The Application and Mechanism Analysis of Enteral Nutrition in Clinical Management of Chronic Diseases
Abstract
1. Introduction
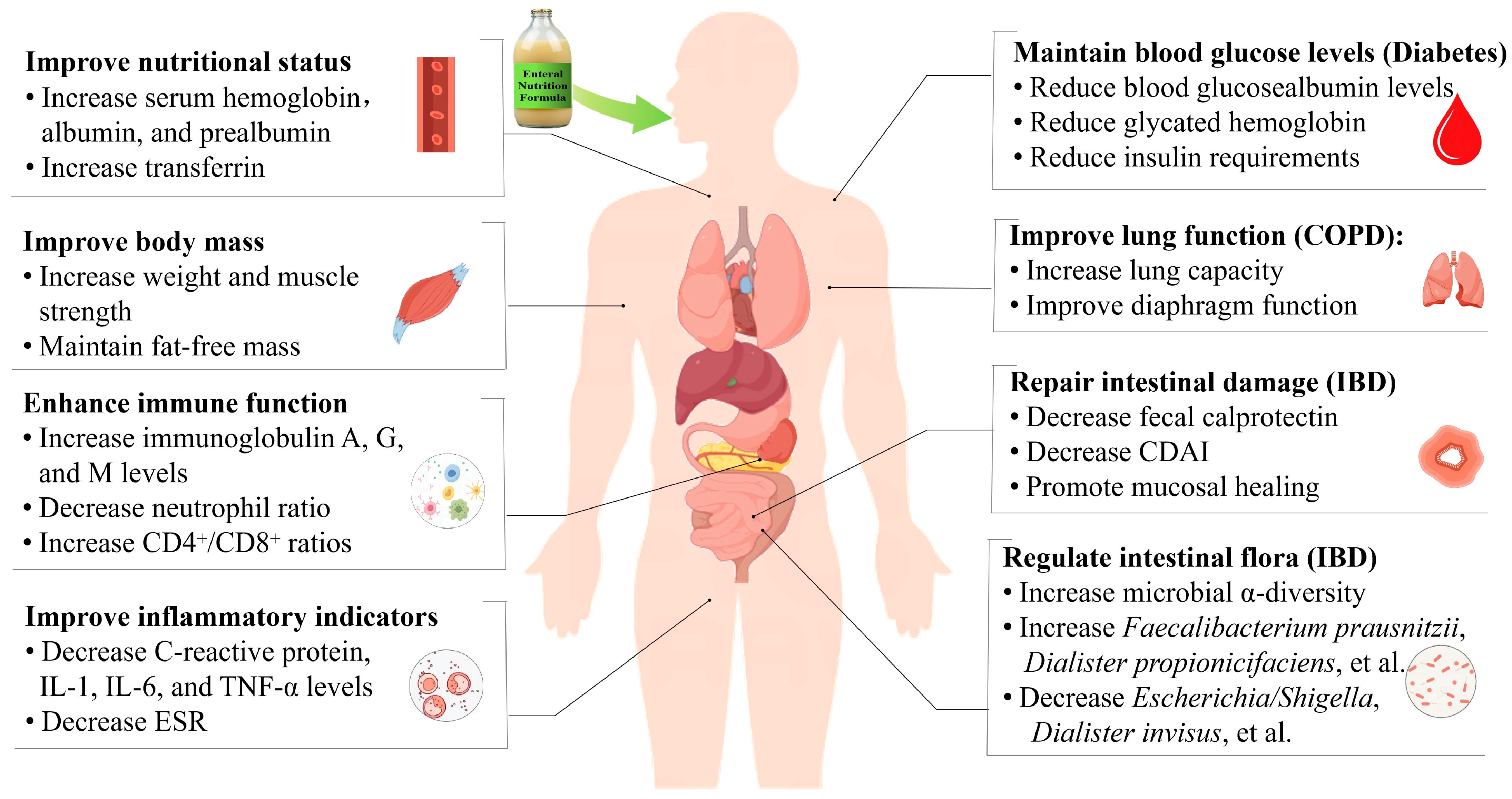
2. The Application of Enteral Nutrition in the Management of Chronic Diseases
3. The Mechanism of Enteral Nutrition in Clinical Nutritional Management for Chronic Diseases
3.1. Cancer
3.2. Kidney Disease
3.3. Diabetes
3.4. Inflammatory Bowel Disease
3.5. Chronic Respiratory Disease
4. Comprehensive Benefit Analysis of Enteral Nutrition in the Management of Chronic Diseases
5. Challenges and Strategies for Enteral Nutrition in Chronic Disease Management
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mckenna, M.; Collins, J.L. Chapter 1 Current Issues and Challenges in Chronic Disease Control. In Chronic Disease Epidemiology and Control, 2nd ed.; Remington, P.L., Brownson, R.C., Wegner, M.V., Eds.; American Public Health Association: Washington, DC, USA, 2010; p. 1. [Google Scholar]
- World Health Organization. Invisible Numbers: The True Extent of Noncommunicable Diseases and What to Do About Them. Available online: https://apps.who.int/iris/rest/bitstreams/1466662/retrieve (accessed on 5 October 2024).
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Jia, R.; Zhao, H.; Yan, X.; Yang, Z. The effect of enteral nutrition nursing intervention on postoperative treatment of chronic critically ill patients: Health prevention data analysis. Prev. Med. 2023, 174, 107635. [Google Scholar] [CrossRef]
- Andersen, S.; Fichera, R.; Banks, M.; Brown, T.; Kennedy, G.; Weber, N.; Williams, D.; Bauer, J. Proactive enteral nutrition for patients undergoing allogeneic stem cell transplantation—Implementation and clinical outcomes. Eur. J. Clin. Nutr. 2024, 78, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation. Available online: https://iris.who.int/handle/10665/42665 (accessed on 5 October 2024).
