Illuminating the Connection: Cutaneous Vitamin D3 Synthesis and Its Role in Skin Cancer Prevention
Abstract
1. Introduction
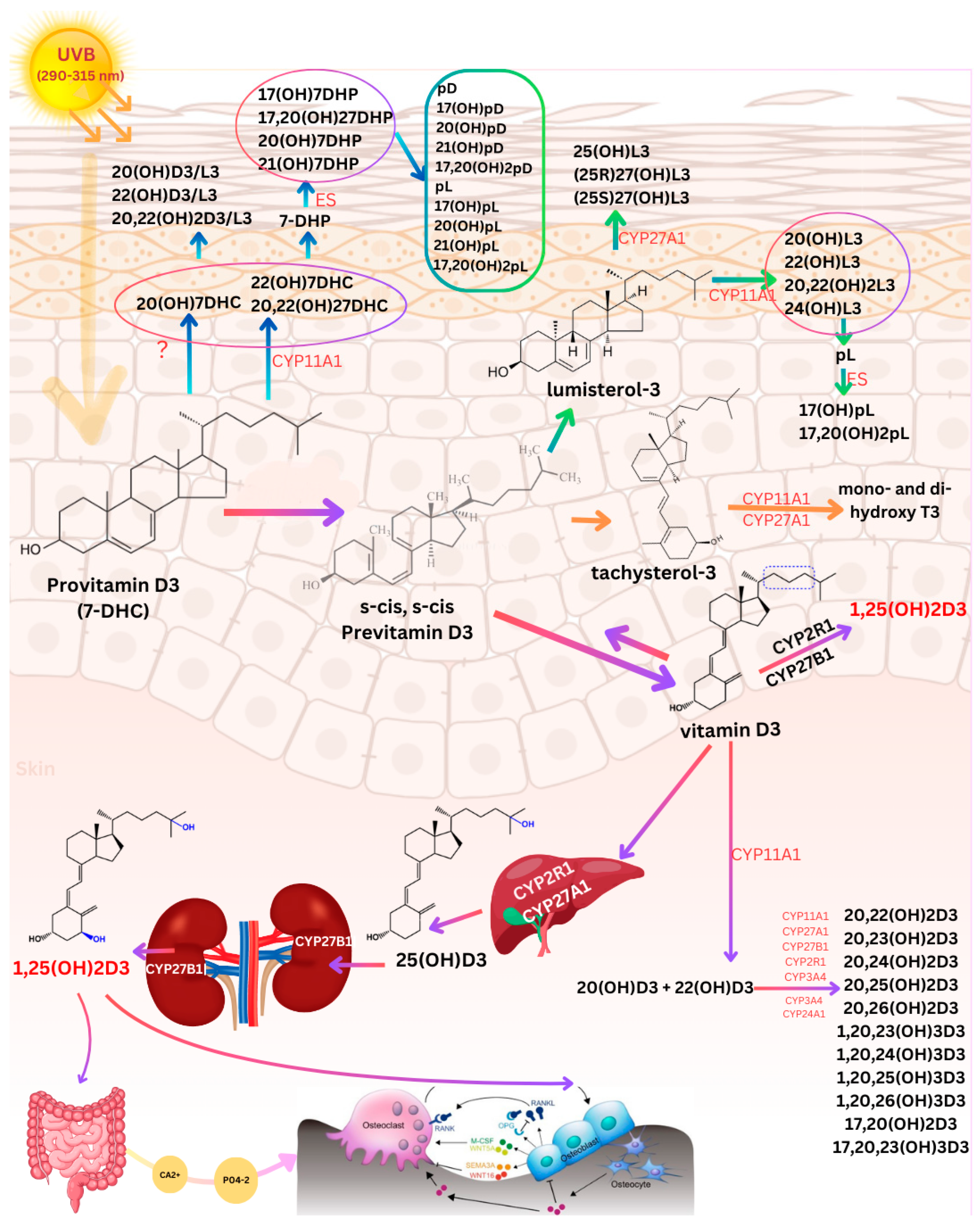
2. Photoproduction and Cutaneous Metabolism of Vitamin D3 and Photoproducts
2.1. Cutaneous Transformation of 7-Dehydrocholesterol to Vitamin D3
2.2. Production and Metabolism of Photoproducts
2.3. Factors Controlling Cutaneous Vitamin D Synthesis
2.4. Metabolism of Vitamin D3
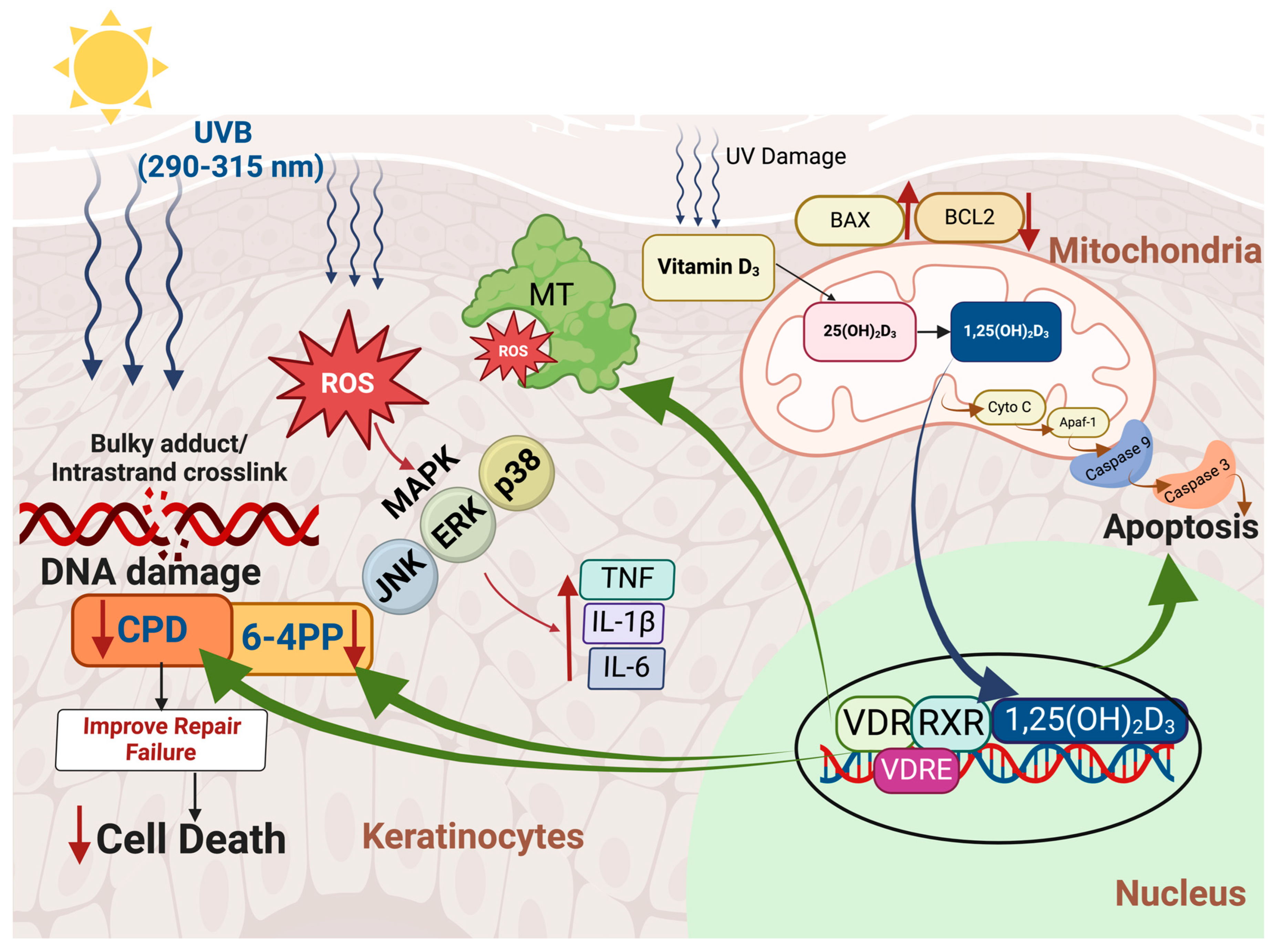
3. Mechanisms Associated with UVA/UVB-Induced Skin Cancers and Vitamin D’s Modulatory Effects
4. Sunlight Dilemma: The Associations Between Sun Exposure, DNA Damage, and Skin Cancer Risk
4.1. 1,25-Dihydroxyvitamin D3 and Its Inhibitory Effects on UVB-Induced Skin Carcinogenesis
4.2. Vitamin D and the Anti-P53 Connection
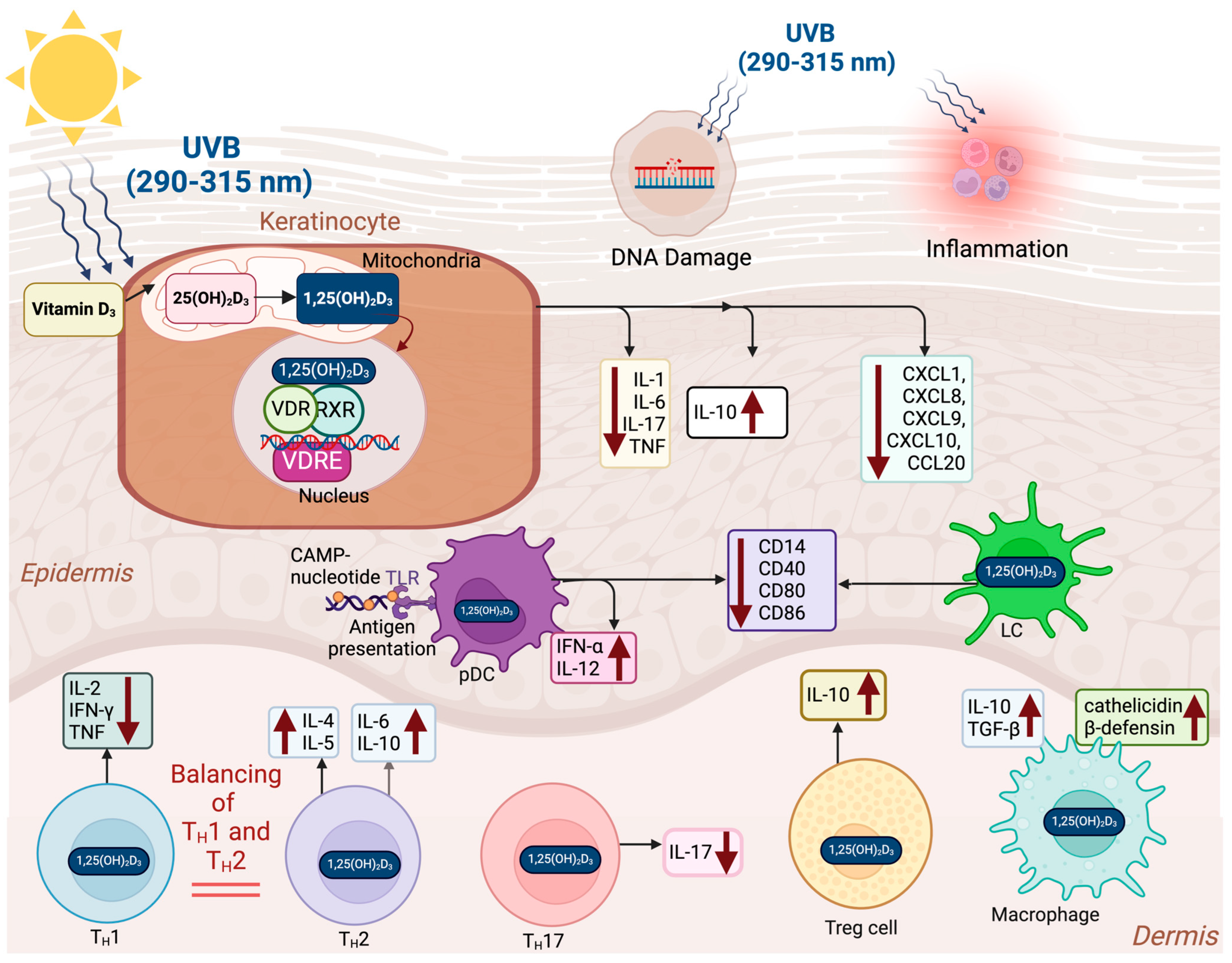
4.3. Immunomodulatory Effects of Vitamin D
5. Balancing Skin Protection and Vitamin D3 Synthesis: Strategies for Safe Sun Exposure
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Dermato-Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed]
- Fell, G.L.; Robinson, K.C.; Mao, J.; Woolf, C.J.; Fisher, D.E. Skin β-endorphin mediates addiction to UV light. Cell 2014, 157, 1527–1534. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wierzbicka, J.M.; Żmijewski, M.A.; Piotrowska, A.; Nedoszytko, B.; Lange, M.; Tuckey, R.C.; Slominski, A.T. Bioactive forms of vitamin D selectively stimulate the skin analog of the hypothalamus-pituitary-adrenal axis in human epidermal keratinocytes. Mol. Cell. Endocrinol. 2016, 437, 312–322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, D.; Fernandez, B.O.; Hamilton, A.; Lang, N.N.; Gallagher, J.M.C.; Newby, D.E.; Feelisch, M.; Weller, R.B. UVA irradiation of human skin vasodilates arterial vasculature and lowers blood pressure independently of nitric oxide synthase. J. Investig. Dermatol. 2014, 134, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Brennemann, J.; McQuarrie, I. Brennemann’s Practice of Pediatrics; W.F. Prior: Oneida, NY, USA, 1946. [Google Scholar]
- Mozołowski, W. Jędrzej Sniadecki (1768–1838) on the Cure of Rickets. Nature 1939, 143, 121. [Google Scholar] [CrossRef]
- Palm, T.A. The geographical distribution and etiology of rickets. Practitioner 1890, 45, 270–342. [Google Scholar]
- Huldschinsky, K. Heilung von Rachitis durch künstliche Höhensonne. Dtsch. Med. Wochenschr. 1919, 45, 712–713. [Google Scholar] [CrossRef]
- Huldschinsky, K. The Ultra-Violet Light Treatment of Rickets; Alpine Press: New Jersey, CA, USA, 1928; pp. 3–19. [Google Scholar]
- Hess, A.F.; Unger, L.J. The cure of infantile rickets by sunlight. J. Am. Med. Assoc. 1921, 77, 39. [Google Scholar]
- Steenbock, H.; Black, A. The reduction of growth-promoting and calcifying properties in a ration by exposure to ultraviolet light. J. Biol. Chem. 1924, 61, 408–422. [Google Scholar]
- Hess, A.F.; Weinstock, M. Antirachitic properties imparted to inert fluids and to green vegetables by ultra-violet irradiation. J. Biol. Chem. 1924, 62, 301–313. [Google Scholar] [CrossRef]
- Mathew, J.; Berger, D.; Tabatabaie, V. Severe Osteomalacia and Fractures Secondary to Vitamin D Deficiency. J. Endocr. Soc. 2021, 5 (Suppl. S1), A221. [Google Scholar] [CrossRef] [PubMed Central]
- Apperly, F.L. The relation of solar radiation to cancer mortality in North American. Cancer Res. 1941, 1, 191–195. [Google Scholar]
- Garland, C.F.; Garland, F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int. J. Epidemiol. 1980, 9, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Garland, F.C.; Garland, C.F.; Gorham, E.D.; Young, J.F. Geographic variation in breast cancer mortality in the United States: A hypothesis involving exposure to solar radiation. Prev Med. 1990, 19, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.; Garland, F.; Shaw, E.; Comstock, G.; Helsing, K.; Gorham, E. Serum 25-hydroxyvitamin D and colon cancer: Eight-year prospective study. Lancet 1989, 334, 1176–1178. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Gorham, E.D.; Mohr, S.B.; Grant, W.B.; Giovannucci, E.L.; Lipkin, M.; Newmark, H.; Holick, M.F.; Garland, F.C. Vitamin D and prevention of breast cancer: Pooled analysis. J. Steroid. Biochem. Mol. Biol. 2007, 103, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, E.; Garland, C. Sunlight, vitamin D, and ovarian cancer mortality rates in US women. Int. J. Epidemiol. 1994, 23, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Negri, M.; Gentile, A.; de Angelis, C.; Montò, T.; Patalano, R.; Colao, A.; Pivonello, R.; Pivonello, C. Vitamin D-Induced Molecular Mechanisms to Potentiate Cancer Therapy and to Reverse Drug-Resistance in Cancer Cells. Nutrients 2020, 12, 1798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohr, S.B.; Garland, C.F.; Gorham, E.D.; Garland, F.C. