.2 | 11 | 11.7 | |
| Fishery | 2 | 2.4 | 0 | 0.0 | |
| Monthly income (Baht) | |||||
| <9000 | 70 | 84.3 | 62 | 66.0 | 0.005 * |
| ≥9000 | 13 | 15.7 | 32 | 34.0 | |
| Smoking | 13 | 15.7 | 4 | 4.3 | 0.010 * |
| Alcohol consumption | 7 | 8.4 | 1 | 1.1 | 0.019 * |
| Sweet consumption a | |||||
| Normal | 7 | 8.4 | 20 | 22.0 | 0.033 * |
| Moderate | 60 | 72.3 | 60 | 65.9 | |
| High | 16 | 19.3 | 11 | 12.1 | |
| Fat Score a | |||||
| Normal | 9 | 11.0 | 17 | 18.7 | <0.001 * |
| Moderate | 31 | 37.8 | 56 | 61.5 | |
| High | 42 | 51.2 | 18 | 19.8 | |
| Variables | Crude Odds for Blood Pb ≥ 3 µg/dL | a Adjusted Odds for Blood Pb ≥ 3 µg/dL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β Coefficients (SE) | ORs | 95% CI | p-Valve | β Coefficients (SE) | ORs | 95% CI | p-Valve | |||
| Lower | Upper | Lower | Upper | |||||||
| Blood cadmium | 1.001 (0.334) | 2.72 | 1.42 | 5.23 | 0.003 * | 1.569 (0.685) | 4.80 | 1.25 | 18.40 | 0.022 * |
| Occupations | ||||||||||
| Unemployment | Ref | Ref | ||||||||
| Color painter | 1.932 (0.672) | 6.90 | 1.85 | 25.76 | 0.004 * | 0.472 (1.135) | 1.60 | 0.17 | 14.82 | 0.678 |
| Merchant | −0.018 (0.663) | 0.98 | 0.27 | 3.60 | 0.978 | −0.670 (1.127) | 0.51 | 0.06 | 4.65 | 0.552 |
| Livestock worker | −0.041 (0.992) | 0.96 | 0.14 | 6.70 | 0.967 | 1.052 (1.746) | 2.86 | 0.09 | 87.78 | 0.547 |
| Laborer | 0.440 (0.598) | 1.55 | 0.48 | 5.02 | 0.462 | 0.826 (1.061) | 2.28 | 0.29 | 18.26 | 0.436 |
| Fishing-net knitting | 2.821 (0.925) | 16.80 | 2.74 | 102.87 | 0.002 * | 3.159 (1.436) | 23.55 | 1.41 | 393.14 | 0.028 * |
| Farmer | 0.269 (0.735) | 1.30 | 0.31 | 5.53 | 0.714 | 2.424 (1.394) | 11.30 | 0.74 | 173.58 | 0.082 |
| Education | ||||||||||
| Primary school | Ref | Ref | ||||||||
| Secondary school | −1.368 (0.425) | 0.25 | 0.11 | 0.59 | 0.001 * | −2.873 (1.013) | 0.06 | 0.01 | 0.41 | 0.005 * |
| University | −2.061 (0.784) | 0.12 | 0.03 | 0.59 | 0.009 * | −2.260 (1.424) | 0.10 | 0.01 | 1.69 | 0.112 |
| Behavior consumption | ||||||||||
| Smoking | 1.430 (0.594) | 4.17 | 1.31 | 13.37 | 0.016 * | 1.938 (1.477) | 0.36 | 0.03 | 3.93 | 0.402 |
| Alcohol drinking | 2.148 (1.080) | 8.56 | 1.03 | 71.16 | 0.047 * | 3.825 (2.039) | 45.85 | 0.84 | 2493.10 | 0.061 |
| Sweet Score | ||||||||||
| Normal | Ref | Ref | ||||||||
| Moderate | 1.050 (0.476) | 2.85 | 1.13 | 7.26 | 0.027 * | 1.227 (0.789) | 3.41 | 0.73 | 16.00 | 0.120 |
| High | 1.425 (0.588) | 4.15 | 1.31 | 13.17 | 0.015 * | 1.792 (1.028) | 6.00 | 0.80 | 45.05 | 0.081 |
| Fat Score | ||||||||||
| Normal | Ref | Ref | ||||||||
| Moderate | 0.045 (0.469) | 1.04 | 0.42 | 2.62 | 0.924 | 0.022 (0.750) | 1.02 | 0.24 | 4.44 | 0.976 |
| High | 1.483 (0.499) | 4.40 | 1.66 | 11.73 | 0.003 * | 1.633 (0.896) | 5.12 | 0.88 | 29.67 | 0.068 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adokwe, J.B.; Pouyfung, P.; Kuraeiad, S.; Wongrith, P.; Inchai, P.; Yimthiang, S.; Satarug, S.; Khamphaya, T. Concurrent Lead and Cadmium Exposure Among Diabetics: A Case-Control Study of Socio-Demographic and Consumption Behaviors. Nutrients 2025, 17, 710. https://doi.org/10.3390/nu17040710
Adokwe JB, Pouyfung P, Kuraeiad S, Wongrith P, Inchai P, Yimthiang S, Satarug S, Khamphaya T. Concurrent Lead and Cadmium Exposure Among Diabetics: A Case-Control Study of Socio-Demographic and Consumption Behaviors. Nutrients. 2025; 17(4):710. https://doi.org/10.3390/nu17040710
Chicago/Turabian StyleAdokwe, Jonah Bawa, Phisit Pouyfung, Saruda Kuraeiad, Paleeratana Wongrith, Puchong Inchai, Supabhorn Yimthiang, Soisungwan Satarug, and Tanaporn Khamphaya. 2025. "Concurrent Lead and Cadmium Exposure Among Diabetics: A Case-Control Study of Socio-Demographic and Consumption Behaviors" Nutrients 17, no. 4: 710. https://doi.org/10.3390/nu17040710
APA StyleAdokwe, J. B., Pouyfung, P., Kuraeiad, S., Wongrith, P., Inchai, P., Yimthiang, S., Satarug, S., & Khamphaya, T. (2025). Concurrent Lead and Cadmium Exposure Among Diabetics: A Case-Control Study of Socio-Demographic and Consumption Behaviors. Nutrients, 17(4), 710. https://doi.org/10.3390/nu17040710









