Subclinical Carotid Disease Is Associated with Low Serum Vitamin D in Nondiabetic Middle-Aged Hypertensive Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Laboratory Measurements and Vitamin D Assessment
2.3. Carotid B-Mode Ultrasonography
| • Coefficient of distensibility (Distensibility) | 2 × (SD-DD)/DD/pulse pressure (10−3/kPa) |
| • Young’s elastic modulus (Young) | DD/(IMT × Distensibility) (103/kPa) |
| • Beta-stiffness (β-stiffness) | Ln (systolic BP/diastolic BP)/[(SD-DD)/DD] |
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Risk factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.B.; Nahas-Neto, J.; Bueloni-Dias, F.; Ferreira Poloni, P.; Lera Orsatti, C.; Aguiar Petri Nahas, E. Vitamin D deficiencyis associated with metabolic syndrome in postmenopusal women. Maturitas 2018, 107, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.; Qayyum, R. Relationship between 25-hydroxyvitamin D and all-cause and cardiovascular disease mortality. Am. J. Med. 2013, 126, 509–514. [Google Scholar] [CrossRef]
- Pilz, S.; Gaksch, M.; Kienreich, K.; Grϋbler, M.; Verheien, N.; Fahrleitner-Pammer, A.; Treiber, G.; Drechsler, C.; Ó Hartaigh, B.; Obermayer-Pietsch, B.; et al. Effects of vitamin D on blood pressure and cardiovascular risk factors: A randomized controlled trial. Hypertension 2015, 65, 1195–1201. [Google Scholar] [CrossRef]
- Polak, J.F.; Szklo, M.; Kronmal, R.A.; Burke, G.L.; Shea, S.; Zavodni, D.H.; O’Leary, D.H. The value of carotid artery plaque and intima-media thickness (IMT) for incident cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2013, 2, e000087. [Google Scholar] [CrossRef]
- van der Meer, I.M.; Bota, M.L.; Hofman, A.; del Sol, A.I.; van der Kuip, D.A.M.; Witterman, J.C.M. Predictive value of noninvasive measurements of atherosclerosis for incident myocardial infarction: The Rotterdam Study. Circulation 2004, 109, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Nambi, V.; Chambless, L.; Folson, A.R.; He, M.; Hu, Y.; Mosley, T.; Volcik, K.; Boerwinkle, E.; Ballantyne, C.M. Carotid intima-media thickness and presence or absence of plaques improve prediction of coronary heart disease. The ARIC (Atherosclerosis Risk in Communities) Study. J. Am. Coll. Cardiol. 2010, 55, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Gariepy, J.; Chironi, G.; Megnien, J.L.; Levenson, J. Intima-media thickness: A new tool for diagnosis and treatment of cardiovascular risk. J. Hypertens. 2002, 20, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guallar, E.; Qiao, Y.; Wasserman, B.A. Is carotid intima-media thickness as predictive as other noninvasive techniques for the detection of coronary artery disease? Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Fallo, F.; Catena, C.; Camozzi, V.; Luisetto, G.; Cosma, C.; Plebani, M.; Lupia, M.; Tona, F.; Sechi, L.A. Low serum 25-hydroxyvitamin D levels are associated with left ventricular hypertrophy in arterial hypertension. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Brosolo, G.; Da Porto, A.; Catena, C.; Sechi, L.A. Arterial stiffening in hypertension: Is it just high blood pressure. Rev. Cardiovasc. Med. 2021, 22, 1073–1075. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.-W.; Liu, J.-H.; Xiao, H.-N.; Yang, Y.-F.; Dong, W.-J.; Zhang, Q.-B.; Liu, L.; He, C.-S.; Wu, B.-H. Relationship between plasma levels of 25-hydroxyvitamin D and arterial stiffness in elderly Chinese with non-dipper hypertension. An observational study. Medicine 2020, 99, 7. [Google Scholar] [CrossRef]
- Kuloglu, O.; Gur, M.; Seker, T.; Kalkan, G.Y.; Sahin, D.Y.; Tanboga, I.H.; Koyunsevewr, N.Y.; Harbaloglu, H.; Turkoglu, C.; Akyol, S.; et al. Serum 25-hydroxyvitamin D level is associated with arterial stiffness, left ventricle hypertrophy, and inflammation in newly diagnosed hypertension. J. Investig. Med. 2013, 61, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Gürel, O.M.; Bilgic, A.; Demircelik, B.; Ozaydin, M.; Bozduman, F.; Aytürk, Z.; Yilmaz, H.; Atar, A.; Selcoki, Y.; Eryonucu, B. The relationship between 25-hydroxyvitamin D levels and ambulatory arterial stiffness index in newly diagnosed and never-treated hypertensive patients. Blood Press. Monit. 2016, 21, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Cakal, S.; Cakal, B.; Karaca, O. Association of vitamin D deficiency with arterial stiffness in newly diagnosed hypertension. Blood Press. Monit. 2021, 26, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Simons, P.C.; Algra, A.; Bots, M.L.; Grobbee, D.E.; van der Graaf, Y. Common carotid intima-media thickness and arterial stiffness: Indicators of cardiovascular risk in high-risk patients. The SMART Study (Second Manifestations of ARTerial disease). Circulation 1999, 100, 951–957. [Google Scholar] [CrossRef]
- Liao, D.; Arnett, D.K.; Tyroler, H.A.; Riley, W.A.; Chambless, L.E.; Szklo, M.; Heiss, G. Arterial stiffness and the development of hypertension. The ARIC study. Hypertension 1999, 34, 201–206. [Google Scholar] [CrossRef]
