Impact of Probiotic Supplementation and High-Intensity Interval Training on Primary Dysmenorrhea: A Double-Blind, Randomized Controlled Trial Investigating Inflammation and Hormonal Modulation
Abstract
:1. Introduction
2. Materials and Methods
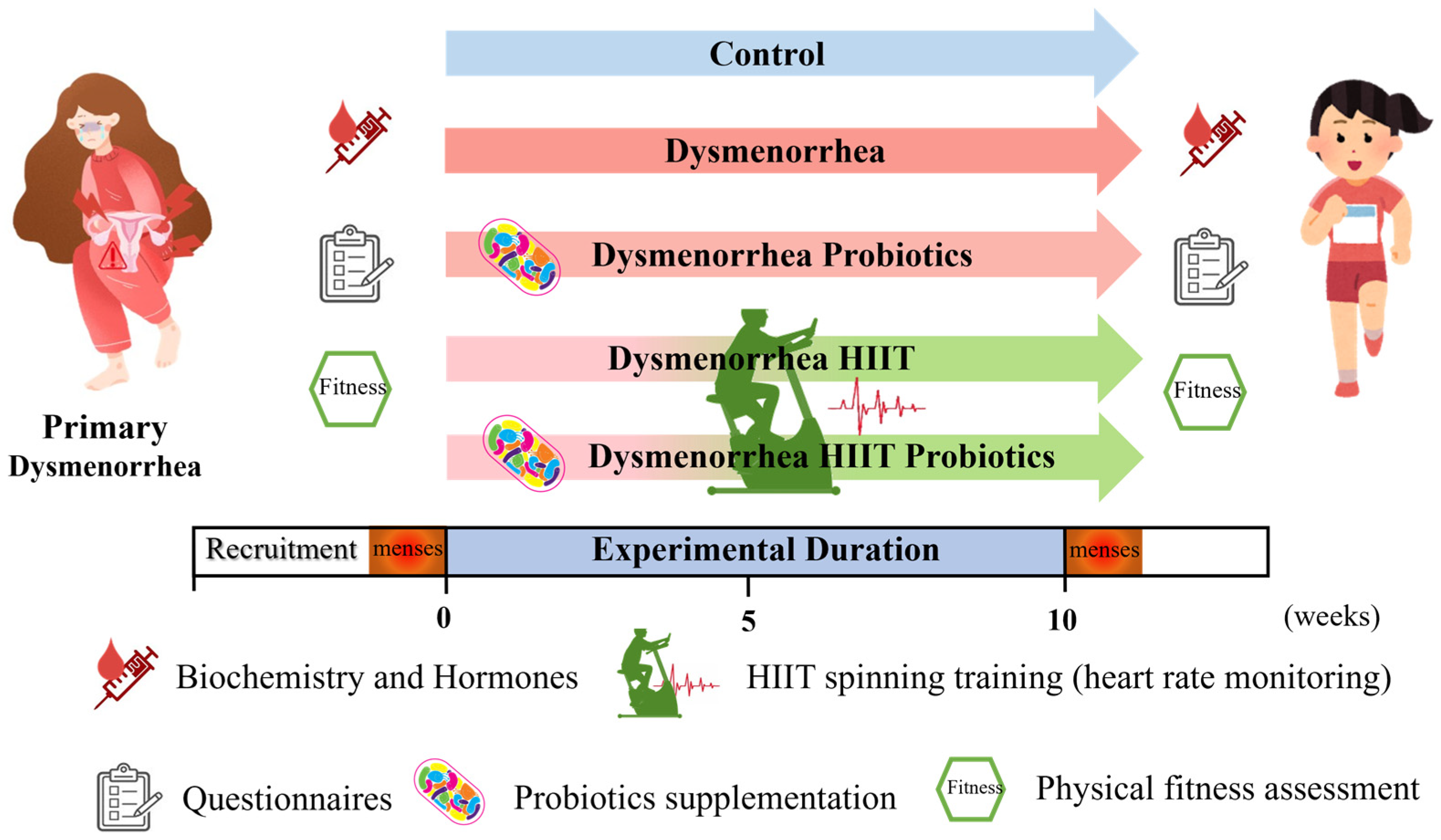
2.1. Experimental Design
2.2. Subjects
2.3. Probiotics
2.4. HIIT Intervention
2.5. Menstrual Questionnaire Surveys
2.6. Hormone, Prostaglandins, and Biochemical Analyses
2.7. Functional Fitness
2.8. Sample Size Calculation
2.9. Statistical Analysis
3. Results
3.1. Anthropometric and Menstrual Characteristics of Participants
3.2. Premenstrual Syndromes with HIIT and Probiotics Implementsation
3.3. Effects of HIIT Based on MDQ and SF-MPQ
3.4. Effects of HIIT and Probiotic Supplementation on Biochemical Variables and Hormones
3.5. Physical Fitness with HIIT and Probiotic Supplementation
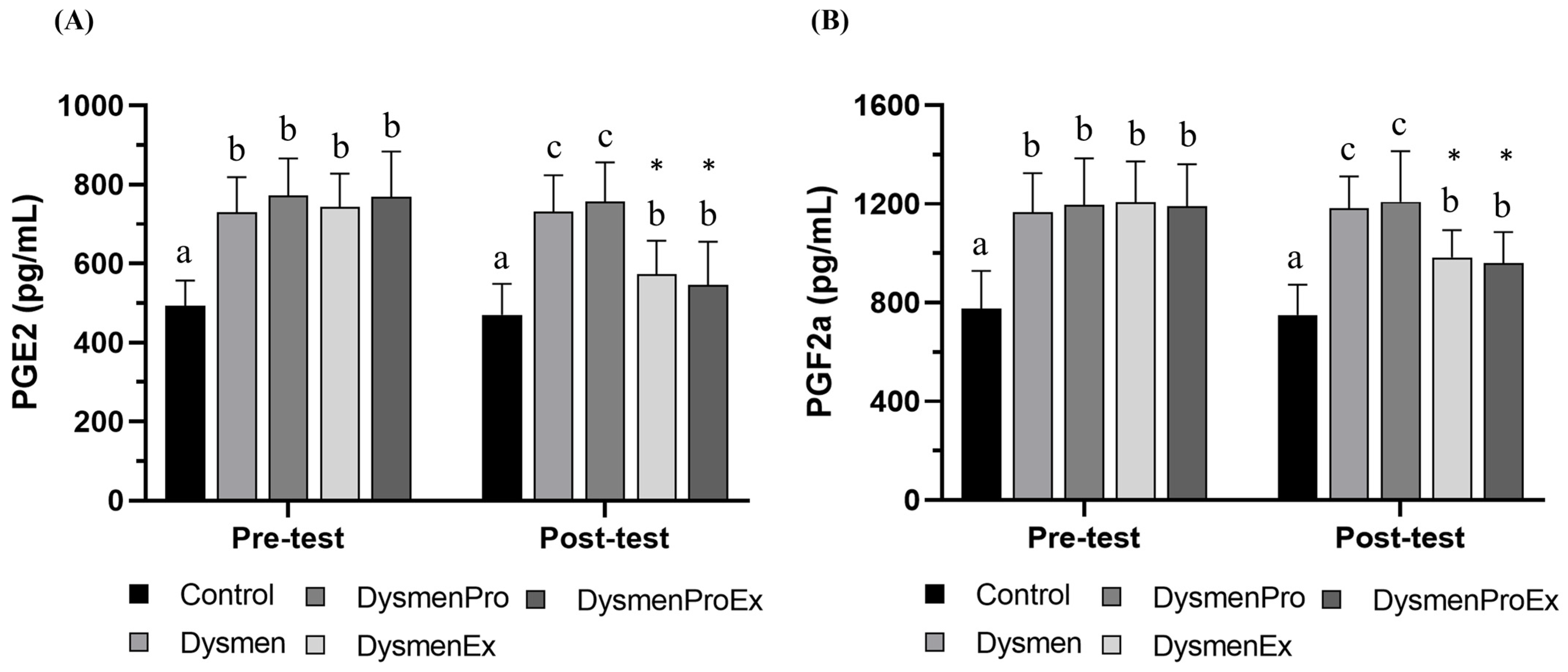
3.6. Effects of HIIT on Prostaglandins Levels
3.7. Correlation Between Hormones, hsCRP, and Prostaglandins Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, E.B.; Chakravarthi, V.P.; Wolfe, M.W.; Rumi, M.A.K. ERβ regulation of gonadotropin responses during folliculogenesis. Int. J. Mol. Sci. 2021, 22, 10348. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, M.J.; Hong, Y.Z.; Li, W.J. Insight into Dysmenorrhea research from 1992 to 2022: A bibliometric analysis. J. Pain Res. 2023, 16, 3591–3611. [Google Scholar] [CrossRef]
- Horvat, M.; Pavan, J.D.; Marinović, L.; Bursać, D.; Ribić, R.; Neuberg, M.; Bursać, D. Prevalence of primary dysmenorrhoea and its impact on academic performance among croatian students during the COVID-19 pandemic. Obs. Gynecol. Int. 2023, 2023, 2953762. [Google Scholar] [CrossRef]
- Gutman, G.; Nunez, A.T.; Fisher, M. Dysmenorrhea in adolescents. Curr. Probl. Pediatr. Adolesc. Health Care 2022, 52, 101186. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Lazzeri, L.; Perelli, F.; Reis, F.M.; Petraglia, F. Dysmenorrhea and related disorders. F1000Research 2017, 6, 1645. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chiang, Y.F.; Lin, Y.J.; Huang, K.C.; Chen, H.Y.; Hamdy, N.M.; Huang, T.C.; Chang, H.Y.; Shieh, T.M.; Huang, Y.J.; et al. Effect of vitamin D supplementation on primary dysmenorrhea: A systematic review and meta-analysis of randomized clinical trials. Nutrients 2023, 15, 2830. [Google Scholar] [CrossRef]
- López-Liria, R.; Torres-Álamo, L.; Vega-Ramírez, F.A.; García-Luengo, A.V.; Aguilar-Parra, J.M.; Trigueros-Ramos, R.; Rocamora-Pérez, P. Efficacy of physiotherapy treatment in primary dysmenorrhea: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 7832. [Google Scholar] [CrossRef] [PubMed]
- Shetty, G.B.; Shetty, B.; Mooventhan, A. Efficacy of acupuncture in the management of primary dysmenorrhea: A randomized controlled trial. J. Acupunct. Meridian Stud. 2018, 11, 153–158. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, F.Y.; Shen, Y.; Xu, J.Y.; Xie, L.Z.; Li, S.Y.; Ding, D.N.; Zhang, D.Q.; Han, F.J. Complementary and alternative medicine for dysmenorrhea caused by endometriosis: A review of utilization and mechanism. Evid. Based Complement. Altern. Med. 2021, 2021, 6663602. [Google Scholar] [CrossRef]
- Armour, M.; Ee, C.C.; Naidoo, D.; Ayati, Z.; Chalmers, K.J.; Steel, K.A.; de Manincor, M.J.; Delshad, E. Exercise for dysmenorrhea. Cochrane Database Syst. Rev. 2019, 9, CD004142. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.J.; Griffiths, M.; Deenmamode, A.H.P.; O’Driscoll, J.M. High-intensity interval training and cardiometabolic health in the general population: A systematic review and meta-analysis of randomised controlled trials. Sports Med. 2023, 53, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Hadjispyrou, S.; Dinas, P.C.; Delitheos, S.M.; Koumprentziotis, I.A.; Chryssanthopoulos, C.; Philippou, A. The effect of high-intensity interval training on mitochondrial-associated indices in overweight and obese adults: A systematic review and meta-analysis. Front. Biosci. (Landmark Ed.) 2023, 28, 281. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, J.V.; Petrov, I.; Tsekoura, D.; Dionyssiotis, Y.; Ferreira, A.S.; Lopes, A.J.; Ljoka, C.; Foti, C. Does group-based high-intensity aerobic interval training improve the inflammatory status in patients with chronic heart failure? Eur. J. Phys. Rehabil. Med. 2022, 58, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Salehpoor, Z.; Rezapourmoghadam, M.; Tanideh, N.; Jahromi, M.K. The effect of pentoxifylline and different types of exercise training on coagulation factors in a rat endometriosis model. Eur. J. Obs. Gynecol. Reprod. Biol. X 2024, 21, 100292. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Dasriya, V.L.; Samtiya, M.; Ranveer, S.; Dhillon, H.S.; Devi, N.; Sharma, V.; Nikam, P.; Puniya, M.; Chaudhary, P.; Chaudhary, V.; et al. Modulation of gut-microbiota through probiotics and dietary interventions to improve host health. J. Sci. Food Agric. 2024, 104, 6359–6375. [Google Scholar] [CrossRef] [PubMed]
- Del Toro-Barbosa, M.; Hurtado-Romero, A.; Garcia-Amezquita, L.E.; García-Cayuela, T. Psychobiotics: Mechanisms of action, evaluation methods and effectiveness in applications with food products. Nutrients 2020, 12, 3896. [Google Scholar] [CrossRef]
- Liu, Y.W.; Liu, W.H.; Wu, C.C.; Juan, Y.C.; Wu, Y.C.; Tsai, H.P.; Wang, S.; Tsai, Y.C. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res. 2016, 1631, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Pan, C.H.; Wei, C.C.; Huang, H.Y. Lactobacillus plantarum PS128 improves physiological adaptation and performance in triathletes through gut microbiota modulation. Nutrients 2020, 12, 2315. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Chiu, C.C.; Lee, M.C.; Huang, W.C. Combination of treadmill aerobic exercise with Bifidobacterium longum OLP-01 supplementation for treatment of high-fat diet-induced obese murine model. Obes. Facts 2021, 14, 306–319. [Google Scholar] [CrossRef]
- Zakaria, I.A.; Mohammed Zain, N.A.; Teik, C.K.; Abu, M.A.; Zainuddin, A.A.; Abdul Aziz, N.H.; Safian, N.; Mohd Mokhtar, N.; Raja Ali, R.A.; Beng Kwang, N.; et al. The role of probiotics in improving menstrual health in women with primary dysmenorrhoea: A randomized, double-blind, placebo-controlled trial (the PERIOD study). Women’s Health 2024, 20, 17455057241234524. [Google Scholar] [CrossRef]
- Huang, W.C.; Chiu, P.C.; Ho, C.H. The sprint-interval exercise using a spinning bike improves physical fitness and ameliorates primary dysmenorrhea symptoms through hormone and inflammation modulations: A randomized controlled trial. J. Sports Sci. Med. 2022, 21, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Kuo, Y.W.; Lin, J.H.; Lin, C.H.; Chen, J.F.; Tsai, S.Y.; Lee, M.C.; Hsu, Y.J.; Huang, C.C.; Tsou, Y.A.; et al. Probiotic Strains Isolated from an Olympic Woman’s Weightlifting Gold Medalist Increase Weight Loss and Exercise Performance in a Mouse Model. Nutrients 2022, 14, 1270. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Hsu, Y.J.; Huang, C.C.; Liu, H.C.; Lee, M.C. Exercise Training Combined with Bifidobacterium longum OLP-01 Supplementation Improves Exercise Physiological Adaption and Performance. Nutrients 2020, 12, 1145. [Google Scholar] [CrossRef]
- Lee, M.C.; Hsu, Y.J.; Chen, M.T.; Kuo, Y.W.; Lin, J.H.; Hsu, Y.C.; Huang, Y.Y.; Li, C.M.; Tsai, S.Y.; Hsia, K.C.; et al. Efficacy of Lactococcus lactis subsp. lactis LY-66 and Lactobacillus plantarum PL-02 in Enhancing Explosive Strength and Endurance: A Randomized, Double-Blinded Clinical Trial. Nutrients 2024, 16, 1921. [Google Scholar] [CrossRef]
