Anticancer and Antioxidant Effects of Bioactive Peptides from Black Soldier Fly Larvae (Hermetia illucens)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Alkali-Soluble BSFL Protein
2.3. Preparation of ASBP-H
2.3.1. Optimization of ASBP-H Conditions Using Antioxidant Assays
2.3.2. Isolation of ASBP-AH
2.4. Characterization of ASBP and ASBP-H
2.4.1. Nutritional Composition Using Proximate Analysis
2.4.2. Amino Acid Analysis
2.4.3. Determination of ASBP and ASBP-H Pattern
2.4.4. Determination of Degree of Hydrolysis (DH)
2.5. Biological Activities of ASBP-AH and Its Fractions
2.5.1. Determination of Antioxidant Activity via Colorimetric Techniques
2.5.2. Determination of Antimutagenicity Using the Salmonella Mutation Assay
2.5.3. Determination of Anti-Inflammatory Activities in Murine Macrophages
2.5.4. Evaluation of Cytotoxicity
2.6. Microarray Analysis
2.7. Western Blot Analysis
2.8. The Identification of Peptide Sequences Using LC-MS/MS
2.9. De Novo Peptide Sequencing
2.10. Statistical Analysis
3. Results
3.1. ASBP Extracted from BSFL
3.2. Optimization of the Enzymatic Hydrolysis for ASBP Peptide Production
3.3. Antioxidant and Anti-Inflammation of ASBP-AH Peptide Fractions
3.4. Antimutagenicity Activity of ASBP-AH Peptide Fractions
3.5. Anticancer Activity of ASBP-AH Fractions in Colon Cancer Cell Lines
3.6. Microarray Analysis in ASBP-AH3-Treated COLO205 Cells
3.7. Amino Acid Compositions of ASBP-AH Fractions
3.8. Characterization of Peptides Derived from ASBP-AH3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Putra, S.N.K.M.; Ishak, N.H.; Sarbon, N.M. Preparation and characterization of physicochemical properties of golden apple snail (Pomacea canaliculata) protein hydrolysate as affected by different proteases. Biocatal. Agric. Biotechnol. 2018, 13, 123–128. [Google Scholar] [CrossRef]
- Mora, L.; Toldrá, F. Advanced enzymatic hydrolysis of food proteins for the production of bioactive peptides. Curr. Opin. Food Sci. 2023, 49, 100973. [Google Scholar] [CrossRef]
- Xu, X.; Qiao, Y.; Shi, B.; Dia, V.P. Alcalase and bromelain hydrolysis affected physicochemical and functional properties and biological activities of legume proteins. Food Struct. 2021, 27, 100178. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Zhang, W.; Li, X. Bioactive peptides for anticancer therapies. Biomater. Transl. 2023, 4, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application (Review). Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Zhu, Q.; Ding, J.; Zhao, L.; Zhang, F.; Lu, J.; Feng, Y.; Wang, J.; Liu, Z.; Kuang, M.; et al. Bioactive peptide relieves glucocorticoid-induced osteoporosis by giant macrocyclic encapsulation. J. Control Release 2024, 369, 75–87. [Google Scholar] [CrossRef]
