Association of Oxidative Stress Markers with Incident Hyperglycemia in Gestational Diabetes Mellitus in an Educational Intervention
Abstract
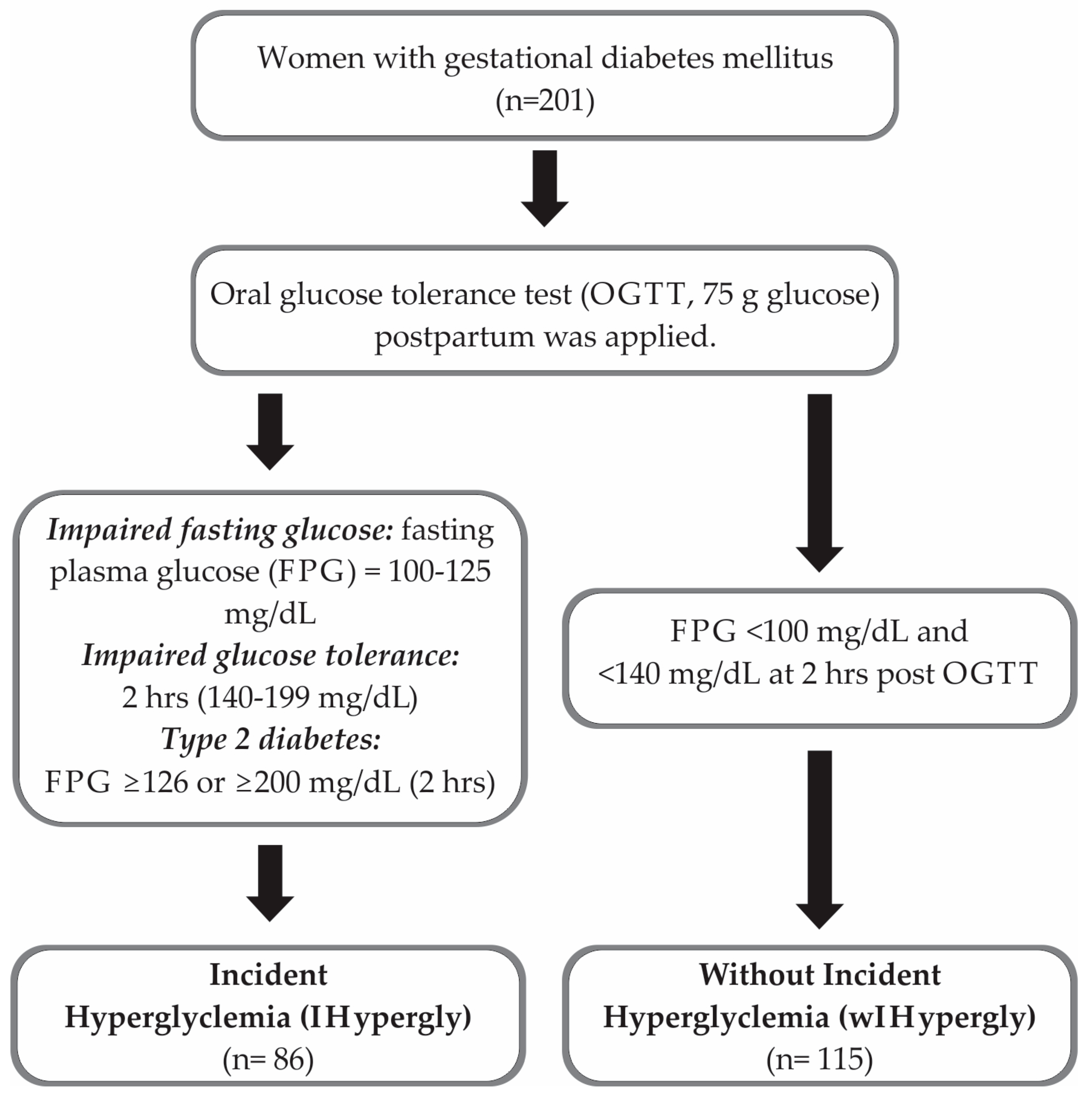
:1. Background
2. Methods
Statistical Analysis
3. Results
Description of the Sample
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Chapter 2: Diagnosis and Classification of diabetes: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48 (Suppl. 1), S27–S49. [Google Scholar]
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Powe, C.E.; Immanuel, J.; Karuranga, S.; et al. IDF Diabetes Atlas: Estimation of global and regional gestational diabetes mellitus prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group`s Criteria. Diabetes Res. Clin. Pract. 2022, 183, 109050. [Google Scholar] [CrossRef] [PubMed]
- Campos-Nonato, I.; Galván-Valencia, O.; Hernández-Barrera, L.; Oviedo-Solis, C.; Barquera, S. Prevalence of obesity and associated risk factors in Mexican adults: Results of the Ensanut 2022. Salud Publica Mex. 2023, 65 (Suppl. I), S238–S247. [Google Scholar] [CrossRef] [PubMed]
- Basto-Abreu, A.; López-Olmedo, N.; Rojas-Martínez, R.; Aguilar-Salinas, C.A.; Moreno-Banda, G.L.; Carnalla, M.; Rivera, J.A.; Romero-Martinez, M.; Barquera, S.; Barrientos-Gutiérrez, T.; et al. Prevalence of prediabetes and diabetes in Mexico. Ensanut 2022. Salud Publica Mex. 2023, 65 (Suppl. I), S163–S168. [Google Scholar] [CrossRef]
- Lappas, M.; Hidden, U.; Desoye, G.; Froehlich, J.; Hauguel-de Mouzon, S.; Jawerbaum, A. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid. Redox Signal. 2011, 15, 3061–3100. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Are oxidative stress activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 2003, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Y.; Chen, R.; Wei, Y.; Feng, Y.; Zheng, W.; Liao, H.; Zhang, Z. Aberrant expression of oxidative stress related proteins affects the pregnancy outcome of gestational diabetes mellitus patients. Am. J. Transl. Res. 2019, 11, 269–279. [Google Scholar] [PubMed]
- Li, H.; Yin, Q.; Li, N.; Ouyang, Z.; Zhong, M. Plasma markers of oxidative stress in patients with gestational diabetes mellitus in the second and third trimester. Obstet. Gynecol. Int. 2016, 2016, 3865454. [Google Scholar] [CrossRef]
- Saucedo, R.; Ortega-Camarillo, C.; Ferreira-Hermosillo, A.; Díaz-Velázquez, M.F.; Meixueiro-Calderón, C.; Valencia-Ortega, J. Role of oxidative stress and inflammation in gestational diabetes mellitus. Antioxidants 2023, 12, 1812. [Google Scholar] [CrossRef]