- Steiner, M.; Macdougall, M.; Brown, E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch. Women’s Ment. Health 2003, 6, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Moos, R.H. The development of a menstrual distress questionnaire. Psychosom. Med. 1968, 30, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.L.; Hung, Y.L.; Chen, H.H.; Wang, Y.J. Auricular acupressure for pain relief in adolescents with dysmenorrhea: A placebo-controlled study. J. Altern. Complement. Med. 2013, 19, 313–318. [Google Scholar] [CrossRef]
- Melzack, R. The short-form McGill pain questionnaire. Pain 1987, 30, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Yang, J.; Wang, R.; Wang, Y. Extracorporeal shock wave therapy for treating primary dysmenorrhea: A randomized controlled trial. Medicine 2021, 100, e23798. [Google Scholar] [CrossRef]
- Lesnak, J.B.; Sluka, K.A. Mechanism of exercise-induced analgesia: What we can learn from physically active animals. Pain Rep. 2020, 5, e850. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Silva, J.; Avila, M.A.; de Oliveira, R.F.; Dedicação, A.C.; Godoy, A.G.; Rodrigues, J.C.; Driusso, P. Prevalence, pain intensity and symptoms associated with primary dysmenorrhea: A cross-sectional study. BMC Women’s Health 2024, 24, 92. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.H.; Hsieh, H.F.; Yang, Y.H.; Chen, H.M.; Hsu, S.C.; Wang, H.H. Influencing factors of dysmenorrhoea among hospital nurses: A questionnaire survey in Taiwan. BMJ Open 2017, 7, e017615. [Google Scholar] [CrossRef] [PubMed]
- Tiranini, L.; Nappi, R.E. Recent advances in understanding/management of premenstrual dysphoric disorder/premenstrual syndrome. Fac. Rev. 2022, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Ross, K. Psychobiotics: Are they the future intervention for managing depression and anxiety? a literature review. Explore 2023, 19, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Itani, R.; Soubra, L.; Karout, S.; Rahme, D.; Karout, L.; Khojah, H.M.J. Primary dysmenorrhea: Pathophysiology, diagnosis, and treatment updates. Korean J. Fam. Med. 2022, 43, 101–108. [Google Scholar] [CrossRef]
- Meaidi, A.; Mascolo, A.; Sessa, M.; Toft-Petersen, A.P.; Skals, R.; Gerds, T.A.; Wessel Skovlund, C.; Morch, L.S.; Rossi, F.; Capuano, A.; et al. Venous thromboembolism with use of hormonal contraception and non-steroidal anti-inflammatory drugs: Nationwide cohort study. BMJ 2023, 382, e074450. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Zhu, S.Y.; Ma, X.; Ding, X.S.; Deng, Y.; Wang, Y.; Sun, A.J. The effect of Ding-kun-dan comparing with Marvelon on primary dysmenorrhea: A prospective, double-blind, multicenter, randomized controlled trial. J. Ethnopharmacol. 2024, 318, 116975. [Google Scholar] [CrossRef] [PubMed]
- Haidari, F.; Homayouni, F.; Helli, B.; Haghighizadeh, M.H.; Farahmandpour, F. Effect of chlorella supplementation on systematic symptoms and serum levels of prostaglandins, inflammatory and oxidative markers in women with primary dysmenorrhea. Eur. J. Obs. Gynecol. Reprod. Biol. 2018, 229, 185–189. [Google Scholar] [CrossRef]
- Lei, K.; Georgiou, E.X.; Chen, L.; Yulia, A.; Sooranna, S.R.; Brosens, J.J.; Bennett, P.R.; Johnson, M.R. Progesterone and the repression of myometrial inflammation: The roles of mkp-1 and the ap-1 system. Mol. Endocrinol. 2015, 29, 1454–1467. [Google Scholar] [CrossRef] [PubMed]
- Roomruangwong, C.; Sirivichayakul, S.; Matsumoto, A.K.; Michelin, A.P.; de Oliveira Semeão, L.; de Lima Pedrão, J.V.; Barbosa, D.S.; Moreira, E.G.; Maes, M. Menstruation distress is strongly associated with hormone-immune-metabolic biomarkers. J. Psychosom. Res. 2021, 142, 110355. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhuang, Y.; Si, S.; Cheng, H.; Alifu, X.; Mo, M.; Zhou, H.; Liu, H.; Yu, Y. The association of reproductive hormones during the menstrual period with primary dysmenorrhea. Int. J. Women’s Health 2023, 15, 1501–1514. [Google Scholar] [CrossRef] [PubMed]
