Associations of Cooking Salt Intake During Pregnancy with Low Birth Weight and Small for Gestational Age Newborns: A Large Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Population and Design
2.2. Salt Intake and Other Dietary Data
2.3. Outcome Ascertainment
2.4. Covariate Assessment
2.5. Statistical Analyses
2.6. Role of the Funding Source
3. Results
3.1. Basic Characteristics of Participants
3.2. Salt Intake and Birth Weight Traits
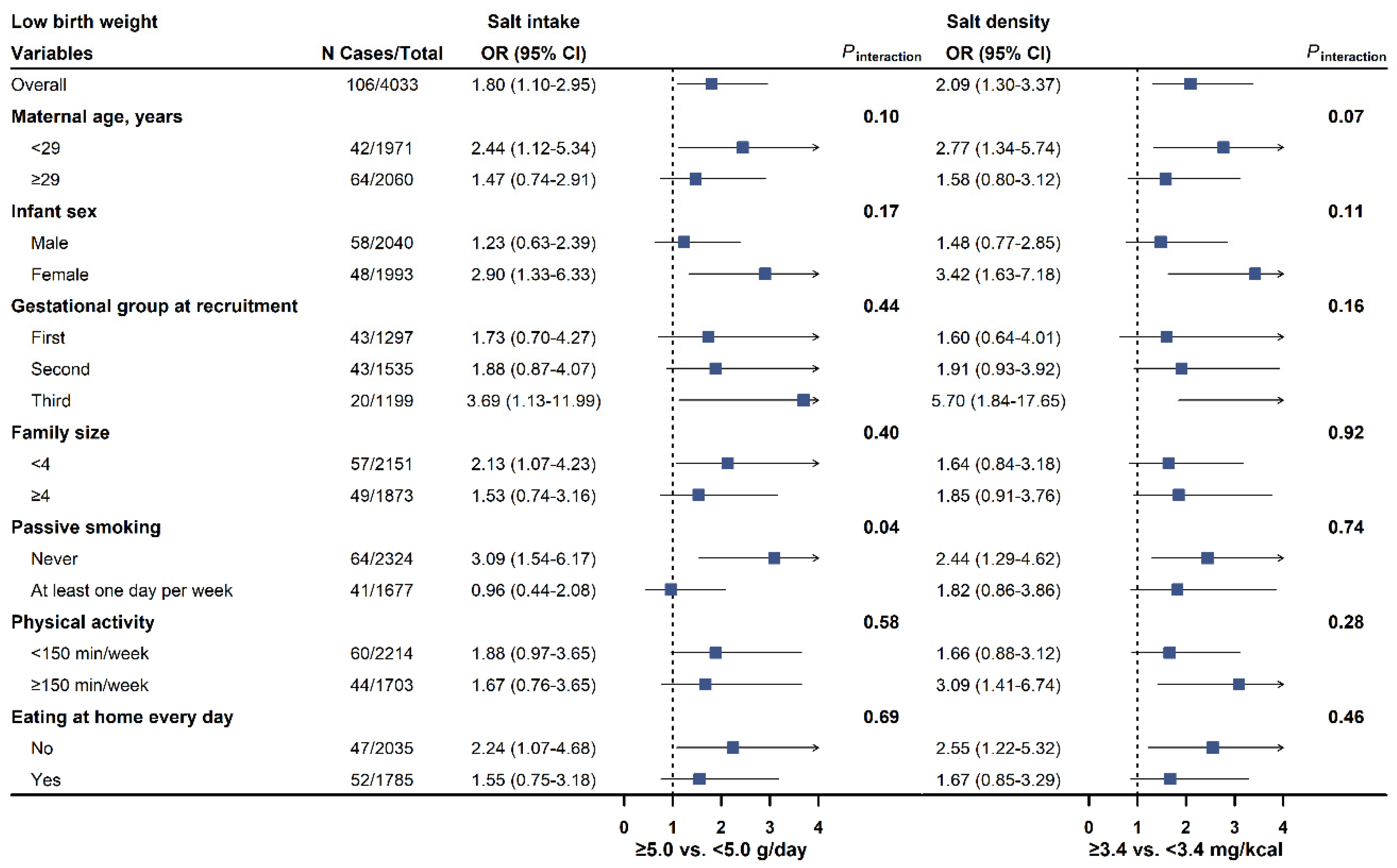
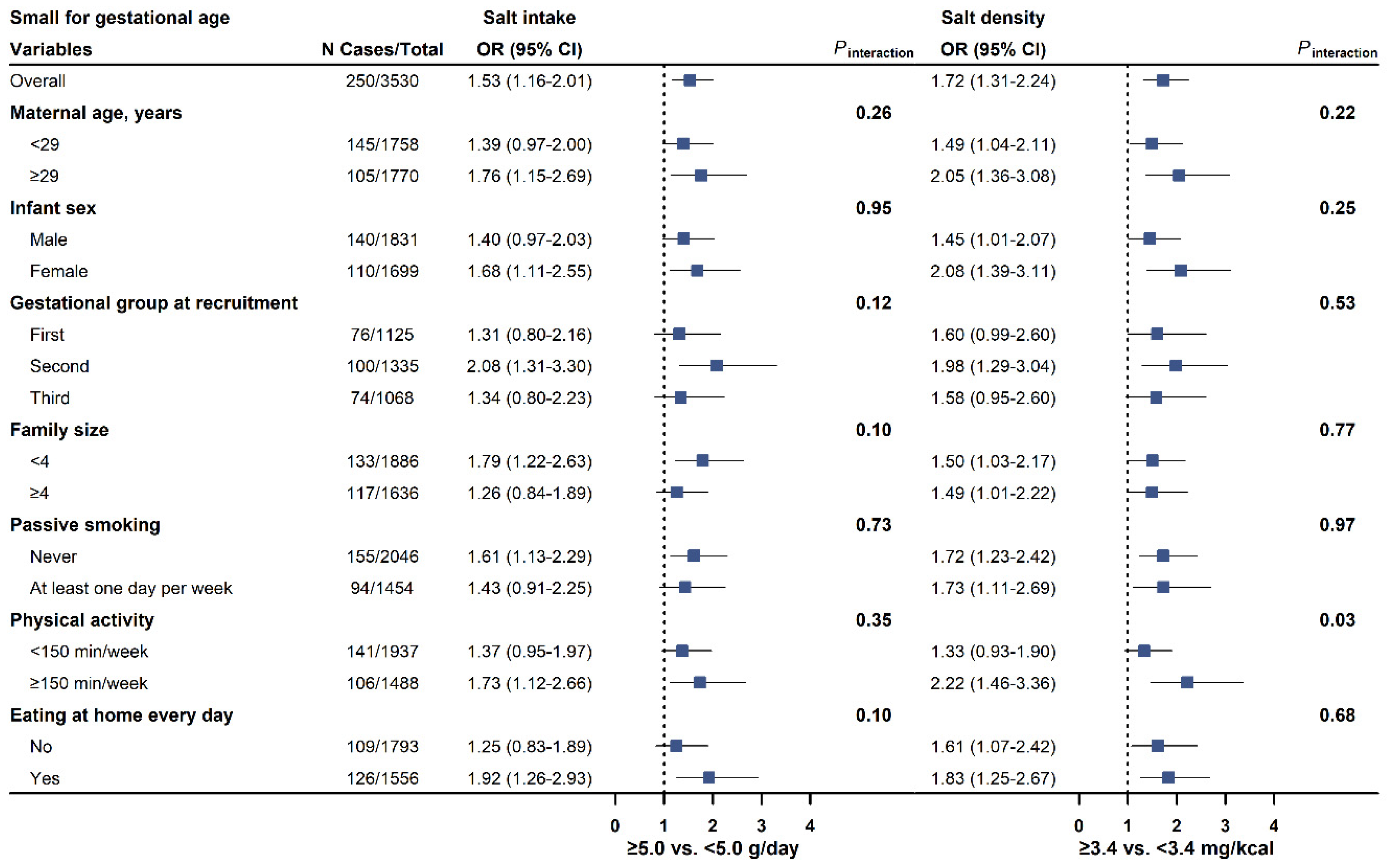
3.3. Subgroup Analyses
3.4. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Guidelines Approved by the Guidelines Review Committee. In Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- World Health Organization. WHO global report on sodium intake reduction. In WHO Global Report on Sodium Intake Reduction; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Reddin, C.; Ferguson, J.; Murphy, R.; Clarke, A.; Judge, C.; Griffith, V.; Alvarez, A.; Smyth, A.; Mente, A.; Yusuf, S.; et al. Global mean potassium intake: A systematic review and Bayesian meta-analysis. Eur. J. Nutr. 2023, 62, 2027–2037. [Google Scholar] [CrossRef] [PubMed]
- Birukov, A.; Andersen, L.B.; Herse, F.; Rakova, N.; Kitlen, G.; Kyhl, H.B.; Golic, M.; Haase, N.; Kräker, K.; Müller, D.N.; et al. Aldosterone, Salt, and Potassium Intakes as Predictors of Pregnancy Outcome, Including Preeclampsia. Hypertension 2019, 74, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Arvizu, M.; Bjerregaard, A.A.; Madsen, M.T.B.; Granström, C.; Halldorsson, T.I.; Olsen, S.F.; Gaskins, A.J.; Rich-Edwards, J.W.; Rosner, B.A.; Chavarro, J.E. Sodium Intake during Pregnancy, but Not Other Diet Recommendations Aimed at Preventing Cardiovascular Disease, Is Positively Related to Risk of Hypertensive Disorders of Pregnancy. J. Nutr. 2020, 150, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, Y.; Zhang, B.; Zheng, T.; Li, J.; Peng, Y.; Zhou, A.; Buka, S.L.; Liu, S.; Zhang, Y.; et al. Impact of the 2017 ACC/AHA Guideline for High Blood Pressure on Evaluating Gestational Hypertension-Associated Risks for Newborns and Mothers. Circ. Res. 2019, 125, 184–194. [Google Scholar] [CrossRef]