- Xue, Z.; Wen, H.; Zhai, L.; Yu, Y.; Li, Y.; Yu, W.; Cheng, A.; Wang, C.; Kou, X. Antioxidant activity and anti-proliferative effect of a bioactive peptide from chickpea (Cicer arietinum L.). Food Res. Int. 2015, 77, 75–81. [Google Scholar] [CrossRef]
- Ortiz-Martinez, M.; Gonzalez de Mejia, E.; García-Lara, S.; Aguilar, O.; Lopez-Castillo, L.M.; Otero-Pappatheodorou, J.T. Antiproliferative effect of peptide fractions isolated from a quality protein maize, a white hybrid maize, and their derived peptides on hepatocarcinoma human HepG2 cells. J. Funct. Foods 2017, 34, 36–48. [Google Scholar] [CrossRef]
- Kuerban, A.; Al-Ghafari, A.; Alghamdi, S.; Quadri Syed, F.; Mirza, M.; Mohammed, F.; Abulnaja, K.; Alshaibi, H.; Alsufiani, H.; Kumosani, T.; et al. Potential antiglycation, antioxidant and antiproliferative activities of Vicia faba peptides. J. Food Meas. Charact. 2020, 14, 2155–2162. [Google Scholar] [CrossRef]
- Wattayagorn, V.; Kongsema, M.; Tadakittisarn, S.; Chumnanpuen, P. Riceberry Rice Bran Protein Hydrolyzed Fractions Induced Apoptosis, Senescence and G1/S Cell Cycle Arrest in Human Colon Cancer Cell Lines. Appl. Sci. 2022, 12, 6917. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.; et al. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef]
- Zhu, Y.; Lao, F.; Pan, X.; Wu, J. Food Protein-Derived Antioxidant Peptides: Molecular Mechanism, Stability and Bioavailability. Biomolecules 2022, 12, 1622. [Google Scholar] [CrossRef]
- FAO. Edible Insects: Future Prospects for Food and Feed Security; FAO: Rome, Italy, 2013. [Google Scholar]
- Cermeño, M.; Bascón, C.; Amigo-Benavent, M.; Felix, M.; FitzGerald, R.J. Identification of peptides from edible silkworm pupae (Bombyx mori) protein hydrolysates with antioxidant activity. J. Funct. Foods 2022, 92, 105052. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Guo, J.; Zheng, Y.; Duan, H.; Liu, G.; Yan, W. Silkworm pupa protein-derived peptides alleviate LPS-induced inflammatory response in RAW264.7 macrophage cells through the NF-κB/MAPK/PI3K-AKT signaling pathway. J. Agric. Food Res. 2024, 16, 101165. [Google Scholar] [CrossRef]
- Singh, S.; Bhat, H.F.; Kumar, S.; Lone, A.B.; Aadil, R.M.; Aït-Kaddour, A.; Hassoun, A.; Proestos, C.; Bhat, Z.F. Ultrasonication and microwave pre-treated locust protein hydrolysates enhanced the storage stability of meat emulsion. Ultrason. Sonochem 2023, 98, 106482. [Google Scholar] [CrossRef]