- Hilger, J.; Friedel, A.; Herr, R.; Rausch, T.; Roos, F.; Wahl, D.A.; Pierroz, D.D.; Weber, P.; Hoffmann, K. A systematic review ofvitamin D status in population worldwide. Br. J. Nutr. 2014, 111, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.P.; von Mühlen, D.; Michos, E.D.; Miller, E.R.; Appel, L.J.; Araneta, M.R.; Barrett-Connor, E. Serum vitamin D, parathyroid hormone levels, and carotid atherosclerosis. Atherosclerosis 2009, 207, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-W.; Chou, R.-H.; Liu, L.-K.; Chen, L.-K.; Huang, P.-H.; Lin, S.-J. The relationship between circulating vitamin D3 and subclinical atherosclerosis in an elderly Asian population. Sci. Rep. 2020, 10, 18704. [Google Scholar]
- Sechi, L.A.; Zingaro, L.; Catena, C.; Perin, A.; De Marchi, S.; Bartoli, E. Lipoprotein(a) and apolipoprotein(a) isoforms and proteinuria in patients with moderate renal failure. Kidney Int. 1999, 56, 1049–1057. [Google Scholar] [CrossRef]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E. 2023 ESH guidelines for the management of arterial hypertension. The task force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [PubMed]
- Catena, C.; Brosolo, G.; Da Porto, A.; Donnini, D.; Bulfone, L.; Vacca, A.; Soardo, G.; Sechi, L.A. Association of non-alcoholic fatty liver disease with left ventricular changes in treatment-näive patients with uncomplicated hypertension. Front. Cardiovasc. Med. 2022, 9, 1030968. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011, 34, S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Catena, C.; Novello, M.; Dotto, L.; De Marchi, S.; Sechi, L.A. Serum lipoprotein(a) concentrations and alcohol consumption inhypertension: Possible relevance for cardiovascular damage. J. Hypertens. 2003, 21, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Brosolo, G.; Da Porto, A.; Bulfone, L.; Vacca, A.; Bertin, N.; Catena, C.; Sechi, L.A. Cortisol secretion and abnormalities of glucose metabolism in nondiabetic patients with hypertension. J. Hypertens. 2024, 42, 227–235. [Google Scholar] [CrossRef]
- Brosolo, G.; Da Porto, A.; Bulfone, L.; Scandolin, L.; Vacca, A.; Bertin, N.; Vivarelli, C.; Sechi, L.A.; Catena, C. Vitamin D deficiency is associated with glycometabolic changes in nondiabetic patients with arterial hypertension. Nutrients 2022, 14, 311. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Bikley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment and prevention of vitamin D deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, S.; Rundek, T.; Ramas, R.; Elkind, M.S.V.; Cho Paik, M.; Boden-Albala, B.; Sacco, R.L. Carotid plaque surface irregularity predicts ischemic stroke the Northern Manhattan Study. Stroke 2006, 37, 2696–2701. [Google Scholar] [CrossRef] [PubMed]
- Rundek, T.; Arif, H.; Boden-Albala, B.; Elkind, M.S.; Paik, M.C.; Sacco, R.L. Carotid plaques, a subclinical precursor of vascular events: The Northern Manhattan Study. Neurology 2008, 70, 1200–1207. [Google Scholar] [CrossRef]