- Nishida, C.; Uauy, R.; Kumanyika, S.; Shetty, P. The joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases: Process, product and policy implications. Public Health Nutr. 2004, 7, 245–250. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Harsanyi, L.; Laviano, A.; Ljungqvist, O.; Soeters, P.; DGEM (German Society for Nutritional Medicine); Jauch, K.W.; Kemen, M.; Hiesmayr, J.M.; et al. ESPEN Guidelines on Enteral Nutrition: Surgery including organ transplantation. Clin. Nutr. 2006, 25, 224–244. [Google Scholar] [CrossRef]
- Urlep, D.; Orel, R.; Kunstek, P.; Benedik, E. Treatment of Active Crohn’s Disease in Children Using Partial Enteral Nutrition Combined with a Modified Crohn’s Disease Exclusion Diet: A Pilot Prospective Cohort Trial on Clinical and Endoscopic Outcomes. Nutrients 2023, 15, 4676. [Google Scholar] [CrossRef]
- Folwarski, M.; Kłęk, S.; Zoubek-Wójcik, A.; Szafrański, W.; Bartoszewska, L.; Figuła, K.; Jakubczyk, M.; Jurczuk, A.; Kamocki, Z.; Kowalczyk, T.; et al. Foods for Special Medical Purposes in Home Enteral Nutrition-Clinical Practice Experience. Multicenter Study. Front. Nutr. 2022, 9, 906186. [Google Scholar] [CrossRef] [PubMed]
- Harkness, L. The History of Enteral Nutrition Therapy: From Raw Eggs and Nasal Tubes to Purified Amino Acids and Early Postoperative Jejunal Delivery. J. Am. Diet Assoc. 2002, 102, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Matarese, L.E.; Gottschlich, M.M. Contemporary Nutrition Support Practice: A Clinical Guide; WB Saunders Company: Philadelphia, PA, USA, 1998; pp. 79–98. [Google Scholar]
- Dudrick, S.J.; Palesty, J.A. Historical highlights of the development of enteral nutrition. Surg. Clin. N. Am. 2011, 91, 945–964. [Google Scholar] [CrossRef] [PubMed]
- Church, A.; Zoeller, S. Enteral nutrition product formulations: A review of available products and indications for use. Nutr. Clin. Pract. 2023, 38, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Silk, D.B. Formulation of enteral diets. Nutrition 1999, 15, 626–632. [Google Scholar] [CrossRef]
- Klepper, C.M.; Moore, J.; Gabel, M.E.; Fleet, S.E.; Kassel, R. Pediatric formulas: Categories, composition, and considerations. Nutr. Clin. Pract. 2023, 38, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.O.; Velapati, S.R.; Patel, J.; Hurt, R.T.; Mundi, M.S. Enteral Nutrition Therapy: Historical Perspective, Utilization, and Complications. Curr. Gastroenterol. Rep. 2024, 26, 200–210. [Google Scholar] [CrossRef] [PubMed]
- White, B.; Svolos, V.; Gervais, L.; Jatkowska, A.; Nichols, B.; MacDonald, J.; Seenan, J.P.; Hansen, R.; Russell, R.K.; Milling, S.; et al. Inflammation-related Proteins Support Diagnosis of Inflammatory Bowel Disease and Are Modified by Exclusive Enteral Nutrition in Children with Crohn’s Disease, Especially of Ileal Phenotype. Inflamm. Bowel Dis. 2024, izae107. [Google Scholar] [CrossRef] [PubMed]
- Gliwska, E.; Guzek, D.; Przekop, Z.; Sobocki, J.; Głąbska, D. Quality of Life of Cancer Patients Receiving Enteral Nutrition: A Systematic Review of Randomized Controlled Trials. Nutrients 2021, 13, 4551. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.J.; Yang, T.M.; Tsai, Y.H. Nutritional supplementation in patients with chronic obstructive pulmonary disease. J. Formos. Med. Assoc. 2016, 115, 595–601. [Google Scholar] [CrossRef]
- Geesala, R.; Gongloor, P.; Recharla, N.; Shi, X.Z. Mechanisms of Action of Exclusive Enteral Nutrition and Other Nutritional Therapies in Crohn’s Disease. Nutrients 2024, 16, 3581. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, D.; Zhang, X.; Yang, J.; Chen, X. Efficacy of early enteral nutrition versus total parenteral nutrition for patients with gastric cancer complicated with diabetes mellitus: A systematic review and meta-analysis. Nutr. Diet 2022, 79, 129–139. [Google Scholar] [CrossRef]
- Ojo, O.; Weldon, S.M.; Thompson, T.; Crockett, R.; Wang, X.H. The Effect of Diabetes-Specific Enteral Nutrition Formula on Cardiometabolic Parameters in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2019, 11, 1905. [Google Scholar] [CrossRef]
- Gabrielli, C.P.; Steemburgo, T. Adequate calorie and protein administration via enteral nutrition may contribute to improved 30-day survival in patients with solid tumors at nutritional risk. Clin. Nutr. ESPEN 2024, 59, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Triantafillidis, J.K.; Papakontantinou, J.; Antonakis, P.; Konstadoulakis, M.M.; Papalois, A.E. Enteral Nutrition in Operated-On Gastric Cancer Patients: An Update. Nutrients 2024, 16, 1639. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Bager, P.; Escher, J.; Forbes, A.; Hébuterne, X.; Hvas, C.L.; Joly, F.; Klek, S.; Krznaric, Z.; Ockenga, J.; et al. ESPEN guideline on Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2023, 42, 352–379. [Google Scholar] [CrossRef] [PubMed]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults with Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef]
- Sabatino, A.; Fiaccadori, E.; Barazzoni, R.; Carrero, J.J.; Cupisti, A.; Waele, E.D.; Jonckheer, J.; Cuerda, C.; Bischoff, S.C. ESPEN practical guideline on clinical nutrition in hospitalized patients with acute or chronic kidney disease. Clin. Nutr. 2024, 43, 2238–2254. [Google Scholar] [CrossRef]
- Rhodes, C. Enteral Nutrition in Adults with Chronic Kidney Disease: Things to Consider. J. Ren. Nutr. 2021, 31, 427–430. [Google Scholar] [CrossRef]
- Barreira, J.V. The Role of Nutrition in Cancer Patients. Nutr. Cancer 2021, 73, 2849–2850. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.H.; Beuren, A.G.; Friedrich, H.J.; Gabrielli, C.P.; Stefani, G.P.; Steemburgo, T. The Importance of Nutrition in Cancer Care: A Narrative Review. Curr. Nutr. Rep. 2024, 13, 950–965. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Han, Z.; Gan, W.; Zhu, L.; Cao, L. Effects of early enteral nutrition on nutritional status and immune function in colon cancer patients during chemotherapy. Curr. Top. Nutraceutical Res. 2024, 22, 703–708. [Google Scholar]
