Gut Microbiota and Autism: Unlocking Connections
Abstract
1. Introduction
- Preclinical findings demonstrate that alterations in microbial composition can modulate social and anxiety-like behaviors in animal models [20].
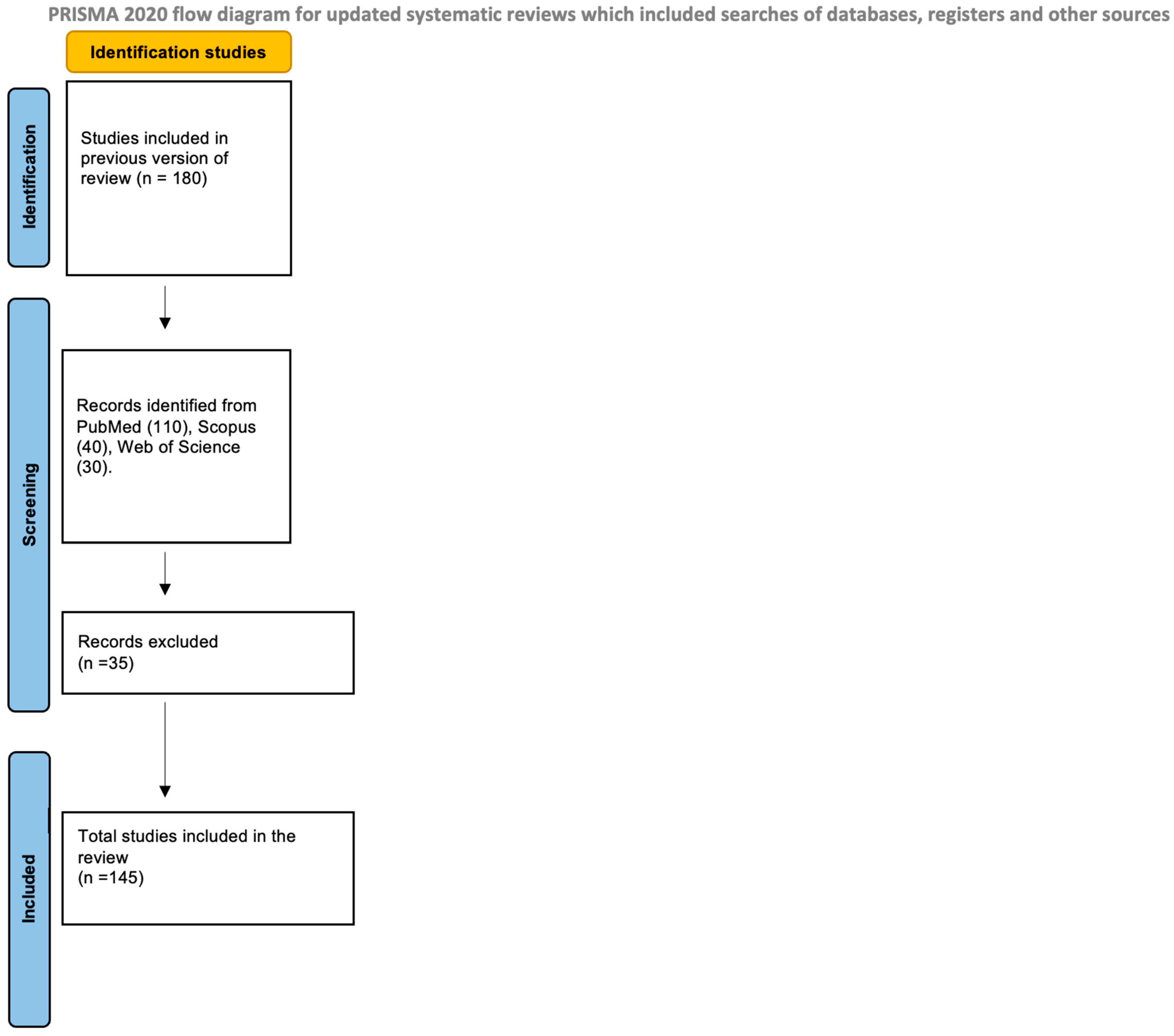
2. Materials and Methods
3. Results and Discussion
3.1. Microbiota–Gut–Brain Axis, Biological Barriers, and Neurodevelopment
3.2. Intestinal Immune Barrier and Blood–Brain Barrier
3.3. Endocrine, Neural, and Metabolic Pathways in Microbiota–Gut–Brain Communication
3.4. Neural Pathways
3.5. Microbial Metabolites and Neurotransmitters
3.6. Microglia, Inflammation, and Neurocognitive Development
3.7. Direct Implications for ASD
3.8. Clinical Evidence: Taxonomy, Metabolomics, Immunology, and Extraintestinal Microbiota
3.8.1. Taxonomic Evidence
- Reduced abundance of beneficial taxa such as Bifidobacterium, Prevotella, and butyrate-producing families (Faecalibacteriaceae, Lachnospiraceae, Ruminococcaceae) [69];
- Increased abundance of potentially pro-inflammatory and neuroactive species, including Clostridium spp., Desulfovibrio, and Veillonella [70].
- These taxa affect the production of key metabolites such as SCFAs, neurotransmitter precursors, and inflammatory mediators, thereby affecting brain function and behavior [71].