- Singh, S.; Bhat, H.F.; Kumar, S.; Manzoor, M.; Lone, A.B.; Verma, P.K.; Aadil, R.M.; Papastavropoulou, K.; Proestos, C.; Bhat, Z.F. Locust protein hydrolysates have the potential to enhance the storage stability of cheese. Curr. Res. Food Sci. 2023, 7, 100561. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, H.; Chen, Y.; Lang, M.; Chen, Y.; Shi, L. Silkworm Pupa Protein Hydrolysate Induces Mitochondria-Dependent Apoptosis and S Phase Cell Cycle Arrest in Human Gastric Cancer SGC-7901 Cells. Int. J. Mol. Sci. 2018, 19, 1013. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Wolf, D.; Gutzeit, H. The black soldier fly, Hermetia illucens—A promising source for sustainable production of proteins, lipids and bioactive substances. Z. Für Naturforschung C 2017, 72, 351–363. [Google Scholar] [CrossRef]
- Souza, T.; Suleria, H. Black soldier fly larvae (Hermetica illucens) as a sustainable source of nutritive and bioactive compounds, and their consumption challenges. Anim. Prod. Sci. 2023, 64, AN23192. [Google Scholar] [CrossRef]
- Zhu, D.; Huang, X.; Tu, F.; Wang, C.; Yang, F. Preparation, antioxidant activity evaluation, and identification of antioxidant peptide from black soldier fly (Hermetia illucens L.) larvae. J. Food Biochem. 2020, 44, e13186. [Google Scholar] [CrossRef] [PubMed]
- Firmansyah, M.; Abduh, M.Y. Production of protein hydrolysate containing antioxidant activity from Hermetia illucens. Heliyon 2019, 5, e02005. [Google Scholar] [CrossRef] [PubMed]
- Pimchan, T.; Hamzeh, A.; Siringan, P.; Thumanu, K.; Hanboonsong, Y.; Yongsawatdigul, J. Antibacterial peptides from black soldier fly (Hermetia illucens) larvae: Mode of action and characterization. Sci. Rep. 2024, 14, 26469. [Google Scholar] [CrossRef] [PubMed]
- Latimer, G.W., Jr. (Ed.) Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Janssen, R.H.; Vincken, J.P.; van den Broek, L.A.; Fogliano, V.; Lakemond, C.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Iwai, K.; Aito-Inoue, M. Identification of food-derived bioactive peptides in blood and other biological samples. J. AOAC Int. 2008, 91, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, F.; Moyano, F.; Díaz-López, M. Use of SDS-page in the assessment of protein hydrolysis by fish digestive enzymes. Aquac. Int. 2002, 9, 255–267. [Google Scholar] [CrossRef]
- Setthaya, P.; Jaturasitha, S.; Ketnawa, S.; Chaiyaso, T.; Sato, K.; Wongpoomchai, R. Influence of Commercial Protease and Drying Process on Antioxidant and Physicochemical Properties of Chicken Breast Protein Hydrolysates. Foods 2021, 10, 2994. [Google Scholar] [CrossRef]
- Dantas, F.G.d.S.; Castilho, P.F.d.; Almeida-Apolonio, A.A.d.; Araújo, R.P.d.; Oliveira, K.M.P.d. Mutagenic potential of medicinal plants evaluated by the Ames Salmonella/microsome assay: A systematic review. Mutat. Res.-Rev. Mutat. Res. 2020, 786, 108338. [Google Scholar] [CrossRef]
