Investigating the Impact of Sorghum on Tau Protein Phosphorylation and Mitochondrial Dysfunction Modulation in Alzheimer’s Disease: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation and Polyphenol Extraction
2.2.2. Preparation of Aβ Oligomer
2.2.3. Cell Culture and Treatments
2.2.4. Preparation of Cell Lysates
2.2.5. Measurements of Intracellular Tau and Phosphorylated Tau
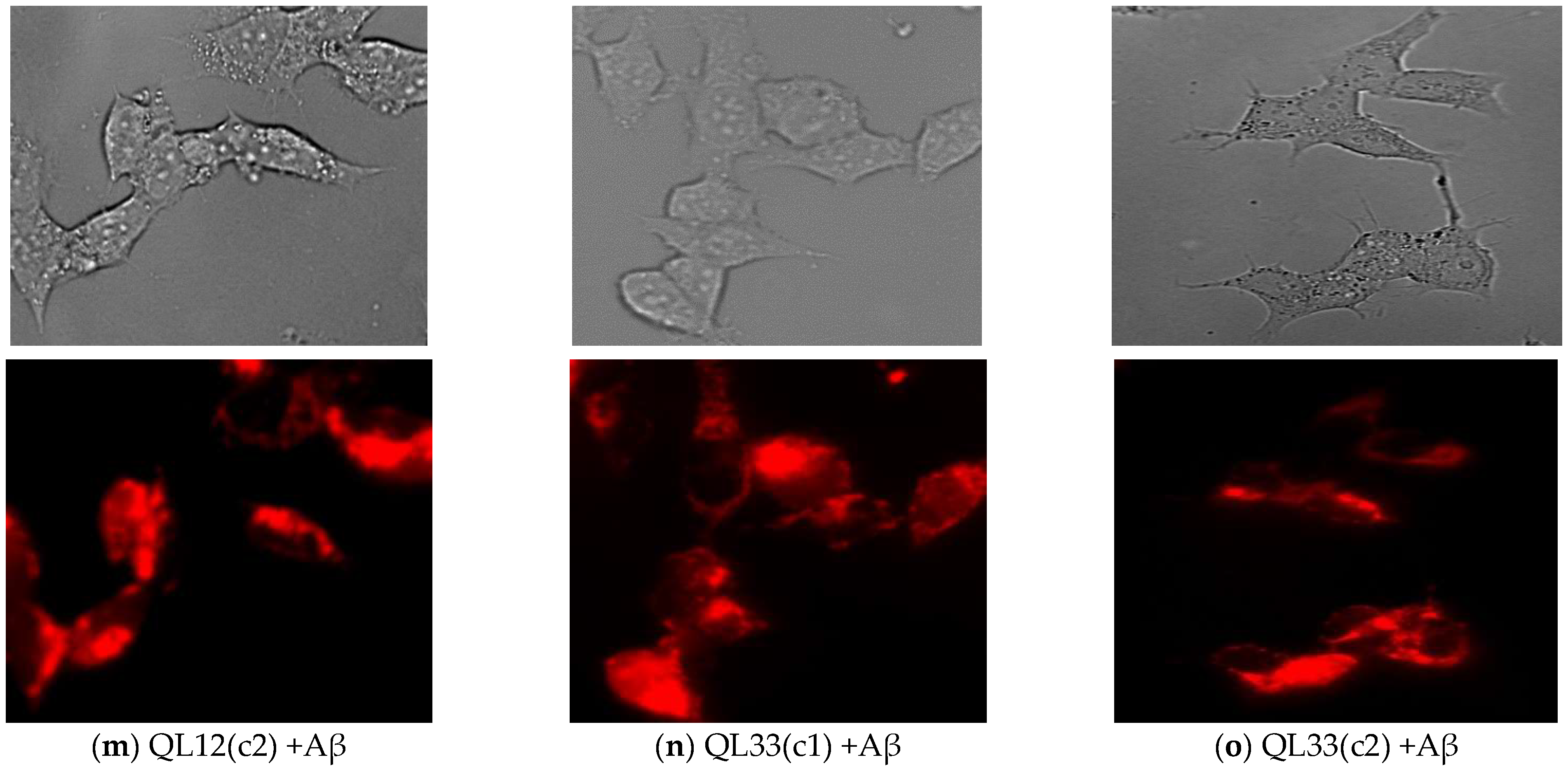
2.2.6. Mitochondrial Membrane Potential (Δψm)
Optimisation of TMRE
Live Cell Imaging
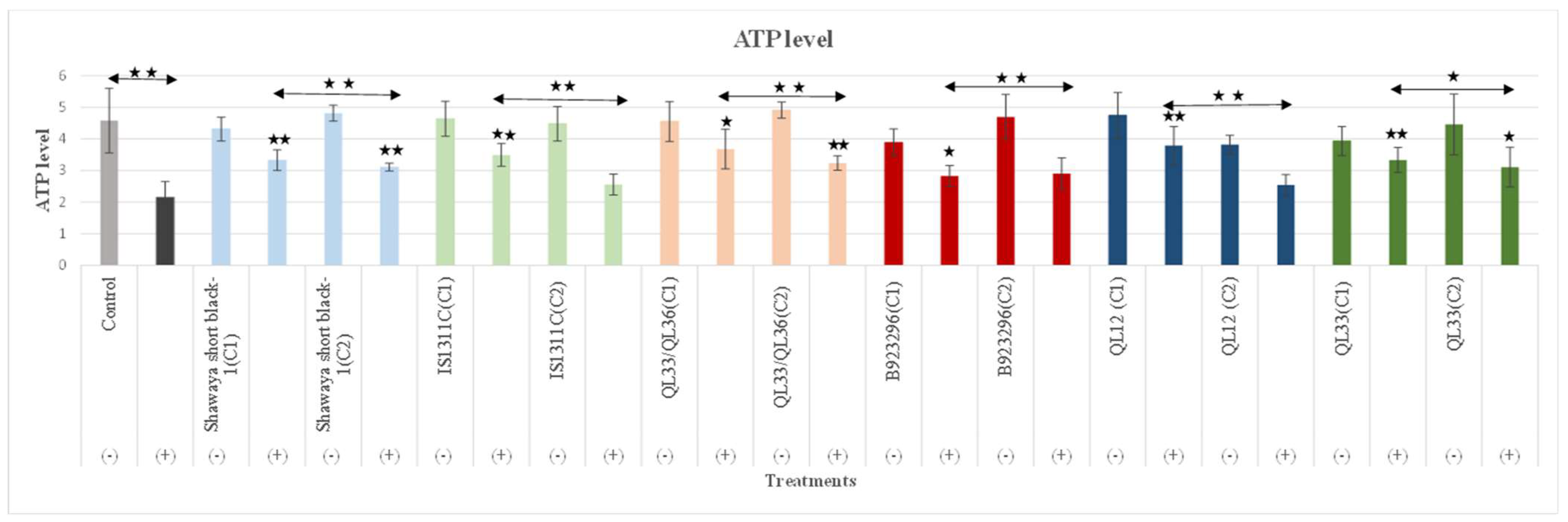
2.2.7. ATP Assay
Optimisation of CellTiter Glo ATP Detection Assay Kit
ATP Assay for Evaluation of Mitochondrial Function
Statistical Analysis
3. Results
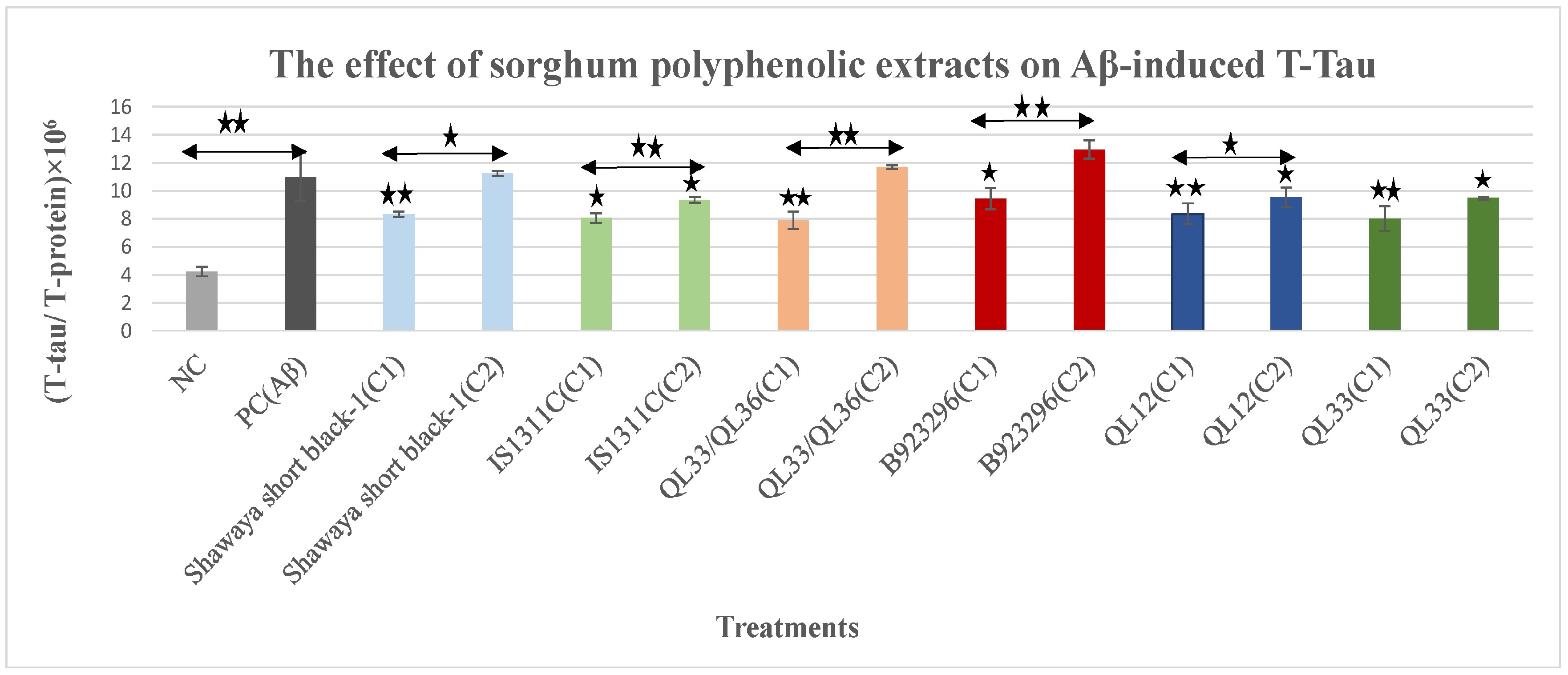
3.1. The Effect of Polyphenol Extract from Sorghum on Aβ42-Induced Total-Tau
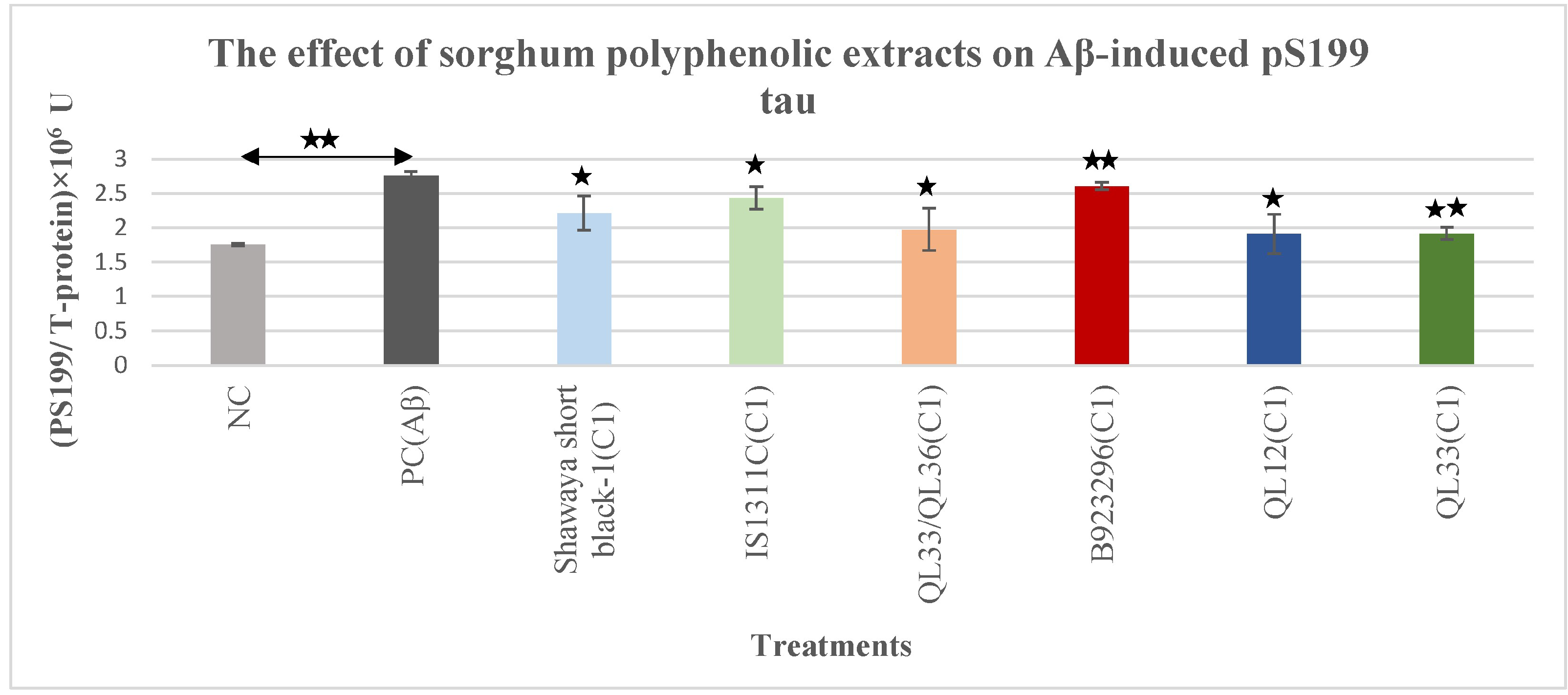
3.2. The Effect of Polyphenol-Rich Extract from Sorghum on Aβ-Induced Phospho-Tau pS199
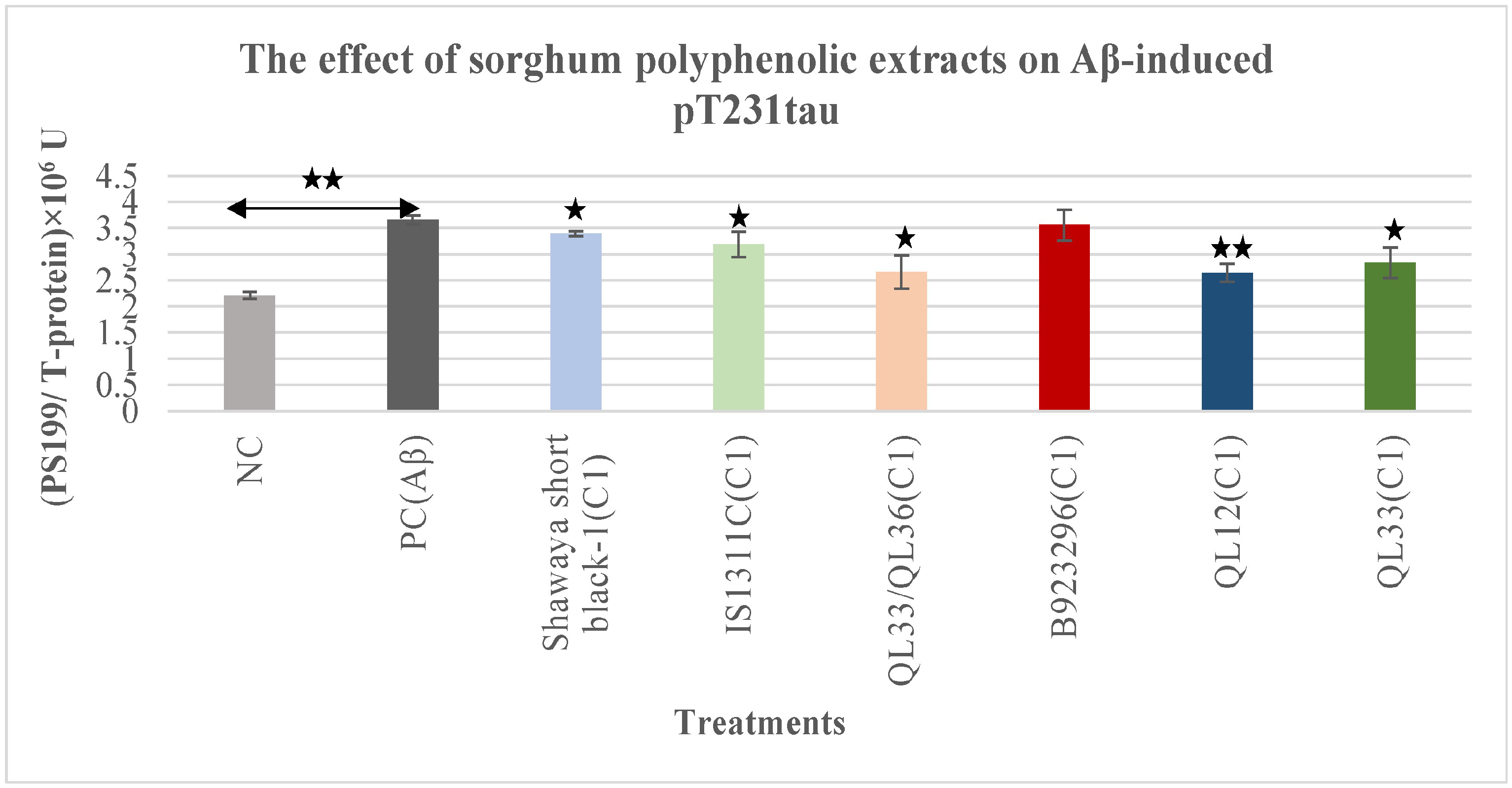
3.3. Effect on Aβ-Induced Phospho-Tau pT231
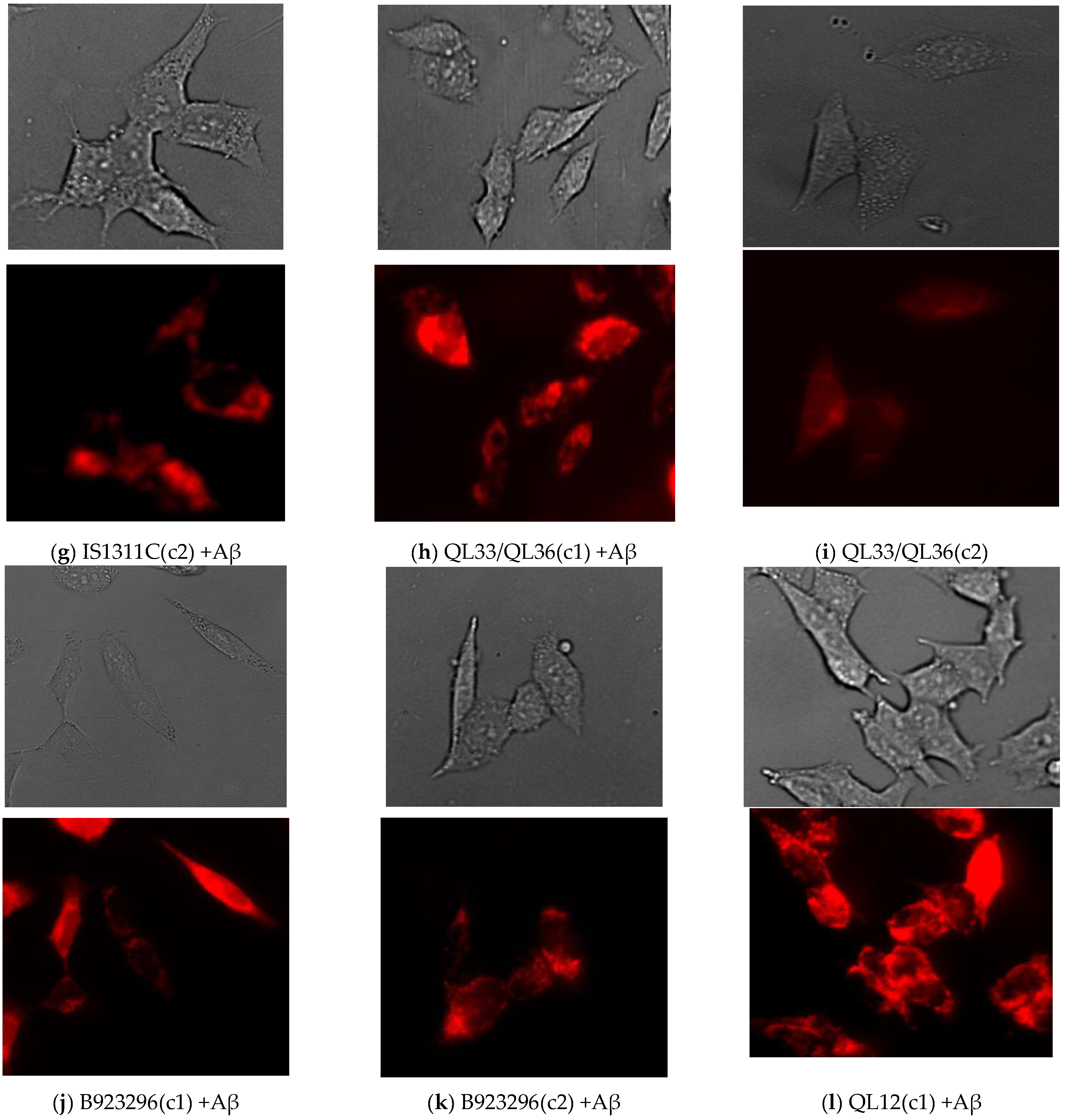
3.4. Effect of Sorghum Extracts on Mitochondrial Membrane Potential (Δψm)
3.5. Effect of Sorghum Extracts on ATP Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mecocci, P.; Boccardi, V.; Cecchetti, R.; Bastiani, P.; Scamosci, M.; Ruggiero, C.; Baroni, M. A Long Journey into Aging, Brain Aging, and Alzheimer’s Disease Following the Oxidative Stress Tracks. J. Alzheimers Dis. 2018, 62, 1319–1335. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Jiang, Z.F. Accumulated amyloid-beta peptide and hyperphosphorylated tau protein: Relationship and links in Alzheimer’s disease. J. Alzheimers Dis. 2009, 16, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Leuner, K.; Müller, W.E.; Reichert, A.S. From Mitochondrial Dysfunction to Amyloid Beta Formation: Novel Insights into the Pathogenesis of Alzheimer’s Disease. Mol. Neurobiol. 2012, 46, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.C.; Emerson, S.; Kesselheim, A.S. Evaluation of Aducanumab for Alzheimer Disease: Scientific Evidence and Regulatory Review Involving Efficacy, Safety, and Futility. JAMA 2021, 325, 1717–1718. [Google Scholar] [CrossRef]
- Canady, V.A. FDA approves first drug therapy for Alzheimer’s in 18 years. Ment. Health Wkly. 2021, 31, 3–4. [Google Scholar] [CrossRef]
- Mietelska-Porowska, A.; Wasik, U.; Goras, M.; Filipek, A.; Niewiadomska, G. Tau protein modifications and interactions: Their role in function and dysfunction. Int. J. Mol. Sci. 2014, 15, 4671–4713. [Google Scholar] [CrossRef]
- Binder, L.I.; Guillozet-Bongaarts, A.L.; Garcia-Sierra, F.; Berry, R.W. Tau, tangles, and Alzheimer’s disease. Biochim. Biophys. Acta 2005, 1739, 216–223. [Google Scholar] [CrossRef]
- Gamblin, T.C.; Chen, F.; Zambrano, A.; Abraha, A.; Lagalwar, S.; Guillozet, A.L.; Lu, M.; Fu, Y.; Garcia-Sierra, F.; LaPointe, N.; et al. Caspase cleavage of tau: Linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2003, 100, 10032–10037. [Google Scholar] [CrossRef]
