Enhancement of Lower Limb Muscle Strength and Reduction of Inflammation in the Elderly: A Randomized, Double-Blind Clinical Trial Comparing Lacticaseibacillus paracasei PS23 Probiotic with Heat-Treated Supplementation
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Design
2.3. Blood Pressure and Body Composition Measurements
2.4. Handgrip Strength Test
2.5. Functional Performance
2.6. Clinical Biochemistry
2.7. Statistical Analysis
3. Results
3.1. The Basic Information of Subjects and Effects of PS23 Supplementation on Blood Pressure and Body Composition in the Elderly
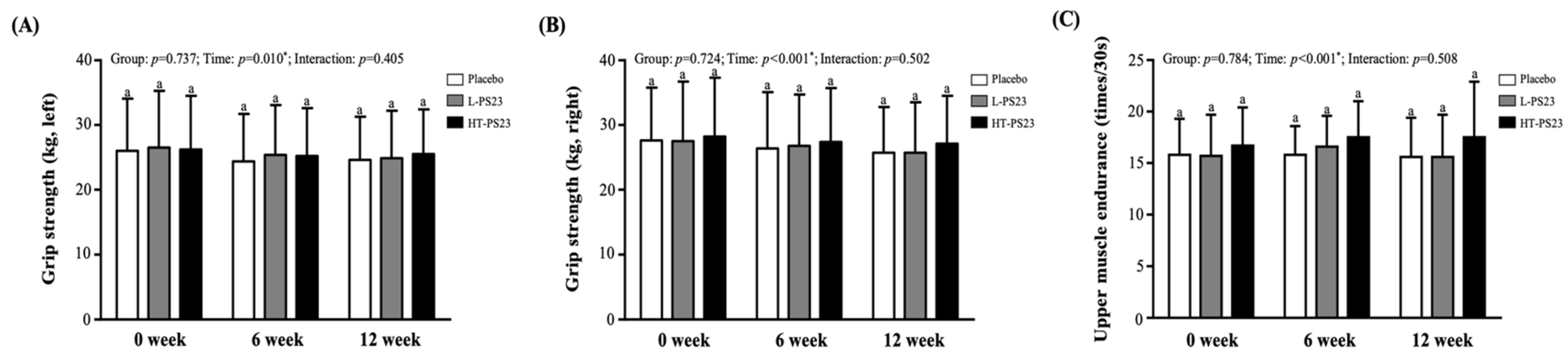
3.2. Effects of PS23 Supplementation on Grip Strength and Upper Limb Endurance in the Elderly
3.3. Effects of PS23 Supplementation on Lower Limb Muscle Strength and Endurance in the Elderly
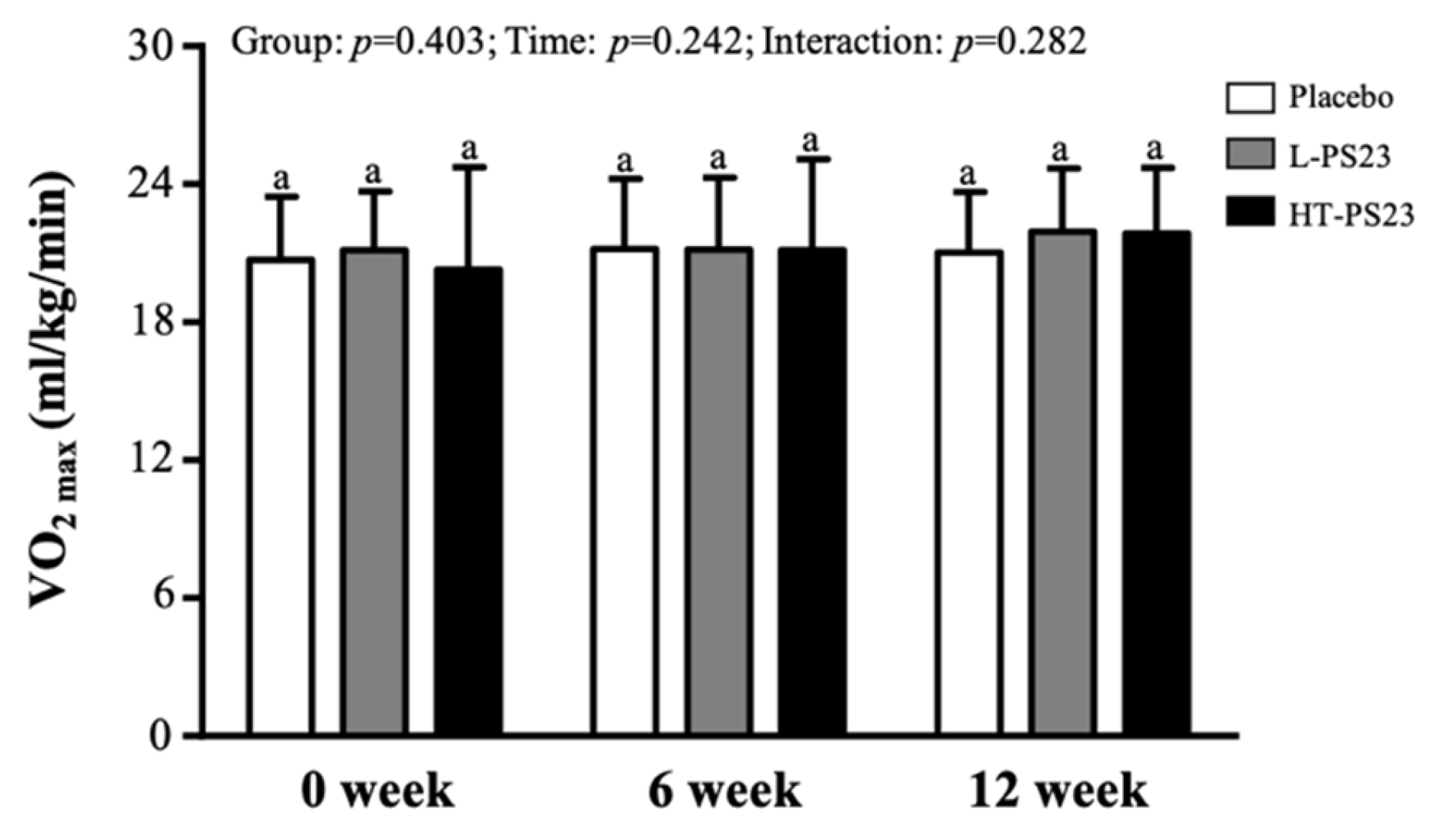
3.4. Effect of PS23 Supplementation on Predicting Maximal Oxygen Uptake (VO2max) in the Elderly
3.5. Effects of PS23 Supplementation on Blood Indicators Related to Muscle Growth, Synthesis, or Inflammation in the Elderly
3.6. Effects of PS23 Supplementation on Biochemical Characteristics in the Elderly
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, S.; Jung, H.W.; Baek, J.Y.; Jang, I.Y.; Lee, E. Sarcopenia as the Mobility Phenotype of Aging: Clinical Implications. J. Bone Metab. 2024, 31, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Melton, L.J., 3rd; Khosla, S.; Crowson, C.S.; O’Connor, M.K.; O’Fallon, W.M.; Riggs, B.L. Epidemiology of sarcopenia. J. Am. Geriatr. Soc. 2000, 48, 625–630. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Buccheri, E.; Dell’Aquila, D.; Russo, M.; Chiaramonte, R.; Musumeci, G.; Vecchio, M. Can artificial intelligence simplify the screening of muscle mass loss? Heliyon 2023, 9, e16323. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, A.; De Vito, G. Muscle strength, power and adaptations to resistance training in older people. Eur. J. Appl. Physiol. 2004, 91, 450–472. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Chambers, E.; Lochlainn, M.N.; Witard, O.C. Mechanisms Linking the Gut-Muscle Axis With Muscle Protein Metabolism and Anabolic Resistance: Implications for Older Adults at Risk of Sarcopenia. Front. Physiol. 2021, 12, 770455. [Google Scholar] [CrossRef] [PubMed]
- Buch, A.; Carmeli, E.; Boker, L.K.; Marcus, Y.; Shefer, G.; Kis, O.; Berner, Y.; Stern, N. Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age—An overview. Exp. Gerontol. 2016, 76, 25–32. [Google Scholar] [CrossRef]
- Vermeiren, S.; Vella-Azzopardi, R.; Beckwée, D.; Habbig, A.K.; Scafoglieri, A.; Jansen, B.; Bautmans, I.; Gerontopole Brussels Study Group. Frailty and prediction of negative health outcomes: A meta-analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1163.e1–1163.e17. [Google Scholar] [CrossRef] [PubMed]
