Augmenting Cognitive Function in the Elderly with Mild Cognitive Impairment Using Probiotic Lacticaseibacillus rhamnosus CBT-LR5: A 12-Week Randomized, Double-Blind, Parallel-Group Non-Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Product
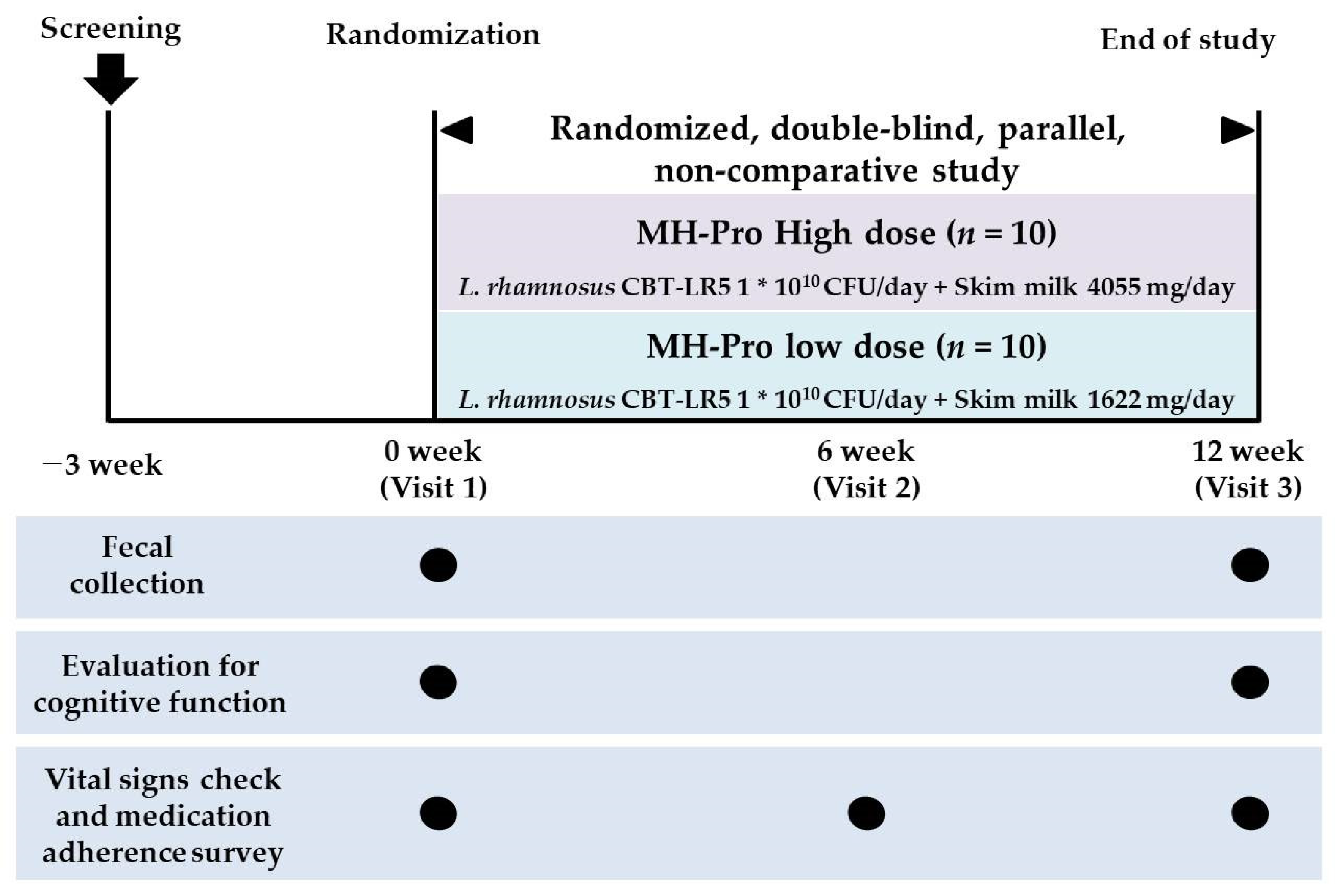
2.2. Study Design
2.3. Inclusion and Exclusion Criteria
2.4. Randomization and Blinding
2.5. Assessment for Cognitive Function and Biochemical Parameters
2.6. Fecal Sample Collection
2.7. Fecal DNA Extraction and Next-Generation Sequencing (NGS) Analysis
2.8. Dietary Intake and Physical Activity Assessment
2.9. Safety Outcome Measurements
2.10. Statistical Analysis
3. Results
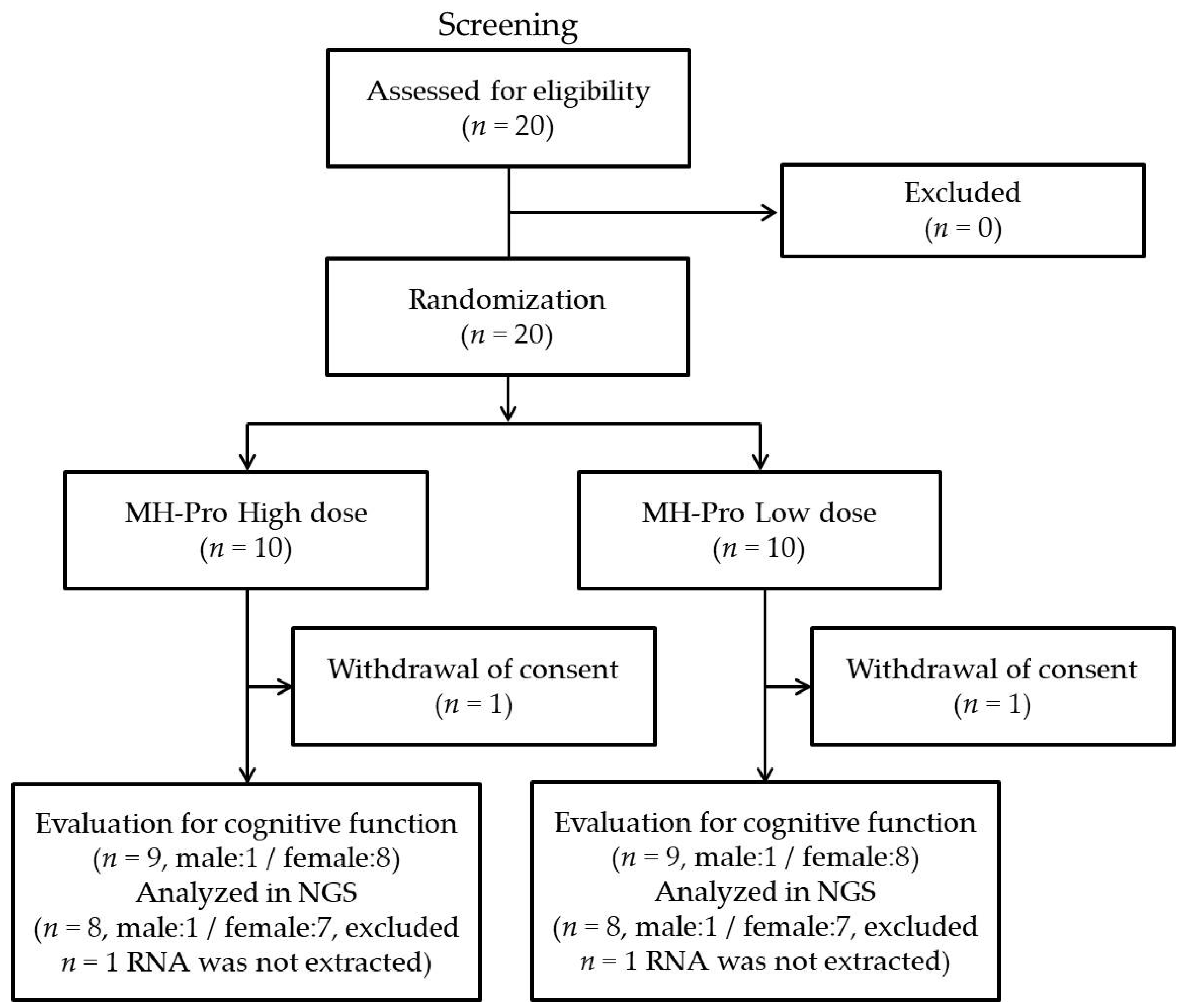
3.1. Participant Recruitment and General Demographic Characteristics
3.2. Primary/Secondary Outcomes for Cognitive Function
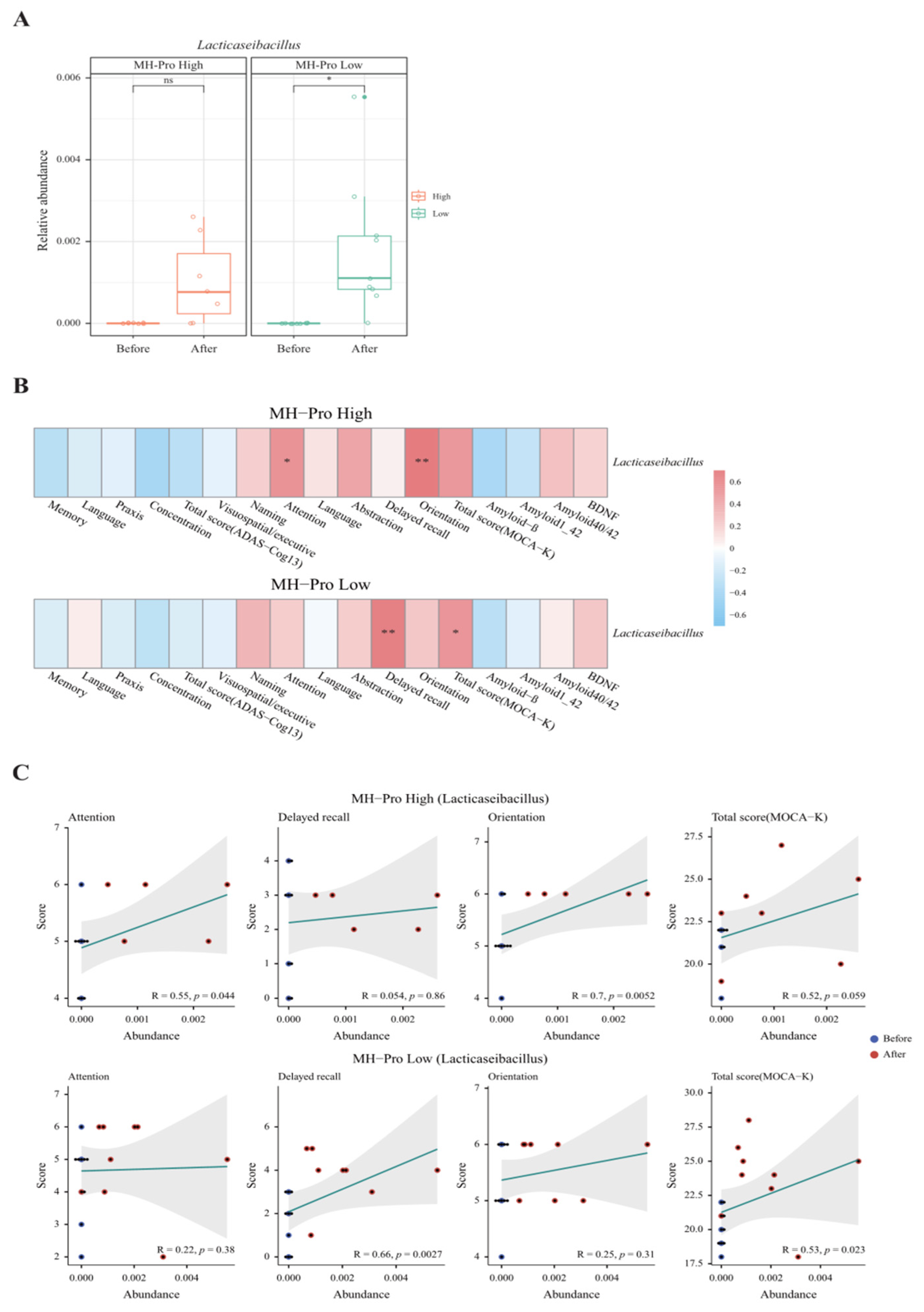
3.3. Gut Microbiota Analysis
3.4. Dietary Intake and Physical Activity
3.5. Safety and Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Ageing and Health; Fact Sheets. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 1 October 2024).
- Central Dementia Center. Korean Dementia Observatory 2023; NMC-2024-0044-10 Report; Central Dementia Center: Seoul, Republic of Korea, 2024. [Google Scholar]
- Alzheimer’s Association2024 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [CrossRef] [PubMed]
- Atri, A. The Alzheimer’s disease clinical spectrum: Diagnosis and management. Med. Clin. 2019, 103, 263–293. [Google Scholar]
- Nanjiba, R. An Updated Review on the Approved and Potential Drugs for the Treatment of Alzheimer’s Disease. Bachelor’s Thesis, Brac University, Dhaka, Bangladesh, 2024. [Google Scholar]
- Ameen, T.B.; Kashif, S.N.; Abbas, S.M.I.; Babar, K.; Ali, S.M.S.; Raheem, A. Unraveling Alzheimer’s: The promise of aducanumab, lecanemab, and donanemab. Egypt. J. Neurol. Psychiatry Neurosurg. 2024, 60, 72. [Google Scholar] [CrossRef]
- Lazarević-Pašti, T. Side effects of Alzheimer’s disease treatment. Curr. Med. Chem. 2023, 30, 2705–2709. [Google Scholar] [CrossRef]
- Martinez-Lopez, S.; Tabone, M.; Clemente-Velasco, S.; Gonzalez-Soltero, M.D.R.; Bailen, M.; de Lucas, B.; Bressa, C.; Dominguez-Balmaseda, D.; Marin-Munoz, J.; Antunez, C.; et al. A systematic review of lifestyle-based interventions for managing Alzheimer’s disease: Insights from randomized controlled trials. J. Alzheimer’s Dis. 2024, 102, 943–966. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, T.; Tu, Y.; Deng, X.; Sigen, A.; Li, Y.; Jing, X.; Wei, L.; Huang, N.; Cheng, Y.; et al. Multidomain interventions for non-pharmacological enhancement (MINE) program in Chinese older adults with mild cognitive impairment: A multicenter randomized controlled trial protocol. BMC Neurol. 2023, 23, 341. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Bock, P.M.; Telo, G.H.; Ramalho, R.; Sbaraini, M.; Leivas, G.; Martins, A.F.; Schaan, B.D. The effect of probiotics, prebiotics or synbiotics on metabolic outcomes in individuals with diabetes: A systematic review and meta-analysis. Diabetologia 2021, 64, 26–41. [Google Scholar] [CrossRef]