3.8.2. Metabolic Evidence
3.8.3. Immune–Inflammatory Tests
3.8.4. Extraintestinal Microbiota
3.8.5. Study Limitations
4. Diet, Microbiota, and Autism: Between Empiricism and Personalization
4.1. The Western Diet and Its Consequences
4.2. Exclusion Diets: Evidence and Controversies
5. Emerging Therapeutic Diets
5.1. Ketogenic Diet (KD)
5.2. Low-Glycemic-Index and Low-FODMAP Diets
5.3. Food Selectivity and Nutritional Deficiencies
6. The Need for Personalized Nutrition
6.1. Maternal Diet, Functional Foods, and the Environment
6.2. Functional Foods and Bioactive Nutrients
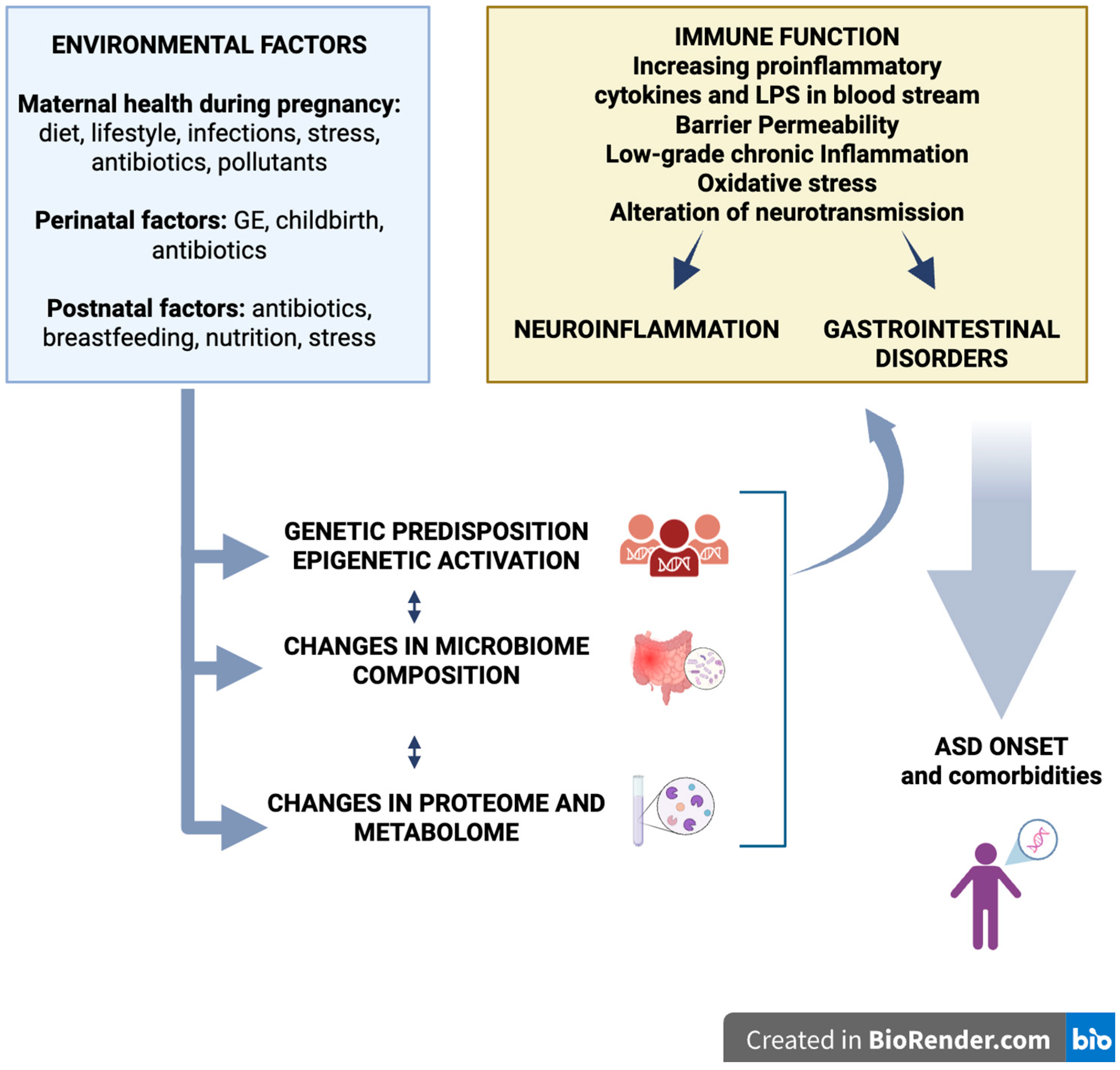
7. Environmental Factors and Epigenetic Modulation
8. Toward Preventive and Translational Approaches
9. Current ASD and Microbiota Limits and Future Prospects
9.1. Methodological Limitations of the Available Studies
9.2. Future Prospects
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shaw, K.A. Maenner, Prevalence and Early Identification of Autism Spectrum Disorder Among Children Aged 4 and 8 Years—Autism and Developmental Disabilities Monitoring Network, 16 Sites, United States, 2022. MMWR. Surveill. Summ. 2025, 74, 1–22. [Google Scholar] [CrossRef]
- De Angelis, M.; Francavilla, R.; Piccolo, M.; De Giacomo, A.; Gobbetti, M. Autism spectrum disorders and intestinal microbiota. Gut Microbes 2015, 6, 207–213. [Google Scholar] [CrossRef]
- Maitin-Shepard, M.; O’tIerney-Ginn, P.; Kraneveld, A.D.; Lyall, K.; Fallin, D.; Arora, M.; Fasano, A.; Mueller, N.T.; Wang, X.; Caulfield, L.E.; et al. Food, nutrition, and autism: From soil to fork. Am. J. Clin. Nutr. 2024, 120, 240–256. [Google Scholar] [CrossRef]
- Alharthi, A.; Alhazmi, S.; Alburae, N.; Bahieldin, A. The Human Gut Microbiome as a Potential Factor in Autism Spectrum Disorder. Int. J. Mol. Sci. 2022, 23, 1363. [Google Scholar] [CrossRef]
- Masini, E.; Loi, E.; Vega-Benedetti, A.F.; Carta, M.; Doneddu, G.; Fadda, R.; Zavattari, P. An Overview of the Main Genetic, Epigenetic and Environmental Factors Involved in Autism Spectrum Disorder Focusing on Synaptic Activity. Int. J. Mol. Sci. 2020, 21, 8290. [Google Scholar] [CrossRef]
- Blair, H.J.; Morales, L.; Cryan, J.F.; Aburto, M.R. Neuroglia and the microbiota-gut-brain axis. Handb. Clin. Neurol. 2025, 209, 171–196. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Biagioli, V.; Volpedo, G.; Riva, A.; Mainardi, P.; Striano, P. From Birth to Weaning: A Window of Opportunity for Microbiota. Nutrients 2024, 16, 272. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhou, B.; Kan, J.; Liu, G.; Zhang, S.; Si, L.; Zhang, X.; Yang, X.; Ma, J.; Cheng, J.; et al. Gut microbiota: Linking nutrition and perinatal depression. Front. Cell. Infect. Microbiol. 2022, 12, 932309. [Google Scholar] [CrossRef]
- Leonardi, L.; Perna, C.; Bernabei, I.; Fiore, M.; Ma, M.; Frankovich, J.; Tarani, L.; Spalice, A. Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) and Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS): Immunological Features Underpinning Controversial Entities. Children 2024, 11, 1043. [Google Scholar] [CrossRef] [PubMed]
- Swedo, S.E.; Frankovich, J.; Murphy, T.K. Murphy, Overview of Treatment of Pediatric Acute-Onset Neuropsychiatric Syndrome. J. Child Adolesc. Psychopharmacol. 2017, 27, 562–565. [Google Scholar] [CrossRef]
- Su, Q.; Wong, O.W.H.; Lu, W.; Wan, Y.; Zhang, L.; Xu, W.; Li, M.K.T.; Liu, C.; Cheung, C.P.; Ching, J.Y.L.; et al. Multikingdom and functional gut microbiota markers for autism spectrum disorder. Nat. Microbiol. 2024, 9, 2344–2355. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, G.; Wan, L.; Liang, Y.; Liu, X.; Yan, H.; Zhang, B.; Yang, G. Effect of fecal microbiota transplantation in children with autism spectrum disorder: A systematic review. Front. Psychiatry 2023, 14, 1123658. [Google Scholar] [CrossRef]
- Kang, D.-W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- McElhanon, B.O.; McCracken, C.; Karpen, S.; Sharp, W.G. Gastrointestinal Symptoms in Autism Spectrum Disorder: A Meta-analysis. Pediatrics 2014, 133, 872–883. [Google Scholar] [CrossRef]
- Ho, L.K.H.; Tong, V.J.W.; Syn, N.; Nagarajan, N.; Tham, E.H.; Tay, S.K.; Shorey, S.; Tambyah, P.A.; Law, E.C.N. Gut microbiota changes in children with autism spectrum disorder: A systematic review. Gut Pathog. 2020, 12, 6. [Google Scholar] [CrossRef]
- Osama, A.; Anwar, A.M.; Ezzeldin, S.; Ahmed, E.A.; Mahgoub, S.; Ibrahim, O.; Ibrahim, S.A.; Abdelhamid, I.A.; Bakry, U.; Diab, A.A.; et al. Integrative multi-omics analysis of autism spectrum disorder reveals unique microbial macromolecules interactions. J. Adv. Res. 2025, 77, 265–279. [Google Scholar] [CrossRef]
- Tao, X.; Li, Z.; Wang, D.; Pu, J.; Liu, Y.; Gui, S.; Zhong, X.; Yang, D.; Zhou, H.; Tao, W.; et al. Perturbations in gut microbiota in autism spectrum disorder: A systematic review. Front. Neurosci. 2025, 19, 1448478. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Cruz, N.J.; Kang, D.-W.; Gandal, M.J.; Wang, B.; Kim, Y.-M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef] [PubMed]