- Pheiffer, C.; Dias, S.; Jack, B.; Malaza, N.; Adam, S. Adiponectin as a potential biomarker for pregnancy disorders. Int. J. Mol. Sci. 2021, 22, 1326. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, Y.H.; Chen, Y.P.; Yuan, X.L.; Wang, J.; Zhu, H.; Lu, C.M. Maternal circulating concentrations of tumor necrosis factor-alpha, leptin, and adiponectin in gestational diabetes mellitus: A systematic review and meta-analysis. Sci. World J. 2014, 2014, 926932. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cheng, Y.; Wang, D.; Chen, H.; Chen, H.; Ming, W.-K.; Wang, Z. Incidence rate of type 2 diabetes mellitus after gestational diabetes mellitus: A systematic review and meta-analysis of 170,139 women. J. Diabetes Res. 2020, 2020, 3076463. [Google Scholar] [CrossRef] [PubMed]
- Bengston, A.M.; Ramos, S.Z.; Savitz, D.A.; Werner, E.F. Risk factors for progression from gestational diabetes to postpartum type 2 diabetes: A review. Clin. Obstet. Gynecol. 2021, 64, 234–243. [Google Scholar] [CrossRef]
- Uusitupa, M.; Khan, T.A.; Viguiliouk, E.; Kahleova, H.; Rivellese, A.A.; Hermansen, K.; Pfeiffer, A.; Thanopoulou, A.; Salas-Salvadó, J.; Schwab, U.; et al. Prevention of type 2 diabetes by lifestyle changes: A systematic review and meta-analysis. Nutrients 2019, 11, 2611. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, Y.; Cui, D.; Li, C.; Ma, R.C.; Li, J.; Yang, X. Effects of lifestyle intervention on long-term risk of diabetes in women with prior gestational diabetes: A systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2021, 22, e13122. [Google Scholar] [CrossRef]
- Aroda, V.R.; Christophi, C.A.; Edelstein, S.L.; Zhang, P.; Herman, W.H.; Barrett-Connor, E.; Delahanty, L.M.; Montez, M.G.; Ackermann, R.T.; Zhuo, X.; et al. Diabetes Prevention Program Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: The Diabetes Prevention Program outcomes study 10-year follow up. J. Clin. Edocrinol. Metab. 2015, 100, 1646–1653. [Google Scholar]
- Huvinen, E.; Koivusalo, S.B.; Meinilä, J.; Valkama, A.; Tiitinen, A.; Rönö, K.; Stach-Lempinen, B.; Eriksson, J.G. Effects of a lifestyle intervention during pregnancy and first postpartum year: Findings from the RADIEL study. J. Clin. Endocrinol. Metab. 2018, 103, 1669–1677. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar]
- Hernández-Avila, M.; Romieu, I.; Parra, S.; Hernández-Avila, J.; Madrigal, H.; Willett, W. Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Pública Mex. 1998, 40, 133–140. [Google Scholar] [PubMed]
- Bourges, H.; Casanueva, E.; Rosado, J.L. Recomendaciones de Ingestión de Nutrimentos para la Población Mexicana; Editorial Médica Panamericana: Ciudad de México, Mexico, 2005. [Google Scholar]
- Rivas, A.; Romero, A.; Mariscal-Arcas, M.; Monteagudo, C.; López, G.; Lorenzo, M.L.; Ocaña-Peinado, F.M.; Olea-Serrano, F. Association between antioxidant quality score (DAQs) and bone mineral density in Spanish women. Nutr. Hosp. 2012, 27, 1886–1893. [Google Scholar] [PubMed]