- Llorens, F.; Villar-Piqué, A.; Candelise, N.; Ferrer, I.; Zerr, I. Tau Protein as a Biological Fluid Biomarker in Neurodegenerative Dementias. In Cognitive Disorders; Sibat, H.F., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Yoshida, H.; Hastie, C.J.; McLauchlan, H.; Cohen, P.; Goedert, M. Phosphorylation of microtubule-associated protein tau by isoforms of c-Jun N-terminal kinase (JNK). J. Neurochem. 2004, 90, 352–358. [Google Scholar] [CrossRef]
- Dong, D.-W.; Zhang, Y.-S.; Yang, W.-Y.; Wang-Qin, R.-Q.; Xu, A.-D.; Ruan, Y.-W. Hyperphosphorylation of tau protein in the ipsilateral thalamus after focal cortical infarction in rats. Brain Res. 2014, 1543, 280–289. [Google Scholar] [CrossRef]
- Mondragón-Rodríguez, S.; Mena, R.; Binder, L.I.; Smith, M.A.; Perry, G.; García-Sierra, F. Conformational changes and cleavage of tau in Pick bodies parallel the early processing of tau found in Alzheimer pathology. Neuropathol. Appl. Neurobiol. 2008, 34, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Arai, H.; Urakami, K.; Ishiguro, K.; Ohno, H.; Hampel, H.; Buerger, K.; Wiltfang, J.; Otto, M.; Kretzschmar, H.; et al. Large-scale, multicenter study of cerebrospinal fluid tau protein phosphorylated at serine 199 for the antemortem diagnosis of Alzheimer’s disease. Ann. Neurol. 2001, 50, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Meredith, J.E., Jr.; Sankaranarayanan, S.; Guss, V.; Lanzetti, A.J.; Berisha, F.; Neely, R.J.; Slemmon, J.R.; Portelius, E.; Zetterberg, H.; Blennow, K.; et al. Characterization of novel CSF Tau and ptau biomarkers for Alzheimer’s disease. PLoS ONE 2013, 8, e76523. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Buerger, K.; Zinkowski, R.; Teipel, S.J.; Goernitz, A.; Andreasen, N.; Sjoegren, M.; DeBernardis, J.; Kerkman, D.; Ishiguro, K.; et al. Measurement of Phosphorylated Tau Epitopes in the Differential Diagnosis of Alzheimer Disease: A Comparative Cerebrospinal Fluid Study. Arch. Gen. Psychiatry 2004, 61, 95–102. [Google Scholar] [CrossRef]
- Schwalbe, M.; Kadavath, H.; Biernat, J.; Ozenne, V.; Blackledge, M.; Mandelkow, E.; Zweckstetter, M. Structural Impact of Tau Phosphorylation at Threonine 231. Structure 2015, 23, 1448–1458. [Google Scholar] [CrossRef]
- Santos, J.R.F.; Bauer, C.; Schuchhardt, J.; Wedekind, D.; Waniek, K.; Lachmann, I.; Wiltfang, J.; Vogelgsang, J. Validation of a prototype tau Thr231 phosphorylation CSF ELISA as a potential biomarker for Alzheimer’s disease. J. Neural Transm. 2019, 126, 339–348. [Google Scholar] [CrossRef]
- Ewers, M.; Buerger, K.; Teipel, S.J.; Scheltens, P.; Schröder, J.; Zinkowski, R.P.; Bouwman, F.H.; Schönknecht, P.; Schoonenboom, N.S.M.; Andreasen, N.; et al. Multicenter assessment of CSF-phosphorylated tau for the prediction of conversion of MCI. Neurology 2007, 69, 2205–2212. [Google Scholar] [CrossRef]
- Buerger, K.; Zinkowski, R.; Teipel, S.J.; Tapiola, T.; Arai, H.; Blennow, K.; Andreasen, N.; Hofmann-Kiefer, K.; DeBernardis, J.; Kerkman, D.; et al. Differential Diagnosis of Alzheimer Disease with Cerebrospinal Fluid Levels of Tau Protein Phosphorylated at Threonine 231. Arch. Neurol. 2002, 59, 1267–1272. [Google Scholar] [CrossRef]