- Qi, D.; Nie, X.-L.; Zhang, J.-J. The effect of probiotics supplementation on blood pressure: A systemic review and meta-analysis. Lipids Health Dis. 2020, 19, 1–11. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Gonzalez, E.G.; Lamas, A.; Mondragon, A.d.C.; Regal, P.; Miranda, J.M. Probiotics as a possible strategy for the prevention and treatment of allergies. A narrative review. Foods 2021, 10, 701. [Google Scholar] [CrossRef]
- Chen, X.; D’Souza, R.; Hong, S.-T. The role of gut microbiota in the gut-brain axis: Current challenges and perspectives. Protein Cell 2013, 4, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. The microbiota–gut–brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Nam, M.S. Bioactive Peptides in Milk and Dairy Products: A Review. Korean J. Food Sci. Anim. Resour. 2015, 35, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H. Milk-derived bioactive peptides: From science to applications. J. Funct. Foods 2009, 1, 177–187. [Google Scholar] [CrossRef]
- Aslam, H.; Marx, W.; Rocks, T.; Loughman, A.; Chandrasekaran, V.; Ruusunen, A.; Dawson, S.L.; West, M.; Mullarkey, E.; Pasco, J.A.; et al. The effects of dairy and dairy derivatives on the gut microbiota: A systematic literature review. Gut Microbes 2020, 12, 1799533. [Google Scholar] [CrossRef]
- Chong, H.X.; Yusoff, N.A.A.; Hor, Y.-Y.; Lew, L.-C.; Jaafar, M.H.; Choi, S.-B.; Yusoff, M.S.B.; Wahid, N.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Lactobacillus plantarum DR7 alleviates stress and anxiety in adults: A randomised, double-blind, placebo-controlled study. Benef. Microbes 2019, 10, 355–373. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; Xue, Z.; Zhai, Q.; et al. Lactobacillus plantarum CCFM8610 alleviates irritable bowel syndrome and prevents gut microbiota dysbiosis: A randomized, double-blind, placebo-controlled, pilot clinical trial. Engineering 2021, 7, 376–385. [Google Scholar] [CrossRef]
- Sanborn, V.; Azcarate-Peril, M.A.; Updegraff, J.; Manderino, L.; Gunstad, J. Randomized clinical trial examining the impact of Lactobacillus rhamnosus GG probiotic supplementation on cognitive functioning in middle-aged and older adults. Neuropsychiatr. Dis. Treat. 2020, 16, 2765–2777. [Google Scholar] [CrossRef]
- Yang, X.; He, X.; Xu, S.; Zhang, Y.; Mo, C.; Lai, Y.; Song, Y.; Yan, Z.; Ai, P.; Qian, Y.; et al. Effect of Lacticaseibacillus paracasei strain Shirota supplementation on clinical responses and gut microbiome in Parkinson’s disease. Food Funct. 2023, 14, 6828–6839. [Google Scholar] [CrossRef]
- Ahn, S.B.; Jun, D.W.; Kang, B.-K.; Lim, J.H.; Lim, S.; Chung, M.-J. Randomized, double-blind, placebo-controlled study of a multispecies probiotic mixture in nonalcoholic fatty liver disease. Sci. Rep. 2019, 9, 5688. [Google Scholar] [CrossRef]