- Guo, H.; Chariyakornkul, A.; Phannasorn, W.; Mahatheeranont, S.; Wongpoomchai, R. Phytochemical Profile and Chemopreventive Properties of Cooked Glutinous Purple Rice Extracts Using Cell-Based Assays and Rat Model. Foods 2022, 11, 2333. [Google Scholar] [CrossRef]
- Vachiraarunwong, A.; Gi, M.; Kiyono, T.; Suzuki, S.; Fujioka, M.; Qiu, G.; Guo, R.; Yamamoto, T.; Kakehashi, A.; Shiota, M.; et al. Characterizing the toxicological responses to inorganic arsenicals and their metabolites in immortalized human bladder epithelial cells. Arch. Toxicol. 2024, 98, 2065–2084. [Google Scholar] [CrossRef]
- Okuno, T.; Gi, M.; Fujioka, M.; Yukimatu, N.; Kakehashi, A.; Takeuchi, A.; Endo, G.; Endo, Y.; Wanibuchi, H. Acetoaceto-o-Toluidide Enhances Cellular Proliferative Activity in the Urinary Bladder of Rats. Toxicol. Sci. 2019, 169, 456–464. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L.; Dossena, A.; Sforza, S. Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res. Int. 2017, 105, 812–820. [Google Scholar] [CrossRef]
- Nielsen, P.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Cho, H.-R.; Lee, S.-O. Novel hepatoprotective peptides derived from protein hydrolysates of mealworm (Tenebrio molitor). Food Res. Int. 2020, 133, 109194. [Google Scholar] [CrossRef] [PubMed]
- Karami, Z.; Akbari-Adergani, B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Fashakin, O.O.; Tangjaidee, P.; Unban, K.; Klangpetch, W.; Khumsap, T.; Sringarm, K.; Rawdkuen, S.; Phongthai, S. Isolation and Identification of Antioxidant Peptides Derived from Cricket (Gryllus bimaculatus) Protein Fractions. Insects 2023, 14, 674. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.B.; Wang, X.F.; Wang, H.Y.; Liu, Y.; Chen, Y. Studies on mechanism of action of anticancer peptides by modulation of hydrophobicity within a defined structural framework. Mol. Cancer Ther. 2011, 10, 416–426. [Google Scholar] [CrossRef]
- Ghadiri, N.; Javidan, M.; Sheikhi, S.; Taştan, Ö.; Parodi, A.; Liao, Z.; Tayybi Azar, M.; Ganjalıkhani-Hakemi, M. Bioactive peptides: An alternative therapeutic approach for cancer management. Front. Immunol. 2024, 15, 1310443. [Google Scholar] [CrossRef] [PubMed]
- Zozo, B.; Wicht, M.; Mshayisa, V.; Wyk, J. The Nutritional Quality and Structural Analysis of Black Soldier Fly Larvae Flour before and after Defatting. Insects 2022, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Xavier, A.A.O.; Mercadante, A.Z.; Garrido-Fernández, J.; Pérez-Gálvez, A. Fat content affects bioaccessibility and efficiency of enzymatic hydrolysis of lutein esters added to milk and yogurt. Food Res. Int. 2014, 65, 171–176. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.-B.; Shim, J.-H.; Abd El-Aty, A.M. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides From Food and By-Products: A Review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef]