- O’Donnell, M.; Mente, A.; Rangarajan, S.; McQueen, M.J.; Wang, X.; Liu, L.; Yan, H.; Lee, S.F.; Mony, P.; Devanath, A.; et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N. Engl. J. Med. 2014, 371, 612–623. [Google Scholar] [CrossRef]
- Ma, Y.; He, F.J.; Sun, Q.; Yuan, C.; Kieneker, L.M.; Curhan, G.C.; MacGregor, G.A.; Bakker, S.J.L.; Campbell, N.R.C.; Wang, M.; et al. 24-Hour Urinary Sodium and Potassium Excretion and Cardiovascular Risk. N. Engl. J. Med. 2022, 386, 252–263. [Google Scholar] [CrossRef]
- Emmerik, N.E.; de Jong, F.; van Elburg, R.M. Dietary Intake of Sodium during Infancy and the Cardiovascular Consequences Later in Life: A Scoping Review. Ann. Nutr. Metab. 2020, 76, 114–121. [Google Scholar] [CrossRef]
- Ma, H.; Xue, Q.; Wang, X.; Li, X.; Franco, O.H.; Li, Y.; Heianza, Y.; Manson, J.E.; Qi, L. Adding salt to foods and hazard of premature mortality. Eur. Heart J. 2022, 43, 2878–2888. [Google Scholar] [CrossRef]
- Cook, N.R.; Appel, L.J.; Whelton, P.K. Sodium Intake and All-Cause Mortality Over 20 Years in the Trials of Hypertension Prevention. J. Am. Coll. Cardiol. 2016, 68, 1609–1617. [Google Scholar] [CrossRef]
- Ando, K. Proper Salt Intake During Pregnancy. Circ. J. Off. J. Jpn. Circ. Soc. 2016, 80, 2094–2095. [Google Scholar] [CrossRef]
- Inoue, M.; Tsuchihashi, T.; Hasuo, Y.; Ogawa, M.; Tominaga, M.; Arakawa, K.; Oishi, E.; Sakata, S.; Ohtsubo, T.; Matsumura, K.; et al. Salt Intake, Home Blood Pressure, and Perinatal Outcome in Pregnant Women. Circ. J. Off. J. Jpn. Circ. Soc. 2016, 80, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Q.; Lumbers, E.R.; Schumacher, T.L.; Collins, C.E.; Rae, K.M.; Pringle, K.G.; Gomeroi Gaaynggal Advisory, C. Maternal Diet Influences Fetal Growth but Not Fetal Kidney Volume in an Australian Indigenous Pregnancy Cohort. Nutrients 2021, 13, 569. [Google Scholar] [CrossRef] [PubMed]
- Bank, T.C.; Grasch, J.L.; Chung, J.; Mercer, B.M.; McNeil, R.B.; Parry, S.; Saade, G.; Shanks, A.; Silver, R.M.; Simhan, H.; et al. “Sodium intake and the development of hypertensive disorders of pregnancy”. Am. J. Obstet. Gynecol. MFM 2023, 5, 101166. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; Aubert, A.M.; Shivappa, N.; Bernard, J.Y.; Mensink-Bout, S.M.; Geraghty, A.A.; Mehegan, J.; Suderman, M.; Polanska, K.; Hanke, W.; et al. Associations of maternal dietary inflammatory potential and quality with offspring birth outcomes: An individual participant data pooled analysis of 7 European cohorts in the ALPHABET consortium. PLoS Med. 2021, 18, e1003491. [Google Scholar] [CrossRef]