- Kossman, D.A.; Williams, N.I.; Domchek, S.M.; Kurzer, M.S.; Stopfer, J.E.; Schmitz, K.H. Exercise lowers estrogen and progesterone levels in premenopausal women at high risk of breast cancer. J. Appl. Physiol. 2011, 111, 1687–1693. [Google Scholar] [CrossRef]
- Otağ, A.; Hazar, M.; Otağ, I.; Beyleroğlu, M. Effect of increasing maximal aerobic exercise on serum gonadal hormones and alpha-fetoprotein in the luteal phase of professional female soccer players. J. Phys. Ther. Sci. 2016, 28, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Matsuda, T.; Ikegami, N.; Funaki, A.; Yamada, M.; Kamemoto, K.; Sakamaki-Sunaga, M. Effects of the menstrual cycle on EPOC and fat oxidation after low-volume high-intensity interval training. J. Sports Med. Phys. Fit. 2023, 63, 1165–1174. [Google Scholar] [CrossRef]
- Bahrami, A.; Bahrami-Taghanaki, H.; Khorasanchi, Z.; Timar, A.; Jaberi, N.; Azaryan, E.; Tayefi, M.; Ferns, G.A.; Sadeghnia, H.R.; Ghayour-Mobarhan, M. Menstrual problems in adolescence: Relationship to serum vitamins A and E, and systemic inflammation. Arch. Gynecol. Obs. 2020, 301, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Lalooha, F.; Nooshabadi, M.R.; Haghighian, H.K. Decreased dysmenorrhea pain in girls by reducing oxidative stress and inflammatory biomarkers following supplementation with oleoylethanolamide: A randomized controlled trial. J. Obs. Gynaecol. Res. 2022, 48, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Vincent, K.; Warnaby, C.; Stagg, C.J.; Moore, J.; Kennedy, S.; Tracey, I. Dysmenorrhoea is associated with central changes in otherwise healthy women. Pain 2011, 152, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.K.; Khan, Z.; Weaver, A.L.; Vaughan, L.E.; Gemzell-Danielsson, K.; Stewart, E.A. Vaginal bromocriptine improves pain, menstrual bleeding and quality of life in women with adenomyosis: A pilot study. Acta Obstet. Gynecol. Scand. 2019, 98, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Atakan, M.M.; Li, Y.; Koşar, Ş.N.; Turnagöl, H.H.; Yan, X. Evidence-based effects of high-intensity interval training on exercise capacity and health: A review with historical perspective. Int. J. Environ. Res. Public Health 2021, 18, 7201. [Google Scholar] [CrossRef]
- Martland, R.; Mondelli, V.; Gaughran, F.; Stubbs, B. Can high-intensity interval training improve physical and mental health outcomes? A meta-review of 33 systematic reviews across the lifespan. J. Sports Sci. 2020, 38, 430–469. [Google Scholar] [CrossRef] [PubMed]
- Henke, E.; Oliveira, V.S.; Silva, I.M.; Schipper, L.; Dorneles, G.; Elsner, V.R.; de Oliveira, M.R.; Romão, P.R.T.; Peres, A. Acute and chronic effects of high intensity interval training on inflammatory and oxidative stress markers of postmenopausal obese women. Transl. Sports Med. 2018, 1, 257–264. [Google Scholar] [CrossRef]
- MacInnis, M.J.; Gibala, M.J. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 2017, 595, 2915–2930. [Google Scholar] [CrossRef]
- Stöggl, T.L.; Björklund, G. High intensity interval training leads to greater improvements in acute heart rate recovery and anaerobic power as high volume low intensity training. Front. Physiol. 2017, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Kieu, N.T.V.; Jung, S.J.; Shin, S.W.; Jung, H.W.; Jung, E.S.; Won, Y.H.; Kim, Y.G.; Chae, S.W. The validity of the ymca 3-minute step test for estimating maximal oxygen uptake in healthy korean and vietnamese adults. J. Lifestyle Med. 2020, 10, 21–29. [Google Scholar] [CrossRef]
- Liu, Y.; Abdullah, B.B.; Abu Saad, H.B. Effects of high-intensity interval training on strength, speed, and endurance performance among racket sports players: A systematic review. PLoS ONE 2024, 19, e0295362. [Google Scholar] [CrossRef] [PubMed]
- Merkouris, E.; Mavroudi, T.; Miliotas, D.; Tsiptsios, D.; Serdari, A.; Christidi, F.; Doskas, T.K.; Mueller, C.; Tsamakis, K. Probiotics’ effects in the treatment of anxiety and depression: A comprehensive review of 2014–2023 clinical trials. Microorganisms 2024, 12, 411. [Google Scholar] [CrossRef] [PubMed]