- Cianci, R.; Pagliari, D.; Piccirillo, C.A.; Fritz, J.H.; Gambassi, G. The Microbiota and Immune System Crosstalk in Health and Disease. Mediat. Inflamm. 2018, 2018, 2912539. [Google Scholar] [CrossRef] [PubMed]
- Weyh, C.; Krüger, K.; Strasser, B. Physical Activity and Diet Shape the Immune System during Aging. Nutrients 2020, 12, 622. [Google Scholar] [CrossRef] [PubMed]
- Siddharth, J.; Chakrabarti, A.; Pannérec, A.; Karaz, S.; Morin-Rivron, D.; Masoodi, M.; Feige, J.N.; Parkinson, S.J. Aging and sarcopenia associate with specific interactions between gut microbes, serum biomarkers and host physiology in rats. Aging 2017, 9, 1698–1720. [Google Scholar] [CrossRef] [PubMed]
- Clements, S.J.; Carding, S.R. Diet, the intestinal microbiota, and immune health in aging. Crit. Rev. Food Sci. Nutr. 2018, 58, 651–661. [Google Scholar] [CrossRef]
- Castro-Mejía, J.L.; Khakimov, B.; Krych, Ł.; Bülow, J.; Bechshøft, R.L.; Højfeldt, G.; Mertz, K.H.; Garne, E.S.; Schacht, S.R.; Ahmad, H.F.; et al. Physical fitness in community-dwelling older adults is linked to dietary intake, gut microbiota, and metabolomic signatures. Aging Cell 2020, 19, e13105. [Google Scholar] [CrossRef]
- Yan, X.; Li, H.; Xie, R.; Lin, L.; Ding, L.; Cheng, X.; Xu, J.; Bai, L.; Qiao, Y. Relationships between sarcopenia, nutrient intake, and gut microbiota in Chinese community-dwelling older women. Arch. Gerontol. Geriatr. 2023, 113, 105063. [Google Scholar] [CrossRef]
- Picca, A.; Fanelli, F.; Calvani, R.; Mulè, G.; Pesce, V.; Sisto, A.; Pantanelli, C.; Bernabei, R.; Landi, F.; Marzetti, E. Gut Dysbiosis and Muscle Aging: Searching for Novel Targets against Sarcopenia. Mediat. Inflamm. 2018, 2018, 7026198. [Google Scholar] [CrossRef]
- Liao, X.; Wu, M.; Hao, Y.; Deng, H. Exploring the Preventive Effect and Mechanism of Senile Sarcopenia Based on “Gut-Muscle Axis”. Front. Bioeng. Biotechnol. 2020, 8, 590869. [Google Scholar] [CrossRef]
- Ma, T.; Shen, X.; Shi, X.; Sakandar, H.A.; Quan, K.; Li, Y.; Jin, H.; Kwok, L.Y.; Zhang, H.; Sun, Z. Targeting gut microbiota and metabolism as the major probiotic mechanism-An evidence-based review. Trends Food Sci. 2023, 138, 178–198. [Google Scholar] [CrossRef]
- Sugimura, Y.; Kanda, A.; Sawada, K.; Wai, K.M.; Tanabu, A.; Ozato, N.; Midorikawa, T.; Hisada, T.; Nakaji, S.; Ihara, K. Association between Gut Microbiota and Body Composition in Japanese General Population: A Focus on Gut Microbiota and Skeletal Muscle. Int. J. Environ. Res. Public Health 2022, 19, 7464. [Google Scholar] [CrossRef] [PubMed]