- Xu, Y.; Wei, F.X. A retrospective study of enteral nutrition on immune and inflammatory factors after liver cancer surgery. Medicine 2021, 100, e27718. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhan, Q. Rehabilitation treatment of enteral nutrition whey protein in lung cancer patients in southern China. Ciência Tecnol. Aliment. 2020, 41, 654–659. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, X.; Zhao, H.; Chen, N.; Wang, J.; Zhuang, H.; Zhang, X. Clinical Application of Enteral Nutrition Combined with Microbial Preparation for Intestinal Preparation in Elderly Patients with Colorectal Cancer. Med. Sci. Monit. 2022, 28, e935366. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, G.; Zhu, L. Home enteral nutrition for postoperative elderly patients with esophageal cancer. Ann. Palliat. Med. 2021, 10, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Kita, R.; Miyata, H.; Sugimura, K.; Tanaka, K.; Makino, T.; Yamashita, K.; Yamasaki, M.; Motoori, M.; Shiraishi, O.; Kimura, Y.; et al. Clinical effect of enteral nutrition support during neoadjuvant chemotherapy on the preservation of skeletal muscle mass in patients with esophageal cancer. Clin. Nutr. 2021, 40, 4380–4385. [Google Scholar] [CrossRef]
- Jałocha, I.; Ławiński, M.; Zadka, K.; Matin, M.; Jachnis, A.; Ukleja, A.; Charuta, A.; Horbańczuk, J.O.; Słodkowski, M.; Atanasov, A.G. The impact of home enteral nutrition planned with the use of indirect calorimetry on the nutritional status and body composition of cancer patients. Curr. Res. Biotechnol. 2024, 7, 100160. [Google Scholar] [CrossRef]
- Yeen, S.F.; Kafadar, M.T.; Gök, M.A. Comparison of Perioperative Standard and Immunomodulating Enteral Nutrition in Patients Received Major Abdominal Cancer Surgery: A Prospective, Randomized, Controlled Clinical Trial. Indian J. Surg. 2020, 82, 828–834. [Google Scholar] [CrossRef]
- Miyata, H.; Yano, M.; Yasuda, T.; Yamasaki, M.; Murakami, K.; Makino, T.; Nishiki, K.; Sugimura, K.; Motoori, M.; Shiraishi, O.; et al. Randomized study of the clinical effects of ω-3 fatty acid-containing enteral nutrition support during neoadjuvant chemotherapy on chemotherapy-related toxicity in patients with esophageal cancer. Nutrition 2017, 33, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Gavazzi, C.; Colatrugliom, S.; Valoriani, F.; Mazzaferro, V.; Sabbatini, A.; Biffi, R.; Mariani, L.; Miceli, R. Impact of home enteral nutrition in malnourished patients with upper gastrointestinal cancer: A multicentre randomised clinical trial. Eur. J. Cancer 2016, 64, 107–112. [Google Scholar] [CrossRef]
- Sezer, S.; Bal, Z.; Tutal, E.; Uyar, M.E.; Acar, N.O. Long-term oral nutrition supplementation improves outcomes in malnourished patients with chronic kidney disease on hemodialysis. JPEN J. Parenter. Enteral. Nutr. 2014, 38, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Kelly, O.J.; Huang, M.C.; Liao, H.Y.; Lin, C.C.; Tung, T.Y.; Cheng, R.W.; Wang, M.Y.; Yalawar, M.; Hwang, S.J. A Low-Protein Diet with a Renal-Specific Oral Nutrition Supplement Helps Maintain Nutritional Status in Patients with Advanced Chronic Kidney Disease. J. Pers. Med. 2021, 11, 1360. [Google Scholar] [CrossRef]
- Moretti, H.D.; Johnson, A.M.; Keeling-Hathaway, T.J. Effects of protein supplementation in chronic hemodialysis and peritoneal dialysis patients. J. Ren. Nutr. 2009, 19, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Lansink, M.; Rouws, C.H.F.C.; van Laere, K.M.J.; Frost, G.S. Administration of a new diabetes specific enteral formula results in an improved 24 h glucose profile in type 2 diabetic patients. Diabetes Res. Clin. Pract. 2009, 84, 259–266. [Google Scholar] [CrossRef]
- Ballesteros Pomar, M.D.; Lardiés Sánchez, B.; Argente Pla, M.; Ramos Carrasco, A.; Suárez Gutiérrez, L.; Yoldi Arrieta, A.; Sorribes Carreras, P.; Gutiérrez Medina, S.; Molina Soria, J.B.; Berrio Miranda, M.; et al. A real-life study of the medium to long-term effectiveness of a hypercaloric, hyperproteic enteral nutrition formula specifically for patients with diabetes on biochemical parameters of metabolic control and nutritional status. Endocrinol. Diabetes Nutr. 2022, 69, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Mesejo, A.; Montejo-González, J.C.; Vaquerizo-Alonso, C.; Lobo-Tamer, G.; Zabarte-Martinez, M.; Herrero-Meseguer, J.I.; Acosta-Escribano, J.; Blesa-Malpica, A.; Martinez-Lozano, F. Diabetes-specific enteral nutrition formula in hyperglycemic, mechanically ventilated, critically ill patients: A prospective, open-label, blind-randomized, multicenter study. Crit. Care 2015, 19, 390. [Google Scholar] [CrossRef] [PubMed]
- Huhmann, M.B.; Yamamoto, S.; Neutel, J.M.; Cohen, S.S.; Ochoa Gautier, J.B. Very high-protein and low-carbohydrate enteral nutrition formula and plasma glucose control in adults with type 2 diabetes mellitus: A randomized crossover trial. Nutr. Diabetes 2018, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, J.; Zhang, Y.; Liu, C. Early enteral nutrition and total parenteral nutrition on the nutritional status and blood glucose in patients with gastric cancer complicated with diabetes mellitus after radical gastrectomy. Exp. Ther. Med. 2018, 16, 321–327. [Google Scholar] [CrossRef]
- Rolandsdotter, H.; Jönsson-Videsäter, K.; Fagerberg, U.L.; Finkel, Y.; Eberhardson, M. Exclusive Enteral Nutrition: Clinical Effects and Changes in Mucosal Cytokine Profile in Pediatric New Inflammatory Bowel Disease. Nutrients 2019, 11, 414. [Google Scholar] [CrossRef]
- Jiang, T.; Jiang, Y.; Jin, Q.; Xu, S.; Fingerhut, A.; Shi, Y.; Zheng, M.; He, Z. Role of perioperative nutritional status and enteral nutrition in predicting and preventing post-operative complications in patients with Crohn’s disease. Front. Nutr. 2023, 9, 1085037. [Google Scholar] [CrossRef]
- Wall, C.L.; Gearry, R.B.; Day, A.S. Treatment of Active Crohn’s Disease with Exclusive and Partial Enteral Nutrition: A Pilot Study in Adults. Inflamm. Intest. Dis. 2018, 2, 219–227. [Google Scholar] [CrossRef]