- Choi, J.; Son, D.; An, S.; Cho, E.; Lim, S.; Lee, H.-J. Effects of Lactiplantibacillus plantarum CBT LP3 and Bifidobacterium breve CBT BR3 supplementation on weight loss and gut microbiota of overweight dogs. Sci. Rep. 2024, 14, 25446. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Park, S.; Paik, J.W.; Chae, S.W.; Kim, D.H.; Jeong, D.G.; Ha, E.; Kim, M.; Hong, G.; Park, S.H.; et al. Efficacy and Safety of Lactobacillus Plantarum C29-Fermented Soybean (DW2009) in Individuals with Mild Cognitive Impairment: A 12-Week, Multi-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019, 11, 305. [Google Scholar] [CrossRef]
- Armstrong, T.; Bull, F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ). J. Public Health 2006, 14, 66–70. [Google Scholar] [CrossRef]
- Didari, T.; Solki, S.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. A systematic review of the safety of probiotics. Expert Opin. Drug Saf. 2014, 13, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Shao, Y.; Zhang, Y.; Zhao, Y.; Han, M.; Gai, Z. In vitro and in vivo genome-based safety evaluation of Lacticaseibacillus rhamnosus LRa05. Food Chem. Toxicol. 2024, 186, 114600. [Google Scholar] [CrossRef] [PubMed]
- Mathipa-Mdakane, M.G.; Thantsha, M.S. Lacticaseibacillus rhamnosus: A Suitable Candidate for the Construction of Novel Bioengineered Probiotic Strains for Targeted Pathogen Control. Foods 2022, 11, 785. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Liu, Y.; Zhong, J.; Zhang, D. Assessing the Safety and Probiotic Characteristics of Lacticaseibacillus rhamnosus X253 via Complete Genome and Phenotype Analysis. Microorganisms 2023, 11, 140. [Google Scholar] [CrossRef]
- Skinner, J.; Carvalho, J.O.; Potter, G.G.; Thames, A.; Zelinski, E.; Crane, P.K.; Gibbons, L.E.; the Alzheimer’s Disease Neuroimaging Initiative. The Alzheimer’s Disease Assessment Scale-Cognitive-Plus (ADAS-Cog-Plus): An expansion of the ADAS-Cog to improve responsiveness in MCI. Brain Imaging Behav. 2012, 6, 489–501. [Google Scholar] [CrossRef]
- Janicki, S.C.; Schupf, N. Hormonal influences on cognition and risk for Alzheimer’s disease. Curr. Neurol. Neurosci. Rep. 2010, 10, 359–366. [Google Scholar] [CrossRef]
- Calvo, M.V.; Kohen, V.L.; Díaz-Mardomingo, C.; García-Herranz, S.; Baliyan, S.; Tomé-Carneiro, J.; Colmenarejo, G.; Visioli, F.; Venero, C.; Fontecha, J. Milk fat globule membrane-enriched milk improves episodic memory: A randomized, parallel, double-blind, placebo-controlled trial in older adults. J. Funct. Foods 2023, 111, 105849. [Google Scholar] [CrossRef]