- Leeuwendaal, N.K.; Stanton, C.; O’toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef]
- Stiemsma, L.T.; Nakamura, R.E.; Nguyen, J.G.; Michels, K.B. Does Consumption of Fermented Foods Modify the Human Gut Microbiota? J. Nutr. 2020, 150, 1680–1692. [Google Scholar] [CrossRef]
- Johnson, A.J.; Vangay, P.; Al-Ghalith, G.A.; Hillmann, B.M.; Ward, T.L.; Shields-Cutler, R.R.; Kim, A.D.; Shmagel, A.K.; Syed, A.N.; Walter, J.; et al. Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. Cell Host Microbe 2019, 25, 789–802.e5. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet–microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, N.; Su, X.; Gao, Y.; Yang, R. Gut-Microbiota-Derived Metabolites Maintain Gut and Systemic Immune Homeostasis. Cells 2023, 12, 793. [Google Scholar] [CrossRef] [PubMed]
- Christovich, A.; Luo, X.M. Gut Microbiota, Leaky Gut, and Autoimmune Diseases. Front. Immunol. 2022, 13, 946248. [Google Scholar] [CrossRef]
- Donald, K.; Finlay, B.B. Early-life interactions between the microbiota and immune system: Impact on immune system development and atopic disease. Nat. Rev. Immunol. 2023, 23, 735–748. [Google Scholar] [CrossRef]
- Fuhler, G. The immune system and microbiome in pregnancy. Best Pract. Res. Clin. Gastroenterol. 2020, 44–45, 101671. [Google Scholar] [CrossRef]
- Daneman, R.; Rescigno, M. The Gut Immune Barrier and the Blood-Brain Barrier: Are They So Different? Immunity 2009, 31, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Carloni, S.; Rescigno, M. Unveiling the gut-brain axis: Structural and functional analogies between the gut and the choroid plexus vascular and immune barriers. Semin. Immunopathol. 2022, 44, 869–882. [Google Scholar] [CrossRef]
- Sterling, K.G.; Dodd, G.K.; Alhamdi, S.; Asimenios, P.G.; Dagda, R.K.; De Meirleir, K.L.; Hudig, D.; Lombardi, V.C. Mucosal Immunity and the Gut-Microbiota-Brain-Axis in Neuroimmune Disease. Int. J. Mol. Sci. 2022, 23, 13328. [Google Scholar] [CrossRef]
- Belvoncikova, P.; Maronek, M.; Gardlik, R. Gut Dysbiosis and Fecal Microbiota Transplantation in Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 10729. [Google Scholar] [CrossRef]
- O’riordan, K.J.; Moloney, G.M.; Keane, L.; Clarke, G.; Cryan, J.F. The gut microbiota-immune-brain axis: Therapeutic implications. Cell Rep. Med. 2025, 6, 101982. [Google Scholar] [CrossRef]
- Carloni, S.; Bertocchi, A.; Mancinelli, S.; Bellini, M.; Erreni, M.; Borreca, A.; Braga, D.; Giugliano, S.; Mozzarelli, A.M.; Manganaro, D.; et al. Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science 2021, 374, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Knox, E.G.; Lynch, C.M.K.; Lee, Y.S.; O’DRiscoll, C.M.; Clarke, G.; Cryan, J.F.; Aburto, M.R. The gut microbiota is important for the maintenance of blood–cerebrospinal fluid barrier integrity. Eur. J. Neurosci. 2023, 57, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Luczynski, P.; McVey Neufeld, K.-A.; Oriach, C.S.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Growing up in a Bubble: Using Germ-Free Animals to Assess the Influence of the Gut Microbiota on Brain and Behavior. Int. J. Neuropsychopharmacol. 2016, 19, pyw020. [Google Scholar] [CrossRef] [PubMed]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The Microbiota–Gut–Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 11245. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 2021, 13, 1894070. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, J.; Qi, Z.; Yang, L.; Du, Z.; Wu, Y.; Song, Q.; Li, X.; Sun, J.; Chen, P.; et al. Prenatal psychological stress mediates vertical transmission of gut microbiome to the next generation affecting offspring depressive-like behaviors and neurotransmitter. BMC Psychol. 2025, 13, 791. [Google Scholar] [CrossRef]
- Biggio, F.; Gorini, G.; Utzeri, C.; Olla, P.; Marrosu, F.; Mocchetti, I.; Follesa, P. Chronic vagus nerve stimulation induces neuronal plasticity in the rat hippocampus. Int. J. Neuropsychopharmacol. 2009, 12, 1209. [Google Scholar] [CrossRef]
- Al Noman, A.; Alhudhaibi, A.M.; Afroza, M.; Tonni, S.D.; Shehab, H.M.; Iba, N.J.; Taha, T.H.; Abdallah, E.M. Neuroplasticity and the microbiome: How microorganisms influence brain change. Front. Microbiol. 2025, 16, 1629349. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, K.; Su, N.; Yuan, C.; Zhang, N.; Hu, X.; Fu, Y.; Zhao, F. Microbiota–gut–brain axis in health and neurological disease: Interactions between gut microbiota and the nervous system. J. Cell. Mol. Med. 2024, 28, e70099. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Grasset, E.; Holm, L.M.; Karsenty, G.; MacPherson, A.J.; Olofsson, L.E.; Bäckhed, F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc. Natl. Acad. Sci. USA 2018, 115, 6458–6463. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Brambilla, P.; Perez, J.; Barale, F.; Schettini, G.; Soares, J.C. GABAergic dysfunction in mood disorders. Mol. Psychiatry 2003, 8, 721–737. [Google Scholar] [CrossRef]