- Walden, K.E.; Moon, J.M.; Hagele, A.M.; Allen, L.E.; Gaige, C.J.; Krieger, J.M.; Jäger, R.; Mumford, P.W.; Pane, M.; Kerksick, C.M. A randomized controlled trial to examine the impact of a multi-strain probiotic on self-reported indicators of depression, anxiety, mood, and associated biomarkers. Front. Nutr. 2023, 10, 1219313. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Fang, Y.; Li, H.; Liu, Y.; Wei, J.; Zhang, S.; Wang, L.; Fan, R.; Wang, L.; Li, S.; et al. Psychobiotic Lactobacillus plantarum JYLP-326 relieves anxiety, depression, and insomnia symptoms in test anxious college via modulating the gut microbiota and its metabolism. Front. Immunol. 2023, 14, 1158137. [Google Scholar] [CrossRef] [PubMed]
- Accettulli, A.; Corbo, M.R.; Sinigaglia, M.; Speranza, B.; Campaniello, D.; Racioppo, A.; Altieri, C.; Bevilacqua, A. Psycho-microbiology, a new frontier for probiotics: An exploratory overview. Microorganisms 2022, 10, 2141. [Google Scholar] [CrossRef]
- Carroquino-Garcia, P.; Jiménez-Rejano, J.J.; Medrano-Sanchez, E.; de la Casa-Almeida, M.; Diaz-Mohedo, E.; Suarez-Serrano, C. Therapeutic exercise in the treatment of primary dysmenorrhea: A systematic review and meta-analysis. Phys. Ther. 2019, 99, 1371–1380. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Control | Dysmen | DysmenPro | DysmenEx | DysmenExPro |
|---|---|---|---|---|---|
| Age (years) | 24.4 ± 6.5 | 24.5 ± 4.8 | 21.8 ± 3.9 | 21.9 ± 2.5 | 24.9 ± 7.4 |
| Height (cm) | 158 ± 4.5 | 161 ± 4.0 | 161 ± 6.2 | 159 ± 4.6 | 162 ± 4.9 |
| Weight (kg) | 54.2 ± 6.2 | 56.0 ± 7.0 | 51.7 ± 5.9 | 54.1 ± 6.4 | 56.0 ± 8.7 |
| BMI (kg/m2) | 21.6 ± 2.3 | 21.7 ± 2.4 | 20.0 ± 2.1 | 21.3 ± 2.4 | 21.3 ± 2.9 |
| Systolic blood pressure (mmHg) | 114 ± 13 | 107 ± 9.6 | 108 ± 9.6 | 115 ± 9.7 | 113 ± 6.5 |
| Diastolic blood pressure (mmHg) | 76 ± 6.1 | 75 ± 7.2 | 71 ± 7.9 | 74 ± 8.0 | 74 ± 6.2 |
| Resting heart rate (bpm) | 80 ± 11.8 | 81 ± 6.5 | 85 ± 10.6 | 79 ± 12.6 | 80 ± 10.9 |
| Menarche age (years) | 12.0 ± 1.2 | 12.1 ± 1.0 | 12.3 ± 1.0 | 12.5 ± 1.2 | 12.3 ± 0.9 |
| Interval of menstrual cycle (days) | 28.1 ± 1.3 | 30.0 ± 1.7 | 30.0 ± 1.8 | 29.0 ± 1.7 | 29.9 ± 3.5 |
| Duration of menstrual cycle (days) | 4.9 ± 1.2 | 5.2 ± 1.1 | 5.5 ± 0.9 | 5.4 ± 1.1 | 5.3 ± 1.1 |
| Menstrual flow + | 1.8 ± 0.6 | 2.2 ± 0.7 | 2.1 ± 0.5 | 1.7 ± 0.6 | 2.0 ± 0.6 |
| Days with dysmenorrhea (during menses) | 0.1 ± 0.3 a | 2.9 ± 1.1 b | 2.9 ± 1.6 b | 2.8 ± 0.6 b | 3.0 ± 1.1 b |
| Painkiller medication (times) | 0.2 ± 0.6 a | 3.8 ± 1.7 b | 3.6 ± 1.1 b | 3.9 ± 1.2 b | 4.1 ± 1.7 b |
| Exercise frequency | |||||
| None | 6 | 5 | 8 | 6 | 5 |
| ≤3 times/week | 4 | 7 | 7 | 7 | 6 |
| ≥4 times/week | 0 | 1 | 0 | 1 | 2 |
| PSST Component | Control Group | Dysmen Group | DysmenPro Group | DysmenEx Group | DysmenExPro Group | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rank Mean | Rank Mean | Rank Mean | Rank Mean | Rank Mean | ||||||
| Symptom | Pre | After | Pre | After | Pre | After | Pre | After | Pre | After |
| Anger/irritability | 1.4 ± 0.5 a | 1.4 ± 0.5 a | 2.3 ± 0.7 ab | 2.4 ± 0.8 b | 2.4 ± 0.7 b | 1.8 ± 0.6 a* | 2.1 ± 0.8 ab | 1.6 ± 0.5 a | 2.4 ± 0.8 b | 1.7 ± 0.6 a* |
| Anxiety/tension | 1.1 ± 0.3 a | 1.2 ± 0.4 a | 1.7 ± 0.9 ab | 2.2 ± 0.8 b | 1.9 ± 0.6 b | 1.7 ± 0.6 a | 1.9 ± 0.9 ab | 1.6 ± 0.6 a | 2.0 ± 0.8 b | 1.7 ± 0.6 a |
| Tearfulness | 1.2 ± 0.4 a | 1.3 ± 0.6 | 1.5 ± 0.7 a | 1.4 ± 0.7 | 2.3 ± 0.9 ab | 1.8 ± 0.6 * | 1.9 ± 1.0 a | 1.6 ± 0.7 | 1.8 ± 1.1 a | 1.7 ± 0.7 |
| Depressed mood | 1.3 ± 0.5 a | 1.3 ± 0.5 a | 2.5 ± 0.7 b | 2.5 ± 0.7 c | 2.5 ± 0.6 b | 1.8 ± 0.7 ab* | 2.4 ± 0.9 b | 1.8 ± 0.7 ab* | 2.4 ± 1.1 b | 1.8 ± 0.6 b* |
| Decreased interest in work activities | 1.3 ± 0.5 a | 1.4 ± 0.5 a | 2.2 ± 0.8 ab | 2.2 ± 0.9 | 2.4 ± 1.1 b | 2.2 ± 0.9 | 2.1 ± 0.9 ab | 1.6 ± 0.5 | 2.1 ± 0.6 ab | 1.7 ± 0.5 |