- Ohsawa, K.; Uchida, N.; Ohki, K.; Nakamura, Y.; Yokogoshi, H. Lactobacillus helveticus–fermented milk improves learning and memory in mice. Nutr. Neurosci. 2015, 18, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, K.; Uchida, N.; Ohki, K.; Yokogoshi, H. Identification of peptides present in sour milk whey that ameliorate scopolamine-induced memory impairment in mice. Int. J. Food Sci. Nutr. 2018, 69, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Yeon, S.-W.; You, Y.S.; Kwon, H.-S.; Yang, E.H.; Ryu, J.-S.; Kang, B.H.; Kang, J.-H. Fermented milk of Lactobacillus helveticus IDCC3801 reduces beta-amyloid and attenuates memory deficit. J. Funct. Foods 2010, 2, 143–152. [Google Scholar] [CrossRef]
- Xu, R. Bioactive peptides in milk and their biological and health implications. Food Rev. Int. 1998, 14, 1–16. [Google Scholar] [CrossRef]
- Bilikiewicz, A.; Gaus, W. Colostrinin (a naturally occurring, proline-rich, polypeptide mixture) in the treatment of Alzheimer’s disease. J. Alzheimer’s Dis. 2004, 6, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Kita, M.; Kobayashi, K.; Obara, K.; Koikeda, T.; Umeda, S.; Ano, Y. Supplementation with Whey Peptide Rich in beta-Lactolin Improves Cognitive Performance in Healthy Older Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Front. Neurosci. 2019, 13, 399. [Google Scholar] [CrossRef]
- Wu, L.; Sun, D. Meta-Analysis of Milk Consumption and the Risk of Cognitive Disorders. Nutrients 2016, 8, 824. [Google Scholar] [CrossRef]
- Anderson, R.C.; Alpass, F.M. Effectiveness of dairy products to protect against cognitive decline in later life: A narrative review. Front. Nutr. 2024, 11, 1366949. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, H.; Ju, Y.; Liu, J.; Wang, M.; Liu, B.; Zhang, Y. Gut microbiota-derived short-chain fatty acids and depression: Deep insight into biological mechanisms and potential applications. Gen. Psychiatr. 2024, 37, e101374. [Google Scholar] [CrossRef]
- Guo, C.; Huo, Y.-J.; Li, Y.; Han, Y.; Zhou, D. Gut-brain axis: Focus on gut metabolites short-chain fatty acids. World J. Clin. Cases 2022, 10, 1754–1763. [Google Scholar] [CrossRef]
- Motataianu, A.; Serban, G.; Andone, S. The Role of Short-Chain Fatty Acids in Microbiota-Gut-Brain Cross-Talk with a Focus on Amyotrophic Lateral Sclerosis: A Systematic Review. Int J. Mol. Sci. 2023, 24, 15094. [Google Scholar] [CrossRef]
- Reimann, F.; Gribble, F.M. G protein-coupled receptors as new therapeutic targets for type 2 diabetes. Diabetologia 2016, 59, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017, 595, 489–503. [Google Scholar] [CrossRef]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Onisiforou, A.; Charalambous, E.G.; Zanos, P. Shattering the Amyloid Illusion: The Microbial Enigma of Alzheimer’s Disease Pathogenesis—From Gut Microbiota and Viruses to Brain Biofilms. Microorganisms 2025, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Neshat, M.; Pourjafar, H.; Jafari, S.M.; Samakkhah, S.A.; Mirzakhani, E. The role of probiotics and prebiotics in modulating of the gut-brain axis. Front Nutr. 2023, 10, 1173660. [Google Scholar] [CrossRef]