- Pagani, L.; Eckert, A. Amyloid-Beta Interaction with Mitochondria. Int. J. Alzheimers Dis. 2011, 2011, 925050. [Google Scholar] [CrossRef]
- Reddy, P.H. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer’s disease. Exp. Neurol. 2009, 218, 286–292. [Google Scholar] [CrossRef]
- Reddy, P.H.; Beal, M.F. Amyloid beta, mitochondrial dysfunction and synaptic damage: Implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol. Med. 2008, 14, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Prabhu, B.M.; Galati, D.F.; Avadhani, N.G.; Anandatheerthavarada, H.K. Accumulation of Amyloid Precursor Protein in the Mitochondrial Import Channels of Human Alzheimer’s Disease Brain Is Associated with Mitochondrial Dysfunction. J. Neurosci. 2006, 26, 9057–9068. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.D.; Parks, J.K. Cytochrome C Oxidase in Alzheimer’s Disease Brain. Purif. Charact. 1995, 45, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.M.; Santana, I.; Swerdlow, R.H.; Oliveira, C.R. Mitochondria dysfunction of Alzheimer’s disease cybrids enhances Aβ toxicity. J. Neurochem. 2004, 89, 1417–1426. [Google Scholar] [CrossRef]
- Bubber, P.; Haroutunian, V.; Fisch, G.; Blass, J.P.; Gibson, G.E. Mitochondrial abnormalities in Alzheimer brain: Mechanistic implications. Ann. Neurol. 2005, 57, 695–703. [Google Scholar] [CrossRef]
- Du Yan, S.; Shi, Y.; Zhu, A.; Fu, J.; Zhu, H.; Zhu, Y.; Gibson, L.; Stern, E.; Collison, K.; Al-Mohanna, F.; et al. Role of ERAB/l-3-Hydroxyacyl-coenzyme A Dehydrogenase Type II Activity in Aβ-induced Cytotoxicity. J. Biol. Chem. 1999, 274, 2145–2156. [Google Scholar] [CrossRef]
- Alikhani, N.; Ankarcrona, M.; Glaser, E. Mitochondria and Alzheimer’s disease: Amyloid-β peptide uptake and degradation by the presequence protease, hPreP. J. Bioenerg. Biomembr. 2009, 41, 447–451. [Google Scholar] [CrossRef]
- Tillement, L.; Lecanu, L.; Papadopoulos, V. Alzheimer’s disease: Effects of β-amyloid on mitochondria. Mitochondrion 2011, 11, 13–21. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef]
- Wobst, H.J.; Sharma, A.; Diamond, M.I.; Wanker, E.E.; Bieschke, J. The green tea polyphenol (−)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Lett. 2015, 589, 77–83. [Google Scholar] [CrossRef]
- Wang, J.; Santa-Maria, I.; Ho, L.; Ksiezak-Reding, H.; Ono, K.; Teplow, D.B.; Pasinetti, G.M. Grape derived polyphenols attenuate tau neuropathology in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2010, 22, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Zhao, D.; Wu, Q.; Simon, J.; Wang, J.; Radu, A.; Pasinetti, G.M. Pine Bark Polyphenolic Extract Attenuates Amyloid-β and Tau Misfolding in a Model System of Alzheimer’s Disease Neuropathology. J. Alzheimers Dis. 2020, 73, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Wang, X.-L.; Wu, J.-M.; Tang, Y.; Qiu, W.-Q.; Shen, X.; Teng, J.-F.; Pan, R.; Zhao, Y.; Yu, L.; et al. Polyphenols isolated from lychee seed inhibit Alzheimer’s disease-associated Tau through improving insulin resistance via the IRS-1/PI3K/Akt/GSK-3β pathway. J. Ethnopharmacol. 2020, 251, 112548. [Google Scholar] [CrossRef]