- Liu, J.; Andrews, J.M.; Sammour, T.; Bryant, R.V.; Grafton, R.; Simpson, E.; Putrus, E.; Nixon, C. Benefits of Exclusive Enteral Nutrition in Adults with Complex Active Crohn’s Disease: A Case Series of 13 Consecutive Patients. Crohn’s Colitis 360 2019, 1, otz044. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, Z.; Diao, N.; Tang, J.; Zhu, X.; Guo, Q.; Chao, K.; Gao, X. Lipidomics reveals significant alterations associated with exclusive enteral nutrition treatment in adult patients with active Crohn’s disease. Ann. Transl. Med. 2022, 10, 1062. [Google Scholar] [CrossRef]
- Falcone, E.L.; Han, Y.; Kreuzburg, S.; Heller, T.; Church, J.A.; Grou, C.; Calderon, V.; Subramanian, P.; Deming, C.; Conlan, S.; et al. Exclusive enteral nutrition induced sustained changes in the microbiota and improved inflammatory bowel disease in a pediatric patient with chronic granulomatous disease. J. Allergy Clin. Immunol. Pract. 2021, 9, 1011–1014. [Google Scholar] [CrossRef]
- Hart, L.; Farbod, Y.; Szamosi, J.C.; Yamamoto, M.; Britz-McKibbin, P.; Halgren, C.; Zachos, M.; Pai, N. Effect of Exclusive Enteral Nutrition and Corticosteroid Induction Therapy on the Gut Microbiota of Pediatric Patients with Inflammatory Bowel Disease. Nutrients 2020, 12, 1691. [Google Scholar] [CrossRef]
- Lv, Y.; Lou, Y.; Liu, A.; Cheng, Q.; Yang, G.; Xu, C.; Luo, Y.; Lou, J.; Yu, J.; Fang, Y.; et al. The impact of exclusive enteral nutrition on the gut microbiome and bile acid metabolism in pediatric Crohn’s disease. Clin. Nutr. 2023, 42, 116–128. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, L.; Chen, Y.; Chen, H. Exclusive enteral nutrition remodels the intestinal flora in patients with active Crohn’s disease. BMC Gastroenterol. 2022, 22, 212. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, X.; Wang, D.; Li, Y. Enteral Nutrition Improves Diaphragmatic Thickness and Prognosis of Mechanically Ventilated Patients with Chronic Obstructive Pulmonary Disease. Curr. Top. Nutraceutical Res. 2021, 19, 333–338. [Google Scholar] [CrossRef]
- Zhang, J.; Dou, Q.; Chen, J.; Liang, Y.; Huang, Z. Analysis of clinical effects of early enteral nutrition standardized treatment process management on patients with acute exacerbation of chronic obstructive pulmonary disease on invasive mechanical ventilation. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2020, 32, 67–71. [Google Scholar]
- Degirmenci, D.; Sahin, H.; Soylu, M. The effect of enteral nutrition support on muscle function capacity and pulmonary functions in malnourished patients with Chronic Obstructive Pulmonary Disease. Prog. Nutr. 2018, 20, 120–127. [Google Scholar]
- Li, Y.; Xie, Y.P.; Li, X.M.; Lu, T. Effects of early standardized enteral nutrition on preventing acute muscle loss in the acute exacerbation of chronic obstructive pulmonary disease patients with mechanical ventilation. World J. Emerg. Med. 2023, 14, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Xiao, D.; Chen, H.; Rao, L.; Xiao, H.; Li, H. Effects of beclomethasone and aminophylline combined with enteral nutrition in chronic obstructive pulmonary disease on nutritional status and immune function in elders. Asia Pac. J. Clin. Nutr. 2021, 30, 60–66. [Google Scholar] [PubMed]
- Nelms, C.L. Optimizing Enteral Nutrition for Growth in Pediatric Chronic Kidney Disease (CKD). Front. Pediatr. 2018, 6, 214. [Google Scholar] [CrossRef] [PubMed]
- Ash, S.; Campbell, K.L.; Bogard, J.; Millichamp, A. Nutrition prescription to achieve positive outcomes in chronic kidney disease: A systematic review. Nutrients 2014, 6, 416–451. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Cano, N.J.; Budde, K.; Chazot, C.; Kovesdy, C.P.; Mak, R.H.; Mehrotra, R.; Raj, D.S.; Sehgal, A.R.; Stenvinkel, P.; et al. Diets and enteral supplements for improving outcomes in chronic kidney disease. Nat. Rev. Nephrol. 2011, 7, 369–384. [Google Scholar] [CrossRef]
- Shen, C.L.; Liebstein, D.; Fernandez, H. Malnutrition and protein energy wasting are associated with severity and progression of pediatric chronic kidney disease. Pediatr. Nephrol. 2024, 39, 243–250. [Google Scholar] [CrossRef]
- Jadeja, Y.P.; Kher, V. Protein energy wasting in chronic kidney disease: An update with focus on nutritional interventions to improve outcomes. Indian J. Endocrinol. Metab. 2012, 16, 246–251. [Google Scholar] [CrossRef]
- Sabatino, A.; Regolisti, G.; Gandolfini, I.; Delsante, M.; Fani, F.; Gregorini, M.C.; Fiaccadori, E. Diet and enteral nutrition in patients with chronic kidney disease not on dialysis: A review focusing on fat, fiber and protein intake. J. Nephrol. 2017, 30, 743–754. [Google Scholar] [CrossRef]
- Gui, L.L.; Xu, S.T.; He, Q.P.; Liu, J.; Liu, Y.; Gou, J.Z.; Liu, Z.H.; Xie, H.L. Nutritional support treatment in critically ill patients with kidney disease: A prospective study. Chin. J. Nephrol. Dial. Transplant. 2013, 22, 207–212. [Google Scholar]
- Marlais, M.; Stojanovic, J.; Jones, H.; Cleghorn, S.; Rees, L. Catch-up growth in children with chronic kidney disease started on enteral feeding after 2 years of age. Pediatr. Nephrol. 2020, 35, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, D.J.; Gast, T.R.; Ferguson, K.B.; Bunchman, T.E.; Barletta, G.M. Nutritional management of hyperkalemic infants with chronic kidney disease, using adult renal formulas. J. Ren. Nutr. 2010, 20, 121–126. [Google Scholar] [CrossRef] [PubMed]
- González Infantino, C.A.; González, C.D.; Sánchez, R.; Presner, N. Hyperglycaemia and hypoalbuminemia as prognostic mortality factors in patients with enteral feeding. Nutrition 2013, 29, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Cortinovis, F.; Cortesi, L.; Sileo, F. Protocol for blood glucose control during enteral nutrition induction in patients with diabetes mellitus. Mediterr. J. Nutr. Metab. 2009, 1, 159–163. [Google Scholar] [CrossRef]
- Qaseem, A.; Chou, R.; Humphrey, L.L.; Shekelle, P. Inpatient glycaemic control: Best practice advice from the clinical guidelines committee of the American College of Physicians. Am. J. Med. Qual. 2014, 29, 95–98. [Google Scholar] [CrossRef]
- Wright, K.; Ojo, O. Foot care for residents with type 2 diabetes. Nurs. Resid. Care 2010, 12, 585–589. [Google Scholar] [CrossRef]