- Snigdha, S.; Ha, K.; Tsai, P.; Dinan, T.G.; Bartos, J.D.; Shahid, M. Probiotics: Potential novel therapeutics for microbiota-gut-brain axis dysfunction across gender and lifespan. Pharmacol. Ther. 2022, 231, 107978. [Google Scholar] [CrossRef]
- Suganya, K.; Koo, B.-S. Gut–Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Beneficially Modulate Microbial and Immune Pathways to Improve Brain Functions. Int. J. Mol. Sci. 2020, 21, 7551. [Google Scholar] [CrossRef]
- Zheng, M.; Ye, H.; Yang, X.; Shen, L.; Dang, X.; Liu, X.; Gong, Y.; Wu, Q.; Wang, L.; Ge, X.; et al. Probiotic Clostridium butyricum ameliorates cognitive impairment in obesity via the microbiota-gut-brain axis. Brain Behav. Immun. 2024, 115, 565–587. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, X.; Zhang, L.; Tang, C.; Meng, F.; Zhou, L.; Zhu, P.; Lu, Z.; Lu, Y. Ingestion of Lacticaseibacillus Rhamnosus Fmb14 prevents depression-like behavior and brain neural activity via the microbiota–gut–brain axis in colitis mice. Food Funct. 2023, 14, 1909–1928. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Wu, J. Impact of food-derived bioactive peptides on gut function and health. Food Res. Int. 2021, 147, 110485. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Augustin, O.; Rivero-Gutiérrez, B.; Mascaraque, C.; Sanchez de Medina, F.S. Food derived bioactive peptides and intestinal barrier function. Int. J. Mol. Sci. 2014, 15, 22857–22873. [Google Scholar] [CrossRef]
- Yaffe, K.; Middleton, L.E.; Lui, L.Y.; Spira, A.P.; Stone, K.; Racine, C.; Ensrud, K.E.; Kramer, J.H. Mild cognitive impairment, dementia, and their subtypes in oldest old women. Arch. Neurol. 2011, 68, 631–636. [Google Scholar] [CrossRef]
- Arenaza-Urquijo, E.M.; Boyle, R.; Casaletto, K.; Anstey, K.J.; Vila-Castelar, C.; Colverson, A.; Palpatzis, E.; Eissman, J.M.; Ng, T.K.S.; Raghavan, S.; et al. Sex and gender differences in cognitive resilience to aging and Alzheimer’s disease. Alzheimer’s Dement. 2024, 20, 5695–5719. [Google Scholar] [CrossRef]
- Pszczolowska, M.; Walczak, K.; Miskow, W.; Mroziak, M.; Kozlowski, G.; Beszlej, J.A.; Leszek, J. Association between Female Reproductive Factors and Risk of Dementia. J. Clin. Med. 2024, 13, 2983. [Google Scholar] [CrossRef]
- Sattler, C.; Toro, P.; Schönknecht, P.; Schröder, J. Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and Alzheimer’s disease. Psychiatry Res. 2012, 196, 90–95. [Google Scholar] [CrossRef]
- Sharp, E.S.; Gatz, M. Relationship Between Education and Dementia: An Updated Systematic Review. Alzheimer Dis. Assoc. Disord. 2011, 25, 289–304. [Google Scholar] [CrossRef]
- Ashton, N.J.; Janelidze, S.; Mattsson-Carlgren, N.; Binette, A.P.; Strandberg, O.; Brum, W.S.; Karikari, T.K.; González-Ortiz, F.; Di Molfetta, G.; Meda, F.J. Differential roles of Aβ42/40, p-tau231 and p-tau217 for Alzheimer’s trial selection and disease monitoring. Nat. Med. 2022, 28, 2555–2562. [Google Scholar] [CrossRef]