- Jentzsch, A.M.; Bachmann, H.; Fürst, P.; Biesalski, H.K. Improved analysis of malondialdehyde in human body fluid. Free Radic. Biol. Med. 1996, 20, 251–256. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lwt Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in Oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar]
- Bao, W.; Yeung, E.; Tobias, D.K.; Hu, F.B.; Vaag, A.A.; Chavarro, J.E.; Mills, J.L.; Grunnet, L.G.; Bowers, K.; Ley, S.H.; et al. Long-term risk of type 2 diabetes mellitus in relation to BMI and weight change among women with a history of gestational diabetes mellitus: A prospective cohort study. Diabetologia 2015, 58, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Li, S.; Chavarro, J.E.; Tobias, D.K.; Zhu, Y.; Hu, F.B.; Zhang, C. Low carbohydrate-diet scores and long-term risk of type 2 diabetes among women with history of gestational diabetes mellitus: A prospective cohort study. Diabetes Care 2016, 39, 43–49. [Google Scholar] [CrossRef]
- Torres-Torres, J.; Monroy-Muñoz, I.E.; Perez-Duran, J.; Solis-Paredes, J.M.; Camacho-Martinez, Z.A.; Baca, D.; Espino-Y-Sosa, S.; Martinez-Portilla, R.; Rojas-Zepeda, L.; Borboa-Olivares, H.; et al. Cellular and Molecular Pathophysiology of Gestational Diabetes. Int. J. Mol. Sci. 2024, 25, 11641. [Google Scholar] [CrossRef]
- Martinez-Martinez, E.; Cachofeiro, V. Oxidative stress in obesity. Antioxidants 2022, 11, 639. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.; Ipekci, S.H.; Kebapcilar, L.; Dede, N.D.; Kurban, S.; Erbay, E.; Gonen, M.S. Effect of Aerobic Exercise Training on MDA and TNF-alpha Levels in Patients with Type 2 Diabetes Mellitus. Int. Sch. Res. Not. 2014, 2014, 820387. [Google Scholar]
- Alghadir, A.H.; Gabr, S.A.; Anwer, S.; Al-Eisa, E. Fatigue and Oxidative Stress Response to Physical Activity in Type 2 Diabetic Patients. Int. J. Diabetes Dev. Ctries. 2016, 36, 59–64. [Google Scholar] [CrossRef]
- Małkowska, P. Positive Effects of Physical Activity on Insulin Signaling. Curr. Issues Mol. Biol. 2024, 46, 5467–5487. [Google Scholar] [CrossRef] [PubMed]
| Baseline | Post-Intervention | |||||
|---|---|---|---|---|---|---|
| IHypergly (n = 86) Median (IQR) | wIHypergly (n = 115) Median (IQR) | p | IHypergly (n = 86) Median (IQR) | wIHypergly (n = 115) Median (IQR) | p | |
| Age (years) | 33 (31–38) | 34 (30–37) | 0.402 | ___ | ___ | ___ |
| Pregestational and post-intervention BMI (Kg/m2) * | 30.37 ± 5.45 | 28.47 ± 4.81 | 0.010 | 31.15 ± 5.52 | 28.77 ± 4.79 | 0.016 |
| BMI Δ (post-intervention-pregestational) (Kg/m2) * | ___ | ___ | ___ | 0.77 ± 2.91 | 0.32 ± 2.89 | 0.570 |
| End of pregnancy BMI (Kg/m2) * | 32.68 ± 5.20 | 31.06 ± 4.23 | 0.016 | ___ | ___ | ___ |
| Weight gained during pregnancy (Kg) * | 5.88 ± 6.10 | 6.38 ± 6.66 | 0.587 | ___ | ___ | ___ |