- Shokri-Mashhadi, N.; Navab, F.; Ansari, S.; Rouhani, M.H.; Hajhashemy, Z.; Saraf-Bank, S. A meta-analysis of the effect of probiotic administration on age-related sarcopenia. Food Sci. Nutr. 2023, 11, 4975–4987. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA 2016, 113, E7554–E7563. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, R.; Li, L. Effects of Probiotic Supplementation on Exercise and the Underlying Mechanisms. Foods 2023, 12, 1787. [Google Scholar] [CrossRef]
- Bienenstock, J.; Gibson, G.; Klaenhammer, T.R.; Walker, W.A.; Neish, A.S. New insights into probiotic mechanisms: A harvest from functional and metagenomic studies. Gut Microbes 2013, 4, 94–100. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Teame, T.; Wang, A.; Xie, M.; Zhang, Z.; Yang, Y.; Ding, Q.; Gao, C.; Olsen, R.E.; Ran, C.; Zhou, Z. Paraprobiotics and Postbiotics of Probiotic Lactobacilli, Their Positive Effects on the Host and Action Mechanisms: A Review. Front. Nutr. 2020, 7, 570344. [Google Scholar] [CrossRef]
- Chen, L.H.; Huang, S.Y.; Huang, K.C.; Hsu, C.C.; Yang, K.C.; Li, L.A.; Chan, C.H.; Huang, H.Y. Lactobacillus paracasei PS23 decelerated age-related muscle loss by ensuring mitochondrial function in SAMP8 mice. Aging 2019, 11, 756–770. [Google Scholar] [CrossRef]
- Cheng, L.H.; Cheng, S.H.; Wu, C.C.; Huang, C.L.; Wen, P.J.; Chang, M.Y.; Tsai, Y.C. Lactobacillus paracasei PS23 dietary supplementation alleviates muscle aging via ghrelin stimulation in d-galactose-induced aging mice. J. Funct. Foods 2021, 85, 104651. [Google Scholar] [CrossRef]
- Rondanelli, M.; Gasparri, C.; Barrile, G.C.; Battaglia, S.; Cavioni, A.; Giusti, R.; Mansueto, F.; Moroni, A.; Nannipieri, F.; Patelli, Z.; et al. Effectiveness of a Novel Food Composed of Leucine, Omega-3 Fatty Acids and Probiotic Lactobacillus paracasei PS23 for the Treatment of Sarcopenia in Elderly Subjects: A 2-Month Randomized Double-Blind Placebo-Controlled Trial. Nutrients 2022, 14, 4566. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Ho, C.S.; Hsu, Y.J.; Huang, C.C. Live and Heat-Killed Probiotic Lactobacillus paracasei PS23 Accelerated the Improvement and Recovery of Strength and Damage Biomarkers after Exercise-Induced Muscle Damage. Nutrients 2022, 14, 4563. [Google Scholar] [CrossRef]
- Lee, M.C.; Hsu, Y.J.; Ho, C.S.; Tsai, Y.S.; Chen, C.C.; Huang, C.C. Supplementation with Lactiplantibacillus brevis GKEX Combined with Resistance Exercise Training Improves Muscle Mass, Strength Performance, and Body Fat Condition in Healthy Humans. Foods 2024, 13, 1030. [Google Scholar] [CrossRef] [PubMed]
- Sports Administration. Fitness Guide 2020. Available online: https://www.fitness.org.tw/direct01.php (accessed on 23 May 2020).