| Decreased interest in home activities | 1.1 ± 0.3 | 1.3 ± 0.5 | 1.5 ± 0.5 | 1.8 ± 0.6 | 2.0 ± 0.9 | 1.5 ± 0.6 | 1.6 ± 0.9 | 1.7 ± 0.6 | 1.8 ± 0.7 | 1.5 ± 0.7 |
| Decreased interest in social activities | 1.2 ± 0.4 | 1.3 ± 0.5 a | 2.1 ± 0.8 | 2.3 ± 0.9 b | 2.1 ± 1.0 | 2.1 ± 0.7 b | 1.9 ± 0.8 | 1.9 ± 0.9 ab | 2.1 ± 0.8 | 1.8 ± 0.6 ab |
| Difficulty concentrating | 1.0 ± 0 a | 1.1 ± 0.3 a | 1.8 ± 0.7 ab | 1.9 ± 0.7 b | 2.1 ± 0.8 b | 1.7 ± 0.7 b | 1.8 ± 0.9 ab | 1.7 ± 0.7 b | 1.9 ± 1.0 ab | 1.8 ± 0.6 b |
| Fatigue/lack of energy | 1.5 ± 0.5 a | 1.4 ± 0.5 a | 2.9 ± 0.9 b | 2.9 ± 0.9 c | 3.0 ± 0.8 b | 2.5 ± 0.7 bc* | 2.6 ± 0.9 ab | 2.1 ± 0.6 b* | 2.9 ± 1.1 b | 2.3 ± 0.5 b |
| Overeating/food cravings | 1.4 ± 0.5 | 1.5 ± 0.7 | 2.2 ± 1.1 | 2.1 ± 0.9 | 2.3 ± 1.1 | 2.1 ± 1.1 | 2.2 ± 0.8 | 2.1 ± 0.8 | 2.3 ± 0.9 | 2.0 ± 1.0 |
| Insomnia | 1.1 ± 0.3 | 1.0 ± 0 | 1.6 ± 0.9 | 1.6 ± 0.9 | 1.3 ± 0.6 | 1.3 ± 0.6 | 1.2 ± 0.6 | 1.4 ± 0.6 | 1.5 ± 0.5 | 1.5 ± 0.5 |
| Hypersomnia | 1.3 ± 0.5 a | 1.4 ± 0.5 a | 2.4 ± 1.1 b | 2.6 ± 1.0 c | 2.6 ± 0.9 b | 2.3 ± 1.0 bc | 2.3 ± 0.9 ab | 1.8 ± 1.0 ab | 2.4 ± 0.7 b | 1.8 ± 0.6 ab* |
| Feeling overwhelmed/out of control | 1.2 ± 0.4 | 1.0 ± 0 a | 1.3 ± 0.6 | 1.3 ± 0.6 ab | 1.9 ± 0.9 | 1.6 ± 0.7 b | 1.5 ± 0.8 | 1.3 ± 0.5 ab | 1.4 ± 0.5 | 1.3 ± 0.5 ab |
| Physical symptoms + | 1.4 ± 0.5 a | 1.4 ± 0.5 a | 2.4 ± 0.7 b | 2.5 ± 0.9 b | 2.6 ± 0.7 b | 2.4 ± 0.6 b | 2.4 ± 0.6 b | 1.9 ± 0.4 a* | 2.5 ± 0.6 b | 1.8 ± 0.4 a* |
| Interference with activities | ||||||||||
| Work productivity/efficiency | 1.1 ± 0.3 a | 1.1 ± 0.3 a | 1.9 ± 0.6 b | 1.8 ± 0.5 b | 2.0 ± 0.7 b | 1.5 ± 0.6 ab | 2.0 ± 0.8 b | 1.4 ± 0.5 ab* | 1.9 ± 0.8 ab | 1.5 ± 0.5 ab* |
| Colleagues/classmate | 1.0 ± 0 | 1.0 ± 0 | 1.4 ± 0.7 | 1.5 ± 0.7 | 1.3 ± 0.5 | 1.2 ± 0.4 | 1.4 ± 0.8 | 1.4 ± 0.5 | 1.3 ± 0.5 | 1.2 ± 0.4 |
| Family relationships | 1.0 ± 0 | 1.0 ± 0 | 1.5 ± 0.8 | 1.3 ± 0.5 | 1.4 ± 0.6 | 1.4 ± 0.7 | 1.2 ± 0.8 | 1.3 ± 0.5 | 1.4 ± 0.5 | 1.3 ± 0.5 |
| Social activities | 1.4 ± 0.5 a | 1.0 ± 0 a | 2.0 ± 0.8 b | 2.1 ± 0.9 b | 1.9 ± 0.6 b | 1.4 ± 0.7 a | 1.8 ± 0.8 ab | 1.5 ± 0.5 a | 1.8 ± 0.7 ab | 1.4 ± 0.5 a* |
| Home responsibilities | 1.0 ± 0 | 1.0 ± 0 | 1.2 ± 0.6 | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.3 ± 0.6 | 1.1 ± 0.3 | 1.1 ± 0.4 | 1.2 ± 0.6 | 1.0 ± 0 |
| Total scores | 22.7 ± 3.4 a | 23.1 ± 3.8 a | 36.7 ± 9.8 b | 37.6 ± 7.6 c | 39.3 ± 8.5 b | 33.7 ± 8.0 bc* | 35.5 ± 9.9 b | 30.7 ± 6.7 b* | 37.2 ± 10 b | 30.8 ± 4.7 b* |
| Distress Component | Control Group | Dysmen Group | DysmenPro Group | DysmenEx Group | DysmenExPro Group | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rank Mean | Rank Mean | Rank Mean | Rank Mean | Rank Mean | ||||||
| Item | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| Cramp | 1.0 ± 0 a | 1.0 ± 0 a | 2.8 ± 0.9 b | 2.7 ± 0.6 c | 2.5 ± 0.9 b | 2.1 ± 0.6 b | 2.7 ± 0.7 b | 2.1 ± 0.7 b* | 2.8 ± 0.8 b | 2.0 ± 0.7 b* |
| Fatigue | 1.7 ± 0.5 a | 1.5 ± 0.5 a | 2.7 ± 0.7 b | 2.7 ± 0.6 d | 2.9 ± 0.7 b | 2.5 ± 0.5 cd* | 2.7 ± 0.6 b | 2.1 ± 0.5 bc* | 2.8 ± 0.7 b | 2.0 ± 0.6 b* |
| Backache | 1.7 ± 0.7 | 1.5 ± 0.5 a | 2.2 ± 1.9 | 2.2 ± 0.8 b | 2.3 ± 0.9 | 2.0 ± 0.9 ab | 2.4 ± 0.6 | 1.8 ± 0.6 ab* | 1.9 ± 0.7 | 1.6 ± 0.5 ab |
| Swelling (chest/abdomen) | 1.8 ± 0.6 | 1.4 ± 0.5 a | 2.1 ± 0.8 | 2.1 ± 0.5 bc | 2.3 ± 0.7 | 2.5 ± 0.9 c | 2.4 ± 0.6 | 1.6 ± 0.5 ab* | 2.3 ± 0.6 | 2.1 ± 0.5 bc |
| Painful or tender breast | 1.2 ± 0.4 a | 1.3 ± 0.5 a | 2.1 ± 0.8 b | 2.0 ± 0.6 b | 2.4 ± 0.8 b | 2.2 ± 1.0 b | 2.4 ± 0.8 b | 1.6 ± 0.5 ab* | 2.3 ± 0.8 b | 1.8 ± 0.8 ab |
| General ache and pains | 1.4 ± 0.5 a | 1.3 ± 0.5 a | 2.5 ± 1.1 b | 2.6 ± 0.9 b | 2.3 ± 0.8 b | 1.6 ± 0.6 a* | 2.4 ± 0.8 b | 1.6 ± 0.6 a* | 2.1 ± 0.9 ab | 1.5 ± 0.6 a* |
| Dizziness | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.7 ± 0.9 | 1.4 ± 0.5 | 1.5 ± 0.9 | 1.6 ± 0.9 | 1.5 ± 0.9 | 1.3 ± 0.5 | 1.5 ± 0.7 | 1.2 ± 0.4 |