- Ren, W.; Yin, J.; Xiao, H.; Chen, S.; Liu, G.; Tan, B.; Li, N.; Peng, Y.; Li, T.; Zeng, B.; et al. Intestinal Microbiota-Derived GABA Mediates Interleukin-17 Expression during Enterotoxigenic Escherichia coli Infection. Front. Immunol. 2017, 7, 685. [Google Scholar] [CrossRef]
- Duranti, S.; Ruiz, L.; Lugli, G.A.; Tames, H.; Milani, C.; Mancabelli, L.; Mancino, W.; Longhi, G.; Carnevali, L.; Sgoifo, A.; et al. Bifidobacterium adolescentis as a key member of the human gut microbiota in the production of GABA. Sci. Rep. 2020, 10, 14112. [Google Scholar] [CrossRef]
- Reyes, R.E.N.; Zhang, Z.; Gao, L.; Asatryan, L. Asatryan, Microbiome meets microglia in neuroinflammation and neurological disorders. Neuroimmunol. Neuroinflamm. 2020, 7, 215–233. [Google Scholar] [CrossRef]
- Davoli-Ferreira, M.; Thomson, C.A.; McCoy, K.D. Microbiota and Microglia Interactions in ASD. Front. Immunol. 2021, 12, 676255. [Google Scholar] [CrossRef] [PubMed]
- Keane, L.; Clarke, G.; Cryan, J.F. A role for microglia in mediating the microbiota–gut–brain axis. Nat. Rev. Immunol. 2025, 25, 847–861. [Google Scholar] [CrossRef]
- Hickman, S.E.; Kingery, N.D.; Ohsumi, T.K.; Borowsky, M.L.; Wang, L.-C.; Means, T.K.; El Khoury, J. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 2013, 16, 1896–1905. [Google Scholar] [CrossRef]
- Dufour, A.; Olya, A.H.; Foulon, S.; Réda, C.; Mokhtari, A.; Faivre, V.; Hua, J.; Bokobza, C.; Griffiths, A.D.; Nghe, P.; et al. Neonatal inflammation impairs developmentally-associated microglia and promotes a highly reactive microglial subset. Brain Behav. Immun. 2025, 123, 466–482. [Google Scholar] [CrossRef]
- Mirarchi, A.; Albi, E.; Arcuri, C. Microglia Signatures: A Cause or Consequence of Microglia-Related Brain Disorders? Int. J. Mol. Sci. 2024, 25, 10951. [Google Scholar] [CrossRef]
- Herrera, M.L.; Paraíso-Luna, J.; Bustos-Martínez, I.; Barco, Á. Targeting epigenetic dysregulation in autism spectrum disorders. Trends Mol. Med. 2024, 30, 1028–1046. [Google Scholar] [CrossRef]
- Eshraghi, A.A.; Liu, G.; Kay, S.-I.S.; Eshraghi, R.S.; Mittal, J.; Moshiree, B.; Mittal, R. Epigenetics and Autism Spectrum Disorder: Is There a Correlation? Front. Cell. Neurosci. 2018, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Becattini, S.; Taur, Y.; Pamer, E.G. Antibiotic-Induced Changes in the Intestinal Microbiota and Disease. Trends Mol. Med. 2016, 22, 458–478. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.; Guarner, F.; Fernandez, L.B.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef]
- Snider, L.A.; Lougee, L.; Slattery, M.; Grant, P.; Swedo, S.E. Antibiotic prophylaxis with azithromycin or penicillin for childhood-onset neuropsychiatric disorders. Biol. Psychiatry 2005, 57, 788–792. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; De Jesus-Laboy, K.M.; Shen, N.; Cox, L.M.; Amir, A.; Gonzalez, A.; Bokulich, N.A.; Song, S.J.; Hoashi, M.; Rivera-Vinas, J.I.; et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med. 2016, 22, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Li, L.; Lagerberg, T.; Chang, Z.; Cortese, S.; Rosenqvist, M.A.; Almqvist, C.; D’onofrio, B.M.; Hegvik, T.-A.; Hartman, C.; Chen, Q.; et al. Maternal pre-pregnancy overweight/obesity and the risk of attention-deficit/hyperactivity disorder in offspring: A systematic review, meta-analysis and quasi-experimental family-based study. Int. J. Epidemiol. 2020, 49, 857–875. [Google Scholar] [CrossRef]
- Sanchez, C.E.; Barry, C.; Sabhlok, A.; Russell, K.; Majors, A.; Kollins, S.H.; Fuemmeler, B.F. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: A meta-analysis. Obes. Rev. 2018, 19, 464–484. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Wang, T.; Chen, B.; Luo, M.; Xie, L.; Lu, M.; Lu, X.; Zhang, S.; Wei, L.; Zhou, X.; Yao, B.; et al. Microbiota-indole 3-propionic acid-brain axis mediates abnormal synaptic pruning of hippocampal microglia and susceptibility to ASD in IUGR offspring. Microbiome 2023, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- Troisi, J.; Autio, R.; Beopoulos, T.; Bravaccio, C.; Carraturo, F.; Corrivetti, G.; Cunningham, S.; Devane, S.; Fallin, D.; Fetissov, S.; et al. Genome, Environment, Microbiome and Metabolome in Autism (GEMMA) Study Design: Biomarkers Identification for Precision Treatment and Primary Prevention of Autism Spectrum Disorders by an Integrated Multi-Omics Systems Biology Approach. Brain Sci. 2020, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Marzal, L.N.; Rojas-Velazquez, D.; Rigters, D.; Prince, N.; Garssen, J.; Kraneveld, A.D.; Perez-Pardo, P.; Lopez-Rincon, A. A robust microbiome signature for autism spectrum disorder across different studies using machine learning. Sci. Rep. 2024, 14, 814. [Google Scholar] [CrossRef]