- Patil, S.P.; Tran, N.; Geekiyanage, H.; Liu, L.; Chan, C. Curcumin-induced upregulation of the anti-tau cochaperone BAG2 in primary rat cortical neurons. Neurosci. Lett. 2013, 554, 121–125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Camilleri, A.; Zarb, C.; Caruana, M.; Ostermeier, U.; Ghio, S.; Högen, T.; Schmidt, F.; Giese, A.; Vassallo, N. Mitochondrial membrane permeabilisation by amyloid aggregates and protection by polyphenols. Biochim. Biophys. Acta (BBA) Biomembr. 2013, 1828, 2532–2543. [Google Scholar] [CrossRef]
- Fuentealba, J.; Dibarrart, A.J.; Fuentes-Fuentes, M.C.; Saez-Orellana, F.; Quiñones, K.; Guzmán, L.; Perez, C.; Becerra, J.; Aguayo, L.G. Synaptic failure and adenosine triphosphate imbalance induced by amyloid-β aggregates are prevented by blueberry-enriched polyphenols extract. J. Neurosci. Res. 2011, 89, 1499–1508. [Google Scholar] [CrossRef]
- Li, G.; Luan, G.; He, Y.; Tie, F.; Wang, Z.; Suo, Y.; Ma, C.; Wang, H. Polyphenol Stilbenes from Fenugreek (Trigonella foenum-graecum L.) Seeds Improve Insulin Sensitivity and Mitochondrial Function in 3T3-L1 Adipocytes. Oxidative Med. Cell. Longev. 2018, 2018, 7634362. [Google Scholar] [CrossRef]
- Sandoval-Acuña, C.; Ferreira, J.; Speisky, H. Polyphenols and mitochondria: An update on their increasingly emerging ROS-scavenging independent actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef]
- Wu, G.; Johnson, S.K.; Bornman, J.F.; Bennett, S.J.; Fang, Z. Changes in whole grain polyphenols and antioxidant activity of six sorghum genotypes under different irrigation treatments. Food Chem. 2017, 214, 199–207. [Google Scholar] [CrossRef]
- Svensson, L.; Sekwati-Monang, B.; Lutz, D.L.; Schieber, A.; Ganzle, M.G. Phenolic acids and flavonoids in nonfermented and fermented red sorghum (Sorghum bicolor (L.) Moench). J. Agric. Food Chem. 2010, 58, 9214–9220. [Google Scholar] [CrossRef]
- Liu, L.; Chen, L.; Abbasi, A.M.; Wang, Z.; Li, D.; Shen, Y. Optimization of extraction of polyphenols from Sorghum moench using response surface methodology, and determination of their antioxidant activities. Trop. J. Pharm. Res. 2018, 17, 619–626. [Google Scholar] [CrossRef]
- Stine, W.B.; Jungbauer, L.; Yu, C.; LaDu, M.J. Preparing synthetic Abeta in different aggregation states. Methods Mol. Biol. 2011, 670, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, N.; Hone, E.; Sohrabi, H.R.; Johnson, S.; Zhong, L.; Chatur, P.; Gunzburg, S.; Martins, R.N.; Fernando, W.M.A.D.B. Sorghum Grain Polyphenolic Extracts Demonstrate Neuroprotective Effects Related to Alzheimer’s Disease in Cellular Assays. Foods 2024, 13, 1716. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Tran, K.C.; Zeng, A.Y.; Massa, S.M.; Longo, F.M. Small molecule modulation of the p75 neurotrophin receptor inhibits multiple amyloid beta-induced tau pathologies. Sci. Rep. 2020, 10, 20322. [Google Scholar] [CrossRef]
- Stancu, I.-C.; Vasconcelos, B.; Terwel, D.; Dewachter, I. Models of β-amyloid induced Tau-pathology: The long and “folded” road to understand the mechanism. Mol. Neurodegener. 2014, 9, 51. [Google Scholar] [CrossRef]