- Sanz-Paris, A.; Álvarez Hernández, J.; Ballesteros-Pomar, M.D.; Botella-Romero, F.; León-Sanz, M.; Martín-Palmero, Á.; Olveira, G. Evidence-based recommendations and expert consensus on enteral nutrition in the adult patient with diabetes mellitus or hyperglycemia. Nutrition 2017, 41, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Buranapin, S.; Siangruangsang, S.; Chantapanich, V.; Hengjeerajarus, N. The comparative study of diabetic specific formula and standard formula on postprandial plasma glucose control in type 2 DM patients. J. Med. Assoc. Thail. 2014, 97, 582–588. [Google Scholar]
- Hise, M.E.; Fuhrman, M.P. The Effect of Diabetes Specific Enteral Formulae on Clinical and Glycaemic Indicators. Pract. Gastroenterol. 2009, 33, 20–36. [Google Scholar]
- Goh, J.; O’Morain, C.A. Review article: Nutrition and adult inflammatory bowel disease. Aliment. Pharmacol. Ther. 2003, 17, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, L.; Buchuk, R.; Lujan, R.; Focht, G.; Greenfeld, S.; Kariv, R.; Weisband, Y.L.; Lederman, N.; Matz, E.; Dotan, I.; et al. Enteral nutrition compared with corticosteroids in children with Crohn’s disease: A long-term nationwide study from the epi-IIRN. Aliment. Pharmacol. Ther. 2024, 60, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Dhillon, A.; Zhang, D.; Sherlock, M.E.; Tondeur, M.; Zachos, M. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2018, 4, CD000542. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Boye, T.L.; Temby, M.; Sojwal, R.S.; Holman, D.R.; Sinha, S.R.; Rogalla, S.R.; Nielsen, O.H. Gut Microbiome in Inflammatory Bowel Disease: Role in Pathogenesis, Dietary Modulation, and Colitis-Associated Colon Cancer. Microorganisms 2022, 10, 1371. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J. The Effect of Protein Nutritional Support on Inflammatory Bowel Disease and Its Potential Mechanisms. Nutrients 2024, 16, 2302. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Ragavan, M.L.; Hemalatha, S. The functional roles of short chain fatty acids as postbiotics in human gut: Future perspectives. Food Sci. Biotechnol. 2023, 33, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Quaderi, S.A.; Hurst, J.R. The unmet global burden of COPD. Glob. Health Epidemiol. Genom. 2018, 3, e4. [Google Scholar] [CrossRef]
- Mete, B.; Pehlivan, E.; Gülbaş, G.; Günen, H. Prevalence of malnutrition in COPD and its relationship with the parameters related to disease severity. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 3307–3312. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Shin, S.H.; Kim, H.; Im, Y.; Cho, J.; Kang, D.; Park, H.Y. Longitudinal BMI change and outcomes in Chronic Obstructive Pulmonary Disease: A nationwide population-based cohort study. Respir. Res. 2024, 25, 150. [Google Scholar] [CrossRef] [PubMed]
- Mador, M.J. Muscle mass, not body weight, predicts outcome in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002, 166, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Tian, Z.; Li, C.; Lin, S. Application of low-carbohydrate, high-fat enteral nutrition in the treatment of patients with acute exacerbation of chronic obstructive pulmonary disease complicated by respiratory failure. Chongqing Med. J. 2018, 47, 103–105. [Google Scholar]
- Zeng, H.; Zeng, X.; Xiong, N.; Wang, L.; Yang, Y.; Wang, L.; Li, H.; Zhao, W. How stroke-related dysphagia relates to quality of life: The mediating role of nutritional status and psychological disorders, and the moderating effect of enteral nutrition mode. Front. Nutr. 2024, 11, 1339694. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Huang, Z.; Hou, W.; Lin, Y.; Yu, J. Prospective study of an adalimumab combined with partial enteral nutrition in the induction period of Crohn’s disease. Inflamm. Res. 2024, 73, 199–209. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yu, F.; Wang, F.; Liu, Q. Impacts of Enteral Nutrition Support Based on Multiform Internet Education Mode on Perioperative Nutritional Indexes and Quality of Life of Patients with Gastric Cancer. Altern. Ther. Health Med. 2024, 30, 232–237. [Google Scholar]
- Du, J.; Wu, X.; Liu, Y.; Lei, L.; Zhao, H.; Chen, Y.; Nie, C. Does enteral nutrition require continuity of management: A randomized controlled study. Ann. Med. Surg. 2024, 86, 3998–4004. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Paris, A.; Martinez-Trufero, J.; Lambea-Sorrosal, J.; Milà-Villarroel, R.; Calvo-Gracia, F.; on behalf of the DIAPOENO Study. Impact of an Oral Nutritional Protocol with Oligomeric Enteral Nutrition on the Quality of Life of Patients with Oncology Treatment-Related Diarrhea. Nutrients 2020, 13, 84. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, L.W.; Qiang, Y.; Cong, Z.Z.; Zheng, C.; Gu, W.F.; Luo, C.; Xie, K.; Shen, Y. Home enteral nutrition for patients with esophageal cancer undergoing esophagectomy: A systematic review and meta-analysis. Front. Nutr. 2022, 9, 895422. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, R.; Zhu, W.; Gong, J.; Zhang, W.; Li, Y.; Gu, L.; Li, N.; Li, J. Effect of exclusive enteral nutrition on health-related quality of life for adults with active Crohn’s disease. Nutr. Clin. Pract. 2013, 28, 499–505. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, W.; Jiang, H.; Zhu, S.; Xu, J.; Bao, W.; Dang, Y.; Wang, M.Y. Economic value of nutritional support methods in gastrointestinal cancer: A quantitative meta-analysis. Asia Pac. J. Clin. Nutr. 2020, 29, 83–93. [Google Scholar] [PubMed]
- Han, Y.Y.; Lai, S.R.; Partridge, J.S.; Wang, M.Y.; Sulo, S.; Tsao, F.W.; Hegazi, R.A. The clinical and economic impact of the use of diabetes-specific enteral formula on ICU patients with type 2 diabetes. Clin. Nutr. 2017, 36, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Lacson, E.J.; Ikizler, T.A.; Lazarus, J.M.; Teng, M.; Hakim, R.M. Potential impact of nutritional intervention on end-stage renal disease hospitalization, death, and treatment costs. J. Ren. Nutr. 2007, 17, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Randolph, S.; Mustad, V.A.; Lee, J.; Sun, J. Economic analysis of a diabetes-specific nutritional meal replacement for patients with type 2 diabetes. Asia Pac. J. Clin. Nutr. 2010, 19, 1–7. [Google Scholar]