| Cold sweat | 1.0 ± 0 | 1.0 ± 0 | 1.5 ± 0.9 | 1.1 ± 0.3 | 1.3 ± 0.5 | 1.2 ± 0.4 | 1.4 ± 0.9 | 1.2 ± 0.4 | 1.5 ± 0.7 | 1.2 ± 0.4 |
| Headache | 1.3 ± 0.5 a | 1.4 ± 0.7 | 2.2 ± 1.1 b | 1.9 ± 1.0 | 1.6 ± 0.9 ab | 1.3 ± 0.6 | 1.5 ± 0.9 ab | 1.5 ± 0.8 | 1.8 ± 0.9 ab | 1.5 ± 0.5 |
| Nausea/vomiting | 1.0 ± 0 | 1.0 ± 0 | 1.5 ± 0.8 | 1.3 ± 0.6 | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.4 ± 0.6 | 1.3 ± 0.5 | 1.4 ± 0.7 | 1.2 ± 0.4 |
| Hot flashes | 1.3 ± 0.5 | 1.3 ± 0.5 ab | 1.5 ± 0.8 | 1.3 ± 0.5 ab | 1.6 ± 0.7 | 1.6 ± 0.7 a | 1.3 ± 0.5 | 1.1 ± 0.3 b | 1.7 ± 0.9 | 1.3 ± 0.5 ab* |
| Muscle stiffness | 1.0 ± 0 | 1.0 ± 0 | 1.2 ± 0.6 | 1.2 ± 0.4 | 1.3 ± 0.6 | 1.3 ± 0.6 | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.2 ± 0.4 | 1.1 ± 0.3 |
| Swelling legs | 1.2 ± 0.4 a | 1.2 ± 0.4 a | 1.9 ± 0.9 ab | 1.4 ± 0.7 ab | 2.1 ± 1.1 b | 1.8 ± 0.8 b | 1.3 ± 0.5 a | 1.3 ± 0.6 a | 1.4 ± 0.9 ab | 1.2 ± 0.4 a |
| Heart pounding | 1.0 ± 0 a | 1.0 ± 0 | 1.5 ± 0.8 b | 1.3 ± 0.6 | 1.3 ± 0.6 ab | 1.3 ± 0.4 | 1.1 ± 0.4 ab | 1.3 ± 0.4 | 1.2 ± 0.4 ab | 1.1 ± 0.3 |
| Skin blemish or disorder | 1.2 ± 0.4 a | 1.5 ± 0.7 a | 2.3 ± 1.0 b | 2.2 ± 1.0 b | 2.5 ± 1.1 b | 2.2 ± 0.9 b | 1.8 ± 0.7 ab | 1.8 ± 0.4 ab | 2.0 ± 0.7 b | 1.7 ± 0.6 ab |
| Numbness | 1.0 ± 0 | 1.0 ± 0 | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.1 ± 0.4 | 1.1 ± 0.3 | 1.0 ± 0 | 1.0 ± 0 | 1.0 ± 0 | 1.1 ± 0.3 |
| Total score | 19.9 ± 1.9 a | 19.5 ± 2.8 a | 30.9 ± 8.4 b | 28.4 ± 4.8 c | 30.2 ± 5.7 b | 27.5 ± 5.2 c* | 28.5 ± 3.8 b | 23.5 ± 4.2 b* | 28.8 ± 5.4 b | 23.5 ± 4.9 b* |
| Short-Form McGill Pain Questionnaire (total score) | 15.2 ± 0.6 a | 15.3 ± 0.9 a | 27.4 ± 5.7 b | 27.3 ± 8.1 c | 27.5 ± 4.8 b | 26.3 ± 4.1 c | 27.5 ± 6.7 b | 21.1 ± 4.3 b* | 27.5 ± 7.0 b | 21.6 ± 6.5 b* |
| Visual analog scale of pain | 0.5 ± 0.7 a | 0.4 ± 0.7 a | 7.6 ± 0.8 b | 7.5 ± 1.0 c | 7.4 ± 0.9 b | 6.9 ± 1.1 c | 7.5 ± 1.4 b | 4.4 ± 1.8 b* | 7.5 ± 1.1 b | 4.3 ± 1.7 b* |
| Control Group | Dysmen Group | DysmenPro Group | DysmenEx Group | DysmenExPro Group | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| AST (U/L) | 14.3 ± 3.3 | 14.3 ± 2.1 | 15.2 ± 2.6 | 14.3 ± 4.3 | 15.6 ± 2.9 | 14.5 ± 3.3 | 14.4 ± 2.6 | 14.1 ± 3.2 | 15.1 ± 2.4 | 14.4 ± 3.7 |
| ALT (U/L) | 6.3 ± 1.9 | 6.1 ± 1.4 | 7.3 ± 1.9 | 6.6 ± 2.7 | 6.9 ± 1.7 | 6.8 ± 2.8 | 6.9 ± 2.3 | 6.8 ± 2.7 | 7.6 ± 1.8 | 6.8 ± 3.4 |
| BUN (mg/dL) | 11.2 ± 1.8 | 11.1 ± 2.8 | 11.1 ± 3.0 | 11.2 ± 1.7 | 11.5 ± 3.7 | 11.6 ± 1.6 | 11.4 ± 2.9 | 11.9 ± 2.7 | 11.4 ± 1.9 | 11.2 ± 2.9 |
| CREA (mg/dL) | 0.68 ± 0.14 | 0.65 ± 0.13 | 0.67 ± 0.09 | 0.64 ± 0.08 | 0.63 ± 0.09 | 0.62 ± 0.11 | 0.64 ± 0.09 | 0.64 ± 0.11 | 0.59 ± 0.13 | 0.63 ± 0.11 |
| TG (mg/dL) | 68.4 ± 17 | 68.0 ± 15 | 69.1 ± 17 | 67.3 ± 15 | 72.7 ± 14 | 70.7 ± 14 | 65.3 ± 18 | 64.9 ± 19 | 67.0 ± 13 | 68.7 ± 14 |
| CHOL (mg/dL) | 172 ± 24 | 172 ± 34 | 175 ± 29 | 170 ± 24 | 173 ± 26 | 171 ± 22 | 168 ± 29 | 169 ± 20 | 170 ± 26 | 171 ± 26 |
| LDH (U/L) | 114 ± 15 | 117 ± 14 | 119 ± 16 | 120 ± 17 | 116 ± 22 | 115 ± 17 | 113 ± 13 | 115 ± 11 | 117 ± 21 | 120 ± 16 |
| CPK (U/L) | 65 ± 29 | 66 ± 22 | 64 ± 29 | 63 ± 25 | 65 ± 25 | 65 ± 20 | 66 ± 32 | 67 ± 27 | 62 ± 22 | 66 ± 25 |
| HsCRP (mg/dL) | 0.044 ± 0.04 a | 0.045 ± 0.04 a | 0.170 ± 0.05 b | 0.186 ± 0.08 c | 0.171 ± 0.06 b | 0.144 ± 0.07 bc | 0.182 ± 0.05 b | 0.108 ± 0.05 b* | 0.177 ± 0.04 b | 0.114 ± 0.03 b* |
| Estradiol (pg/mL) | 147 ± 26 a | 144 ± 21 a | 187 ± 25 b | 188 ± 26 b | 183 ± 27 b | 166 ± 29 ab* | 181 ± 27 b | 137 ± 25 a* | 180 ± 31 b | 136 ± 31 a* |
| FSH (mIU/mL) | 3.2 ± 1.2 | 3.2 ± 1.1 | 3.1 ± 1.3 | 3.4 ± 1.6 | 3.0 ± 1.2 | 3.0 ± 1.3 | 3.1 ± 1.1 | 3.3 ± 0.9 | 3.5 ± 1.3 | 3.7 ± 2.0 |
| LH (mIU/mL) | 5.1 ± 1.8 | 4.9 ± 1.7 | 5.0 ± 1.5 | 5.3 ± 1.5 | 4.9 ± 2.1 | 5.0 ± 2.0 | 5.5 ± 2.6 | 6.2 ± 2.4 | 5.0 ± 2.4 | 5.4 ± 2.3 |