- Zheng, W.H.; Bastianetto, S.; Mennicken, F.; Ma, W.; Kar, S. Amyloid β peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience 2002, 115, 201–211. [Google Scholar] [CrossRef]
- Ma, Q.L.; Yang, F.; Rosario, E.R.; Ubeda, O.J.; Beech, W.; Gant, D.J.; Chen, P.P.; Hudspeth, B.; Chen, C.; Zhao, Y.; et al. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: Suppression by omega-3 fatty acids and curcumin. J. Neurosci. 2009, 29, 9078–9089. [Google Scholar] [CrossRef]
- Huang, H.-C.; Tang, D.; Xu, K.; Jiang, Z.-F. Curcumin attenuates amyloid-β-induced tau hyperphosphorylation in human neuroblastoma SH-SY5Y cells involving PTEN/Akt/GSK-3β signaling pathway. J. Recept. Signal Transduct. 2014, 34, 26–37. [Google Scholar] [CrossRef]
- Kim, D.I.; Lee, K.H.; Gabr, A.A.; Choi, G.E.; Kim, J.S.; Ko, S.H.; Han, H.J. Aβ-Induced Drp1 phosphorylation through Akt activation promotes excessive mitochondrial fission leading to neuronal apoptosis. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 2820–2834. [Google Scholar] [CrossRef]
- Alonso, A.C.; Li, B.; Grundke-Iqbal, I.; Iqbal, K. Mechanism of tau-induced neurodegeneration in Alzheimer disease and related tauopathies. Curr. Alzheimer Res. 2008, 5, 375–384. [Google Scholar] [CrossRef]
- Chukwu, J.E.; Pedersen, J.T.; Pedersen, L.Ø.; Volbracht, C.; Sigurdsson, E.M.; Kong, X.-P. Tau Antibody Structure Reveals a Molecular Switch Defining a Pathological Conformation of the Tau Protein. Sci. Rep. 2018, 8, 6209. [Google Scholar] [CrossRef] [PubMed]
- Llorens, F.; Schmitz, M.; Karch, A.; Cramm, M.; Lange, P.; Gherib, K.; Varges, D.; Schmidt, C.; Zerr, I.; Stoeck, K. Comparative analysis of cerebrospinal fluid biomarkers in the differential diagnosis of neurodegenerative dementia. Alzheimer’s Dement. 2016, 12, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Schoonenboom, N.S.; Reesink, F.E.; Verwey, N.A.; Kester, M.I.; Teunissen, C.E.; van de Ven, P.M.; Pijnenburg, Y.A.; Blankenstein, M.A.; Rozemuller, A.J.; Scheltens, P.; et al. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology 2012, 78, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Lewczuk, P.; Riederer, P.; O’Bryant, S.E.; Verbeek, M.M.; Dubois, B.; Visser, P.J.; Jellinger, K.A.; Engelborghs, S.; Ramirez, A.; Parnetti, L.; et al. Cerebrospinal fluid and blood biomarkers for neurodegenerative dementias: An update of the Consensus of the Task Force on Biological Markers in Psychiatry of the World Federation of Societies of Biological Psychiatry. World J. Biol. Psychiatry 2018, 19, 244–328. [Google Scholar] [CrossRef]
- Gong, E.J.; Park, H.R.; Kim, M.E.; Piao, S.; Lee, E.; Jo, D.-G.; Chung, H.Y.; Ha, N.-C.; Mattson, M.P.; Lee, J. Morin attenuates tau hyperphosphorylation by inhibiting GSK3β. Neurobiol. Dis. 2011, 44, 223–230. [Google Scholar] [CrossRef]
- Pillai, J.; Khrestian, M.; Bekris, L.; Safar, J.; Leverenz, J. Elevated cerebrospinal fluid total Tau levels reflects predominant cortical involvement in Alzheimer’s disease (P1.093). Neurology 2017, 88, P1.093. [Google Scholar] [CrossRef]