- Islam, M.; Huang, Y.; Islam, S.; Fan, B.; Tong, L.; Wang, F. Influence of the Degree of Hydrolysis on Functional Properties and Antioxidant Activity of Enzymatic Soybean Protein Hydrolysates. Molecules 2022, 27, 6110. [Google Scholar] [CrossRef]
- Merz, M.; Eisele, T.; Berends, P.; Appel, D.; Rabe, S.; Blank, I.; Stressler, T.; Fischer, L. Flavourzyme, an Enzyme Preparation with Industrial Relevance: Automated Nine-Step Purification and Partial Characterization of Eight Enzymes. J. Agric. Food Chem. 2015, 63, 5682–5693. [Google Scholar] [CrossRef] [PubMed]
- Vogelsang-O’Dwyer, M.; Sahin, A.W.; Arendt, E.K.; Zannini, E. Enzymatic Hydrolysis of Pulse Proteins as a Tool to Improve Techno-Functional Properties. Foods 2022, 11, 1307. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gao, P.; Zhang, Y.; Wang, X.; Lu, S.; Yue, C.; Bai, C.; Wu, W.; Zhang, Y.; Zhao, Z. Measurement of degree of hydrolysis and molecular weight distribution of protein hydrolysates by liquid chromatography-mass spectrometry. Talanta 2024, 268, 125347. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A. Production of bioactive peptides using enzymatic hydrolysis and identification antioxidative peptides from patin (Pangasius sutchi) sarcoplasmic protein hydolysate. J. Funct. Foods 2014, 9, 280–289. [Google Scholar] [CrossRef]
- Rivera-Jiménez, J.; Berraquero-García, C.; Pérez-Gálvez, R.; García-Moreno, P.J.; Espejo-Carpio, F.J.; Guadix, A.; Guadix, E.M. Peptides and protein hydrolysates exhibiting anti-inflammatory activity: Sources, structural features and modulation mechanisms. Food Funct. 2022, 13, 12510–12540. [Google Scholar] [CrossRef] [PubMed]
- Dixon, K.; Kopras, E. Genetic alterations and DNA repair in human carcinogenesis. Semin. Cancer Biol. 2004, 14, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Neudecker, T.; Henschler, D. Mutagenicity of chloroolefins in the Salmonella/mammalian microsome test: III. Metabolic activation of the allylic chloropropenes allyl chloride, 1,3-dichloropropene, 2,3-dichloro-1-propene, 1,2,3-trichloropropene, 1,1,2,3-tetrachloro-2-propene and hexachloropropene by S9 mix via two different metabolic pathways. Mutat. Res./Genet. Toxicol. 1986, 170, 1–9. [Google Scholar] [CrossRef]
- Jumeri; Kim, S.M. Antioxidant and anticancer activities of enzymatic hydrolysates of solitary tunicate (Styela clava). Food Sci. Biotechnol. 2011, 20, 1075–1085. [Google Scholar] [CrossRef]