- Borrelli, O.; Cordischi, L.; Cirulli, M.; Paganelli, M.; Labalestra, V.; Uccini, S.; Russo, P.M.; Cucchiara, S. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: A randomized controlled open-label trial. Clin. Gastroenterol. Hepatol. 2006, 4, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.; Banks, M.; Hughes, B.G.; Lin, C.; Kenny, L.; Bauer, J. Tube feeding during treatment for head and neck cancer—Adherence and patient reported barriers. Oral Oncol. 2017, 72, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Hirai, F.; Ishida, T.; Takeshima, F.; Yamamoto, S.; Yoshikawa, I.; Ashizuka, S.; Inatsu, H.; Mitsuyama, K.; Sou, S.; Iwakiri, R.; et al. Effect of a concomitant elemental diet with maintenance anti-tumor necrosis factor-α antibody therapy in patients with Crohn’s disease: A multicenter, prospective cohort study. J. Gastroenterol. Hepatol. 2019, 34, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Kozeniecki, M.; Fritzshall, R. Enteral Nutrition for Adults in the Hospital Setting. Nutr. Clin. Pract. 2015, 30, 634–651. [Google Scholar] [CrossRef] [PubMed]
- Vrijens, B.; De Geest, S.; Hughes, D.A.; Przemyslaw, K.; Demonceau, J.; Ruppar, T.; Dobbels, F.; Fargher, E.; Morrison, V.; Lewek, P.; et al. A new taxonomy for describing and defining adherence to medications. Br. J. Clin. Pharmacol. 2012, 73, 691–705. [Google Scholar] [CrossRef]
- Elfadil, O.M.; Patel, A.; Joly, F.; Lal, S.; Bozzetti, F.; Cuerda, C.; Jeppesen, P.B.; Gossum, A.V.; Wanten, G.; Szczepanek, K.; et al. Patients’ and caregivers’ perspective on challenges and outcomes with tube feeding: Analysis of home enteral nutrition survey data. Clin. Nutr. ESPEN 2024, 61, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Suluhan, D.; Yildiz, D.; Surer, I.; Fidanci Eren, B.; Balamtekin, N. Effect of Gastrostomy Tube Feeding Education on Parents of Children with Gastrostomy. Nutr. Clin. Pract. 2021, 36, 1220–1229. [Google Scholar] [CrossRef]
- Mohamed, E.O.; Ewy, M.; Patel, J.; Patel, I.; Mundi, M.S. Growing use of home enteral nutrition: A great tool in nutrition practice toolbox. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Heidegger, C.P.; Graf, S.; Perneger, T.; Genton, L.; Oshima, T.; Pichard, C. The burden of diarrhea in the intensive care unit (ICU-BD). A survey and observational study of the caregivers’ opinions and workload. Int. J. Nurs. Stud. 2016, 59, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Semerci, R.; Pars, H. Complications of pediatric enteral nutrition at home: A systematic review of quantitative research. Clin. Sci. Nutr. 2024, 6, 27. [Google Scholar] [CrossRef]
- Wanden-Berghe, C.; Patino-Alonso, M.C.; Galindo-Villardón, P.; Sanz-Valero, J. Complications Associated with Enteral Nutrition: CAFANE Study. Nutrients 2019, 11, 2041. [Google Scholar] [CrossRef] [PubMed]
- Montejo, J.C. Enteral nutrition-related gastrointestinal complications in critically ill patients: A multicenter study. The Nutritional and Metabolic Working Group of the Spanish Society of Intensive Care Medicine and Coronary Units. Crit. Care. Med. 1999, 27, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, N.C.; Kamel, A.Y.; Shoulders, B.R.; Rosenthal, M.D.; Murray-Casanova, I.M.; Brakenridge, S.C.; Moore, F.A. Nonocclusive mesenteric ischemia: A rare but lethal complication of enteral nutrition in critically ill patients. Nutr. Clin. Pract. 2022, 37, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Pars, H.; Soyer, T. Home Gastrostomy Feeding Education Program: Effects on the Caregiving Burden, Knowledge, and Anxiety Level of Mothers. JPEN J. Parenter. Enter. Nutr. 2020, 44, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Chen, Y.; Yang, L.; Li, W.; Zhou, Z.; Li, L.; Xiao, Y.; Zhao, J.; Li, L.; Xia, Y. Nursing Practice Based on Evidence-Based Concepts to Prevent Enteral Nutrition Complications for Critically Ill Neurosurgical Patients. Front. Surg. 2022, 9, 857877. [Google Scholar] [CrossRef]
- Mundi, M.S.; Velapati, S.; Kuchkuntla, A.R.; Hurt, R.T. Reduction in Healthcare Utilization with Transition to Peptide-Based Diets in Intolerant Home Enteral Nutrition Patients. Nutr. Clin. Pract. 2020, 35, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.; Smith, A.; Stelter, A.; Uhing, M.; Blom, K.; Goday, P.S. Reining in Nasogastric Tubes: Implementation of a Pediatric Bridle Program. J. Pediatr. Nurs. 2021, 61, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.W.; Chua, A.Y.T.; Yin, K.N.; McDonald, K.; Radley, R.; Phelps, S.; Cleeve, S.; Charlesworth, P. Optimal management of gastrojejunal tube in the ENFit era—Interventions that changed practice. J. Pediatr. Surg. 2021, 56, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.O.; Linch, F.B.; Seegmiller, S.L.; Hurt, R.T.; Mundi, M.S.; Neisen, M.J. Safety and effectiveness of radiologic and endoscopic percutaneous gastrostomy placement: A randomized study. JPEN J. Parenter. Enteral. Nutr. 2022, 46, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Benton, K.; Thomson, I.; Isenring, E.; Smithers, B.M.; Agarwal, E. An investigation into the nutritional status of patients receiving an Enhanced Recovery After Surgery (ERAS) protocol versus standard care following Oesophagectomy. Support. Care Cancer 2018, 26, 2057–2062. [Google Scholar] [CrossRef]
- Stow, R.; Ives, N.; Smith, C.; Rick, C.; Rushton, A. A cluster randomised feasibility trial evaluating nutritional interventions in the treatment of malnutrition in care home adult residents. Trials 2015, 16, 433. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Tandon, P.; Gohel, T.; Corrigan, M.L.; Coughlin, K.L.; Shatnawei, A.; Chatterjee, S.; Kirby, D.F. Gastrointestinal Manifestations, Malnutrition, and Role of Enteral and Parenteral Nutrition in Patients with Scleroderma. J. Clin. Gastroenterol. 2015, 49, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Sulistyo, A.; Abrahao, A.; Freitas, M.E.; Ritsma, B.; Zinman, L. Enteral tube feeding for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst. Rev. 2023, 8, CD004030. [Google Scholar]