- Lee, M.C.; Tu, Y.T.; Lee, C.C.; Tsai, S.C.; Hsu, H.Y.; Tsai, T.Y.; Liu, T.H.; Young, S.L.; Lin, J.S.; Huang, C.C. Lactobacillus plantarum TWK10 Improves Muscle Mass and Functional Performance in Frail Older Adults: A Randomized, Double-Blind Clinical Trial. Microorganisms 2021, 9, 1466. [Google Scholar] [CrossRef]
- Li, F.; Chang, C.H.; Ho, C.A.; Wu, C.Y.; Yeh, H.C.; Chan, Y.S.; Cheng, J.Y.; ChangChien, W.S.; Ho, C.S. The Determination of Step Frequency in 3-min Incremental Step-in-Place Tests for Predicting Maximal Oxygen Uptake from Heart Rate Response in Taiwanese Adults. Int. J. Environ. Res. Public Health 2022, 19, 563. [Google Scholar] [CrossRef]
- Nucci, R.A.B.; Filho, V.A.N.; Jacob-Filho, W.; Otoch, J.P.; Pessoa, A.F.M. Role of Nutritional Supplements on Gut-Muscle Axis Across Age: A Mini-Review. Cell Physiol. Biochem. 2023, 57, 161–168. [Google Scholar] [PubMed]
- Prokopidis, K.; Giannos, P.; Kirwan, R.; Ispoglou, T.; Galli, F.; Witard, O.C.; Triantafyllidis, K.K.; Kechagias, K.S.; Morwani-Mangnani, J.; Ticinesi, A.; et al. Impact of probiotics on muscle mass, muscle strength and lean mass: A systematic review and meta-analysis of randomized controlled trials. J. Cachexia Sarcopenia Muscle 2023, 14, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Handajani, Y.S.; Turana, Y.; Hengky, A.; Hamid, G.; Schroeder-Butterfill, E.; Kristian, K. Probiotics supplementation or probiotic-fortified products on sarcopenic indices in older adults: Systematic review and meta-analysis from recent randomized controlled trials. Front. Aging 2024, 5, 1307762. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, C.S.; Garde, E.; Reislev, N.L.; Wimmelmann, C.L.; Bieler, T.; Ziegler, A.K.; Gylling, A.T.; Dideriksen, K.J.; Siebner, H.R.; Mortensen, E.L.; et al. Physical activity as intervention for age-related loss of muscle mass and function: Protocol for a randomised controlled trial (the LISA study). BMJ Open 2016, 6, e012951. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, B.C.; Murch, S.J.; Weaver, C.M.; Jafari, M. Editorial: Plant Foods and Dietary Supplements: Building Solid Foundations for Clinical Trials. Front. Nutr. 2022, 9, 881688. [Google Scholar] [CrossRef] [PubMed]
- Bullo, V.; Roma, E.; Gobbo, S.; Duregon, F.; Bergamo, M.; Bianchini, G.; Doria, E.; Cugusi, L.; Blasio, A.D.; Bocalini, D.S.; et al. Lower Limb Strength Profile in Elderly with Different Pathologies: Comparisons with Healthy Subjects. Geriatrics 2020, 5, 83. [Google Scholar] [CrossRef] [PubMed]
- de Jong, J.C.B.C.; Attema, B.J.; van der Hoek, M.D.; Verschuren, L.; Caspers, M.P.M.; Kleemann, R.; van der Leij, F.R.; van den Hoek, A.M.; Nieuwenhuizen, A.G.; Keijer, J. Sex differences in skeletal muscle-aging trajectory: Same processes, but with a different ranking. Geroscience 2023, 45, 2367–2386. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.J.; Patten, C.; Reid, K.F.; Carabello, R.J.; Phillips, E.M.; Fielding, R.A. Impaired voluntary neuromuscular activation limits muscle power in mobility-limited older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 495–502. [Google Scholar] [CrossRef]
- Candow, D.G.; Chilibeck, P.D. Differences in size, strength, and power of upper and lower body muscle groups in young and older men. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Quan, M.; Cao, Z.B. Effect of vitamin D supplementation on upper and lower limb muscle strength and muscle power in athletes: A meta-analysis. PLoS ONE 2019, 14, e0215826. [Google Scholar] [CrossRef]