- Yeo, E.T.Y.; Wong, K.W.L.; See, M.L.; Wong, K.Y.; Gan, S.Y.; Chan, E.W.L. Piper sarmentosum Roxb. confers neuroprotection on beta-amyloid (Abeta)-induced microglia-mediated neuroinflammation and attenuates tau hyperphosphorylation in SH-SY5Y cells. J. Ethnopharmacol. 2018, 217, 187–194. [Google Scholar] [CrossRef]
- Sul, D.; Kim, H.-S.; Lee, D.; Joo, S.S.; Hwang, K.W.; Park, S.-Y. Protective effect of caffeic acid against beta-amyloid-induced neurotoxicity by the inhibition of calcium influx and tau phosphorylation. Life Sci. 2009, 84, 257–262. [Google Scholar] [CrossRef]
- Kim, J.H.; Wang, Q.; Choi, J.M.; Lee, S.; Cho, E.J. Protective role of caffeic acid in an Abeta25-35-induced Alzheimer’s disease model. Nutr. Res. Pract. 2015, 9, 480–488. [Google Scholar] [CrossRef]
- Silva, T.; Mohamed, T.; Shakeri, A.; Rao, P.P.N.; Soares da Silva, P.; Remião, F.; Borges, F. Repurposing nitrocatechols: 5-Nitro-α-cyanocarboxamide derivatives of caffeic acid and caffeic acid phenethyl ester effectively inhibit aggregation of tau-derived hexapeptide AcPHF6. Eur. J. Med. Chem. 2019, 167, 146–152. [Google Scholar] [CrossRef]
- Sousa, A.; Araújo, P.; Azevedo, J.; Cruz, L.; Fernandes, I.; Mateus, N.; de Freitas, V. Antioxidant and antiproliferative properties of 3-deoxyanthocyanidins. Food Chem. 2016, 192, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Awika, J.M.; Rooney, L.W.; Waniska, R.D. Properties of 3-deoxyanthocyanins from sorghum. J. Agric. Food Chem. 2004, 52, 4388–4394. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, X.; Zhang, N.; Ji, Z.; Ma, Z.; Fu, Q.; Qu, R.; Ma, S. Ferulic acid attenuates diabetes-induced cognitive impairment in rats via regulation of PTP1B and insulin signaling pathway. Physiol. Behav. 2017, 182, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, T.; Li, P.; Wei, N.; Zhao, Z.; Liang, H.; Ji, X.; Chen, W.; Xue, M.; Wei, J. The Ambiguous Relationship of Oxidative Stress, Tau Hyperphosphorylation, and Autophagy Dysfunction in Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2015, 2015, 352723. [Google Scholar] [CrossRef]
- Peterson, D.W.; George, R.C.; Scaramozzino, F.; LaPointe, N.E.; Anderson, R.A.; Graves, D.J.; Lew, J. Cinnamon Extract Inhibits Tau Aggregation Associated with Alzheimer’s Disease In Vitro. J. Alzheimers Dis. 2009, 17, 585–597. [Google Scholar] [CrossRef]
- Snow, A.D.; Castillo, G.M.; Nguyen, B.P.; Choi, P.Y.; Cummings, J.A.; Cam, J.; Hu, Q.; Lake, T.; Pan, W.; Kastin, A.J.; et al. The Amazon rain forest plant Uncaria tomentosa (cat’s claw) and its specific proanthocyanidin constituents are potent inhibitors and reducers of both brain plaques and tangles. Sci. Rep. 2019, 9, 561. [Google Scholar] [CrossRef]
- Pasinetti, G.M. Novel role of red wine-derived polyphenols in the prevention of Alzheimer’s disease dementia and brain pathology: Experimental approaches and clinical implications. Planta Med. 2012, 78, 1614–1619. [Google Scholar] [CrossRef]
- Resende, R.; Ferreiro, E.; Pereira, C.; Oliveira, C.R. ER stress is involved in Aβ-induced GSK-3β activation and tau phosphorylation. J. Neurosci. Res. 2008, 86, 2091–2099. [Google Scholar] [CrossRef]