- Mikeš, O.; Brantsæter, A.L.; Knutsen, H.K.; Torheim, L.E.; Bienertová Vašků, J.; Pruša, T.; Čupr, P.; Janák, K.; Dušek, L.; Klánová, J. Dietary patterns and birth outcomes in the ELSPAC pregnancy cohort. J. Epidemiol. Community Health 2022, 76, 613–619. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Zhao, Y.; Chen, F.; Mi, B.; Zhou, J.; Chen, Y.; Wang, D.; Pei, L. Geographical variations in maternal dietary patterns during pregnancy associated with birth weight in Shaanxi province, Northwestern China. PLoS ONE 2021, 16, e0254891. [Google Scholar] [CrossRef]
- Cook, N.R.; He, F.J.; MacGregor, G.A.; Graudal, N. Sodium and health-concordance and controversy. BMJ 2020, 369, m2440. [Google Scholar] [CrossRef]
- Tan, M.; He, F.J.; Wang, C.; MacGregor, G.A. Twenty-Four-Hour Urinary Sodium and Potassium Excretion in China: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2019, 8, e012923. [Google Scholar] [CrossRef]
- Anderson, C.A.; Appel, L.J.; Okuda, N.; Brown, I.J.; Chan, Q.; Zhao, L.; Ueshima, H.; Kesteloot, H.; Miura, K.; Curb, J.D.; et al. Dietary sources of sodium in China, Japan, the United Kingdom, and the United States, women and men aged 40 to 59 years: The INTERMAP study. J. Am. Diet Assoc. 2010, 110, 736–745. [Google Scholar] [CrossRef]
- Jachimowicz-Rogowska, K.; Winiarska-Mieczan, A. Initiatives to Reduce the Content of Sodium in Food Products and Meals and Improve the Population’s Health. Nutrients 2023, 15, 2393. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, S.; Cui, X.; Song, Q.; Shi, Z.; Su, J.; Zang, J. Effects of Dietary Patterns during Pregnancy on Preterm Birth: A Birth Cohort Study in Shanghai. Nutrients 2021, 13, 2367. [Google Scholar] [CrossRef]
- Zhuang, P.; Mao, L.; Wu, F.; Wang, J.; Jiao, J.; Zhang, Y. Cooking Oil Consumption Is Positively Associated with Risk of Type 2 Diabetes in a Chinese Nationwide Cohort Study. J. Nutr. 2020, 150, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Z.J. China Food Composition Tables Standard Edition; Peking University Medical Press: Beijing, China, 2018. [Google Scholar]