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia 2008, 51, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Munger, K.L.; Zhang, S.M.; O’Reilly, E.; Hernán, M.A.; Olek, M.J.; Willett, W.C.; Ascherio, A. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004, 62, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Welham, J.; Chant, D.; Torrey, E.F.; McGrath, J. A systematic review and meta-analysis of Northern Hemisphere season of birth studies in schizophrenia. Schizophr. Bull. 2003, 29, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Coury, S.M.; Lombroso, A.; Avila-Quintero, V.J.; Taylor, J.H.; Flores, J.M.; Szejko, N.; Bloch, M.H. Systematic review and meta-analysis: Season of birth and schizophrenia risk. Schizophr. Res. 2023, 252, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Scragg, R.; Sowers, M.; Bell, C.; Third National Health and Nutrition Examination Survey. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004, 27, 2813–2818. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Atkins, A.; Downes, M.; Wei, Z. Vitamin D in Diabetes: Uncovering the Sunshine Hormone’s Role in Glucose Metabolism and Beyond. Nutrients 2023, 15, 1997. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ceglia, L.; Chadha, C.; Chatterjee, R.; et al. D2d Research Group. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 520–530. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melamed, M.L.; Muntner, P.; Michos, E.D.; Uribarri, J.; Weber, C.; Sharma, J.; Raggi, P. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: Results from NHANES 2001 to 2004. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1179–1185. [Google Scholar] [CrossRef]
- Grübler, M.R.; März, W.; Pilz, S.; Grammer, T.B.; Trummer, C.; Müllner, C.; Schwetz, V.; Pandis, M.; Verheyen, N.; Tomaschitz, A.; et al. Vitamin-D concentrations, cardiovascular risk and events—A review of epidemiological evidence. Rev. Endocr. Metab. Disord. 2017, 18, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dudenkov, D.V.; Mara, K.C.; Maxson, J.A.; Thacher, T.D. Serum 25-hydroxyvitamin D values and risk of incident cardiovascular disease: A population-based retrospective cohort study. J. Steroid. Biochem. Mol. Biol. 2021, 213, 105953. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, A.K.; Lal, A.; Kumar, V.; Singhal, M.; Billot, L.; Gupta, K.L.; Banerjee, D.; Jha, V. A Randomized Trial of Vitamin D Supplementation on Vascular Function in CKD. J. Am. Soc. Nephrol. 2017, 28, 3100–3108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Acharya, P.; Dalia, T.; Ranka, S.; Sethi, P.; Oni, O.A.; Safarova, M.S.; Parashara, D.; Gupta, K.; Barua, R.S. The Effects of Vitamin D Supplementation and 25-Hydroxyvitamin D Levels on the Risk of Myocardial Infarction and Mortality. J. Endocr. Soc. 2021, 5, bvab124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holick, M.F. Revisiting Vitamin D Guidelines: A Critical Appraisal of the Literature. Endocr. Pract. 2024, 30, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D. Vitamin D: Production, Metabolism, and Mechanisms of Action. In Endotext; De Groot, L.J., Chrousos, G., Dungan, K., Feingold, K.R., Grossman, A., Hershman, J.M., Koch, C., Korbonits, M., McLachlan, R., New, M., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.M.; Manson, J.E.; Costenbader, K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022, 376, e066452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costenbader, K.H.; Cook, N.R.; Lee, I.M.; Hahn, J.; Walter, J.; Bubes, V.; Kotler, G.; Yang, N.; Friedman, S.; Alexander, E.K.; et al. Vitamin D and Marine n-3 Fatty Acids for Autoimmune Disease Prevention: Outcomes Two Years After Completion of a Double-Blind, Placebo-Controlled Trial. Arthritis Rheumatol. 2024, 76, 973–983. [Google Scholar] [CrossRef]
- Demay, M.B.; Pittas, A.G.; Bikle, D.D.; Diab, D.L.; Kiely, M.E.; Lazaretti-Castro, M.; Lips, P.; Mitchell, D.M.; Murad, M.H.; Powers, S.; et al. Vitamin D for the Prevention of Disease: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2024, 109, 1907–1947. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Staten, M.A.; Knowler, W.C.; Nelson, J.; Vickery, E.M.; LeBlanc, E.S.; Neff, L.M.; Park, J.; Pittas, A.G.; D2d Research Group. Intratrial Exposure to Vitamin D and New-Onset Diabetes Among Adults with Prediabetes: A Secondary Analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care 2020, 43, 2916–2922. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pittas, A.G.; Kawahara, T.; Jorde, R.; Dawson-Hughes, B.; Vickery, E.M.; Angellotti, E.; Nelson, J.; Trikalinos, T.A.; Balk, E.M. Vitamin D and Risk for Type 2 Diabetes in People with Prediabetes: A Systematic Review and Meta-analysis of Individual Participant Data from 3 Randomized Clinical Trials. Ann. Intern. Med. 2023, 176, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.; Willemze, R.; de Gruijl, F.R.; Bavinck, J.N.B.; Bajdik, C.D. The influence of painful sunburns and lifetime of sun exposure on the risk of actinic keratoses, seborrheic warts, melanocytic nevi, atypical nevi and skin cancer. J. Investig. Dermatol. 