- Golpich, M.; Amini, E.; Mohamed, Z.; Azman Ali, R.; Mohamed Ibrahim, N.; Ahmadiani, A. Mitochondrial Dysfunction and Biogenesis in Neurodegenerative diseases: Pathogenesis and Treatment. CNS Neurosci. Ther. 2017, 23, 5–22. [Google Scholar] [CrossRef]
- Moreira, P.I.; Carvalho, C.; Zhu, X.; Smith, M.A.; Perry, G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2010, 1802, 2–10. [Google Scholar] [CrossRef]
- Qi, G.; Mi, Y.; Fan, R.; Zhao, B.; Ren, B.; Liu, X. Tea polyphenols ameliorates neural redox imbalance and mitochondrial dysfunction via mechanisms linking the key circadian regular Bmal1. Food Chem. Toxicol. 2017, 110, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pozo, C.; Mizgier, M.L.; Speisky, H.; Gotteland, M. Differential protective effects of quercetin, resveratrol, rutin and epigallocatechin gallate against mitochondrial dysfunction induced by indomethacin in Caco-2 cells. Chem. Biol. Interact. 2012, 195, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, E.; Sanchez-Gomez, M.V.; Ruiz, A.; Cavaliere, F.; Ortiz-Sanz, C.; Quintela-Lopez, T.; Capetillo-Zarate, E.; Sole-Domenech, S.; Matute, C. Mangiferin and Morin Attenuate Oxidative Stress, Mitochondrial Dysfunction, and Neurocytotoxicity, Induced by Amyloid Beta Oligomers. Oxid. Med. Cell. Longev. 2018, 2018, 2856063. [Google Scholar] [CrossRef]
- Kausar, S.; Wang, F.; Cui, H. The Role of Mitochondria in Reactive Oxygen Species Generation and Its Implications for Neurodegenerative Diseases. Cells 2018, 7, 274. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Q.; Sun, X.-B.; Xu, Y.-X.; Zhao, H.; Zhu, Q.-Y.; Zhu, C.-Q. Astaxanthin upregulates heme oxygenase-1 expression through ERK1/2 pathway and its protective effect against beta-amyloid-induced cytotoxicity in SH-SY5Y cells. Brain Res. 2010, 1360, 159–167. [Google Scholar] [CrossRef]
- Yu, H.; Yao, L.; Zhou, H.; Qu, S.; Zeng, X.; Zhou, D.; Zhou, Y.; Li, X.; Liu, Z. Neuroprotection against Aβ25–35-induced apoptosis by Salvia miltiorrhiza extract in SH-SY5Y cells. Neurochem. Int. 2014, 75, 89–95. [Google Scholar] [CrossRef]
- Zeng, K.-W.; Ko, H.; Yang, H.O.; Wang, X.-M. Icariin attenuates β-amyloid-induced neurotoxicity by inhibition of tau protein hyperphosphorylation in PC12 cells. Neuropharmacology 2010, 59, 542–550. [Google Scholar] [CrossRef]
- Schofield, J.H.; Schafer, Z.T. Mitochondrial Reactive Oxygen Species and Mitophagy: A Complex and Nuanced Relationship. Antioxid. Redox Signal. 2021, 34, 517–530. [Google Scholar] [CrossRef]
- Tan, M.A.; An, S.S.A. Neuroprotective potential of the oxindole alkaloids isomitraphylline and mitraphylline in human neuroblastoma SH-SY5Y cells. 3 Biotech 2020, 10, 517. [Google Scholar] [CrossRef]
- Arbex, P.M.; Moreira, M.E.d.C.; Toledo, R.C.L.; de Morais Cardoso, L.; Pinheiro-Sant’ana, H.M.; Benjamin, L.d.A.; Licursi, L.; Carvalho, C.W.P.; Queiroz, V.A.V.; Martino, H.S.D. Extruded sorghum flour (Sorghum bicolor L.) modulate adiposity and inflammation in high fat diet-induced obese rats. J. Funct. Foods 2018, 42, 346–355. [Google Scholar] [CrossRef]