- Catena, C.; Colussi, G.; Url-Michitsch, M.; Nait, F.; Sechi, L.A. Subclinical carotid artery disease and plasma homocysteine levels in patients with hypertension. J. Am. Soc. Hypertens. 2015, 9, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.H.; Korcarz, C.E.; Todd Hurst, R.; Lonn, E.; Kendall, C.B.; Mohler, E.R.; Najjar, S.S.; Rembold, C.M.; Post, S. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A Consensus Statement from the American Society of Echocardiography Carotid Intima-Media Thickness task Force. J. Am. Soc. Echocardiogr. 2008, 21, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, E.; Bozec, E.; Gemignani, V.; Faita, F.; Giannarelli, C.; Ghiadoni, L.; Demi, L.; Boutouyrie, P.; Laurent, S. Assessment of carotid stiffness and intima-media thickness from ultrasound data: Comparison between two methods. J. Ultrasound. Med. 2010, 29, 1169–1175. [Google Scholar] [CrossRef]
- Catena, C.; Colussi, G.; Fagotto, V.; Sechi, L.A. Decreased fibrinolytic activity is associated with carotid artery stiffening in arterial hypertension. J. Res. Med. Sci. 2017, 22, 57. [Google Scholar] [CrossRef] [PubMed]
- Bots, M.L.; Dijk, J.M.; Oren, A.; Grobbee, D.E. Carotid intima-media thickness, arterial stiffness and risk of cardiovascular disease: Current evidence. J. Hypertens. 2002, 20, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Aminbakhsh, A.; Manani, G.B. Carotid intima-media thickness measurement: What defines an abnormality? A systematic review. Clin. Invest. Med. 1992, 22, 149–157. [Google Scholar]
- Polak, J.F.; Pencina, M.J.; Meisner, A.; Pencina, K.M.; Brown, L.S.; Wolf, P.A.; D’Agostino, R.B.S. Associations of carotid artery intima-media thickness (IMT) with risk factors and prevalence of cardiovascular disease: Comparison of mean common artery IMT with maximum internal carotid artery IMT. J. Ultrasound Med. 2010, 29, 1759–1768. [Google Scholar] [CrossRef]
- Kablak-Ziembicka, A.; Przewlocki, T.; Sokolowski, A.; Tracz, W.; Podolee, P. Carotid intima-media thickness, hs-CRP and TNF-a are independently associated with cardiovascular event risk in patients with atherosclerotic occlusive disease. Atherosclerosis 2011, 214, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, V.; Minonne, R.; De Matthaeis, A.; Annese, M.A.; Tabacco, P.; D’Arcangelo, P.; D’Amico, G.; Scillitani, A. Carotid intima-media thickness is not associated with vitamin D and PTH levels in patients admitted to an Internal Medicine Department. Endocrine 2014, 47, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, V.; Mehrpour, M.; Ghoreishi, A.; Kamali, K.; Zamani, B. The association between serum 25-hydroxyvitamin D levels and subclinical atherosclerosis in healthy population. Curr. J. Neurol. 2020, 19, 53–58. [Google Scholar] [CrossRef]