- Ferrreira, L.; Gobbi, S.; Gobbi, L.T. An explanatory mechanism for the different decline in limb strength in older women. Arch. Gerontol. Geriatr. 2009, 49, 373–377. [Google Scholar] [CrossRef]
- Foulstone, E.J.; Savage, P.B.; Crown, A.L.; Holly, J.M.; Stewart, C.E. Role of insulin-like growth factor binding protein-3 (IGFBP-3) in the differentiation of primary human adult skeletal myoblasts. J. Cell Physiol. 2003, 195, 70–79. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Lee, Y.H.; Choi, I. Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases. Cells 2020, 9, 1773. [Google Scholar] [CrossRef] [PubMed]
- Firth, S.M.; Baxter, R.C. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 2002, 23, 824–854. [Google Scholar] [CrossRef]
- LeRoith, D.; Holly, J.M.P.; Forbes, B.E. Insulin-like growth factors: Ligands, binding proteins, and receptors. Mol. Metab. 2021, 52, 101245. [Google Scholar] [CrossRef]
- Valadez-Bustos, N.; Escamilla-Silva, E.M.; García-Vázquez, F.J.; Gallegos-Corona, M.A.; Amaya-Llano, S.L.; Ramos-Gómez, M. Oral Administration of Microencapsulated B. Longum BAA-999 and Lycopene Modulates IGF-1/IGF-1R/IGFBP3 Protein Expressions in a Colorectal Murine Model. Int. J. Mol. Sci. 2019, 20, 4275. [Google Scholar] [CrossRef]
- Conley, K.E.; Jubrias, S.A.; Esselman, P.C. Oxidative capacity and ageing in human muscle. J. Physiol. 2000, 526, 203–210. [Google Scholar] [CrossRef]
- Antuña, E.; Cachán-Vega, C.; Bermejo-Millo, J.C.; Potes, Y.; Caballero, B.; Vega-Naredo, I.; Coto-Montes, A.; Garcia-Gonzalez, C. Inflammaging: Implications in Sarcopenia. Int. J. Mol. Sci. 2022, 23, 15039. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- Sadrzadeh-Yeganeh, H.; Elmadfa, I.; Djazayery, A.; Jalali, M.; Heshmat, R.; Chamary, M. The effects of probiotic and conventional yoghurt on lipid profile in women. Br. J. Nutr. 2010, 103, 1778–1783. [Google Scholar] [CrossRef]
- Asemi, Z.; Zare, Z.; Shakeri, H.; Sabihi, S.S.; Esmaillzadeh, A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann. Nutr. Metab. 2013, 63, 1–9. [Google Scholar] [CrossRef]
- Karamali, M.; Eghbalpour, S.; Rajabi, S.; Jamilian, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Keneshlou, F.; Mirhashemi, S.M.; Chamani, M.; Gelougerdi, S.H.; et al. Effects of Probiotic Supplementation on Hormonal Profiles, Biomarkers of Inflammation and Oxidative Stress in Women With Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Arch. Iran. Med. 2018, 21, 1–7. [Google Scholar]
- Wood, R.I.; Stanton, S.J. Testosterone and sport: Current perspectives. Horm. Behav. 2012, 61, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Liva, S.M.; Voskuhl, R.R. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J. Immunol. 2001, 167, 2060–2067. [Google Scholar] [CrossRef]
- Poutahidis, T.; Springer, A.; Levkovich, T.; Qi, P.; Varian, B.J.; Lakritz, J.R.; Ibrahim, Y.M.; Chatzigiagkos, A.; Alm, E.J.; Erdman, S.E. Probiotic microbes sustain youthful serum testosterone levels and testicular size in aging mice. PLoS ONE 2014, 9, e84877. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.J.; Park, J.M.; Kwon, Y.J.; Kim, K.; Park, S.Y.; Kim, I.; Lim, J.H.; Kim, B.K.; Kim, B.Y. Immunostimulatory Effect of Heat-Killed Probiotics on RAW264.7 Macrophages. J. Microbiol. Biotechnol. 2022, 32, 638–644. [Google Scholar] [CrossRef]
- Fiore, W.; Arioli, S.; Guglielmetti, S. The Neglected Microbial Components of Commercial Probiotic Formulations. Microorganisms 2020, 8, 1177. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Cheng, L.H.; Liu, Y.W.; Jeng, O.J.; Lee, Y.K. Gerobiotics: Probiotics targeting fundamental aging processes. Biosci. Microbiota Food Health 2021, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]