| Systolic blood pressure (mm Hg) * | 116.57 ± 12.54 | 113.93 ± 10.44 | 0.206 | |||
| Diastolic blood pressure (mm Hg) * | 75.96 ± 9.50 | 73.86 ± 8.58 | 0.197 | |||
| Physically active, n (%) ^ | 55 (64) | 83 (73.5) | 0.150 | 41 (47.7) | 59 (51.3) | 0.611 |
| Actively smoking, n (%) ^ | 10 (11.8) | 11 (9.6) | 0.616 | 10 (11.6) | 14 (12.2) | 0.906 |
| Vitamin supplementation, n (%) ^ | 66 (76.7) | 89 (77.4) | 0.914 | ___ | ___ | ___ |
| Gestational age (weeks) | 38.00 (37.05–39) | 38.30 (38–39) | 0.103 | |||
| Apgar score | 9 (9–9) | 9 (9–9) | 0.862 | |||
| Breastfeeding, n (%) ^ | ___ | ___ | ___ | 44 (51.2) | 73 (63.5) | 0.080 |
| Baseline | Post-Intervention | |||||
|---|---|---|---|---|---|---|
| IHypergly (n = 86) Median (IQR) | wIHypergly (n = 115) Median (IQR) | p | IHypergly (n = 86) Median (IQR) | wIHypergly (n = 115) Median (IQR) | p | |
| Fasting plasma glucose (mg/dL) | 69 (59–83.50) | 70 (61.75–85.25) | 0.866 | 101 (94–108.25) | 86 (77–93) | <0.001 |
| Glucose 2 h (mg/dL) post OGTT | ___ | ___ | ___ | 143 (113–169) | 96 (86–117) | <0.001 |
| Triglycerides (mg/dL) | 348 (282.50–429.50) | 327 (277–410) | 0.163 | 153 (106.75–219) | 125 (86–174) | 0.001 |
| LDL-cholesterol (mg/dL) * | 138.45 ± 43.04 | 141.38 ± 37.59 | 0.609 | 122.73 ± 30.68 | 113.08 ± 30.05 | 0.929 |
| Fasting plasma Insulin (μU/mL) | 13.10 (8.32–25.70) | 10.40 (6.82–18.72) | 0.072 | 11.60 (7.60–17.60) | 7.60 (5.10–10.30) | <0.001 |
| Insulin 2 h (μU/mL) post OGTT | ___ | ___ | ___ | 60.75 (36.15–94.27) | 31.55 (16.92–54.85) | <0.001 |
| HbA1c (%) | 5.85 (5.50–6.12) | 5.60 (5.30–5.80) | <0.001 | 5.8 (5.50–6.10) | 5.50 (5.20–5.70) | <0.001 |
| HOMA-IR | 2.13 (1.40–5.30) | 1.82 (1.10–4.12) | 0.196 | 2.98 (1.77–4.86) | 1.53 (1.02–2.25) | <0.001 |
| Baseline | Post-Intervention | |||||
|---|---|---|---|---|---|---|
| IHypergly (n = 86) Median (IQR) | wIHypergly (n = 115) Median (IQR) | p | IHypergly (n = 86) Median (IQR) | wIHypergly (n = 115) Median (IQR) | p | |
| Energy (Kcals) | 1836.82 (1489.44–2330.01) | 2008.68 (1623.69–2396.22) | 0.237 | 1694.83 (1370.53–2183.88) | 1788.55 (1406.18–2307.81) | 0.374 |
| Proteins (% TE) | 13.89 (12.76–15.55) | 14.31 (13.32–15.81) | 0.196 | 18.45 (15.59–22.93) | 17.93 (14.46–21.35) | 0.359 |
| Carbohydrates (% TE) * | 50.94 ± 6.79 | 50.36 ± 6.78 | 0.550 | 47.84 ± 10.57 | 51.06 ± 10.32 | 0.032 |
| Fats (% TE) * | 36.41 ± 5.67 | 37 ± 6.36 | 0.496 | 33.08 ± 11.84 | 30.83 ± 9.74 | 0.105 |
| Sugary foods (more than once a week), n (%) ^ | 36 (41.9) | 32 (27.8) | 0.037 | ___ | ___ | ___ |
| DAQs, 0 (very poor quality) to 5 (high quality) ^ | 3 (2–4.25) | 4 (3–5) | 0.131 | 2 (1–3) | 2 (1–3) | 0.401 |
| Baseline | Post-Intervention | |||||
|---|---|---|---|---|---|---|
| IHypergly (n = 86) Median (IQR) | wIHypergly (n = 115) Median (IQR) | p | IHypergly (n = 86) Median (IQR) | wIHypergly (n = 115) Median (IQR) | p | |