- Sypniewska, G.; Pollak, J.; Strozeki, P.; Kretowicz, M.; Janikowski, G.; Mankowska-Cyl, A.; Pater, A.; Manitius, J. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am. J. Hypertens. 2014, 27, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Gepner, A.D.; Colangelo, L.A.; Blondon, M.; Korcarz, C.E.; de Boer, I.H.; Kestenbaum, B.; Siscovick, D.S.; Kaufman, J.D.; Liu, K.; Stein, J.H. 25-hydroxyvitamin D and parathyroid hormone levels do not predict changes in carotid arterial stiffness: The Multi-Ethnic Study of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1102–1109. [Google Scholar] [CrossRef]
- Giallauria, F.; Milaneschi, Y.; Tanaka, T.; Maggio, M.; Canepa, M.; Elango, P.; Vigorito, C.; Lakatta, E.G.; Ferrucci, L.; Strait, J. Arterial stiffness and vitamin D levels: The Baltimore longitudinal study of aging. J. Clin. Endocrinol. Metab. 2012, 97, 3717–3723. [Google Scholar] [CrossRef]
- Seker, T.; Gür, M.; Kuloglu, O.; Kalkan, G.Y.; Sahin, D.Y.; Turkoglu, C.; Elbasan, Z.; Baykan, A.O.; Gozubuyuk, G.; Cayli, M. Serum 25-hydroxyvitamin D is associated with both arterial and ventricular stiffness in healthy subjects. J. Cardiol. 2013, 62, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Suthar, O.P.; Mathur, S.; Gupta, V.; Agarwal, H.; Mathur, A.; Singh, P.; Sharma, S.L. Study of correlation of serum vitamin D levels with arterial stiffness and cardiovascular morbidity in elderly individuals of western Rajasthan. J. Assoc. Physicians India 2018, 66, 18–21. [Google Scholar] [PubMed]
- McGreevy, C.; Barry, M.; Davenport, C.; Byrne, B.; Donaghy, C.; Collier, G.; Tormey, W.; Smith, D.; Bennett, K.; Williams, D. The effect of vitamin D supplementation on arterial stiffness in an elderly community-based population. J. Am Soc. Hypertens. 2015, 9, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Valcheva, P.; Cardus, A.; Panizo, S.; Parisi, E.; Bozic, M.; Lopez Novoa, J.M.; Dusso, A.; Fernandez, E.; Valdivielso, J.M. Lack of vitamin D receptor causes stress-induced premature senescence in vascular smooth muscle cells through enhanced local angiotensin-II signals. Atherosclerosis 2014, 235, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Zhu, X.; Guo, Y.; Yang, Y.; Jiang, Y.; Liu, B. Active vitamin D regulates macrophage M1/M2 phenotypes via the STAT-1-TREM-1 pathway in diabetic nephropathy. J. Cell. Physiol. 2019, 234, 6917–6926. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, V.; Kasapoglu, P.; Zamani, A.; Basiri, Z.; Tahamoli-Roudsari, A.; Alahgholi-Hajibehzad, M. Vitamin D3 inhibits the proliferation of T helper cells, downregulate CD4(+) T cell cytokines and upregulate inhibitory markers. Hum. Immunol. 2018, 79, 439–445. [Google Scholar] [CrossRef]