| Characteristics | Time | Placebo | L-PS23 | HT-PS23 | Group (G) | Time (T) | G × T |
|---|---|---|---|---|---|---|---|
| Body composition | |||||||
| BW (kg) | 0 week | 60.8 ± 10.5 a | 59.6 ± 9.9 a | 61.6 ± 10.1 a | 0.748 | 0.302 | 0.494 |
| 6 week | 60.6 ± 10.5 a | 59.6 ± 9.9 a | 61.6 ± 10.3 a | ||||
| 12 week | 60.8 ± 10.7 a | 59.8 ± 10.1 a | 61.6 ± 10.2 a | ||||
| BMI (kg/m2) | 0 week | 23.6 ± 3.2 a | 23.3 ± 2.7 a | 24.0 ± 3.3 a | 0.471 | 0.235 | 0.493 |
| 6 week | 23.5 ± 3.3 a | 23.3 ± 2.7 a | 24.0 ± 3.3 a | ||||
| 12 week | 23.6 ± 3.3 a | 23.4 ± 2.8 a | 24.0 ± 3.3 a | ||||
| Muscle mass (kg) | 0 week | 22.7 ± 5.0 a | 22.8 ± 4.3 a | 23.4 ± 4.5 a | 0.767 | 0.132 | 0.638 |
| 6 week | 22.7 ± 4.9 a | 23.0 ± 4.3 a | 23.5 ± 4.4 a | ||||
| 12 week | 22.7 ± 5.0 a | 23.0 ± 4.2 a | 23.5 ± 4.4 a | ||||
| Fat mass (%) | 0 week | 30.9 ± 5.8 a | 28.8 ± 6.5 a | 29.5 ± 7.0 a | 0.482 | 0.115 | 0.379 |
| 6 week | 30.6 ± 5.7 a | 28.6 ± 6.2 a | 29.2 ± 6.8 a | ||||
| 12 week | 30.1 ± 6.0 a | 28.7 ± 6.4 a | 29.4 ± 6.7 a | ||||
| Blood pressure | |||||||
| SBP (mmHg) | 0 week | 134.1 ± 15.3 a | 140.2 ± 20.7 a | 133.9 ± 18.2 a | 0.582 | 0.062 | 0.637 |
| 6 week | 130.6 ± 14.6 a | 135.0 ± 19.5 a | 132.1 ± 19.9 a | ||||
| 12 week | 134.9 ± 17.5 a | 136.1 ± 19.1 a | 134.9 ± 23.4 a | ||||
| DBP (mmHg) | 0 week | 78.5 ± 9.3 a | 76.9 ± 11.5 a | 76.4 ± 10.3 a | 0.722 | 0.018 * | 0.911 |
| 6 week | 76.3 ± 8.2 a | 73.8 ± 13.8 a | 73.6 ± 10.3 a | ||||
| 12 week | 77.0 ± 11.0 a | 76.5 ± 11.8 a | 76.4 ± 14.1 a | ||||
| HR (bpm) | 0 week | 76.2 ± 10.9 a | 75.4 ± 9.3 a | 74.2 ± 10.2 a | 0.486 | 0.675 | 0.397 |
| 6 week | 78.1 ± 14.1 a | 74.2 ± 10.2 a | 74.5 ± 10.3 a | ||||
| 12 week | 77.6 ± 10.9 a | 74.3 ± 11.0 a | 76.0 ± 11.1 a | ||||
| Characteristics | Time | Placebo | L-PS23 | HT-PS23 | Group (G) | Time (T) | G × T |
|---|---|---|---|---|---|---|---|
| Insulin (μU/mL) | 0 week | 8.10 ± 3.16 a | 8.27 ± 3.63 a | 7.91 ± 3.84 a | 0.830 | 0.204 | 0.286 |
| 6 week | 7.46 ± 3.30 a | 8.00 ± 3.69 a | 7.76 ± 4.23 a | ||||
| 12 week | 7.13 ± 3.46 a | 8.02 ± 3.29 a | 8.13 ± 5.44 a | ||||
| IGF-1 (ng/mL) | 0 week | 107.6 ± 39.2 a | 101.8 ± 46.5 a | 100.3 ± 34.1 a | 0.977 | <0.001 * | 0.031 * |
| 6 week | 106.4 ± 36.6 a | 106.1 ± 47.3 a | 101.8 ± 30.4 a | ||||
| 12 week | 110.0 ± 33.2 a | 121.2 ± 56.1 a | 117.4 ± 38.3 a | ||||
| IGFBP-3 (pg/mL) | 0 week | 2488 ± 495 a | 2366 ± 656 a | 2417 ± 673 a | 0.433 | <0.001 * | 0.002 * |
| 6 week | 2425 ± 632 a | 2498 ± 722 a | 2550 ± 826 a | ||||
| 12 week | 2394 ± 497 a | 2666 ± 612 a | 2883 ± 756 b | ||||
| Ghrelin (pmol/L) | 0 week | 922 ± 345 a | 928 ± 376 a | 927 ± 322 a | 0.514 | 0.009 * | 0.344 |
| 6 week | 1053 ± 264 a | 995 ± 189 a | 982 ± 208 a | ||||
| 12 week | 1062 ± 330 a | 985 ± 291 a | 927 ± 209 a | ||||
| HsCRP (mg/dL) | 0 week | 0.09 ± 0.07 a | 0.09 ± 0.07 a | 0.10 ± 0.08 a | 0.503 | 0.185 | 0.001 * |
| 6 week | 0.09 ± 0.07 a | 0.09 ± 0.07 a | 0.10 ± 0.06 a | ||||
| 12 week | 0.13 ± 0.09 b | 0.09 ± 0.07 a | 0.09 ± 0.07 a | ||||
| Myeloperoxidase (ng/mL) | 0 week | 255 ± 88 a | 246 ± 82 a | 254 ± 102 a | 0.606 | 0.994 | 0.622 |
| 6 week | 258 ± 95 a | 236 ± 85 a | 257 ± 107 a | ||||
| 12 week | 267 ± 91 a | 236 ± 88 a | 250 ± 93 a | ||||
| GDF-15 (pg/mL) | 0 week | 895 ± 425 a | 960 ± 466 a | 1039 ± 557 a | 0.433 | 0.023 | 0.406 |
| 6 week | 843 ± 582 a | 866 ± 417 a | 999 ± 561 a | ||||