2003, 120, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Kricker, A.; Armstrong, B.K.; English, D.R. Sun exposure and non-melanocytic skin cancer. Cancer Causes Control. 1994, 5, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.K.; Kricker, A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B 2001, 63, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Rass, K.; Reichrath, J. UV damage and DNA repair in malignant melanoma and nonmelanoma skin cancer. Adv. Exp. Med. Biol. 2008, 624, 162–178. [Google Scholar] [CrossRef] [PubMed]
- Sample, A.; He, Y.Y. Mechanisms and prevention of UV-induced melanoma. Photodermatol. Photoimmunol. Photomed. 2018, 34, 13–24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weller, R.B. Sunlight: Time for a Rethink? J. Investig. Dermatol. 2024, 144, 1724–1732. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Biological Effects of Sunlight, Ultraviolet Radiation, Visible Light, Infrared Radiation and Vitamin D for Health. Anticancer Res. 2016, 36, 1345–1356. [Google Scholar] [PubMed]
- Keegan, R.J.; Lu, Z.; Bogusz, J.M.; Williams, J.E.; Holick, M.F. Photobiology of vitamin D in mushrooms and its bioavailability in humans. Dermatoendocrinology 2013, 5, 165–176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mattila, P.H.; Piironen, V.I.; Uusi-Rauva, E.J.; Koivistoinen, P.E. Vitamin D contents in edible mushrooms. J. Agric. Food Chem. 1994, 42, 2449–2453. [Google Scholar] [CrossRef]
- Holick, M.F.; Tian, X.Q.; Allen, M. Evolutionary importance for the membrane enhancement of the production of vitamin D3 in the skin of poikilothermic animals. Proc. Natl. Acad. Sci. USA 1995, 92, 3124–3126. [Google Scholar] [CrossRef] [PubMed]
- Fieser, L.D.; Fieser, M. Vitamin D. In Anonymous Steroids; Reinhold: New York, NY, USA, 1959; pp. 90–168. [Google Scholar]
- Jacobs, H.J.C.; Boamsma, F.; Havinga, E.; Van Der Gen, A. The photochemistry of previtamin D and tachysterol. Recl. Trav. Chim. Pays-Bas. Belg. 1977, 96, 113–117. [Google Scholar] [CrossRef]
- Tian, X.Q.; Holick, M.F. A liposomal model that mimics the cutaneous production of vitamin D3. J. Biol. Chem. 1999, 274, 4174–4179. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; MacLaughlin, J.A.; Clark, M.B.; Holick, S.A.; Potts, J.T., Jr.; Anderson, R.R.; Blank, I.H.; Parrish, J.A.; Elias, P. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science 1980, 210, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.G.; Matsuoka, L.Y.; Hollis, B.W.; Hu, Y.Z.; Wortsman, J. Human plasma transport of vitamin D after its endogenous synthesis. J. Clin. Investig. 1993, 91, 2552–2555. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Slominski, A.T. Chapter 3—Photobiology of Vitamin D. In Feldman and Pike’s Vitamin D, 5th ed.; Hewison, M., Bouillon, R., Giovannucci, E., Goltzman, D., Meyer, M., Welsh, J., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 27–45. ISBN 9780323913867. [Google Scholar] [CrossRef]
- Slominski, A.; Kim, T.K.; Zmijewski, M.A.; Janjetovic, Z.; Li, W.; Chen, J.; Kusniatsova, E.I.; Semak, I.; Postlethwaite, A.; Miller, D.D.; et al. Novel vitamin D photoproducts and their precursors in the skin. Dermatoendocrinology 2013, 5, 7–19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Skobowiat, C.; Oak, A.S.; Kim, T.K.; Yang, C.H.; Pfeffer, L.M.; Tuckey, R.C.; Slominski, A.T. Noncalcemic 20-hydroxyvitamin D3 inhibits human melanoma growth in in vitro and in vivo models. Oncotarget 2017, 8, 9823–9834. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cui, A.; Xiao, P.; Ma, Y.; Fan, Z.; Zhou, F.; Zheng, J.; Zhang, L. Prevalence, trend, and predictor analyses of vitamin D deficiency in the US population, 2001–2018. Front Nutr. 2022, 9, 965376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsuoka, L.Y.; Ide, L.; Wortsman, J.; MacLaughlin, J.A.; Holick, M.F. Sunscreens suppress cutaneous vitamin D3 synthesis. J. Clin. Endocrinol. Metab. 1987, 64, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.R.; Kline, L.; Holick, M.F. Influence of Season and Latitude on the Cutaneous Synthesis of Vitamin D3: Exposure to Winter Sunlight in Boston and Edmonton Will Not Promote Vitamin D3 Synthesis in Human Skin. J. Clin. Endocrinol. Metab. 1988, 67, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C.; Lu, Z.; Sauter, E. Vitamin D and Skin Physiology: A D-Lightful Story. J. Bone Miner. Res. 2007, 22 (Suppl. S2), V28–V33. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Matsuoka, L.Y.; Wortsman, J. Age, vitamin D, and solar ultraviolet. Lancet 1989, 334, 1104–1105. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The One-Hundred-Year Anniversary of the Discovery of the Sunshine Vitamin D3: Historical, Personal Experience and Evidence-Based Perspectives. Nutrients 2023, 15, 593. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. Med. J. Clin. Endocrinol. Metab.2011, 96, 1911–1930. https://doi.org/10.1210/jc.2011-0385. Erratum in J. Clin. Endocrinol. Metab.2011, 96, 3908. Erratum in J. Clin. Endocrinol. Metab. 2024, 5, dgae373. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Nutrigenomics of Vitamin D. Nutrients 2019, 11, 676. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, J.; Pei, L.; Evans, R.M. Nuclear receptors: Decoding metabolic disease. FEBS Lett. 2008, 582, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.M. The nuclear receptor superfamily: A rosetta stone for physiology. Mol. Endocrinol. 2005, 19, 1429–1438. [Google Scholar] [CrossRef]
- Hoeksema, M.A.; de Winther, M.P. Epigenetic regulation of monocyte and macrophage function. Antioxid. Redox Signal. 2016, 25, 758–774. [Google Scholar] [CrossRef]
- Pike, J.W.; Meyer, M.B. Fundamentals of vitamin D hormone-regulated gene expression. J. Steroid Biochem. Mol. Biol. 2014, 144, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Hii, C.S.; Ferrante, A. The Non-Genomic Actions of Vitamin, D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Gilchrest, B.A.; Eller, M.S.; Geller, A.C.; Yaar, M. The pathogenesis of melanoma induced by ultraviolet radiation. N. Engl. J. Med. 1999, 340, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Fan, W.; Rokohl, A.C.; Guo, Y.; Chen, H.; Gao, T.; Kakkassery, V.; Heindl, L.M. Narrative review: Mechanism of ultraviolet radiation-induced basal cell carcinoma. Front. Oral Maxillofac. Med. 2023, 5, 9. [Google Scholar] [CrossRef]
- Brash, D.E.; Rudolph, J.A.; Simon, J.A.; Lin, A.; McKenna, G.J.; Baden, H.P.; Ponten, J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 1991, 88, 10124–10128. [Google Scholar] [CrossRef]
- Cleaver, J.E. Defective repair replication of DNA in xeroderma pigmentosum. Nature 1968, 218, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, S.I.; Kraemer, K.H. Xeroderma pigmentosum-bridging a gap between clinic and laboratory. Photodermatol. Photoimmunol. Photomed. 2001, 17, 47–54. [Google Scholar] [CrossRef]
- Sarasin, A. The molecular pathways of ultraviolet-induced carcinogenesis. Mutat. Res. Mol. Mech. Mutagen. 1999, 428, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Vechtomova, Y. UV Radiation in DNA Damage and Repair. Encyclopedia. Available online: https://encyclopedia.pub/entry/17565 (accessed on 9 March 2024).
- Kastenhuber, E.R.; Lowe, S.W. Putting p53 in Context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benjamin, C.L.; Ananthaswamy, H.N. p53 and the pathogenesis of skin cancer. Toxicol. Appl. Pharmacol. 2007, 224, 241–248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berg, R.J.; van Kranen, H.J.; Rebel, H.G.; de Vries, A.; van Vloten, W.A.; Van Kreijl, C.F.; van der Leun, J.C.; de Gruijl, F.R. Early p53 alterations in mouse skin carcinogenesis by UVB radiation: Immunohistochemical detection of mutant p53 protein in clusters of preneoplastic epidermal cells. Proc. Natl. Acad. Sci. USA 1996, 93, 274–278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cleaver, J.E.; Crowley, E. UV damage, DNA repair and skin carcinogenesis. Front. Biosci. 2002, 7, d1024–d1043. [Google Scholar] [CrossRef] [PubMed]
- Luze, H.; Nischwitz, S.P.; Zalaudek, I.; Müllegger, R.; Kamolz, L.P. DNA repair enzymes in sunscreens and their impact on photoageing—A systematic review. Photodermatol. Photoimmunol. Photomed. 2020, 36, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Seebode, C.; Lehmann, J.; Emmert, S. Photocarcinogenesis and Skin Cancer Prevention Strategies. Anticancer Res. 2016, 36, 1371–1378. [Google Scholar] [PubMed]
- Tang, X.; Yang, T.; Yu, D.; Xiong, H.; Zhang, S. Current insights and future perspectives of ultraviolet radiation (UV) exposure: Friends and foes to the skin and beyond the skin. Environ. Int. 2024, 185, 108535. [Google Scholar] [CrossRef] [PubMed]
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The impact of ultraviolet radiation on skin photoaging—Review of in vitro studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.L.; Lim, H.W.; Mohammad, T.F. Sunscreens and Photoaging: A Review of Current Literature. Am. J. Clin. Dermatol. 2021, 22, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Ambagaspitiya, S.S.; Appuhamillage, G.A.; Dassanayake, R.S. Impact of vitamin D on ultraviolet-induced photoaging and skin diseases. Explor. Med. 2024, 5, 363–383. [Google Scholar] [CrossRef]