- Devanarayan, V.; Doherty, T.; Charil, A.; Sachdev, P.; Ye, Y.; Murali, L.K.; Llano, D.A.; Zhou, J.; Reyderman, L.; Hampel, H.; et al. Plasma pTau217 predicts continuous brain amyloid levels in preclinical and early Alzheimer’s disease. Alzheimer’s Dement. 2024, 20, 5617–5628. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, S.; Tideman, P.; Cullen, N.; Zetterberg, H.; Blennow, K.; the Alzheimer’s Disease Neuroimaging Initiative; Dage, J.L.; Stomrud, E.; Janelidze, S.; Mattsson-Carlgren, N.; et al. Prediction of future Alzheimer’s disease dementia using plasma phospho-tau combined with other accessible measures. Nat. Med. 2021, 27, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Pichet Binette, A.; Palmqvist, S.; Bali, D.; Farrar, G.; Buckley, C.J.; Wolk, D.A.; Zetterberg, H.; Blennow, K.; Janelidze, S.; Hansson, O. Combining plasma phospho-tau and accessible measures to evaluate progression to Alzheimer’s dementia in mild cognitive impairment patients. Alzheimer’s Res. Ther. 2022, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Solomon, A.; Ahtiluoto, S.; Ngandu, T.; Lehtisalo, J.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. The Finnish geriatric intervention study to prevent cognitive impairment and disability (FINGER): Study design and progress. Alzheimer’s Dement. 2013, 9, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Stephen, R. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (Finger): Findings from the Structural Brain Mri Sub-Study. Ph.D. Thesis, Itä-Suomen yliopisto, Kuopio, Finland, 2020. [Google Scholar]
| MH-Pro High Dose (n = 10) | MH-Pro Low Dose (n = 10) | Total (n = 20) | p-Value (1) | ||
|---|---|---|---|---|---|
| Sex | Male | 1 (10.0) | 1 (10.0) | 2 (10.0) | >0.99 (3) |
| Female | 9 (90.0) | 9 (90.0) | 18 (90.0) | ||
| Age (years) | 68.50 ± 2.59 | 69.40 ± 3.53 | 68.95 ± 3.05 | 0.52 | |
| Education (years) | 12.30 ± 3.33 | 11.70 ± 2.98 | 12.0 ± 3.09 | 0.68 | |
| Height (cm) | 156.20 ± 4.94 | 153.80 ± 5.69 | 155.0 ± 5.33 | 0.33 | |
| Weight (kg) | 58.25 ± 5.11 | 58.42 ± 7.22 | 58.34 ± 6.09 | 0.95 | |
| BMI (kg/m2) | 23.86 ± 1.45 | 24.66 ± 2.45 | 24.26 ± 2.0 | 0.39 | |
| Systolic blood pressure (mmHg) | 125.20 ± 15.75 | 129.60 ± 15.39 | 127.40 ± 15.32 | 0.54 | |
| Diastolic blood pressure (mmHg) | 74.0 ± 9.30 | 75.10 ± 9.52 | 74.55 ± 9.17 | 0.80 | |
| Pulse rate (beats/min) | 72.20 ± 10.02 | 72.20 ± 8.97 | 72.20 ± 9.25 | >0.99 | |
| Alcoholic drinking status, n (%) | 2 (20.0) | 2 (20.0) | 4 (20.0) | >0.99 (2) | |
| Amount of alcohol status (g/week) | 8.0 ± 8.49 | 7.20 ± 5.09 | 7.60 ± 5.73 | 0.92 | |
| Smoking status, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | |
| Comorbidities, n (%) | Hypertension | 5 (35.7) | 2 (14.3) | 7 (50.0) | 0.50 (3) |
| Dyslipidemia | 4 (28.6) | 3 (21.4) | 7 (50.0) | ||
| Medications, n (%) | Hypertension | 7 (41.2) | 3 (21.4) | 10 (32.3) | 0.30 (3) |
| Dyslipidemia | 4 (23.5) | 4 (28.6) | 8 (25.8) | ||
| Vaccination (COVID-19, influenza) | 2 (11.8) | 0 (0.0) | 2 (6.4) | ||