- Zeng, Q.; Hu, Y.; Xie, L.; Zhang, X.; Huang, Y.; Ye, J.; Wang, S.; Xu, J. Gut microbiota diversity and composition in children with autism spectrum disorder: Associations with symptom severity. PeerJ 2025, 13, e19528. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef]
- Lewandowska-Pietruszka, Z.; Figlerowicz, M.; Mazur-Melewska, K. Microbiota in Autism Spectrum Disorder: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 16660. [Google Scholar] [CrossRef]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Macfabe, D.F. Short-chain fatty acid fermentation products of the gut microbiome: Implications in autism spectrum disorders. Microb. Ecol. Health Dis. 2012, 23, 19260. [Google Scholar] [CrossRef]
- Fiore, A.; Murray, P.J. Tryptophan and indole metabolism in immune regulation. Curr. Opin. Immunol. 2021, 70, 7–14. [Google Scholar] [CrossRef]
- Wyatt, M.; Greathouse, K.L. Targeting Dietary and Microbial Tryptophan-Indole Metabolism as Therapeutic Approaches to Colon Cancer. Nutrients 2021, 13, 1189. [Google Scholar] [CrossRef]
- Meeking, M.M.; MacFabe, D.F.; Mepham, J.R.; Foley, K.A.; Tichenoff, L.J.; Boon, F.H.; Kavaliers, M.; Ossenkopp, K.-P. Propionic acid induced behavioural effects of relevance to autism spectrum disorder evaluated in the hole board test with rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 97, 109794. [Google Scholar] [CrossRef]
- Guetterman, H.M.; Huey, S.L.; Knight, R.; Fox, A.M.; Mehta, S.; Finkelstein, J.L. Vitamin B-12 and the Gastrointestinal Microbiome: A Systematic Review. Adv. Nutr. 2022, 13, 530–558. [Google Scholar] [CrossRef]
- Cao, X.; Liu, K.; Liu, J.; Liu, Y.-W.; Xu, L.; Wang, H.; Zhu, Y.; Wang, P.; Li, Z.; Wen, J.; et al. Dysbiotic Gut Microbiota and Dysregulation of Cytokine Profile in Children and Teens With Autism Spectrum Disorder. Front. Neurosci. 2021, 15, 635925. [Google Scholar] [CrossRef]
- Careaga, M.; Rogers, S.; Hansen, R.L.; Amaral, D.G.; Van de Water, J.; Ashwood, P. Immune Endophenotypes in Children With Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 434–441. [Google Scholar] [CrossRef]
- De Sales-Millán, A.; Aguirre-Garrido, J.F.; González-Cervantes, R.M.; Velázquez-Aragón, J.A. Microbiome–Gut–Mucosal–Immune–Brain Axis and Autism Spectrum Disorder (ASD): A Novel Proposal of the Role of the Gut Microbiome in ASD Aetiology. Behav. Sci. 2023, 13, 548. [Google Scholar] [CrossRef]
- Fasano, A. Leaky Gut and Autoimmune Diseases. Clin. Rev. Allergy Immunol. 2012, 42, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Manghi, P.; Filosi, M.; Zolfo, M.; Casten, L.G.; Garcia-Valiente, A.; Mattevi, S.; Heidrich, V.; Golzato, D.; Perini, S.; Thomas, A.M.; et al. Large-scale metagenomic analysis of oral microbiomes reveals markers for autism spectrum disorders. Nat. Commun. 2024, 15, 9743. [Google Scholar] [CrossRef] [PubMed]
- Mengist, B.; Lotfaliany, M.; Pasco, J.A.; Agustini, B.; Berk, M.; Forbes, M.; Lane, M.M.; Orchard, S.G.; Ryan, J.; Owen, A.J.; et al. The risk associated with ultra-processed food intake on depressive symptoms and mental health in older adults: A target trial emulation. BMC Med. 2025, 23, 172. [Google Scholar] [CrossRef]
- Abdollahpour, N.; Fard, S.A.M.; Salahmanesh, A.; Hatamzadeh, H.; Moeini, R.; Soflaei, S.S.; Seifi, N.; Ghayour-Mobarhan, M. The Association between Ultra-Processed Foods and Depression, Anxiety and Sleep in Adults: A Cross-Sectional Study in Iran. Food Sci. Nutr. 2025, 13, e70316. [Google Scholar] [CrossRef]
- Lane, M.M.; Gamage, E.; Travica, N.; Dissanayaka, T.; Ashtree, D.N.; Gauci, S.; Lotfaliany, M.; O’neil, A.; Jacka, F.N.; Marx, W. Ultra-Processed Food Consumption and Mental Health: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2022, 14, 2568. [Google Scholar] [CrossRef]
- Delaroque, C.; Rytter, H.; Bonazzi, E.; Huillet, M.; Ellero-Simatos, S.; Chatonnat, E.; Hao, F.; Patterson, A.; Chassaing, B. Maternal emulsifier consumption alters the offspring early-life microbiota and goblet cell function leading to long-lasting diseases susceptibility. Nat. Commun. 2025, 16, 6954. [Google Scholar] [CrossRef]
- Panyod, S.; Wu, W.-K.; Chang, C.-T.; Wada, N.; Ho, H.-C.; Lo, Y.-L.; Tsai, S.-P.; Chen, R.-A.; Huang, H.-S.; Liu, P.-Y.; et al. Common dietary emulsifiers promote metabolic disorders and intestinal microbiota dysbiosis in mice. Commun. Biol. 2024, 7, 749. [Google Scholar] [CrossRef]
- Holder, M.K.; Peters, N.V.; Whylings, J.; Fields, C.T.; Gewirtz, A.T.; Chassaing, B.; de Vries, G.J. Dietary emulsifiers consumption alters anxiety-like and social-related behaviors in mice in a sex-dependent manner. Sci. Rep. 2019, 9, 172. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Mazloomi, S.N.; Talebi, S.; Mehrabani, S.; Bagheri, R.; Ghavami, A.; Zarpoosh, M.; Mohammadi, H.; Wong, A.; Nordvall, M.; Kermani, M.A.H.; et al. The association of ultra-processed food consumption with adult mental health disorders: A systematic review and dose-response meta-analysis of 260,385 participants. Nutr. Neurosci. 2023, 26, 913–931. [Google Scholar] [CrossRef] [PubMed]