| 12 week | 909 ± 635 a | 880 ± 430 a | 1059 ± 631 a | ||||
| IL-6 (pg/mL) | 0 week | 2.24 ± 0.88 a | 2.46 ± 0.77 a | 2.44 ± 0.80 a | 0.205 | <0.001 * | <0.001 * |
| 6 week | 2.24 ± 0.88 a | 2.18 ± 0.81 a | 2.11 ± 0.80 a | ||||
| 12 week | 2.41 ± 1.00 b | 1.77 ± 0.84 a | 1.47 ± 0.72 a | ||||
| IL-10 (pg/mL) | 0 week | 0.70 ± 0.41 b | 0.48 ± 0.36 a | 0.46 ± 0.26 a | 0.905 | 0.048 * | <0.001 * |
| 6 week | 0.56 ± 0.43 a | 0.46 ± 0.35 a | 0.41 ± 0.32 a | ||||
| 12 week | 0.39 ± 0.29 a | 0.64 ± 0.53 b | 0.68 ± 0.38 b | ||||
| TNF-α (pg/mL) | 0 week | 0.20 ± 0.06 a | 0.19 ± 0.06 a | 0.21 ± 0.08 a | 0.592 | <0.001 * | 0.370 |
| 6 week | 0.24 ± 0.07 a | 0.23 ± 0.06 a | 0.24 ± 0.07 a | ||||
| 12 week | 0.24 ± 0.08 a | 0.22 ± 0.07 a | 0.22 ± 0.07 a | ||||
| Testosterone (ng/mL) | 0 week | 1.70 ± 1.26 a | 1.60 ± 0.84 a | 1.72 ± 1.26 a | 0.221 | 0.001 * | <0.001 * |
| 6 week | 1.75 ± 1.25 a | 1.56 ± 0.88 a | 2.19 ± 1.44 b | ||||
| 12 week | 1.60 ± 1.20 a | 1.62 ± 0.91 a | 2.29 ± 1.58 b | ||||
| Cortisol (μg/dL) | 0 week | 8.82 ± 2.65 a | 9.53 ± 3.00 a | 10.02 ± 2.98 b | 0.544 | 0.082 | 0.599 |
| 6 week | 8.83 ± 3.05 a | 9.03 ± 2.82 a | 9.64 ± 4.20 a | ||||
| 12 week | 8.72 ± 2.94 a | 8.88 ± 2.94 a | 8.86 ± 3.14 a | ||||
| HGH (ng/mL) | 0 week | 1.02 ± 0.59 a | 0.89 ± 0.46 a | 0.99 ± 0.61 a | 0.863 | 0.968 | 0.313 |
| 6 week | 1.03 ± 0.60 a | 0.93 ± 0.46 a | 0.97 ± 0.55 a | ||||
| 12 week | 0.88 ± 0.56 a | 0.98 ± 0.48 a | 1.03 ± 0.69 a | ||||
| 25(OH)D (ng/mL) | 0 week | 24.4 ± 4.7 a | 26.4 ± 8.2 a | 26.3 ± 8.0 a | 0.331 | <0.001 * | 0.688 |
| 6 week | 25.4 ± 4.8 a | 28.5 ± 8.3 a | 27.9 ± 7.6 a | ||||
| 12 week | 24.4 ± 4.9 a | 26.4 ± 8.7 a | 26.2 ± 8.6 a | ||||
| DHEA-S (μg/dL) | 0 week | 103.6 ± 69.6 a | 113.3 ± 50.5 a | 106.0 ± 47.6 a | 0.519 | <0.001 * | 0.007 * |
| 6 week | 109.7 ± 61.1 a | 125.4 ± 57.5 a | 116.2 ± 51.7 a | ||||
| 12 week | 118.4 ± 65.3 a | 140.6 ± 70.2 a | 142.5 ± 56.8 a | ||||
| Cathepsin D (ng/mL) | 0 week | 24.5 ± 6.5 a | 24.7 ± 10.1 a | 25.0 ± 8.6 a | 0.425 | 0.198 | 0.125 |
| 6 week | 24.4 ± 6.3 a | 26.4 ± 9.1 a | 26.8 ± 7.7 a | ||||
| 12 week | 22.7 ± 6.7 a | 26.7 ± 9.2 a | 26.4 ± 9.6 a | ||||
| CysC (mg/L) | 0 week | 0.88 ± 0.15 a | 0.89 ± 0.13 a | 0.91 ± 0.19 a | 0.355 | 0.005 * | 0.529 |
| 6 week | 0.84 ± 0.13 a | 0.86 ± 0.15 a | 0.91 ± 0.24 a | ||||
| 12 week | 0.82 ± 0.15 a | 0.86 ± 0.18 a | 0.90 ± 0.24 a |
| Characteristics | Time | Placebo | L-PS23 | HT-PS23 | Group (G) | Time (T) | G × T |
|---|---|---|---|---|---|---|---|
| AST (U/L) | 0 week | 22 ± 5 a | 22 ± 4 a | 20 ± 3 a | 0.199 | 0.047 * | 0.686 |
| 6 week | 21 ± 5 a | 21 ± 5 a | 20 ± 4 a | ||||
| 12 week | 21 ± 6 a | 22 ± 5 a | 20 ± 4 a | ||||
| ALT (U/L) | 0 week | 18 ± 7 a | 17 ± 7 a | 17 ± 5 a | 0.594 | 0.014 * | 0.858 |
| 6 week | 17 ± 6 a | 16 ± 6 a | 16 ± 4 a | ||||
| 12 week | 17 ± 8 a | 16 ± 6 a | 16 ± 4 a | ||||
| BUN (mg/dL) | 0 week | 16.6 ± 3.0 a | 16.7 ± 4.5 a | 16.6 ± 4.3 a | 0.594 | 0.074 | 0.858 |
| 6 week | 16.7 ± 3.7 a | 16.3 ± 3.7 a | 16.6 ± 4.1 a | ||||
| 12 week | 16.3 ± 3.4 a | 16.3 ± 3.7 a | 16.6 ± 4.1 a | ||||
| CREA (mg/dL) | 0 week | 0.80 ± 0.15 a | 0.81 ± 0.23 a | 0.81 ± 0.17 a | 0.984 | 0.057 | 0.758 |
| 6 week | 0.79 ± 0.15 a | 0.79 ± 0.23 a | 0.79 ± 0.18 a | ||||
| 12 week | 0.79 ± 0.16 a | 0.78 ± 0.24 a | 0.80 ± 0.16 a | ||||
| UA (mg/dL) | 0 week | 5.1 ± 1.4 a | 5.2 ± 1.0 a | 5.2 ± 1.0 a | 0.623 | 0.115 | 0.491 |
| 6 week | 4.9 ± 1.4 a | 5.0 ± 1.0 a | 5.2 ± 0.9 a | ||||
| 12 week | 4.9 ± 1.3 a | 4.9 ± 0.9 a | 5.3 ± 1.1 a | ||||