- Watson, M.; Holman, D.M.; Maguire-Eisen, M. Ultraviolet Radiation Exposure and Its Impact on Skin Cancer Risk. Semin. Oncol. Nurs. 2016, 32, 241–254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Richards, T.B.; Johnson, C.J.; Tatalovich, Z.; Cockburn, M.; Eide, M.J.; Henry, K.A.; Lai, S.-M.; Cherala, S.S.; Huang, Y.; Ajani, U.A. Association between cutaneous melanoma incidence rates among white U.S. residents and county-level estimates of solar ultraviolet exposure. J. Am. Acad. Dermatol. 2011, 65 (Suppl. S1), S50.e1–S50.e9. [Google Scholar] [CrossRef][Green Version]
- Tatalovich, Z.; Wilson, J.P.; Mack, T.; Yan, Y.; Cockburn, M. The objective assessment of lifetime cumulative ultraviolet exposure for determining melanoma risk. J. Photochem. Photobiol. B. 2006, 85, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.R.; Harris, J.K.; Rodriguez-Galindo, C.; Johnson, K.J. Incidence of childhood and adolescent melanoma in the United States: 1973–2009. Pediatrics 2013, 131, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, X.; Zhang, L. Recent global patterns in skin cancer incidence, mortality, and prevalence. Chin. Med. J. 2024; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fukunaga-Kalabis, M.; Li, L.; Herlyn, M. Developmental pathways activated in melanocytes and melanoma. Arch. Biochem. Biophys. 2014, 563, 13–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herlyn, M.; Thurin, J.; Balaban, G.; Bennicelli, J.; Herlyn, D.; Elder, D.; Bondi, E.; Guerry, D.; Nowell, P.; Clark, W.; et al. Characteristics of cultured human melanocytes isolated from different stages of tumor progression. Cancer Res. 1985, 45 Pt 2, 5670–5676. [Google Scholar] [PubMed]
- Paluncic, J.; Kovacevic, Z.; Jansson, P.J.; Kalinowski, D.; Merlot, A.M.; Huang, M.L.; Lok, H.C.; Sahni, S.; Lane, D.J.; Richardson, D.R. Roads to melanoma: Key pathways and emerging players in melanoma progression and oncogenic signaling. Biochim. et Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Fahradyan, A.; Howell, A.C.; Wolfswinkel, E.M.; Tsuha, M.; Sheth, P.; Wong, A.K. Updates on the Management of Non-Melanoma Skin Cancer (NMSC). Healthcare 2017, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, K. Squamous Cell and Basal Cell Carcinoma of the Skin: Diagnosis and Treatment. In Encyclopedia of Cancer. 3; Boffetta, P., Hainaut, P., Eds.; Academic Press: Boston, NY, USA, 2019; pp. 412–416. [Google Scholar]
- Hall, E.T.; Fernandez-Lopez, E.; Silk, A.W.; Dummer, R.; Bhatia, S. Immunologic Characteristics of Nonmelanoma Skin Cancers: Implications for Immunotherapy. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Didona, D.; Paolino, G.; Bottoni, U.; Cantisani, C. Non Melanoma Skin Cancer Pathogenesis Overview. Biomedicines 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Dika, E.; Scarfì, F.; Ferracin, M.; Broseghini, E.; Marcelli, E.; Bortolani, B.; Campione, E.; Riefolo, M.; Ricci, C.; Lambertini, M. Basal Cell Carcinoma: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 5572. [Google Scholar] [CrossRef] [PubMed]
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef]
- Leiter, U.; Keim, U.; Eigentler, T.; Katalinic, A.; Holleczek, B.; Martus, P.; Garbe, C. Incidence, Mortality, and Trends of Nonmelanoma Skin Cancer in Germany. J. Investig. Dermatol. 2017, 137, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- The Skin Cancer Foundation. Skin Cancer Information. Skin Cancer Facts & Statistics. Available online: https://www.skincancer.org/skin-cancer-information/skin-cancer-facts/ (accessed on 25 October 2024).
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Picconi, O.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur. J. Cancer 2005, 41, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; He, Y.Y. Ultraviolet radiation-induced non-melanoma skin cancer: Regulation of DNA damage repair and inflammation. Genes Dis. 2014, 1, 188–198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garland, F.C.; Garland, C.F. Occupational sunlight exposure and melanoma in the U.S. Navy. Arch. Environ. Heal. Int. J. 1990, 45, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, P.G.; Epstein, E.; Nielsen, K.; Landin-Olsson, M.; Ingvar, C.; Olsson, H. Avoidance of sun exposure as a risk factor for major causes of death: A competing risk analysis of the Melanoma in Southern Sweden cohort. J. Intern. Med. 2016, 280, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Krause, R.; Roth, H.J.; Kaase, H.; Stange, R.; Holick, M.F. Vitamin D Status in Chronic Kidney Disease—UVB Irradiation Is Superior to Oral Supplementation. Anticancer Res. 2016, 36, 1397–1401. [Google Scholar] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pèrez, A.; Chen, T.C.; Turner, A.; Raab, R.; Bhawan, J.; Poche, P.; Holick, M.F. Efficacy and safety of topical calcitriol (1,25-dihydroxyvitamin d3) for the treatment of psoriasis. Br. J. Dermatol. 1996, 134, 238–246 PMID: 8746336. [Google Scholar] [CrossRef] [PubMed]