- Rondinella, D.; Raoul, P.C.; Valeriani, E.; Venturini, I.; Cintoni, M.; Severino, A.; Galli, F.S.; Mora, V.; Mele, M.C.; Cammarota, G.; et al. The Detrimental Impact of Ultra-Processed Foods on the Human Gut Microbiome and Gut Barrier. Nutrients 2025, 17, 859. [Google Scholar] [CrossRef]
- Rytter, H.; Naimi, S.; Wu, G.; Lewis, J.; Duquesnoy, M.; Vigué, L.; Tenaillon, O.; Belda, E.; Vazquez-Gomez, M.; Touly, N.; et al. In vitro microbiota model recapitulates and predicts individualised sensitivity to dietary emulsifier. Gut 2025, 74, 761–774. [Google Scholar] [CrossRef]
- Wang, C.; Huang, Z.; Yu, K.; Ding, R.; Ye, K.; Dai, C.; Xu, X.; Zhou, G.; Li, C. High-Salt Diet Has a Certain Impact on Protein Digestion and Gut Microbiota: A Sequencing and Proteome Combined Study. Front. Microbiol. 2017, 8, 1838. [Google Scholar] [CrossRef] [PubMed]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L.; et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Miranda, P.M.; De Palma, G.; Serkis, V.; Lu, J.; Louis-Auguste, M.P.; McCarville, J.L.; Verdu, E.F.; Collins, S.M.; Bercik, P. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome 2018, 6, 57. [Google Scholar] [CrossRef]
- Keller, A.; Rimestad, M.L.; Rohde, J.F.; Petersen, B.H.; Korfitsen, C.B.; Tarp, S.; Lauritsen, M.B.; Händel, M.N. The Effect of a Combined Gluten- and Casein-Free Diet on Children and Adolescents with Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 470. [Google Scholar] [CrossRef] [PubMed]
- Mearin, M.L.; Agardh, D.; Antunes, H.; Al-toma, A.; Auricchio, R.; Castillejo, G.; Catassi, C.; Ciacci, C.; Discepolo, V.; Dolinsek, J.; et al. ESPGHAN Position Paper on Management and Follow-up of Children and Adolescents With Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 369–386. [Google Scholar] [CrossRef]
- Simón, E.; Molero-Luis, M.; Fueyo-Díaz, R.; Costas-Batlle, C.; Crespo-Escobar, P.; Montoro-Huguet, M.A. The Gluten-Free Diet for Celiac Disease: Critical Insights to Better Understand Clinical Outcomes. Nutrients 2023, 15, 4013. [Google Scholar] [CrossRef]
- Shabbir, I.; Liu, K.; Riaz, B.; Rahim, M.F.; Zhong, S.; Aweya, J.J.; Cheong, K.-L. Investigating the Therapeutic Potential of the Ketogenic Diet in Modulating Neurodegenerative Pathophysiology: An Interdisciplinary Approach. Nutrients 2025, 17, 1268. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y. The role of nutrition and gut microbiome in childhood brain development and behavior. Front. Nutr. 2025, 12, 1590172. [Google Scholar] [CrossRef]
- Lim, J.-M.; Letchumanan, V.; Tan, L.T.-H.; Hong, K.-W.; Wong, S.-H.; Ab Mutalib, N.-S.; Lee, L.-H.; Law, J.W.-F. Ketogenic Diet: A Dietary Intervention via Gut Microbiome Modulation for the Treatment of Neurological and Nutritional Disorders (a Narrative Review). Nutrients 2022, 14, 3566. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Chen, Y.; Gong, X.; Chen, Z.; Zhang, X. Ketogenic Diet and Gut Microbiota: Exploring New Perspectives on Cognition and Mood. Foods 2025, 14, 1215. [Google Scholar] [CrossRef]
- Monda, A.; La Torre, M.E.; Messina, A.; Di Maio, G.; Monda, V.; Moscatelli, F.; De Stefano, M.; La Marra, M.; Di Padova, M.; Dipace, A.; et al. Exploring the ketogenic diet’s potential in reducing neuroinflammation and modulating immune responses. Front. Immunol. 2024, 15, 1425816. [Google Scholar] [CrossRef]
- Watt, C.; Sanchez-Rangel, E.; Hwang, J.J. Glycemic Variability and CNS Inflammation: Reviewing the Connection. Nutrients 2020, 12, 3906. [Google Scholar] [CrossRef] [PubMed]
- Bertuccioli, A.; Cardinali, M.; Di Pierro, F.; Zonzini, G.B.; Matera, M.R. Ketogenic and Low FODMAP Diet in Therapeutic Management of a Young Autistic Patient with Epilepsy and Dysmetabolism Poorly Responsive to Therapies: Clinical Response and Effects of Intestinal Microbiota. Int. J. Mol. Sci. 2022, 23, 8829. [Google Scholar] [CrossRef] [PubMed]
- Koc, F.; Arendt, E.; Coffey, A.; Ross, R.P.; Stanton, C. Impact of low FODMAP sourdough bread on gut microbiota using an in vitro colonic fermentation model. Front. Microbiol. 2024, 15, 1496022. [Google Scholar] [CrossRef]
- Nogay, N.H.; Walton, J.; Roberts, K.M.; Nahikian-Nelms, M.; Witwer, A.N. The Effect of the Low FODMAP Diet on Gastrointestinal Symptoms, Behavioral Problems and Nutrient Intake in Children with Autism Spectrum Disorder: A Randomized Controlled Pilot Trial. J. Autism Dev. Disord. 2021, 51, 2800–2811. [Google Scholar] [CrossRef]
- Amadi, C.N.; Orish, C.N.; Frazzoli, C.; Orisakwe, O.E. Dietary interventions for autism spectrum disorder: An updated systematic review of human studies. Psychiatriki 2022, 33, 228–242. [Google Scholar] [CrossRef]