| Glucose (mg/dL) | 0 week | 88 ± 18 a | 88 ± 12 a | 88 ± 16 a | 0.998 | 0.780 | 0.981 |
| 6 week | 89 ± 20 a | 89 ± 12 a | 89 ± 23 a | ||||
| 12 week | 89 ± 17 a | 88 ± 12 a | 88 ± 13 a | ||||
| HbA1c (%) | 0 week | 5.8 ± 0.6 a | 5.8 ± 0.4 a | 5.9 ± 0.5 a | 0.870 | 0.054 | 0.921 |
| 6 week | 5.8 ± 0.7 a | 5.8 ± 0.4 a | 5.9 ± 0.5 a | ||||
| 12 week | 5.8 ± 0.6 a | 5.9 ± 0.4 a | 5.9 ± 0.5 a | ||||
| TG (mg/dL) | 0 week | 110 ± 40 a | 112 ± 49 a | 107 ± 33 a | 0.912 | 0.177 | 0.942 |
| 6 week | 108 ± 39 a | 112 ± 56 a | 106 ± 46 a | ||||
| 12 week | 106 ± 36 a | 105 ± 48 a | 104 ± 40 a | ||||
| TC (mg/dL) | 0 week | 189 ± 31 a | 182 ± 23 a | 190 ± 33 a | 0.588 | 0.068 | 0.823 |
| 6 week | 186 ± 30 a | 178 ± 24 a | 183 ± 34 a | ||||
| 12 week | 182 ± 38 a | 178 ± 28 a | 185 ± 41 a | ||||
| HDL (mg/dL) | 0 week | 59.3 ± 12.9 a | 58.9 ± 11.8 a | 58.7 ± 10.4 a | 0.972 | 0.302 | 0.394 |
| 6 week | 58.4 ± 13.7 a | 58.4 ± 11.5 a | 56.7 ± 11.8 a | ||||
| 12 week | 58.6 ± 15.6 a | 57.3 ± 12.2 a | 59.2 ± 14.4 a | ||||
| LDL (mg/dL) | 0 week | 113.3 ± 26.1 a | 106.8 ± 22.5 a | 114.0 ± 29.7 a | 0.611 | 0.004 * | 0.682 |
| 6 week | 110.5 ± 28.8 a | 103.4 ± 22.4 a | 108.4 ± 27.5 a | ||||
| 12 week | 105.7 ± 28.6 a | 103.5 ± 24.1 a | 107.5 ± 32.4 a | ||||
| Ca (mg/dL) | 0 week | 9.3 ± 0.6 a | 9.2 ± 0.6 a | 9.2 ± 0.5 a | 0.872 | 0.128 | 0.358 |
| 6 week | 9.1 ± 0.6 a | 9.0 ± 0.5 a | 9.0 ± 0.6 a | ||||
| 12 week | 9.0 ± 0.8 a | 9.1 ± 0.6 a | 9.2 ± 0.6 a |
| Items | L-PS23 | HT-PS23 |
|---|---|---|
| Enhancement of lower limb muscle strength | Yes | Yes (greater improvement compared to L-PS23) |
| Reduction of inflammatory markers (e.g., IL-6, CRP) | Yes | Yes (more pronounced effect on IL-6 and CRP reduction) |
| Increase in anti-inflammatory marker (IL-10) | Yes | Yes (slightly higher increase compared to L-PS23) |
| Increase in testosterone levels | No significant effect | Yes (significant increase observed) |
| Effects on muscle mass | No significant change | No significant change |
| Stability and ease of storage | Limited by viability of live cells | High (due to heat inactivation) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-C.; Hsu, Y.-J.; Yang, H.-J.; Huang, C.-C. Enhancement of Lower Limb Muscle Strength and Reduction of Inflammation in the Elderly: A Randomized, Double-Blind Clinical Trial Comparing Lacticaseibacillus paracasei PS23 Probiotic with Heat-Treated Supplementation. Nutrients 2025, 17, 463. https://doi.org/10.3390/nu17030463
Lee M-C, Hsu Y-J, Yang H-J, Huang C-C. Enhancement of Lower Limb Muscle Strength and Reduction of Inflammation in the Elderly: A Randomized, Double-Blind Clinical Trial Comparing Lacticaseibacillus paracasei PS23 Probiotic with Heat-Treated Supplementation. Nutrients. 2025; 17(3):463. https://doi.org/10.3390/nu17030463
Chicago/Turabian StyleLee, Mon-Chien, Yi-Ju Hsu, Hung-Jen Yang, and Chi-Chang Huang. 2025. "Enhancement of Lower Limb Muscle Strength and Reduction of Inflammation in the Elderly: A Randomized, Double-Blind Clinical Trial Comparing Lacticaseibacillus paracasei PS23 Probiotic with Heat-Treated Supplementation" Nutrients 17, no. 3: 463. https://doi.org/10.3390/nu17030463
APA StyleLee, M.-C., Hsu, Y.-J., Yang, H.-J., & Huang, C.-C. (2025). Enhancement of Lower Limb Muscle Strength and Reduction of Inflammation in the Elderly: A Randomized, Double-Blind Clinical Trial Comparing Lacticaseibacillus paracasei PS23 Probiotic with Heat-Treated Supplementation. Nutrients, 17(3), 463. https://doi.org/10.3390/nu17030463