- Kragballe, K. Calcipotriol: A new drug for topical psoriasis treatment. Pharmacol. Toxicol. 1995, 77, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Trémezaygues, L.; Reichrath, J. Vitamin D analogs in the treatment of psoriasis: Where are we standing and where will we be going? Dermatoendocrinology 2011, 3, 180–186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kragballe, K.; Gjertsen, B.T.; de Hoop, D.; Karlsmark, T.; van de Kerhof, P.C.M.; Larko, O.; Nioboer, C.; Peterson, R.; Strand, A.; Tikjøb, G. Double-blind right/left comparison of calcipotriol and betametasone valerate in treatment of psoriasis vulgaris. Lancet 1991, 337, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.N.; Ashton, R.E.; Marks, R.; Harris, R.I.; BerthJones, J. Topical maxacalcitol for the treatment of psoriasis vulgaris: A placebo-controlled, double-blind, dosefinding study with active comparator. Br. J. Dermatol. 1999, 141, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, Y.; Ohkawara, A.; Ohkido, M.; Harada, S.; Tamaki, K.; Nakagawa, H.; Hori, Y.; Nishiyama, S. Long-term safety and efficacy of high-concentration (20 microg/g) tacalcitol ointment in psoriasis vulgaris. Eur. J. Dermatol. 2002, 12, 463–468. [Google Scholar] [PubMed]
- Van De Kerkhof, P.; Berth-Jones, J.; Griffiths, C.; Harrison, P.; Honigsmann, H.; Marks, R.; Roelandts, R.; Schopf, E.; Trompke, C. Longterm efficacy and safety of tacalcitol ointment in patients with chronic plaque psoriasis. Br. J. Dermatol. 2002, 146, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Katayama, I.; Ohkawara, A.; Ohkido, M.; Harada, S.; Tamaki, K.; Nakagawa, H. High-concentration (20 μg/g) tacalcitol ointment therapy on refractory psoriasis vulgaris with low response to topical corticosteroids. Eur. J. Dermatol. 2002, 12, 553–557. [Google Scholar] [PubMed]
- Kragballe, K. Treatment of psoriasis with calcipotriol and other vitamin D analogues. J. Am. Acad. Dermatol. 1992, 27, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Persons, K.; Lu, Z.; Mathieu, J.S.; Holick, M.F. An evaluation of the biologic activity vitamin D receptor binding affinity of the photoisomers of vitamin, D.3.; previtamin D3. J. Nutr. Biochem. 2000, 11, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.S.; Sequeira, V.B.; Dixon, K.M.; Gordon-Thomson, C.; Pobre, K.; Dilley, A.; Mizwicki, M.T.; Norman, A.W.; Feldman, D.; Halliday, G.M.; et al. Photoprotection by 1alpha,25-dihydroxyvitamin D and analogs: Further studies on mechanisms and implications for UV-damage. J. Steroid. Biochem. Mol. Biol. 2010, 121, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Dixon, K.M.; Norman, A.W.; Sequeira, V.B.; Mohan, R.; Rybchyn, M.S.; Reeve, V.E.; Halliday, G.M.; Mason, R.S. 1α,25(OH)2-vitamin D and a nongenomic vitamin D analogue inhibit ultraviolet radiation–induced skin carcinogenesis. Cancer Prev. Res. 2011, 4, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Becklund, B.R.; Severson, K.S.; Vang, S.V.; DeLuca, H.F. UV radiation suppresses experimental autoimmune encephalomyelitis independent of vitamin D production. Proc. Natl. Acad. Sci. USA 2010, 107, 6418–6423. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trémezaygues, L.; Seifert, M.; Tilgen, W.; Reichrath, J. 1,25-dihydroxyvitamin D(3) protects human keratinocytes against UV-B-induced damage: In vitro analysis of cell viability/proliferation, DNA-damage and -repair. Dermatoendocrinology 2009, 1, 239–245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karasawa, M.; Hosoi, J.; Hashiba, H.; Nose, K.; Tohyama, C.; Abe, E.; Suda, T.; Kuroki, T. Regulation of metallothionein gene expression by 1 alpha,25-dihydroxyvitamin D3 in cultured cells and in mice. Proc. Natl. Acad. Sci. USA 1987, 84, 8810–8813. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanada, K.; Sawamura, D.; Tamai, K.; Baba, T.; Hashimoto, I.; Muramatsu, T.; Miura, N.; Naganuma, A. Novel function of metallothionein in photoprotection: Metallothionein-null mouse exhibits reduced tolerance against ultraviolet B injury in the skin. J. Investig. Dermatol. 1998, 111, 582–585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Courtois, S.J.; Segaert, S.; Degreef, H.; Bouillon, R.; Garmyn, M. Ultraviolet B suppresses vitamin D receptor gene expression in keratinocytes. Biochem. Biophys. Res. Commun. 1998, 246, 64–69. [Google Scholar] [CrossRef] [PubMed]
- De Haes, P.; Garmyn, M.; Degreef, H.; Vantieghem, K.; Bouillon, R. Segaert1,25-Dihydroxyvitamin D3 inhibits ultraviolet B-induced apoptosis, Jun kinase activation, and interleukin-6 production in primary human keratinocytes. J. Cell. Biochem. 2003, 89, 663–673. [Google Scholar] [CrossRef] [PubMed]