| Countries/Regions | Disease Type | Type of Clinical Study Design | Intervention Time | Intervention Method | Conclusion | Reference |
|---|---|---|---|---|---|---|
| Poland | Oropharyngeal cancer and digestive system cancer | Prospective, observational study | 3 months | Enteral nutrition | Enteral nutrition can effectively maintain body mass (weight, triceps skinfold thickness), composition (fat-free mass, body cell mass), and nutritional status (albumin levels). | [40] |
| Turkey | Abdominal cancer | Randomized, controlled trial | 37 days | Immunomodulatory enteral nutrition | Immunomodulatory enteral nutrition can reduce the incidence of surgical site infections, pneumonia, urinary tract infections, and shorten the length of hospital stay in patients undergoing abdominal tumor surgery. | [41] |
| Japan | Esophageal cancer | Randomized, open-label clinical trial. | 15 days | Enteral nutrition rich in ω3 fatty acids | The administration of enteral nutrition rich in ω3 fatty acids has been shown to reduce the frequency of chemotherapy-induced mucosal toxicity, such as stomatitis and diarrhea, and exhibits a hepatoprotective effect during chemotherapy. | [42] |
| China | Colon Cancer | Double-blind random sampling approach. | 6 months | Enteral nutrition | The levels of serum hemoglobin, albumin, prealbumin, immunoglobulin A, G, and M in the enteral nutrition group were significantly increased. Conversely, the levels of serum interleukin-1, interleukin-8, and tumor necrosis factor-α were decreased. | [34] |
| China | Liver cancer | Retrospective study | 3 days | Enteral nutrition | The differences in CD4+, CD8+, interleukin-1, interleukin-6, tumor necrosis factor-α, the time to first flatulence, and the time to first defecation were all statistically significant. | [35] |
| China | Lung cancer | Randomized, controlled trial | 2 weeks | Enteral nutrition | Increases in body weight, triceps skin fold thickness (TSP), mid-arm muscle circumference (MAMC), total protein, albumin, and hemoglobin were observed, along with decreases in white blood cell count and the incidence of adverse reactions. | [36] |
| China | Colorectal Cancer | Randomized, controlled trial | 3 days | Enteral nutrition | The total white blood cell count, neutrophil ratio, C-reactive protein levels, IL-6 levels, and postoperative complication rates were significantly lower than those in the control group, while the serum albumin, prealbumin, and transferrin levels were superior to those in the control group. | [37] |
| China | Esophageal cancer | Randomized, controlled trial | 8 weeks | Enteral nutrition | The Enteral nutrition group had significantly higher Body Mass Index (BMI), Scored Patient-Generated Subjective Global Assessment (PG-SGA) scores, serum albumin levels, serum prealbumin levels, CD4 and CD8 T-cell counts, CD4/CD8 ratios, immunoglobulin A, G, and M levels compared to the control group. | [38] |
| Japan | Esophageal cancer | Randomized, controlled trial | 17 days | Enteral nutrition | Enteral nutrition supports the inhibition of skeletal muscle mass loss in esophageal cancer patients during neoadjuvant chemotherapy. | [39] |
| Italy | Upper gastrointestinal cancer | Multicentre randomised clinical trial | 6 months | Enteral nutrition | Helps maintain weight without any safety issues or negative impacts on quality of life. | [43] |
| Turkey | Chronic Kidney Disease | Randomized, controlled trial | 6 months | Renal-specific oral nutritional supplement | Renal-specific oral nutritional supplement improved patients’ serum albumin levels and anthropometric indicators, and reduced the dose of erythropoietin. | [44] |
| China | Chronic Kidney Disease | Prospective, multicenter, single-arm, and open-label study | 6 months | Renal-specific oral nutritional supplement | Renal-specific oral nutritional supplement increased patients’ energy intake and maintained their serum albumin levels, nutritional status, and quality of life. Their body weight and grip strength significantly increased, while the glomerular filtration rate slightly decreased. | [45] |
| USA | Kidney Disease | Randomized crossover design trial | 12 months | Enteral nutrition | Improved serum nutrition indicators, resulting in reduced hospital admission frequency and length of stay. | [46] |
| The Netherlands | Type 2 diabetes mellitus | Randomized, controlled, double-blind, cross-over study | 1 day | Specific high-protein, high-calorie enteral nutrition formula | The use of diabetes-specific formulas can significantly improve the 24-h and postprandial blood glucose levels in diabetic patients. | [47] |
| Spain | Diabetes | Multicenter, prospective, observational, real-life study | 24 weeks | Specific high-protein, high-calorie enteral nutrition formula | The use of a specific high-protein, high-calorie enteral nutrition formula resulted in a decrease in the proportion of malnourished patients from 78.6% to 29.9%. Blood glucose and glycated hemoglobin levels were significantly reduced, while weight, BMI, albumin, prealbumin, and transferrin levels were significantly increased. C-reactive protein levels were significantly decreased, and the C-reactive protein/albumin ratio was reduced. Gastrointestinal tolerance was good, with only a few patients experiencing moderate to severe symptoms. | [48] |
| Spain | Diabetes | Prospective, open-label, blind-randomized, multicenter study | 4 weeks | Diabetes-specific formula | Compared with the standard control group, the diabetes-specific formula significantly reduced insulin requirements, blood glucose levels, capillary blood glucose levels, and the incidence of ventilator-associated tracheobronchitis or pneumonia. | [49] |
| USA | Type 2 diabetes mellitus | Randomized crossover trial | 4 h | High-protein and low-carbohydrate enteral nutrition formula | A high-protein and low-carbohydrate enteral nutrition formula can significantly improve glycemic control in patients with type 2 diabetes, without significant effects on insulin response. | [50] |