| Variables | All Patients (n = 223) | 25(OH)D ≥30 ng/mL (n = 92) | 25(OH)D 21–29 ng/mL (n = 51) | 25(OH)D <21 ng/mL (n = 80) | p Value |
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| Age, y | 50 ± 13 | 46 ± 12 | 52 ± 14 | 53 ± 12 | 0.001 |
| Males, n (%) | 120 (54) | 47 (51) | 31 (61) | 42 (53) | 0.515 |
| BMI, kg/m2 | 27.5 ± 4.7 | 27.0 ± 5.0 | 27.5 ± 4.3 | 28.4 ± 4.8 | 0.158 |
| Systolic BP, mm Hg | 149 ± 18 | 146 ± 18 | 148 ± 19 | 152 ± 17 | 0.089 |
| Diastolic BP, mm Hg | 92 ± 13 | 92 ± 11 | 90 ± 12 | 93 ± 14 | 0.400 |
| Duration of hypertension, y | 8 ± 9 | 6 ± 8 | 8 ± 8 | 10 ± 11 | 0.019 |
| Antihypertensive Tx, n (%) | 129 (58) | 46 (50) | 31 (61) | 52 (65) | 0.124 |
| Alcohol consumption, g/day | 9 ± 18 | 8 ± 16 | 11 ± 23 | 8 ± 14 | 0.551 |
| Smokers, n (%) | 52 (23) | 21 (23) | 12 (24) | 19 (24) | 0.989 |
| Physically active, n (%) | 53 (24) | 22 (24) | 16 (31) | 15 (19) | 0.254 |
| Season | |||||
| Spring, n (%) | 65 (29) | 22 (24) | 15 (29) | 28 (35) | 0.280 |
| Summer, n (%) | 30 (13) | 20 (22) | 6 (12) | 4 (5) | 0.005 |
| Fall, n (%) | 58 (26) | 27 (29) | 10 (20) | 21 (26) | 0.445 |
| Winter, n (%) | 70 (31) | 23 (25) | 20 (39) | 27 (34) | 0.183 |
| Summer/autumn | 88 (39) | 47 (51) | 16 (31) | 25 (31) | 0.012 |
| Blood biochemistries | |||||
| Creatinine, mg/dL | 0.92 ± 0.22 | 0.93 ± 0.17 | 0.89 ± 0.17 | 0.92 ± 0.29 | 0.579 |
| GFR, mL/min·1.73 m2 | 100 ± 27 | 102 ± 27 | 101 ± 25 | 97 ± 29 | 0.467 |
| Sodium, mmol/L | 141 ± 2 | 141 ± 2 | 141 ± 3 | 141 ± 3 | 1.000 |
| Potassium, mmol/L | 4.07 ± 0.38 | 4.04 ± 0.35 | 4.11 ± 0.41 | 4.07 ± 0.40 | 0.576 |
| Glucose, mg/dL | 91 ± 13 | 89 ± 9 | 92 ± 12 | 93 ± 11 | 0.037 |
| Glycated hemoglobin, % | 5.6 ± 0.6 | 5.5 ± 0.4 | 5.6 ± 0.5 | 5.6 ± 0.8 | 0.466 |
| Insulin, µUI/mL | 7.3 (4.1–11.5) | 6.3 (4.0–10.0) | 6.8 (3.5–11.6) | 8.8 (49–12.0) | 0.044 |
| HOMA index | 1.60 (0.85–2.62) | 1.33 (0.84–1.99) | 1.51 (0.73–2.78) | 1.95 (1.13–3.01) | 0.044 |
| Triglycerides, mg/dL | 117 ± 66 | 105 ± 55 | 120 ± 63 | 129 ± 77 | 0.055 |
| Cholesterol, mg/dL | 198 ± 41 | 197 ± 41 | 196 ± 43 | 201 ± 39 | 0.739 |
| HDL cholesterol, mg/dL | 57 ± 19 | 60 ± 18 | 56 ± 16 | 55 ± 21 | 0.188 |
| LDL cholesterol, mg/dL | 118 ± 36 | 115 ± 34 | 115 ± 37 | 122 ± 37 | 0.376 |
| Renin, mUI/mL | 9.7 (5.0–19.8) | 10.9 (5.9–19.7) | 8.9 (6.2–18.0) | 8.9 (4.6–19.4) | 0.771 |
| Aldosterone, pg/mL | 123 ± 78 | 123 ± 82 | 109 ± 72 | 131 ± 77 | 0.296 |
| 25(OH)D, mg/mL | 25.4 (16.4–36.0) | 38.0 (34.0–55.1) | 24.5 (22.4–26.0) | 14.4 (10.6–17.7) | <0.001 |