- Ghaly, G.; Tallima, H.; Dabbish, E.; Badr ElDin, N.; Abd El-Rahman, M.K.; Ibrahim, M.A.A.; Shoeib, T. Anti-Cancer Peptides: Status and Future Prospects. Molecules 2023, 28, 1148. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.Y.; Tseng, Y.J.; Kao, H.J.; Chen, C.H.; Yang, H.H.; Weng, S.L. Identification of subtypes of anticancer peptides based on sequential features and physicochemical properties. Sci. Rep. 2021, 11, 13594. [Google Scholar] [CrossRef]
- Shamova, O.; Orlov, D.; Stegemann, C.; Czihal, P.; Hoffmann, R.; Brogden, K.; Kolodkin, N.; Sakuta, G.; Tossi, A.; Sahl, H.-G.; et al. ChBac3.4: A Novel Proline-Rich Antimicrobial Peptide from Goat Leukocytes. Int. J. Pept. Res. Ther. 2009, 15, 31–42. [Google Scholar] [CrossRef]
- Jobin, M.L.; Blanchet, M.; Henry, S.; Chaignepain, S.; Manigand, C.; Castano, S.; Lecomte, S.; Burlina, F.; Sagan, S.; Alves, I.D. The role of tryptophans on the cellular uptake and membrane interaction of arginine-rich cell penetrating peptides. Biochim. Biophys. Acta 2015, 1848, 593–602. [Google Scholar] [CrossRef]
- Sarah, R.D.; Michelle, W.; Frederick, H.; David, A.P. Anticancer α-Helical Peptides and Structure/Function Relationships Underpinning Their Interactions with Tumour Cell Membranes. Curr. Protein Pept. Sci. 2006, 7, 487–499. [Google Scholar] [CrossRef]
- Dai, Y.; Cai, X.; Shi, W.; Bi, X.; Su, X.; Pan, M.; Li, H.; Lin, H.; Huang, W.; Qian, H. Pro-apoptotic cationic host defense peptides rich in lysine or arginine to reverse drug resistance by disrupting tumor cell membrane. Amino Acids 2017, 49, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Sinpru, B.; Sroichak, T.; Archa, P.; Thongpea, S.; Paengkoum, S.; Purba, R.A.P.; et al. Nutritional Composition of Black Soldier Fly Larvae (Hermetia illucens L.) and Its Potential Uses as Alternative Protein Sources in Animal Diets: A Review. Insects 2022, 13, 831. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, X.; Li, M.; Gong, G.; Liu, W.; Li, T.; Zuo, H.; Li, W.; Gao, F.; Liu, H. Cdh1-mediated Skp2 degradation by dioscin reprogrammes aerobic glycolysis and inhibits colorectal cancer cells growth. EBioMedicine 2020, 51, 102570. [Google Scholar] [CrossRef]
- William, J.N.G.; Dhar, R.; Gundamaraju, R.; Sahoo, O.S.; Pethusamy, K.; Raj, A.; Ramasamy, S.; Alqahtani, M.S.; Abbas, M.; Karmakar, S. SKping cell cycle regulation: Role of ubiquitin ligase SKP2 in hematological malignancies. Front. Oncol. 2024, 14, 1288501. [Google Scholar] [CrossRef]
- Alao, J.P. The regulation of cyclin D1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol. Cancer 2007, 6, 24. [Google Scholar] [CrossRef] [PubMed]
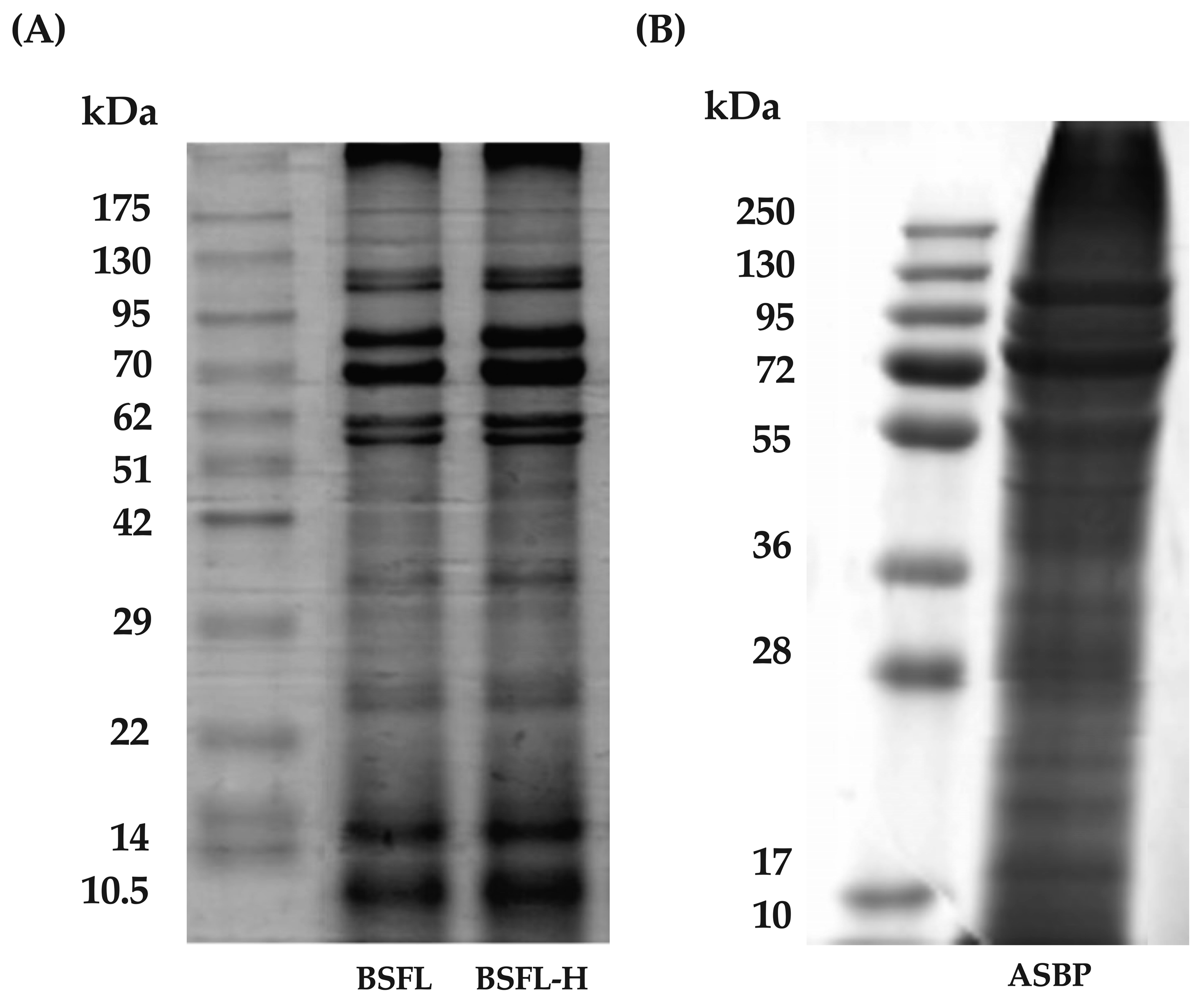

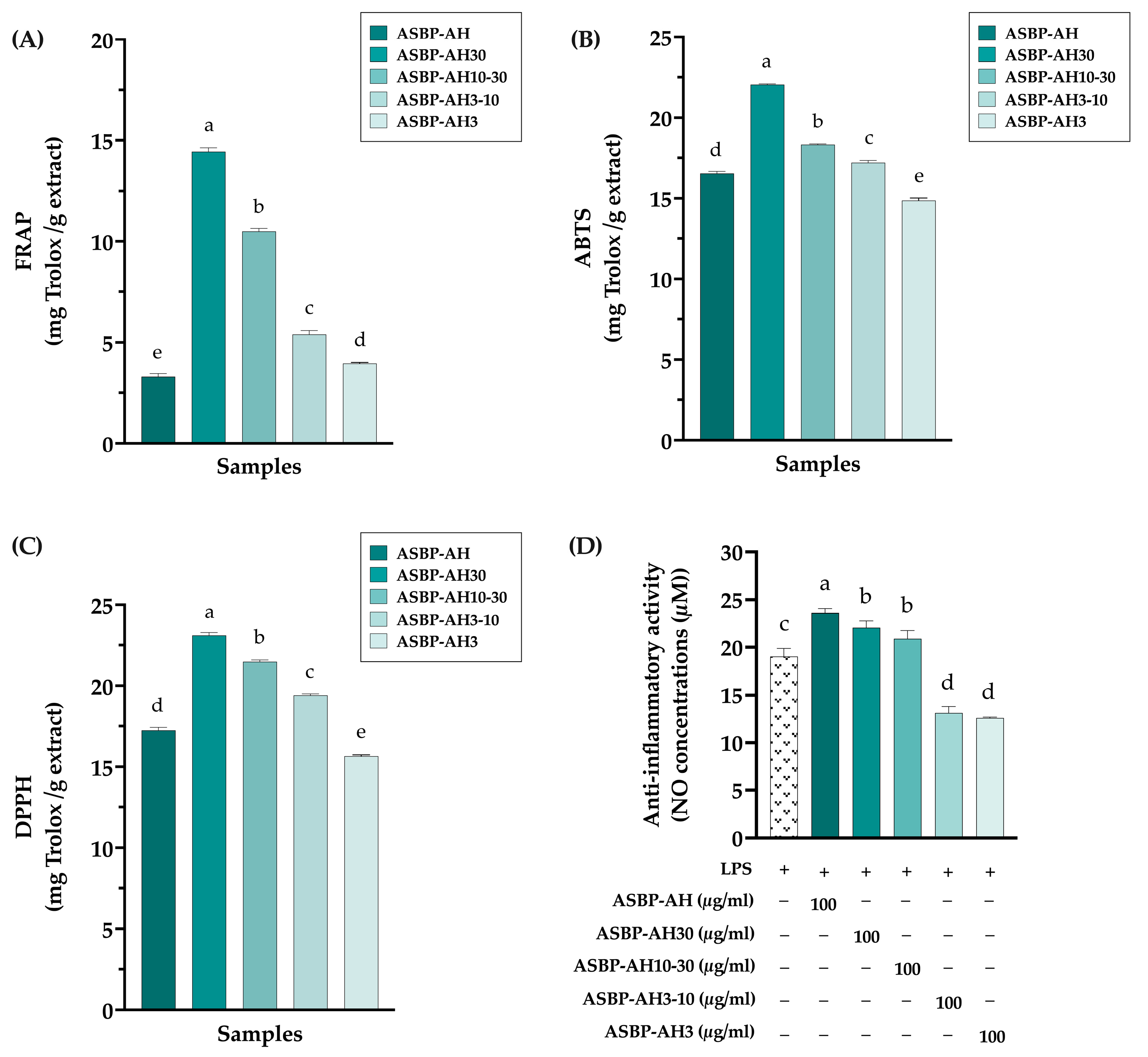


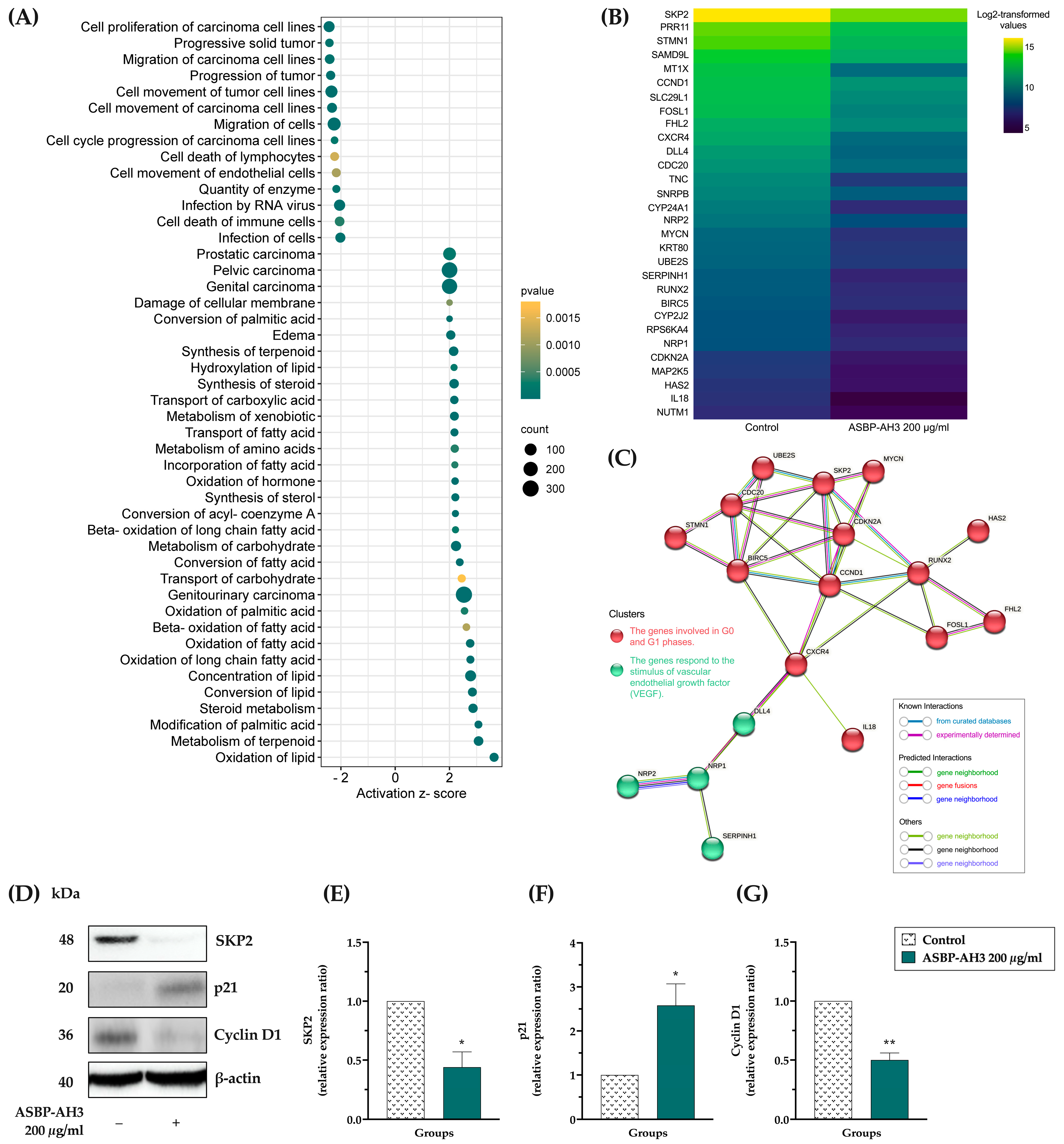
| Samples | Proximate Compositions of BSFL (%) | |||
|---|---|---|---|---|
| Crude Protein | Fat | Ash | Moisture | |
| BSFL | 55.45 ± 0.02 c | 18.45 ± 0.10 a | 7.77 ± 0.63 b | 5.62 ± 0.03 c |