| Prolactin (ng/mL) | 12.7 ± 5.0 | 15.2 ± 3.6 a | 20.7 ± 6.2 | 25.3 ± 8.7 c | 21.2 ± 6.8 | 25.4 ± 5.2 c | 22.1 ± 7.8 | 20.0 ± 5.5 b | 21.3 ± 4.7 | 20.4 ± 4.0 b |
| Progesterone (ng/mL) | 12.3 ± 4.4 b | 13.1 ± 4.0 c | 4.8 ± 3.5 a | 4.8 ± 2.3 a | 4.4 ± 2.9 a | 5.7 ± 2.3 a | 4.1 ± 2.7 a | 10.4 ± 2.9 b* | 4.0 ± 2.8 a | 10.8 ± 2.7 b* |
| Cortisol (μg/dL) | 11.3 ± 2.7 b | 13.1 ± 3.5 c | 6.4 ± 2.4 a | 6.7 ± 3.8 a | 6.5 ± 2.3 a | 7.5 ± 2.6 a | 7.0 ± 2.6 a | 10.5 ± 2.6 b* | 7.2 ± 2.5 a | 10.6 ± 3.2 b* |
| Control Group | Dysmen Group | DysmenPro Group | DysmenEx Group | DysmenExPro Group | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Grip strength (kg) | 26.7 ± 5.5 | 25.4 ± 4.6 | 26.4 ± 2.5 | 25.0 ± 3.7 | 24.9 ± 4.2 | 24.1 ± 4.6 | 25.3 ± 4.6 | 26.1 ± 4.3 | 25.6 ± 5.4 | 25.3 ± 4.6 |
| Standing long jump (cm) | 154 ± 22 | 154 ± 20 a | 155 ± 16 | 154 ± 13 a | 157 ± 21 | 154 ± 18 a | 158 ± 29 | 170 ± 24 *b | 160 ± 19 | 171 ± 16 *b |
| Standing triple jumps (cm) | 434 ± 79 | 424 ± 80 a | 437 ± 58 | 420 ± 60 a | 431 ± 55 | 428 ± 57 a | 452 ± 68 | 483 ± 71 *b | 431 ± 33 | 478 ± 33 *b |
| 3 min step test | 52 ± 5.6 | 52 ± 4.2 a | 51 ± 4.9 | 52 ± 5.0 a | 52 ± 5.6 | 52 ± 5.9 a | 52 ± 7.8 | 59 ± 9.7 *b | 52 ± 5.5 | 58 ± 7.4 *b |
| Bent-knee sit-up | ||||||||||
| 30 s | 17.8 ± 5.0 | 15.8 ± 2.9 | 17.7 ± 6.2 | 16.9 ± 4.4 | 17.6 ± 5.3 | 18.1 ± 2.3 | 18.4 ± 5.5 | 19.6 ± 6.1 | 17.5 ± 3.7 | 17.2 ± 6.1 |
| 60 s | 31.1 ± 8.6 | 31.7 ± 7.8 | 30.4 ± 11 | 29.0 ± 11 | 32.7 ± 10 | 31.9 ± 10 | 33.1 ± 10 | 32.3 ± 10 | 33.0 ± 8.4 | 32.3 ± 11 |
| MDQ | SF-MPQ | PGE2 | PGF2α | Cortisol | Progesterone | Estradiol | HsCRP | Prolactin | |
|---|---|---|---|---|---|---|---|---|---|
| MDQ | 1 | 0.771 ** | 0.379 ** | 0.447 ** | −0.320 * | −0.522 ** | 0.235 | 0.408 ** | 0.278 * |
| SF-MPQ | 0.771 ** | 1 | 0.508 ** | 0.588 ** | −0.364 ** | −0.493 ** | 0.174 | 0.317 * | 0.378 ** |
| PGE2 | 0.379 ** | 0.508 ** | 1 | 0.497 ** | −0.489 ** | −0.593 ** | 0.469 ** | 0.467 ** | 0.495 ** |
| PGF2α | 0.447 ** | 0.588 ** | 0.497 ** | 1 | −0.401 ** | −0.578 ** | 0.395 ** | 0.549 ** | 0.315 * |
| Cortisol | −0.320 * | −0.364 ** | −0.489 ** | −0.401 ** | 1 | 0.328 * | −0.322 * | −0.373 * | −0.256 * |
| Progesterone | −0.522 ** | −0.493 ** | −0.593 ** | −0.579 ** | 0.328 * | 1 | −0.333 ** | −0.627 ** | −0.439 ** |
| Estradiol | 0.235 | 0.174 | 0.469 ** | 0.395 ** | −0.322 * | −0.333 ** | 1 | 0.278 * | 0.321 ** |
| HsCRP | 0.408 ** | 0.317 * | 0.467 ** | 0.549 ** | −0.373 * | −0.627 ** | 0.278 * | 1 | 0.180 |
| Prolactin | 0.278 * | 0.378 ** | 0.495 ** | 0.315 * | −0.256 * | −0.439 ** | 0.321 * | 0.180 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.-Y.; Chen, H.-Y.; Ho, C.-H.; Huang, W.-C. Impact of Probiotic Supplementation and High-Intensity Interval Training on Primary Dysmenorrhea: A Double-Blind, Randomized Controlled Trial Investigating Inflammation and Hormonal Modulation. Nutrients 2025, 17, 622. https://doi.org/10.3390/nu17040622
Yang M-Y, Chen H-Y, Ho C-H, Huang W-C. Impact of Probiotic Supplementation and High-Intensity Interval Training on Primary Dysmenorrhea: A Double-Blind, Randomized Controlled Trial Investigating Inflammation and Hormonal Modulation. Nutrients. 2025; 17(4):622. https://doi.org/10.3390/nu17040622
Chicago/Turabian StyleYang, Min-Yi, Hao-Yu Chen, Chi-Hong Ho, and Wen-Ching Huang. 2025. "Impact of Probiotic Supplementation and High-Intensity Interval Training on Primary Dysmenorrhea: A Double-Blind, Randomized Controlled Trial Investigating Inflammation and Hormonal Modulation" Nutrients 17, no. 4: 622. https://doi.org/10.3390/nu17040622
APA StyleYang, M.-Y., Chen, H.-Y., Ho, C.-H., & Huang, W.-C. (2025). Impact of Probiotic Supplementation and High-Intensity Interval Training on Primary Dysmenorrhea: A Double-Blind, Randomized Controlled Trial Investigating Inflammation and Hormonal Modulation. Nutrients, 17(4), 622. https://doi.org/10.3390/nu17040622