- Perez-Ternero, C.; Werner, C.M.; Nickel, A.G.; Herrera, M.D.; Motilva, M.-J.; Böhm, M.; Alvarez de Sotomayor, M.; Laufs, U. Ferulic acid, a bioactive component of rice bran, improves oxidative stress and mitochondrial biogenesis and dynamics in mice and in human mononuclear cells. J. Nutr. Biochem. 2017, 48, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yang, C.; Liu, R.; Ni, W. Effects of three phenolic compounds on mitochondrial function and root vigor of cotton (Gossypium hirsutum L.) seedling roots. Acta Physiol. Plant. 2019, 41, 60. [Google Scholar] [CrossRef]
- Chen, J.-L.; Duan, W.-J.; Luo, S.; Li, S.; Ma, X.-H.; Hou, B.-N.; Cheng, S.-Y.; Fang, S.-H.; Wang, Q.; Huang, S.-Q.; et al. Ferulic acid attenuates brain microvascular endothelial cells damage caused by oxygen-glucose deprivation via punctate-mitochondria-dependent mitophagy. Brain Res. 2017, 1666, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Cagide, F.; Benfeito, S.; Soares, P.; Garrido, J.; Baldeiras, I.; Ribeiro, J.A.; Pereira, C.M.; Silva, A.F.; Andrade, P.B.; et al. Development of a Mitochondriotropic Antioxidant Based on Caffeic Acid: Proof of Concept on Cellular and Mitochondrial Oxidative Stress Models. J. Med. Chem. 2017, 60, 7084–7098. [Google Scholar] [CrossRef]
- Kumaran, K.S.; Prince, P.S.M. Caffeic acid protects rat heart mitochondria against isoproterenol-induced oxidative damage. Cell Stress Chaperones 2010, 15, 791–806. [Google Scholar] [CrossRef]
- Barros Silva, R.; Santos, N.A.G.; Martins, N.M.; Ferreira, D.A.S.; Barbosa, F.; Oliveira Souza, V.C.; Kinoshita, Â.; Baffa, O.; Del-Bel, E.; Santos, A.C. Caffeic acid phenethyl ester protects against the dopaminergic neuronal loss induced by 6-hydroxydopamine in rats. Neuroscience 2013, 233, 86–94. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezaee, N.; Hone, E.; Sohrabi, H.; Abdulraheem, R.; Johnson, S.K.; Gunzburg, S.; Martins, R.N.; Fernando, W.M.A.D.B. Investigating the Impact of Sorghum on Tau Protein Phosphorylation and Mitochondrial Dysfunction Modulation in Alzheimer’s Disease: An In Vitro Study. Nutrients 2025, 17, 516. https://doi.org/10.3390/nu17030516
Rezaee N, Hone E, Sohrabi H, Abdulraheem R, Johnson SK, Gunzburg S, Martins RN, Fernando WMADB. Investigating the Impact of Sorghum on Tau Protein Phosphorylation and Mitochondrial Dysfunction Modulation in Alzheimer’s Disease: An In Vitro Study. Nutrients. 2025; 17(3):516. https://doi.org/10.3390/nu17030516
Chicago/Turabian StyleRezaee, Nasim, Eugene Hone, Hamid Sohrabi, Rasheed Abdulraheem, Stuart K. Johnson, Stuart Gunzburg, Ralph N. Martins, and W. M. A. D. Binosha Fernando. 2025. "Investigating the Impact of Sorghum on Tau Protein Phosphorylation and Mitochondrial Dysfunction Modulation in Alzheimer’s Disease: An In Vitro Study" Nutrients 17, no. 3: 516. https://doi.org/10.3390/nu17030516
APA StyleRezaee, N., Hone, E., Sohrabi, H., Abdulraheem, R., Johnson, S. K., Gunzburg, S., Martins, R. N., & Fernando, W. M. A. D. B. (2025). Investigating the Impact of Sorghum on Tau Protein Phosphorylation and Mitochondrial Dysfunction Modulation in Alzheimer’s Disease: An In Vitro Study. Nutrients, 17(3), 516. https://doi.org/10.3390/nu17030516