- De Haes, P.; Garmyn, M.; Verstuyf, A.; De Clercq, P.; Vandewalle, M.; Vantieghem, K.; Degreef, H.; Bouillon, R.; Segaert, S.; Bouillon, S. SegaertTwo 14-epi-analogues of 1,25-dihydroxyvitamin D3 protect human keratinocytes against the effects of UVB. Arch. Dermatol. Res. 2004, 295, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Trémezaygues, L.; Sticherling, M.; Pföhler, C.; Friedrich, M.; Meineke, V.; Seifert, M.; Tilgen, W.; Reichrath, J. Cutaneous photosynthesis of vitamin D: An evolutionary highly conserved endocrine system that protects against environmental hazards including UV-radiation and microbial infections. Anticancer Res. 2006, 26, 2743–2748. [Google Scholar] [PubMed]
- Wong, G.; Gupta, R.; Dixon, K.; Deo, S.; Choong, S.; Halliday, G.; Bishop, J.; Ishizuka, S.; Norman, A.; Posner, G.; et al. 1,25-Dihydroxyvitamin D and three low-calcemic analogs decrease UV-induced DNA damage via the rapid response pathway. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 567–570. [Google Scholar] [CrossRef] [PubMed]
- De Haes, P.; Garmyn, M.; Verstuyf, A.; De Clercq, P.; Vandewalle, M.; Degreef, H.; Vantieghem, K.; Bouillon, R. Segaert, S 1,25-Dihydroxyvitamin D3 and analogues protect primary human keratinocytes against UVB-induced DNA damage. J. Photochem. Photobiol. B 2005, 78, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Nemazannikova, N.; Antonas, K.; Dass, C.R. Role of vitamin D metabolism in cutaneous tumour formation and progression. J. Pharm. Pharmacol. 2013, 65, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Hager, G.; Formanek, M.; Gedlicka, C.; Thurnher, D.; Knerer, B.; Kornfehl, J. 1,25(OH)2 vitamin D3 induces elevated expression of the cell cycle-regulating genes P21 and P27 in squamous carcinoma cell lines of the head and neck. Acta Otolaryngol. 2001, 121, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Trémezaygues, L.; Seifert, M.; Vogt, T.; Tilgen, W.; Reichrath, J. 1,25-dihydroxyvitamin D3 modulates effects of ionizing radiation (IR) on human keratinocytes: In vitro analysis of cell viability/proliferation, DNA-damage and -repair. J. Steroid Biochem. Mol. Biol. 2010, 121, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Vasilovici, A.F.; Grigore, L.E.; Ungureanu, L.; Fechete, O.; Candrea, E.; Trifa, A.P.; Vișan, S.; Șenilă, S.; Cosgarea, R. Vitamin D receptor polymorphisms and melanoma (Review). Oncol. Lett. 2019, 17, 4162–4169. [Google Scholar] [CrossRef] [PubMed]
- Denzer, N.; Vogt, T.; Reichrath, J. Vitamin D receptor (VDR) polymorphisms and skin cancer: A systematic review. Dermato-Endocrinology 2011, 3, 205–210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, X.Z.; Yang, B.H.; Yu, G.H.; Liu, S.Z.; Yuan, Z.Y. Polymorphisms in the vitamin D receptor (VDR) genes and skin cancer risk in European population: A meta-analysis. Arch. Dermatol. Res. 2014, 306, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Birke, M.; Schöpe, J.; Wagenpfeil, S.; Vogt, T.; Reichrath, J. Association of Vitamin D Receptor Gene Polymorphisms with Melanoma Risk: A Meta-analysis and Systematic Review. Anticancer. Res. 2020, 40, 583–595. [Google Scholar] [CrossRef]
- Kanno, K.; Akutsu, T.; Ohdaira, H.; Suzuki, Y.; Urashima, M. Effect of vitamin D supplements on relapse or death in a p53-immunoreactive subgroup with digestive tract cancer: Post hoc analysis of the AMATERASU randomized clinical trial. JAMA Netw. Open 2023, 6, e2328886. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The Death D-Fying Vitamin D3 for Digestive Tract Cancers-The p53 Antibody Connection. JAMA Netw. Open 2023, 6, e2328883. [Google Scholar] [CrossRef] [PubMed]
- Sabapathy, K.; Lane, D.P. Understanding p53 functions through p53 antibodies. J. Mol. Cell Biol. 2019, 11, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Stambolsky, P.; Tabach, Y.; Fontemaggi, G.; Weisz, L.; Maor-Aloni, R.; Sigfried, Z.; Shiff, I.; Kogan, I.; Shay, M.; Kalo, E.; et al. Modulation of the vitamin D3 response by cancer-associated mutant p53. Cancer Cell. 2010, 17, 273–285. [Google Scholar] [CrossRef]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Espié, M.; Wassermann, J.; de Kermadec, E.; Lalloum, M.; Coussy, F. Vitamin D and cancers. Presse Med. 2013, 42, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Sui, D.; Wang, Y.; Liu, H.; Chiang, Y.-J.; Ross, M.I.; Gershenwald, J.E.; Cormier, J.N.; Royal, R.E.; Lucci, A.; et al. Association of Vitamin D Levels with Outcome in Patients with Melanoma After Adjustment For C-Reactive Protein. J. Clin. Oncol. 2016, 34, 1741–1747. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davies, J.R.; Field, S.; Randerson-Moor, J.; Harland, M.; Kumar, R.; Anic, G.M.; Nagore, E.; Hansson, J.; Höiom, V.; Jönsson, G.; et al. An inherited variant in the gene coding for vitamin D-binding protein and survival from cutaneous melanoma: A BioGenoMEL study. Pigment. Cell Melanoma Res. 2014, 27, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arrones, O.M.; Zegeer, J.; Gerbo, M.; Manrique-Silva, E.; Requena, C.; Traves, V.; Nagore, E. Decreased vitamin D serum levels at melanoma diagnosis are associated with tumor ulceration and high tumor mitotic rate. Melanoma Res. 2019, 29, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Moro, R.; Sánchez-Silva, A.; Aguerralde-Martin, M.; González-Cuevas, R.; Peruilh-Bagolini, L.; Traves, V.; Manrique-Silva, E.; Requena, C.; Nagore, E. Prognostic Value of Vitamin D Serum Levels in Cutaneous Melanoma. Actas Dermo-Sifiliograficas 2022, 113, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Haddad, S.; Weise, J.J.N.; Wagenpfeil, S.; Vogt, T.; Reichrath, J. Malignant Melanoma: Vitamin D Status as a Risk and Prognostic Factor—Meta-analyses and Systematic Review. Anticancer. Res. 2025, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Di Meglio, P.; Qin, J.Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdallah, F.; Mijouin, L.; Pichon, C. Skin immune landscape: Inside and outside the organism. Mediat. Inflamm. 2017, 2017, 5095293. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, J.A.S. Organization of the skin immune system and compartmentalized immune responses in infectious diseases. Clin. Microbiol. Rev. 2019, 32, e00034-18. [Google Scholar] [CrossRef] [PubMed]
- Maciel, T.T.; Moura, I.C.; Hermine, O. The role of mast cells in cancers. F1000Prime Rep. 2015, 7, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murdaca, G.; Allegra, A.; Tonacci, A.; Musolino, C.; Ricciardi, L.; Gangemi, S. Mast Cells and Vitamin D Status: A Clinical and Biological Link in the Onset of Allergy and Bone Diseases. Biomedicines 2022, 10, 1877. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sigmundsdottir, H.; Pan, J.; Debes, G.F.; Alt, C.; Habtezion, A.; Soler, D.; Butcher, E.C. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat. Immunol. 2007, 8, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Khoo, A.-L.; Koenen, H.J.; Michels, M.; Ooms, S.; Bosch, M.; Netea, M.G.; Joosten, I.; van der Ven, A.J. High-dose vitamin D3 supplementation is a requisite for modulation of skin-homing markers on regulatory T cells in HIV-infected patients. AIDS Res. Hum. Retroviruses 2013, 29, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Dam, T.N.; Møller, B.; Hindkjaer, J.; Kragballe, K. The vitamin D3 analog calcipotriol suppresses the number and antigen-presenting function of Langerhans cells in normal human skin. J. Investig. Dermatol. Symp. Proc. 1996, 1, 72–77. [Google Scholar] [PubMed]
- Penna, G.; Adorini, L. 1 alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 2000, 164, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Klug-Micu, G.M.; Stenger, S.; Sommer, A.; Liu, P.T.; Krutzik, S.R.; Modlin, R.L.; Fabri, M. CD40 ligand and interferon-g induce an antimicrobial response against Mycobacterium tuberculosis in human monocytes. Immunology 2013, 139, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Cai, J.; Li, Y.; Yang, R. 1,25-dihydroxy-vitamin D3 induces macrophage polarization to M2 by upregulating T-cell Ig-mucin-3 expression. Mol. Med. Rep. 2019, 19, 3707–3713. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, C.; Neale, R.E.; Lucas, R.M. Skin cancer and vitamin D: An update. Melanoma Manag. 2015, 2, 51–61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- The British Association of Dermatologists. Available online: https://www.bad.org.uk/bad-response-to-weighing-the-benefits-and-risks-of-sunlight-exposure-by-nice/ (accessed on 30 December 2024).
- Cancer Council Australia. Available online: https://www.cancer.org.au/cancer-information/causes-and-prevention/sun-safety/vitamin-d (accessed on 30 December 2024).
- The World Health Organization. Available online: https://www.who.int/news-room/questions-and-answers/item/radiation-protecting-against-skin-cancer (accessed on 30 December 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uçar, N.; Holick, M.F. Illuminating the Connection: Cutaneous Vitamin D3 Synthesis and Its Role in Skin Cancer Prevention. Nutrients 2025, 17, 386. https://doi.org/10.3390/nu17030386
Uçar N, Holick MF. Illuminating the Connection: Cutaneous Vitamin D3 Synthesis and Its Role in Skin Cancer Prevention. Nutrients. 2025; 17(3):386. https://doi.org/10.3390/nu17030386
Chicago/Turabian StyleUçar, Nazlı, and Michael F. Holick. 2025. "Illuminating the Connection: Cutaneous Vitamin D3 Synthesis and Its Role in Skin Cancer Prevention" Nutrients 17, no. 3: 386. https://doi.org/10.3390/nu17030386
APA StyleUçar, N., & Holick, M. F. (2025). Illuminating the Connection: Cutaneous Vitamin D3 Synthesis and Its Role in Skin Cancer Prevention. Nutrients, 17(3), 386. https://doi.org/10.3390/nu17030386