| None | 4 (23.5) | 7 (50.0) | 11 (35.5) | ||
| CERAD-K (score) | Word list memory | 19.60 ± 2.41 | 19.40 ± 2.88 | 19.50 ± 2.59 | 0.87 |
| Word list recall | 5.70 ± 1.34 | 5.90 ± 0.88 | 5.80 ± 1.11 | 0.70 | |
| Word list recognition | 8.20 ± 1.03 | 7.90 ± 0.99 | 8.05 ± 1.0 | 0.52 | |
| MOCA-K | Total score (score) | 20.30 ± 1.89 | 19.70 ± 1.64 | 20.0 ± 1.75 | 0.46 |
| BDI (score) | 9.20 ± 3.79 | 8.20 ± 4.42 | 8.70 ± 4.04 | 0.59 |
| MH-Pro High Dose (n = 9) | MH-Pro Low Dose (n = 9) | p-Value (2) | ||
|---|---|---|---|---|
| Memory (score) | Baseline | 11.44 ± 4.72 | 13.44 ± 3.43 | 0.32 |
| Visit 3 | 10.78 ± 2.59 | 11.44 ± 3.84 | 0.39 | |
| Change from baseline | −0.67 ± 3.43 | −2.0 ± 3.0 | ||
| p-value (1) | 0.58 | 0.08 | ||
| Language (score) | Baseline | 1.89 ± 1.27 | 1.67 ± 1.50 | 0.74 |
| Visit 3 | 0.89 ± 0.93 | 1.33 ± 1.0 | 0.42 | |
| Change from baseline | −1.0 ± 1.41 | −0.33 ± 1.94 | ||
| p-value (1) | 0.067 | 0.62 | ||
| Praxis (score) | Baseline | 1.33 ± 0.71 | 1.89 ± 1.36 | 0.29 |
| Visit 3 | 1.33 ± 0.71 | 1.67 ± 0.87 | 0.72 | |
| Change from baseline | 0.0 ± 0.71 | −0.22 ± 1.64 | ||
| p-value (1) | >0.99 | 0.70 | ||
| Concentration (score) | Baseline | 2.11 ± 1.05 | 1.67 ± 0.71 | 0.31 |
| Visit 3 | 1.33 ± 0.87 | 1.33 ± 0.87 | 0.32 | |
| Change from baseline | −0.78 ± 0.97 | −0.33 ± 0.87 | ||
| p-value (1) | 0.04 | 0.28 | ||
| Total score (score) | Baseline | 16.78 ± 6.46 | 18.67 ± 3.87 | 0.46 |
| Visit 3 | 14.33 ± 3.32 | 15.78 ± 4.68 | 0.83 | |
| Change from baseline | −2.44 ± 4.13 | −2.89 ± 4.37 | ||
| p-value (1) | 0.11 | 0.08 |
| Variables (Score) | MH-Pro High Dose (n = 9) | MH-Pro Low Dose (n = 9) | p-Value (2) | |
|---|---|---|---|---|
| Visuospatial/ executive | Baseline | 3.22 ± 1.09 | 3.56 ± 0.73 | 0.46 |
| Visit 3 | 3.56 ± 1.01 | 3.67 ±0.87 | 0.63 | |
| Change from baseline | 0.33 ± 1.0 | 0.11 ± 0.93 | ||
| p-value (1) | 0.35 | 0.73 | ||
| Naming | Baseline | 2.89 ± 0.33 | 2.33 ± 0.71 | 0.06 |
| Visit 3 | 3.0 ± 0.0 | 2.89 ± 0.33 | 0.05 | |
| Change from baseline | 0.11 ± 0.33 | 0.56 ±0.53 | ||
| p-value (1) | 0.35 | 0.01 | ||
| Attention | Baseline | 4.44 ± 1.01 | 4.44 ± 1.24 | >0.99 |
| Visit 3 | 5.44 ± 0.73 | 4.89 ± 1.36 | 0.43 | |
| Change from baseline | 1.0 ± 1.41 | 0.44 ± 1.51 | ||
| p-value (1) | 0.07 | 0.40 | ||
| Language | Baseline | 1.78 ± 0.67 | 2.0 ± 0.71 | 0.50 |
| Visit 3 | 1.89 ± 0.93 | 2.0 ± 0.71 | 0.81 | |
| Change from baseline | 0.11 ± 0.78 | 0.0 ± 1.12 | ||
| p-value (1) | 0.68 | >0.99 | ||
| Abstraction | Baseline | 1.0 ± 0.50 | 0.78 ± 0.44 | 0.33 |
| Visit 3 | 1.0 ± 0.71 | 1.22 ± 0.67 | 0.15 | |
| Change from baseline | 0.0 ±0.50 | 0.44 ± 0.73 | ||
| p-value (1) | >0.99 | 0.10 | ||
| Delayed recall | Baseline | 2.22 ±1.30 | 1.56 ± 1.33 | 0.30 |
| Visit 3 | 2.56 ±1.24 | 3.56 ±1.33 | 0.03 | |
| Change from baseline | 0.33 ± 1.41 | 2.0 ± 1.50 | ||
| p-value (1) | 0.50 | 0.009 | ||
| Orientation | Baseline | 5.11 ± 0.60 | 5.33 ± 0.71 | 0.48 |