- Ferrara, R.; Iovino, L.; Ricci, L.; Avallone, A.; Latina, R.; Ricci, P. Food selectivity and autism: A systematic review. World J. Clin. Pediatr. 2025, 14, 101974. [Google Scholar] [CrossRef] [PubMed]
- Al-Beltagi, M. Nutritional management and autism spectrum disorder: A systematic review. World J. Clin. Pediatr. 2024, 13, 99649. [Google Scholar] [CrossRef]
- Pérez-Cabral, I.D.; Bernal-Mercado, A.T.; Islas-Rubio, A.R.; Suárez-Jiménez, G.M.; Robles-García, M.Á.; Puebla-Duarte, A.L.; Del-Toro-Sánchez, C.L. Exploring Dietary Interventions in Autism Spectrum Disorder. Foods 2024, 13, 3010. [Google Scholar] [CrossRef]
- Chong, P.F.; Torio, M.; Fujii, F.; Hirata, Y.; Matsuoka, W.; Sonoda, Y.; Ichimiya, Y.; Yada, Y.; Kaku, N.; Ishimura, M.; et al. Critical vitamin deficiencies in autism spectrum disorder: Reversible and irreversible outcomes. Eur. J. Clin. Nutr. 2022, 76, 1618–1621. [Google Scholar] [CrossRef] [PubMed]
- Bordeleau, M.; de Cossío, L.F.; Chakravarty, M.M.; Tremblay, M. From Maternal Diet to Neurodevelopmental Disorders: A Story of Neuroinflammation. Front. Cell. Neurosci. 2021, 14, 612705. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Konnikova, L.; Brodin, P.; Mysorekar, I.U.; Collado, M.C. The maternal gut microbiome in pregnancy: Implications for the developing immune system. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 35–45. [Google Scholar] [CrossRef]
- Biagioli, V.; Matera, M.; Ramenghi, L.A.; Falsaperla, R.; Striano, P. Microbiome and Pregnancy Dysbiosis: A Narrative Review on Offspring Health. Nutrients 2025, 17, 1033. [Google Scholar] [CrossRef]
- Biete, M.; Vasudevan, S. Gestational diabetes mellitus: Impacts on fetal neurodevelopment, gut dysbiosis, and the promise of precision medicine. Front. Mol. Biosci. 2024, 11, 1420664. [Google Scholar] [CrossRef] [PubMed]
- Nohesara, S.; Abdolmaleky, H.M.; Dickerson, F.; Pinto-Tomás, A.A.; Jeste, D.V.; Thiagalingam, S. Maternal Gut Microbiome-Mediated Epigenetic Modifications in Cognitive Development and Impairments: A New Frontier for Therapeutic Innovation. Nutrients 2024, 16, 4355. [Google Scholar] [CrossRef]
- Basak, S.; Mallick, R.; Sree, B.N.; Duttaroy, A.K. Placental Epigenome Impacts Fetal Development: Effects of Maternal Nutrients and Gut Microbiota. Nutrients 2024, 16, 1860. [Google Scholar] [CrossRef]
- Hasebe, K.; Kendig, M.D.; Kaakoush, N.O.; Tajaddini, A.; Hesam-Shariati, S.; Westbrook, R.F.; Morris, M.J. Pregnancy-related changes in microbiome are disrupted by obesogenic diet exposure: Implications for offspring microbiome development. Food Funct. 2025, 16, 4023–4034. [Google Scholar] [CrossRef]
- Sajdel-Sulkowska, E.M. The Impact of Maternal Gut Microbiota during Pregnancy on Fetal Gut–Brain Axis Development and Life-Long Health Outcomes. Microorganisms 2023, 11, 2199. [Google Scholar] [CrossRef]
- Abuljadayel, D.; Alotibi, A.; Alqothmi, K.; Basingab, F.; Alhazmi, S.; Almuhammadi, A.; Alharthi, A.; Alyoubi, R.; Bahieldin, A. Gut microbiota of children with autism spectrum disorder and healthy siblings: A comparative study. Exp. Ther. Med. 2024, 28, 430. [Google Scholar] [CrossRef] [PubMed]
- Naspolini, N.F.; Schüroff, P.A.; Figueiredo, M.J.; Sbardellotto, G.E.; Ferreira, F.R.; Fatori, D.; Polanczyk, G.V.; Campos, A.C.; Taddei, C.R. The Gut Microbiome in the First One Thousand Days of Neurodevelopment: A Systematic Review from the Microbiome Perspective. Microorganisms 2024, 12, 424. [Google Scholar] [CrossRef] [PubMed]
- Momeni, N.; Mousavi, S.N.; Chiti, H.; Heidarzadeh, S. A maternal sweet diet is associated with the gut dysbiosis in the first trimester of pregnancy. BMC Nutr. 2024, 10, 162. [Google Scholar] [CrossRef]
- Lopez-Tello, J.; Kiu, R.; Schofield, Z.; Zhang, C.X.; van Sinderen, D.; Le Gall, G.; Hall, L.J.; Sferruzzi-Perri, A.N. Maternal gut Bifidobacterium breve modifies fetal brain metabolism in germ-free mice. Mol. Metab. 2024, 88, 102004. [Google Scholar] [CrossRef]
- Hudobenko, J.; Di Gesù, C.M.; Mooz, P.R.; Petrosino, J.; Putluri, N.; Ganesh, B.P.; Rebeles, K.; Blixt, F.W.; Venna, V.R.; McCullough, L.D. Maternal dysbiosis produces long-lasting behavioral changes in offspring. Mol. Psychiatry 2025, 30, 1847–1858. [Google Scholar] [CrossRef]
- Martinat, M.; Rossitto, M.; Di Miceli, M.; Layé, S. Perinatal Dietary Polyunsaturated Fatty Acids in Brain Development, Role in Neurodevelopmental Disorders. Nutrients 2021, 13, 1185. [Google Scholar] [CrossRef] [PubMed]
- Dighriri, I.M.; Alsubaie, A.M.; Hakami, F.M.; Hamithi, D.M.; Alshekh, M.M.; Khobrani, F.A.; Dalak, F.E.; Hakami, A.A.; Alsueaadi, E.H.; Alsaawi, L.S.; et al. Effects of Omega-3 Polyunsaturated Fatty Acids on Brain Functions: A Systematic Review. Cureus 2022, 14, e30091. [Google Scholar] [CrossRef]
- Obuchowska, A.; Gorczyca, K.; Standyło, A.; Obuchowska, K.; Kimber-Trojnar, Ż.; Wierzchowska-Opoka, M.; Leszczyńska-Gorzelak, B. Effects of Probiotic Supplementation during Pregnancy on the Future Maternal Risk of Metabolic Syndrome. Int. J. Mol. Sci. 2022, 23, 8253. [Google Scholar] [CrossRef]