- Zong, X.N.; Li, H.; Zhang, Y.Q.; Wu, H.H.; Zhao, G.L.; Working Committee of the China National Newborn Growth Standards Project. Construction of China national newborn growth standards based on a large low-risk sample. Sci. Rep. 2021, 11, 16093. [Google Scholar] [CrossRef] [PubMed]
- Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [CrossRef]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Goñi, L.; Cuervo, M.; Santiago, S.; Zazpe, I.; Martínez, J.A. Parity implications for anthropometrical variables, lifestyle behaviors and dietary habits in pregnant women. An. Sist. Sanit. Navar. 2014, 37, 349–362. [Google Scholar] [CrossRef]
- White, I.R.; Royston, P.; Wood, A.M. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 2011, 30, 377–399. [Google Scholar] [CrossRef]
- Naska, A.; Lagiou, A.; Lagiou, P. Dietary assessment methods in epidemiological research: Current state of the art and future prospects. F1000Research 2017, 6, 926. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes for Sodium and Potassium. The National Academies Collection: Reports funded by National Institutes of Health. In Dietary Reference Intakes for Sodium and Potassium; Oria, M., Harrison, M., Stallings, V.A., Eds.; National Academies Press (US): Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- Lerchl, K.; Rakova, N.; Dahlmann, A.; Rauh, M.; Goller, U.; Basner, M.; Dinges, D.F.; Beck, L.; Agureev, A.; Larina, I.; et al. Agreement between 24-hour salt ingestion and sodium excretion in a controlled environment. Hypertension 2015, 66, 850–857. [Google Scholar] [CrossRef]
- Elijovich, F.; Weinberger, M.H.; Anderson, C.A.; Appel, L.J.; Bursztyn, M.; Cook, N.R.; Dart, R.A.; Newton-Cheh, C.H.; Sacks, F.M.; Laffer, C.L. Salt Sensitivity of Blood Pressure: A Scientific Statement From the American Heart Association. Hypertension 2016, 68, e7–e46. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Jeong, Y.S.; Koo, K.A.; Yang, J.I.; Park, Y.K. Maternal nutrition intervention focused on the adjustment of salt and sugar intake can improve pregnancy outcomes. Food Sci. Nutr. 2020, 8, 3900–3911. [Google Scholar] [CrossRef] [PubMed]
- Getaneh, T.; Negesse, A.; Dessie, G.; Desta, M. The impact of pregnancy induced hypertension on low birth weight in Ethiopia: Systematic review and meta-analysis. Ital. J. Pediatr. 2020, 46, 174. [Google Scholar] [CrossRef] [PubMed]