| Visit 3 | 5.78 ± 0.44 | 5.56 ± 0.53 | 0.24 | |
| Change from baseline | 0.67 ± 0.87 | 0.22 ± 0.67 | ||
| p-value (1) | 0.05 | 0.35 | ||
| Total score | Baseline | 20.67 ± 1.58 | 20.0 ± 1.41 | 0.36 |
| Visit 3 | 23.22 ± 2.49 | 23.78 ± 2.91 | 0.42 | |
| Change from baseline | 2.56 ± 3.43 | 3.78 ± 2.77 | ||
| p-value (1) | 0.06 | 0.004 |
| MH-Pro High Dose (n = 9) | MH-Pro Low Dose (n = 9) | p-Value (2) | ||
|---|---|---|---|---|
| Amyloid-β1–40 (pg/mL) | Baseline | 205.94 ± 22.26 | 202.83 ± 39.67 | 0.84 |
| Visit 3 | 193.28 ± 15.41 | 196.04 ± 28.40 | 0.73 | |
| Change from baseline | −12.66 ± 25.73 | −6.79 ± 44.09 | ||
| p-value (1) | 0.18 | 0.66 | ||
| Amyloid-β 1–42 (pg/mL) | Baseline | 12.66 ± 6.98 | 9.28 ± 8.72 | 0.38 |
| Visit 3 | 12.33 ± 7.29 | 9.26 ± 5.14 | 0.88 | |
| Change from baseline | −0.33 ± 3.83 | −0.02 ± 4.47 | ||
| p-value (1) | 0.80 | 0.99 | ||
| Amyloid-β1 40/42 | Baseline | 20.47 ± 10.0 | 39.16 ± 28.12 | 0.09 |
| Visit 3 | 20.30 ± 9.85 | 29.29 ± 18.70 | 0.04 | |
| Change from baseline | −0.17 ± 6.88 | −9.87 ± 10.98 | ||
| p-value (1) | 0.94 | 0.02 | ||
| BDNF (pg/mL) | Baseline | 30,055.56 ± 5669.46 | 27,200.0 ± 11,995.42 | 0.53 |
| Visit 3 | 30,800.0 ± 6957.73 | 32,255.56 ± 6427.89 | 0.31 | |
| Change from baseline | 744.44 ± 3354.52 | 5055.56 ± 11,698.94 | ||
| p-value (1) | 0.52 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.-J.; Cho, K.; Jung, E.-S.; Son, D.; Byun, J.-S.; Kim, S.-I.; Chae, S.-W.; Yang, J.-C.; Lee, S.-O.; Lim, S. Augmenting Cognitive Function in the Elderly with Mild Cognitive Impairment Using Probiotic Lacticaseibacillus rhamnosus CBT-LR5: A 12-Week Randomized, Double-Blind, Parallel-Group Non-Comparative Study. Nutrients 2025, 17, 691. https://doi.org/10.3390/nu17040691
Jung S-J, Cho K, Jung E-S, Son D, Byun J-S, Kim S-I, Chae S-W, Yang J-C, Lee S-O, Lim S. Augmenting Cognitive Function in the Elderly with Mild Cognitive Impairment Using Probiotic Lacticaseibacillus rhamnosus CBT-LR5: A 12-Week Randomized, Double-Blind, Parallel-Group Non-Comparative Study. Nutrients. 2025; 17(4):691. https://doi.org/10.3390/nu17040691
Chicago/Turabian StyleJung, Su-Jin, Kyohee Cho, Eun-Soo Jung, Dooheon Son, Jong-Seon Byun, Song-In Kim, Soo-Wan Chae, Jong-Chul Yang, Seung-Ok Lee, and Sanghyun Lim. 2025. "Augmenting Cognitive Function in the Elderly with Mild Cognitive Impairment Using Probiotic Lacticaseibacillus rhamnosus CBT-LR5: A 12-Week Randomized, Double-Blind, Parallel-Group Non-Comparative Study" Nutrients 17, no. 4: 691. https://doi.org/10.3390/nu17040691
APA StyleJung, S.-J., Cho, K., Jung, E.-S., Son, D., Byun, J.-S., Kim, S.-I., Chae, S.-W., Yang, J.-C., Lee, S.-O., & Lim, S. (2025). Augmenting Cognitive Function in the Elderly with Mild Cognitive Impairment Using Probiotic Lacticaseibacillus rhamnosus CBT-LR5: A 12-Week Randomized, Double-Blind, Parallel-Group Non-Comparative Study. Nutrients, 17(4), 691. https://doi.org/10.3390/nu17040691