| BSFL-H | 64.53 ± 0.31 b | 6.66 ± 0.52 b | 8.90 ± 0.44 a | 10.31 ± 0.08 a |
| ASBP | 77.26 ± 0.03 a | 2.21 ± 0.20 c | 9.35 ± 0.05 a | 8.48 ± 0.16 c |
| Amino Acid Groups | Amino Acid Compositions (% Amino Acid/g Sample) | ||||
|---|---|---|---|---|---|
| ASBP-AH | ASBP-AH30 | ASBP-AH10-30 | ASBP-AH3-10 | ASBP-AH3 | |
| Positively Charged Side-Chain Amino Acid | |||||
| Arginine (Arg) | 1.92 ± 0.04 a | 1.96 ± 0.47 a | 1.92 ± 0.39 a | 1.87 ± 0.33 a | 1.54 ± 0.12 a |
| Histidine (His) | 2.02 ± 0.23 a | 2.15 ± 0.10 a | 2.13 ± 0.12 a | 2.13 ± 0.20 a | 2.22 ± 0.01 a |
| Lysine (Lys) | 6.05 ± 0.91 a | 5.70 ± 0.16 a | 6.22 ± 0.50 a | 5.52 ± 0.89 a | 5.59 ± 0.24 a |
| Negatively Charged Side-Chain Amino Acid | |||||
| Asparagine (Asp) | 13.00 ± 5.26 a | 12.12 ± 1.66 a | 12.08 ± 1.95 a | 12.30 ± 2.88 a | 8.98 ± 1.63 a |
| Glutamic acid (Glu) | 14.61 ± 1.01 a | 10.53 ± 0.46 a | 10.64 ± 0.50 a | 12.84 ± 4.68 a | 11.08 ± 1.27 a |
| Polar Uncharged Side-Chain Amino Acid | |||||
| Serine (Ser) | 6.32 ± 0.67 a | 5.77 ± 0.88 a | 5.63 ± 0.45 a | 6.27 ± 0.24 a | 5.22 ± 0.39 a |
| Threonine (Thr) | 3.56 ± 0.36 a | 3.25 ± 0.41 a | 3.39 ± 0.28 a | 3.27 ± 0.60 a | 3.61 ± 0.32 a |
| Hydroxyproline (Hyp) | 0.29 ± 0.09 a | 0.14 ± 0.14 ab | 0.17 ± 0.09 ab | 0.10 ± 0.06 b | 0.10 ± 0.07 b |
| Hydrophobic Amino Acid | |||||
| Alanine (Ala) | 9.37 ± 0.98 b | 12.32 ± 1.50 ab | 12.11 ± 1.68 ab | 11.60 ± 2.72 ab | 15.59 ± 2.79 a |
| Methionine (Met) | 2.66 ± 0.42 a | 2.19 ± 0.87 a | 2.38 ± 0.69 a | 1.98 ± 0.24 a | 2.23 ± 0.54 a |
| Glycine (Gly) | 8.37 ± 1.81 a | 8.70 ± 1.40 a | 8.79 ± 1.27 a | 8.70 ± 0.80 a | 9.47 ± 0.52 a |
| Proline (Pro) | 6.19 ± 0.83 a | 6.29 ± 1.07 a | 7.24 ± 1.51 a | 7.48 ± 1.19 a | 6.81 ± 0.17 a |
| BCAA | |||||
| Valine (Val) | 6.02 ± 0.31 a | 6.68 ± 0.20 a | 6.42 ± 0.04 a | 6.15 ± 0.37 a | 6.36 ± 0.67 a |
| Isoleucine (Ile) | 4.24 ± 0.19 a | 3.98 ± 0.56 a | 3.79 ± 0.60 a | 3.50 ± 0.03 a | 3.65 ± 0.42 a |
| Leucine (Leu) | 7.42 ± 0.59 a | 7.27 ± 1.16 a | 6.79 ± 1.01 a | 6.43 ± 0.39 a | 7.72 ± 0.57 a |
| Aromatic AA | |||||
| Phenylalanine (Phe) | 3.12 ± 0.35 a | 3.78 ± 0.56 a | 3.59 ± 0.42 a | 3.30 ± 0.25 a | 3.16 ± 0.17 a |
| Tyrosine (Tyr) | 4.86 ± 1.46 b | 7.18 ± 1.72 a | 6.71 ± 0.98 ab | 6.57 ± 0.35 ab | 6.67 ± 0.46 ab |
| EAA | 36.99 ± 1.42 a | 36.95 ± 1.79 a | 36.63 ± 1.39 a | 34.14 ± 2.81 a | 36.08 ± 1.37 a |
| Hydrophobic AA | 52.23 ± 4.67 b | 58.38 ± 0.68 ab | 57.82 ± 0.81 ab | 55.70 ± 5.85 ab | 61.67 ± 1.91 a |
| Aromatic AA | 7.98 ± 1.11 b | 10.95 ± 2.28 a | 10.30 ± 1.39 ab | 9.87 ± 0.60 ab | 9.84 ± 0.64 ab |
| BCAA | 17.67 ± 0.48 a | 17.93 ± 1.51 a | 17.00 ± 1.57 a | 16.07 ± 0.78 a | 17.74 ± 1.67 a |