- Kyozuka, H.; Fukusda, T.; Murata, T.; Yamaguchi, A.; Kanno, A.; Yasuda, S.; Sato, A.; Ogata, Y.; Kuse, M.; Hosoya, M.; et al. Impact of preconception sodium intake on hypertensive disorders of pregnancy: The Japan Environment and Children’s study. Pregnancy Hypertens. 2021, 23, 66–72. [Google Scholar] [CrossRef]
- Nielsen, L.H.; Ovesen, P.; Hansen, M.R.; Brantlov, S.; Jespersen, B.; Bie, P.; Jensen, B.L. Changes in the renin-angiotensin-aldosterone system in response to dietary salt intake in normal and hypertensive pregnancy. A randomized trial. J. Am. Soc. Hypertens. JASH 2016, 10, 881–890.e884. [Google Scholar] [CrossRef]
- Gennari-Moser, C.; Khankin, E.V.; Schüller, S.; Escher, G.; Frey, B.M.; Portmann, C.B.; Baumann, M.U.; Lehmann, A.D.; Surbek, D.; Karumanchi, S.A.; et al. Regulation of placental growth by aldosterone and cortisol. Endocrinology 2011, 152, 263–271. [Google Scholar] [CrossRef]
- Todkar, A.; Di Chiara, M.; Loffing-Cueni, D.; Bettoni, C.; Mohaupt, M.; Loffing, J.; Wagner, C.A. Aldosterone deficiency adversely affects pregnancy outcome in mice. Pflug. Arch. Eur. J. Physiol. 2012, 464, 331–343. [Google Scholar] [CrossRef]
- Wang, X.; Ma, H.; Kou, M.; Tang, R.; Xue, Q.; Li, X.; Harlan, T.S.; Heianza, Y.; Qi, L. Dietary Sodium Intake and Risk of Incident Type 2 Diabetes. Mayo Clin. Proc. 2023, 464, 331–343. [Google Scholar] [CrossRef]
- Ye, W.; Luo, C.; Huang, J.; Li, C.; Liu, Z.; Liu, F. Gestational diabetes mellitus and adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ 2022, 377, e067946. [Google Scholar] [CrossRef]
- You, J. Dietary Change, Nutrient Transition and Food Security in Fast-Growing China; Edward Elgar Publishing: Cheltenham, UK, 2014; pp. 204–245. [Google Scholar]
- Du, S.; Batis, C.; Wang, H.; Zhang, B.; Zhang, J.; Popkin, B.M. Understanding the patterns and trends of sodium intake, potassium intake, and sodium to potassium ratio and their effect on hypertension in China. Am. J. Clin. Nutr. 2014, 99, 334–343. [Google Scholar] [CrossRef]
| Salt Intake | |||||
|---|---|---|---|---|---|
| Overall | <5.0 g/day | 5.0–10.0 g/day | ≥10.0 g/day | p | |
| N | 4267 | 2228 | 1465 | 574 | |
| Maternal age, mean (SD), years | 29.6 (4.4) | 29.8 (4.3) | 29.5 (4.5) | 28.8 (4.6) | <0.001 |