| 1,25(OH)D, pg/mL | 59 (43–95.0) | 79 (53–128) | 52 (36–64) | 50 (34–67) | <0.001 |
| PTH, pg/mL | 62 (44–81) | 53 (39–69) | 60 (47–77) | 73 (60–101) | <0.001 |
| Calcium, mg/dL | 9.2 ± 0.5 | 9.1 ± 0.5 (60–101) | 9.2 ± 0.5 | 9.2 ± 0.4 | 0.310 |
| Phosphate, mmol/L | 1.04 ± 0.26 | 1.05 ± 0.24 | 1.05 ± 0.19 | 1.04 ± 0.31 | 0.962 |
| Magnesium, mmol/L | 0.86 ± 0.17 | 0.86 ± 0.15 | 0.84 ± 0.08 | 0.87 ± 0.23 | 0.623 |
| Variables | Spring (n = 65) | Summer (n = 30) | Fall (n = 58) | Winter (n = 70) | p Value |
|---|---|---|---|---|---|
| Blood biochemistries | |||||
| 25(OH)D, mg/mL | 22.4 (11.2–33.5) | 33.6 (24.9–54.8) | 28.0 (18.9–78.0) | 22.7 (15.0–33.4) | <0.001 |
| 1,25(OH)D, pg/mL | 57 (43–77) | 67 (62–93) | 58 (39–109) | 53 (40–97) | 0.281 |
| PTH, pg/mL | 67 (56–84) | 55 (44–68) | 59 (41–72) | 64 (43–81) | 0.154 |
| Calcium, mg/dL | 9.3 ± 0.5 | 9.1 ± 0.5 | 9.2 ± 0.4 | 9.2 ± 0.4 | 0.214 |
| Phosphate, mmol/L | 1.09 ± 0.33 | 0.99 ± 0.15 | 1.04 ± 0.28 | 1.03 ± 0.18 | 0.303 |
| Magnesium, mmol/L | 0.88 ± 0.25 | 0.85 ± 0.05 | 0.87 ± 0.18 | 0.83 ± 0.08 | 0.347 |
| Variables | r | p Value | Variables | r | p Value | |
|---|---|---|---|---|---|---|
| Age | −0.248 | <0.001 | HOMA index | −0.146 | 0.032 | |
| BMI | −0.116 | 0.090 | 1-25(OH)D3 | 0.387 | <0.001 | |
| Systolic BP | −0.092 | 0.190 | PTH | −0.362 | <0.001 | |
| Diastolic BP | −0.018 | 0.797 | Calcium | −0.121 | 0.076 | |
| Duration hypertension | −0.186 | 0.006 | Phosphate | 0.025 | 0.724 | |
| Alcohol consumption | −0.016 | 0.819 | Magnesium | −0.068 | 0.331 | |
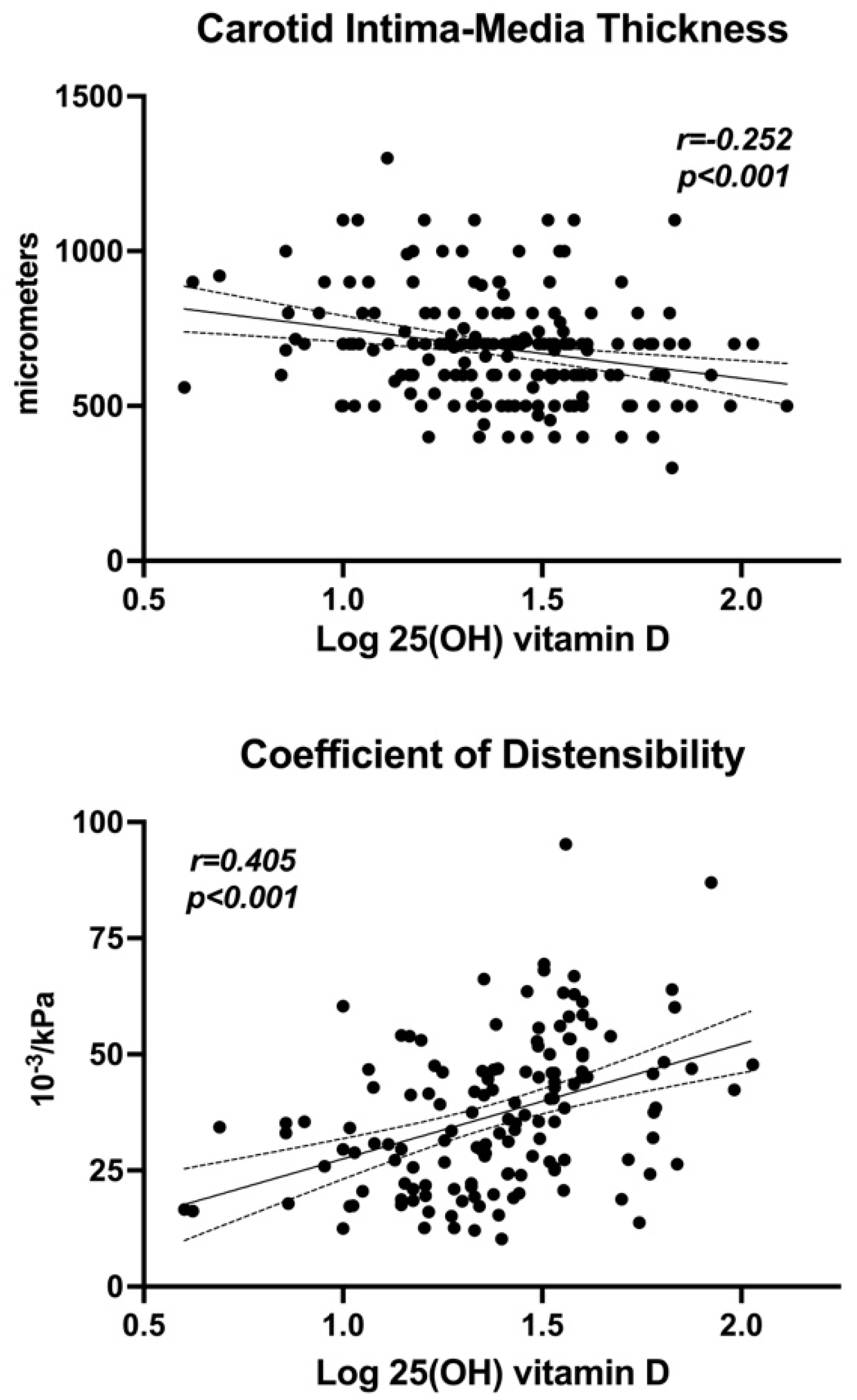
| GFR | −0.008 | 0.906 | Carotid IMT | −0.252 | <0.001 | |
| Glucose | −0.232 | <0.001 | Distensibility | 0.405 | <0.001 | |
| Glycated hemoglobin | −0.092 | 0.237 | Young | −0.189 | 0.025 | |
| Insulin | −0.113 | 0.099 | Beta-stiffness | −0.365 | <0.001 |
| Carotid IMT | Coefficient of Distensibility | Young’s Elastic Modulus | Beta-Stiffness | |||||
|---|---|---|---|---|---|---|---|---|
| r | p Value | r | p Value | r | p Value | r | p Value | |
| Clinical characteristics | ||||||||
| Age | 0.524 | <0.001 | −0.360 | <0.001 | 0.359 | <0.001 | 0.296 | <0.001 |
| BMI | 0.001 | 0.984 | −0.280 | <0.001 | 0.196 | 0.021 | 0.292 | <0.001 |
| Systolic BP | 0.242 | <0.001 | −0.200 | <0.018 | 0.249 | 0.003 | 0.077 | 0.365 |
| Diastolic BP | 0.106 | 0.152 | −0.064 | 0.451 | 0.055 | 0.519 | 0.027 | 0.754 |
| Duration of hypertension | 0.250 | <0.001 | −0.154 | 0.038 | 0.261 | 0.002 | 0.126 | 0.066 |
| Alcohol consumption | 0.132 | 0.077 | −0.110 | 0.132 | 0.132 | 0.077 | 0.069 | 0.427 |
| Biochemical variables | ||||||||
| GFR | −0.047 | 0.514 | 0.109 | 0.198 | −0.070 | 0.408 | −0.128 | 0.067 |