| Peptides | Amino Acid Sequence | Mass (Da) | %Hydrophobic AA | %Positive Charge Side Chains AA |
|---|---|---|---|---|
| AKAKYK | Ala-Lys-Ala-Lys-Tyr-Lys | 707.4330 | 50% | 50% |
| GWWTKK | Gly-Trp-Trp-Thr-Lys-Lys | 804.4282 | 33% | 33% |
| TLVPVMDLK | Thr-Leu-Val-Phe-Val-Met-Asp-Leu-Lys | 1014.5832 | 67% | 11% |
| KNVSLVMPK | Lys-Asn-Val-Ser-Leu-Val-Met-Phe-Lys | 1014.5945 | 56% | 22% |
| QQQFDRKNK | Gln-Gln-Gln-Phe-Asp-Arg-Lys-Asn-Lys | 1190.6155 | 11% | 33% |
| YFMVLVVMLFHR | Tyr-Phe-Met-Val-Leu-Val-Val-Met-Leu-Phe-His-Arg | 1553.8301 | 83% | 17% |
| NEVKFVYR | Asn-Glu-Val-Lys-Phe-Val-Tyr-Arg | 1053.5608 | 50% | 25% |
| APLATHGMYK | Ala-Pro-Leu-Ala-Thr-His-Gly-Met-Tyr-Lys | 1087.5535 | 50% | 20% |
| FALSLLMMR | Phe-Ala-Leu-Ser-Lys-Lys-Met-Met-Arg | 1080.5823 | 56% | 33% |
| TGPVEDCAK | Thr-Gly-Pro-Val-Glu-Asp-Cys-Ala-Lys | 918.4131 | 22% | 11% |
| FYLPVTMWCDK | Phe-Tyr-Leu-Pro-Val-Thr-Met-Trp-Cys-Asp-Lys | 1401.6526 | 55% | 9% |
| VDPLLSNVALSAPLVR | Val-Asp-Pro-Lys-Lys-Ser-Asn-Val-Ala-Lys-Ser-Ala-Pro-Lys-Val-Arg | 1662.9668 | 31% | 31% |
| KDVGLTYFDFK | Lys-Asn-Val-Gly-Leu-Thr-Tyr-Phe-Asp-Phe-Lys | 1331.6760 | 45% | 18% |
| HALLTSER | His-Ala-Lys-Lys-Thr-Ser-Glu-Arg | 925.4981 | 13% | 50% |
| APLAYSTPLLK | Ala-Pro-Leu-Ala-Tyr-Ser-Thr-Pro-Leu-Leu-Lys | 1172.6804 | 55% | 9% |
| QSVNHK | Gln-Ser-Val-Asn-His-Lys | 711.3664 | 17% | 33% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Praseatsook, K.; Vachiraarunwong, A.; Taya, S.; Setthaya, P.; Sato, K.; Wanibuchi, H.; Wongpoomchai, R.; Dejkriengkraikul, P.; Gi, M.; Yodkeree, S. Anticancer and Antioxidant Effects of Bioactive Peptides from Black Soldier Fly Larvae (Hermetia illucens). Nutrients 2025, 17, 645. https://doi.org/10.3390/nu17040645
Praseatsook K, Vachiraarunwong A, Taya S, Setthaya P, Sato K, Wanibuchi H, Wongpoomchai R, Dejkriengkraikul P, Gi M, Yodkeree S. Anticancer and Antioxidant Effects of Bioactive Peptides from Black Soldier Fly Larvae (Hermetia illucens). Nutrients. 2025; 17(4):645. https://doi.org/10.3390/nu17040645
Chicago/Turabian StylePraseatsook, Kwanchanok, Arpamas Vachiraarunwong, Sirinya Taya, Phatthawin Setthaya, Kenji Sato, Hideki Wanibuchi, Rawiwan Wongpoomchai, Pornngarm Dejkriengkraikul, Min Gi, and Supachai Yodkeree. 2025. "Anticancer and Antioxidant Effects of Bioactive Peptides from Black Soldier Fly Larvae (Hermetia illucens)" Nutrients 17, no. 4: 645. https://doi.org/10.3390/nu17040645
APA StylePraseatsook, K., Vachiraarunwong, A., Taya, S., Setthaya, P., Sato, K., Wanibuchi, H., Wongpoomchai, R., Dejkriengkraikul, P., Gi, M., & Yodkeree, S. (2025). Anticancer and Antioxidant Effects of Bioactive Peptides from Black Soldier Fly Larvae (Hermetia illucens). Nutrients, 17(4), 645. https://doi.org/10.3390/nu17040645