| Infants sex, male, n (%) | 2190 (51.3) | 1160 (52.1) | 753 (51.4) | 277 (48.3) | 0.27 |
| Maternal domicile location, n (%) | 0.03 | ||||
| Northern China | 695 (16.3) | 360 (16.2) | 234 (16.0) | 101 (17.6) | |
| Shanghai surrounding area | 2789 (65.4) | 1496 (67.1) | 928 (63.3) | 365 (63.6) | |
| Southern China | 783 (18.4) | 372 (16.7) | 303 (20.7) | 108 (18.8) | |
| Baseline gestational periods, n (%) | <0.001 | ||||
| First trimester | 1369 (32.1) | 801 (36.0) | 400 (27.3) | 168 (29.3) | |
| Second trimester | 1630 (38.2) | 804 (36.1) | 586 (40.0) | 240 (41.8) | |
| Third trimester | 1268 (29.7) | 623 (28.0) | 479 (32.7) | 166 (28.9) | |
| Maternal Education, <13 years, n (%) | 1316 (30.8) | 569 (25.5) | 525 (35.8) | 222 (38.7) | <0.001 |
| Household income, yuan/year, n (%) | <0.001 | ||||
| <100,000 | 727 (17.0) | 310 (13.9) | 295 (20.1) | 122 (21.3) | |
| 100,000–350,000 | 3041 (71.3) | 1603 (71.9) | 1029 (70.2) | 409 (71.3) | |
| ≥350,000 | 499 (11.7) | 315 (14.1) | 141 (9.6) | 43 (7.5) | |
| Pre-pregnancy BMI, kg/m2, n (%) | 0.48 | ||||
| <18.5 | 553 (13.0) | 299 (13.4) | 174 (11.9) | 80 (13.9) | |
| 18.5–23.9 | 3002 (70.4) | 1551 (69.6) | 1055 (72.0) | 396 (69.0) | |
| ≥24.0 | 712 (16.7) | 378 (17.0) | 236 (16.1) | 98 (17.1) | |
| Maternal height, mean (SD), cm | 160.9 (5.2) | 161.0 (5.1) | 160.7 (5.2) | 160.8 (5.3) | 0.24 |
| Alcohol drinking, never, n (%) | 3842 (90.0) | 2002 (89.9) | 1314 (89.7) | 526 (91.6) | 0.38 |
| Passive smoking, never, n (%) | 2488 (58.3) | 1311 (58.8) | 843 (57.5) | 334 (58.2) | 0.73 |
| Leisure-time physical activity, n (%) | 0.09 | ||||
| <150 min/week | 2347 (55.0) | 1213 (54.4) | 794 (54.2) | 340 (59.2) | |
| ≥150 min/week | 1920 (45.0) | 1015 (45.6) | 671 (45.8) | 234 (40.8) | |
| Gestational age at birth, weeks | 38.9 (1.3) | 38.9 (1.3) | 38.9 (1.4) | 39.0 (1.3) | 0.07 |
| Eating at home every day, yes, n (%) | 1898 (44.5) | 738 (33.1) | 818 (55.8) | 342 (59.6) | <0.001 |
| Total energy intake, mean (SD), kcal/day | 1890.6 (725.2) | 1791.1 (694.2) | 1958.1 (722.4) | 2104.4 (783.2) | <0.001 |
| Fruits, mean (SD), g a | 281.4 (226.0) | 275.5 (226.3) | 283.6 (216.9) | 298.6 (246.0) | 0.08 |
| Vegetables, mean (SD), g a | 221.1 (183.8) | 213.8 (178.6) | 228.2 (189.8) | 230.9 (187.1) | 0.03 |