| Reduced glutathione (GSH) (µM) | 11.93 (10.50–15.08) | 11.45 (8.83–13.83) | 0.018 | 14.52 (12.85–16.21) | 12.17 (8.71–14.55) | <0.001 |
| Malondialdehyde (MDA) (nmol) | 35.81 (33.77–39.28) | 34.69 (13.57–37.96) | 0.001 | 11.63 (10.81–38.98) | 40.51 (36.94–55.10) | <0.001 |
| Antioxidant capacity (DPPH) (%) | 39.86 (33.29–46.41) | 40.71 (32.62–49.22) | 0.500 | 41.98 (36.37–52.91) | 40.00 (33.90–46.38) | 0.007 |
| Carbonylated proteins (nmol/mL) | 34.31 (28.97–40.96) | 31.59 (27.04–36.59) | 0.033 | 29.54 (24.43–33.92) | 30.68 (26.59–34.54) | 0.096 |
| Adiponectin (pg/mL) | 3993.77 (3371.17–4334.83) | 3620.53 (2746.84–4054.90) | 0.001 | 3133.03 (2805.04–3369.43) | 2868.38 (2570.18–3183.28) | 0.001 |
| RR | 95% CI | p | |
|---|---|---|---|
| Pregestational BMI (kg/m2) | 1.085 | 1.015–1.161 | 0.017 |
| HbA1c (%) | 3.103 | 1.550–6.213 | 0.001 |
| Malondialdehyde (nmol) | 1.033 | 1.006–1.060 | 0.015 |
| DAQs | 0.776 | 0.607–0.991 | 0.042 |
| Sugary foods (more than once a week) | 2.221 | 1.069–4.615 | 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Martínez, M.L.; Gómez-Díaz, R.A.; Valdez González, A.L.; Ángeles Mejía, S.; Mondragón González, R.; Díaz Flores, M.; Saldaña Espinoza, R.C.; Ramírez-García, L.A.; Díaz Velázquez, M.F.; Wacher, N.H. Association of Oxidative Stress Markers with Incident Hyperglycemia in Gestational Diabetes Mellitus in an Educational Intervention. Nutrients 2025, 17, 680. https://doi.org/10.3390/nu17040680
Ruiz-Martínez ML, Gómez-Díaz RA, Valdez González AL, Ángeles Mejía S, Mondragón González R, Díaz Flores M, Saldaña Espinoza RC, Ramírez-García LA, Díaz Velázquez MF, Wacher NH. Association of Oxidative Stress Markers with Incident Hyperglycemia in Gestational Diabetes Mellitus in an Educational Intervention. Nutrients. 2025; 17(4):680. https://doi.org/10.3390/nu17040680
Chicago/Turabian StyleRuiz-Martínez, Mónica L., Rita A. Gómez-Díaz, Adriana Leticia Valdez González, Selene Ángeles Mejía, Rafael Mondragón González, Margarita Díaz Flores, Ricardo César Saldaña Espinoza, Luz Angélica Ramírez-García, Mary Flor Díaz Velázquez, and Niels H. Wacher. 2025. "Association of Oxidative Stress Markers with Incident Hyperglycemia in Gestational Diabetes Mellitus in an Educational Intervention" Nutrients 17, no. 4: 680. https://doi.org/10.3390/nu17040680
APA StyleRuiz-Martínez, M. L., Gómez-Díaz, R. A., Valdez González, A. L., Ángeles Mejía, S., Mondragón González, R., Díaz Flores, M., Saldaña Espinoza, R. C., Ramírez-García, L. A., Díaz Velázquez, M. F., & Wacher, N. H. (2025). Association of Oxidative Stress Markers with Incident Hyperglycemia in Gestational Diabetes Mellitus in an Educational Intervention. Nutrients, 17(4), 680. https://doi.org/10.3390/nu17040680