| Glucose | 0.137 | 0.053 | −0.188 | 0.025 | 0.107 | 0.206 | 0.190 | 0.024 |
| Glycated hemoglobin | 0.203 | 0.013 | −0.135 | 0.055 | 0.192 | 0.039 | 0.162 | 0.053 |
| HOMA index | 0.013 | 0.855 | −0.273 | 0.001 | 0.110 | 0.202 | 0.228 | 0.007 |
| Triglycerides | 0.089 | 0.213 | −0.174 | 0.039 | 0.087 | 0.309 | 0.165 | 0.052 |
| Total cholesterol | 0.183 | 0.008 | −0.032 | 0.706 | 0.099 | 0.245 | 0.080 | 0.343 |
| HDL cholesterol | −0.122 | 0.092 | 0.134 | 0.115 | −0.027 | 0.752 | −0.116 | 0.171 |
| LDL cholesterol | 0.099 | 0.171 | −0.048 | 0.576 | 0.090 | 0.289 | 0.092 | 0.280 |
| Carotid IMT | Coefficient of Distensibility | ||||||
|---|---|---|---|---|---|---|---|
| β | F Value | p Value | β | F Value | p Value | ||
| Age | 6.20 | 35.21 | <0.001 | Age | −0.34 | 10.41 | 0.002 |
| BMI | 3.43 | 2.39 | 0.124 | BMI | −0.73 | 6.07 | 0.015 |
| Systolic BP | 1.84 | 7.95 | 0.006 | Systolic BP | −0.15 | 4.11 | 0.045 |
| Duration of hypertension | 0.53 | 0.13 | 0.715 | Duration of hypertension | 0.25 | 2.43 | 0.122 |
| Glycated hemoglobin | 3.58 | 0.02 | 0.899 | Glucose | 0.01 | 0.07 | 0.993 |
| Total cholesterol | 0.23 | 0.42 | 0.420 | HOMA index | −0.11 | 0.16 | 0.677 |
| Log 25(OH)D | −166.8 | 8.56 | 0.004 | Triglycerides | −0.02 | 0.91 | 0.341 |
| Log PTH | −129.7 | 4.32 | 0.040 | Log 25(OH)D | 18.24 | 10.17 | 0.002 |
| Summer/Fall | −3.18 | 1.97 | 0.091 | Log PTH | −7.66 | 1.31 | 0.255 |
| Summer/Fall | 0.19 | 1.87 | 0.161 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulfone, L.; Vacca, A.; Brosolo, G.; Da Porto, A.; Bertin, N.; Vivarelli, C.; Catena, C.; Sechi, L.A. Subclinical Carotid Disease Is Associated with Low Serum Vitamin D in Nondiabetic Middle-Aged Hypertensive Patients. Nutrients 2025, 17, 480. https://doi.org/10.3390/nu17030480
Bulfone L, Vacca A, Brosolo G, Da Porto A, Bertin N, Vivarelli C, Catena C, Sechi LA. Subclinical Carotid Disease Is Associated with Low Serum Vitamin D in Nondiabetic Middle-Aged Hypertensive Patients. Nutrients. 2025; 17(3):480. https://doi.org/10.3390/nu17030480
Chicago/Turabian StyleBulfone, Luca, Antonio Vacca, Gabriele Brosolo, Andrea Da Porto, Nicole Bertin, Cinzia Vivarelli, Cristiana Catena, and Leonardo A. Sechi. 2025. "Subclinical Carotid Disease Is Associated with Low Serum Vitamin D in Nondiabetic Middle-Aged Hypertensive Patients" Nutrients 17, no. 3: 480. https://doi.org/10.3390/nu17030480
APA StyleBulfone, L., Vacca, A., Brosolo, G., Da Porto, A., Bertin, N., Vivarelli, C., Catena, C., & Sechi, L. A. (2025). Subclinical Carotid Disease Is Associated with Low Serum Vitamin D in Nondiabetic Middle-Aged Hypertensive Patients. Nutrients, 17(3), 480. https://doi.org/10.3390/nu17030480