| Sodium, mean (SD), mg a | 2804.5 (1432.0) | 2798.9 (1411.6) | 2826.6 (1452.8) | 2770.0 (1458.5) | 0.70 |
| Multivitamin, yes, n (%) | 1782 (41.8) | 935 (42.0) | 603 (41.2) | 244 (42.5) | 0.82 |
| Calcium, yes, n (%) | 1752 (41.1) | 870 (39.0) | 643 (43.9) | 239 (41.6) | 0.01 |
| Folic acid, yes, n (%) | 2636 (61.8) | 1399 (62.8) | 873 (59.6) | 364 (63.4) | 0.10 |
| Gestational diabetes, yes, n (%) | 244 (5.7) | 114 (5.1) | 92 (6.3) | 38 (6.6) | 0.20 |
| Pregnancy-induced hypertension, yes, n (%) | 106 (2.5) | 52 (2.3) | 40 (2.7) | 14 (2.4) | 0.75 |
| Nulliparity, yes, n (%) | 2482 (58.2) | 1307 (58.7) | 821 (56.0) | 354 (61.7) | 0.05 |
| Natural labor, yes, n (%) | 2251 (52.8) | 1190 (53.4) | 746 (50.9) | 315 (54.9) | 0.18 |
| Gestational age at delivery, n (%) | 0.004 | ||||
| Pre-term | 170 (4.0) | 97 (4.4) | 58 (4.0) | 15 (2.6) | |
| Term | 4077 (95.5) | 2124 (95.3) | 1402 (95.7) | 551 (96.0) | |
| Post-term | 20 (0.5) | 7 (0.3) | 5 (0.3) | 8 (1.4) | |
| Salt Intake (g/day) | Salt Density (mg/kcal) b | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | <5.0 | 5.0–10.0 | ≥10.0 | p-Trend c | T1 (<1.9) | T2 (1.9 to 3.7) | T3 (≥3.7) | p-Trend |
| Low birth weight g | ||||||||
| N cases/Total | 47/2122 | 42/1372 | 17/539 | 31/1347 | 32/1342 | 43/1344 | ||
| Model 1 d | 1.00 (ref) | 1.41 (0.92–2.15) | 1.49 (0.85–2.62) | 0.13 | 1.00 (ref) | 1.04 (0.63–1.72) | 1.45 (0.90–2.31) | 0.09 |
| Model 2 e | 1.00 (ref) | 1.75 (1.04–2.94) | 2.13 (1.08–4.21) | 0.02 | 1.00 (ref) | 0.96 (0.52–1.77) | 1.99 (1.14–3.48) | 0.006 |
| Model 3 f | 1.00 (ref) | 1.72 (1.01–2.91) | 2.06 (1.02–4.13) | 0.04 | 1.00 (ref) | 0.93 (0.50–1.73) | 1.91 (1.08–3.36) | 0.01 |
| Small for gestational age | ||||||||
| N cases/Total | 105/1861 | 99/1191 | 46/478 | 68/1174 | 67/1176 | 115/1180 | ||
| Model 1 | 1.00 (ref) | 1.49 (1.12–1.98) | 1.71 (1.19–2.46) | 0.003 | 1.00 (ref) | 0.98 (0.69–1.38) | 1.69 (1.23–2.31) | <0.001 |
| Model 2 | 1.00 (ref) | 1.50 (1.12–2.01) | 1.73 (1.20–2.51) | 0.003 | 1.00 (ref) | 0.99 (0.69–1.40) | 1.70 (1.24–2.34) | <0.001 |
| Model 3 | 1.00 (ref) | 1.46 (1.09–1.97) | 1.69 (1.16–2.47) | 0.006 | 1.00 (ref) | 0.96 (0.68–1.37) | 1.63 (1.18–2.25) | <0.001 |
| Macrosomia g | ||||||||
| N cases/Total | 106/2181 | 93/1423 | 35/557 | 75/1391 | 80/1390 | 79/1380 | ||