| China | Gastric cancer complicated with diabetes mellitus | Randomized, controlled trial | 8 days | Enteral nutrition | Early enteral nutrition support helps patients maintain good nutritional status, reduces postoperative complications, stabilizes blood glucose levels, facilitates earlier postoperative mobilization, shortens hospital stays, and lowers costs. | [51] |
| Sweden | Crohn’s Disease | Prospective cohort study | 6 weeks | Enteral nutrition | The erythrocyte sedimentation rate (ESR), C-reactive protein, and fecal calprotectin are significantly decreased, while hemoglobin, albumin levels, and body weight are significantly increased. Colonoscopy shows promotion of mucosal healing. | [52] |
| China | Crohn’s Disease | Retrospective cohort study | 21 weeks | Enteral nutrition | Preoperative serum levels of albumin, prealbumin, and hemoglobin are elevated, and the incidence of postoperative complications is reduced. | [53] |
| New Zealand | Crohn’s Disease | Prospective non-randomized pilot study | 8 weeks | Enteral nutrition | The levels of inflammatory markers C-reactive protein and fecal calprotectin decrease, while the levels of nutritional markers serum insulin-like growth factor 1 (IGF-1) and albumin increase. | [54] |
| Australian | Crohn’s Disease | Retrospective analysis | 6 weeks | Enteral nutrition | C-reactive protein levels decrease, serum albumin levels increase, body weight increases, and the need for surgical intervention as well as postoperative complications are reduced. | [55] |
| China | Crohn’s Disease | Prospective cohort study | 12 weeks | Enteral nutrition | CDAI, C-reactive protein, ESR, and platelet counts are significantly reduced, while albumin and hemoglobin levels are increased. Colonoscopy shows promotion of mucosal healing. | [56] |
| Canada | Inflammatory Bowel Disease | - | 10 weeks | Enteral nutrition | Faecalibacterium prausnitzii, Dialister propionicifaciens, and Parabacteroides merdae are significantly increased, while Escherichia/Shigella, Dialister invisus, and Negativibacillus are significantly decreased. Fecal microbial α-diversity is also significantly increased. | [57] |
| Canada | Inflammatory Bowel Disease | Prospective cohort study | 8 weeks | Enteral nutrition | The abundance of Blautia, Sellimonas, and uncharacterized bacteria from the family Ruminococcaceae increases, while the abundance of Granulicatella, Haemophilus, and Streptococcus decreases. | [58] |
| China | Crohn’s Disease | Prospective single-center cohort study | 8 weeks | Enteral nutrition | The Pediatric Crohn’s Disease Activity Index (PCDAI) score and calprotectin levels decrease, while the microbiome and bile acid metabolism return to normal levels. The relative expression of Firmicutes phylum, Flavonifractor, and Clostridium V increases. | [59] |
| China | Crohn’s Disease | Cohort Study | 8 weeks | Enteral nutrition | ESR, C-reactive protein, and CDAI significantly decrease, while serum albumin levels increase. The abundance of Firmicutes, Ruminococcus, Lachnospiraceae, Anaerotruncus, Flavonifractor, and Novosphingobium significantly increases, while the abundance of Proteobacteria decreases. | [60] |
| China | Chronic Obstructive Pulmonary Disease | Randomized, controlled trial | 14 days | Enteral nutrition | Significantly improves the nutritional status and diaphragmatic function of patients, inhibits inflammatory responses, shortens the duration of mechanical ventilation, and enhance clinical treatment efficacy and prognosis. | [61] |
| China | Chronic Obstructive Pulmonary Disease | Randomized, controlled trial | - | Enteral nutrition | Improves patients’ energy metabolism and alleviates respiratory muscle fatigue during and after weaning from mechanical ventilation, without increasing the incidence of related complications. | [62] |
| Turkey | Chronic Obstructive Pulmonary Disease | Rospective, controlled, randomized trial | 8 days | Enteral nutrition | Increased patients’ grip strength and forced expiratory volume in second (FEV1). | [63] |
| China | Chronic Obstructive Pulmonary Disease | Observational study | 28 days | Enteral nutrition | Early standardized enteral nutrition can prevent acute muscle loss and intensive care unit-acquired weakness (ICU-AW) in patients with acute exacerbations of chronic obstructive pulmonary disease (AECOPD). | [64] |
| China | Chronic Obstructive Pulmonary Disease | Randomized, controlled trial | 4 weeks | Enteral nutrition | Compared with the control group, patients in the enteral nutrition group showed significant improvements in partial pressure of carbon dioxide, forced expiratory volume in one second/forced vital capacity, and partial pressure of oxygen. The levels of immunoglobulin A, G, and M, as well as the number of CD4+/CD8+ and CD4+/CD3+ T cells were higher in the EN group than in the control group. Additionally, compared with the control group, the enteral nutrition group had increased levels of inflammatory factors, such as tumor necrosis factor-α and interleukin-1 β, while the level of IL-6 was decreased. The serum total protein, albumin, and transferrin levels were significantly higher in the enteral nutrition group than in the control group. | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Wang, J. The Application and Mechanism Analysis of Enteral Nutrition in Clinical Management of Chronic Diseases. Nutrients 2025, 17, 450. https://doi.org/10.3390/nu17030450
Li Q, Wang J. The Application and Mechanism Analysis of Enteral Nutrition in Clinical Management of Chronic Diseases. Nutrients. 2025; 17(3):450. https://doi.org/10.3390/nu17030450
Chicago/Turabian StyleLi, Qingye, and Jing Wang. 2025. "The Application and Mechanism Analysis of Enteral Nutrition in Clinical Management of Chronic Diseases" Nutrients 17, no. 3: 450. https://doi.org/10.3390/nu17030450
APA StyleLi, Q., & Wang, J. (2025). The Application and Mechanism Analysis of Enteral Nutrition in Clinical Management of Chronic Diseases. Nutrients, 17(3), 450. https://doi.org/10.3390/nu17030450