| Model 1 | 1.00 (ref) | 1.37 (1.03–1.83) | 1.35 (0.91–2.00) | 0.09 | 1.00 (ref) | 1.08 (0.78–1.50) | 1.08 (0.78–1.50) | 0.68 |
| Model 2 | 1.00 (ref) | 1.34 (1.00–1.80) | 1.28 (0.85–1.93) | 0.17 | 1.00 (ref) | 1.08 (0.77–1.50) | 1.02 (0.73–1.42) | >0.99 |
| Model 3 | 1.00 (ref) | 1.29 (0.96–1.75) | 1.22 (0.80–1.85) | 0.30 | 1.00 (ref) | 1.05 (0.76–1.47) | 0.98 (0.69–1.38) | 0.83 |
| Large for gestational age | ||||||||
| N cases/Total | 367/2123 | 274/1366 | 94/526 | 248/1354 | 246/1355 | 241/1306 | ||
| Model 1 | 1.00 (ref) | 1.21 (1.02–1.44) | 1.07 (0.83–1.37) | 0.40 | 1.00 (ref) | 1.00 (0.82–1.21) | 1.04 (0.85–1.26) | 0.69 |
| Model 2 | 1.00 (ref) | 1.20 (1.00–1.43) | 1.04 (0.81–1.34) | 0.55 | 1.00 (ref) | 1.00 (0.82–1.22) | 1.03 (0.84–1.25) | 0.79 |
| Model 3 | 1.00 (ref) | 1.15 (0.96–1.38) | 0.98 (0.75–1.27) | 0.97 | 1.00 (ref) | 0.98 (0.80–1.20) | 0.99 (0.81–1.22) | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Wang, Z.; Xiao, Z.; Feng, C.; Sun, Z.; Mao, D.; Zhou, P.; Yuan, C.; Zhao, D.; Shang, W.; et al. Associations of Cooking Salt Intake During Pregnancy with Low Birth Weight and Small for Gestational Age Newborns: A Large Cohort Study. Nutrients 2025, 17, 642. https://doi.org/10.3390/nu17040642
Li T, Wang Z, Xiao Z, Feng C, Sun Z, Mao D, Zhou P, Yuan C, Zhao D, Shang W, et al. Associations of Cooking Salt Intake During Pregnancy with Low Birth Weight and Small for Gestational Age Newborns: A Large Cohort Study. Nutrients. 2025; 17(4):642. https://doi.org/10.3390/nu17040642
Chicago/Turabian StyleLi, Tongtong, Zhengyuan Wang, Zilin Xiao, Chengwu Feng, Zhuo Sun, Dou Mao, Puchen Zhou, Caimei Yuan, Danyang Zhao, Wanning Shang, and et al. 2025. "Associations of Cooking Salt Intake During Pregnancy with Low Birth Weight and Small for Gestational Age Newborns: A Large Cohort Study" Nutrients 17, no. 4: 642. https://doi.org/10.3390/nu17040642
APA StyleLi, T., Wang, Z., Xiao, Z., Feng, C., Sun, Z., Mao, D., Zhou, P., Yuan, C., Zhao, D., Shang, W., Liu, Y., Yuan, C., Hong, L., Zang, J., & Zong, G. (2025). Associations of Cooking Salt Intake During Pregnancy with Low Birth Weight and Small for Gestational Age Newborns: A Large Cohort Study. Nutrients, 17(4), 642. https://doi.org/10.3390/nu17040642