- Vauzour, D. Dietary Polyphenols as Modulators of Brain Functions: Biological Actions and Molecular Mechanisms Underpinning Their Beneficial Effects. Oxidative Med. Cell. Longev. 2012, 2012, 914273. [Google Scholar] [CrossRef]
- Luk, B.; Veeraragavan, S.; Engevik, M.; Balderas, M.; Major, A.; Runge, J.; Luna, R.A.; Versalovic, J. Postnatal colonization with human “infant-type” Bifidobacterium species alters behavior of adult gnotobiotic mice. PLoS ONE 2018, 13, e0196510. [Google Scholar] [CrossRef]
- Wong, C.B.; Huang, H.; Ning, Y.; Xiao, J. Probiotics in the New Era of Human Milk Oligosaccharides (HMOs): HMO Utilization and Beneficial Effects of Bifidobacterium longum subsp. infantis M-63 on Infant Health. Microorganisms 2024, 12, 1014. [Google Scholar] [CrossRef]
- Cukrowska, B.; Bierła, J.B.; Zakrzewska, M.; Klukowski, M.; Maciorkowska, E. The Relationship between the Infant Gut Microbiota and Allergy. The Role of Bifidobacterium breve and Prebiotic Oligosaccharides in the Activation of Anti-Allergic Mechanisms in Early Life. Nutrients 2020, 12, 946. [Google Scholar] [CrossRef]
- Bojarczuk, A.; Skąpska, S.; Khaneghah, A.M.; Marszałek, K. Health benefits of resistant starch: A review of the literature. J. Funct. Foods 2022, 93, 105094. [Google Scholar] [CrossRef]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 303, G1288–G1295. [Google Scholar] [CrossRef]
- Veniaminova, E.; Cespuglio, R.; Cheung, C.W.; Umriukhin, A.; Markova, N.; Shevtsova, E.; Lesch, K.-P.; Anthony, D.C.; Strekalova, T. Autism-Like Behaviours and Memory Deficits Result from a Western Diet in Mice. Neural Plast. 2017, 2017, 9498247. [Google Scholar] [CrossRef]
- Chunchai, T.; Thunapong, W.; Yasom, S.; Wanchai, K.; Eaimworawuthikul, S.; Metzler, G.; Lungkaphin, A.; Pongchaidecha, A.; Sirilun, S.; Chaiyasut, C.; et al. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J. Neuroinflamm. 2018, 15, 11. [Google Scholar] [CrossRef]
- Leo, A.; De Caro, C.; Mainardi, P.; Tallarico, M.; Nesci, V.; Marascio, N.; Striano, P.; Russo, E.; Constanti, A.; De Sarro, G.; et al. Increased efficacy of combining prebiotic and postbiotic in mouse models relevant to autism and depression. Neuropharmacology 2021, 198, 108782. [Google Scholar] [CrossRef] [PubMed]
- Vendrik, K.E.W.; Ooijevaar, R.E.; De Jong, P.R.C.; Laman, J.D.; van Oosten, B.W.; Van Hilten, J.J.; Ducarmon, Q.R.; Keller, J.J.; Kuijper, E.J.; Contarino, M.F. Fecal Microbiota Transplantation in Neurological Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Gavito-Covarrubias, D.; Ramírez-Díaz, I.; Guzmán-Linares, J.; Limón, I.D.; Manuel-Sánchez, D.M.; Molina-Herrera, A.; Coral-García, M.Á.; Anastasio, E.; Anaya-Hernández, A.; López-Salazar, P.; et al. Epigenetic mechanisms of particulate matter exposure: Air pollution and hazards on human health. Front. Genet. 2024, 14, 1306600. [Google Scholar] [CrossRef]
- Perera, F.; Miao, Y.; Ross, Z.; Rauh, V.; Margolis, A.; Hoepner, L.; Riley, K.W.; Herbstman, J.; Wang, S. Prenatal exposure to air pollution during the early and middle stages of pregnancy is associated with adverse neurodevelopmental outcomes at ages 1 to 3 years. Environ. Health 2024, 23, 95. [Google Scholar] [CrossRef] [PubMed]
- Stepanyan, A.; Petrackova, A.; Hakobyan, S.; Savara, J.; Davitavyan, S.; Kriegova, E.; Arakelyan, A. Long-term environmental metal exposure is associated with hypomethylation of CpG sites in NFKB1 and other genes related to oncogenesis. Clin. Epigenetics 2023, 15, 126. [Google Scholar] [CrossRef]
- Kurita, H.; Ohuchi, K.; Inden, M. Effects of Environmental Non-Essential Toxic Heavy Metals on Epigenetics During Development. Toxics 2025, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Rubas, N.C.; Torres, A.; Maunakea, A.K. The Gut Microbiome and Epigenomic Reprogramming: Mechanisms, Interactions, and Implications for Human Health and Disease. Int. J. Mol. Sci. 2025, 26, 8658. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Lloyd, D.; Maciver, S.K.; Khan, N.A. Gut microbiome-derived metabolites and epigenetic modulation as potential countermeasures to acute stress. Discov. Med. 2025, 2, 280. [Google Scholar] [CrossRef]
| Intervention | Main Effects on the Microbiota | Reported Clinical/Metabolic Outcomes |
|---|---|---|
| Western Diet (WD) | ↓ Bifidobacterium, ↓ Prevotella, ↓ butyrate-producing; ↑ Clostridium spp., ↑ Desulfovibrio | ↑ intestinal permeability, ↑ inflammation; (preclinical) [135] |
| Diet rich in fiber, vegetables, polyphenols | ↑ Akkermansia, ↑ F. prausnitzii, ↑ SCFAs, ↑ Bifidobacterium | Improved gut barrier, ↓ inflammation, cognitive benefits [132] |
| Fermented foods | ↑ Lactobacillus, ↑ Bifidobacterium, ↑ diversity | Reduction in inflammation, improvement of GI symptoms [21] |
| Exclusion diets (GFCF, other) | Variable effects; possible ↓ diversity; ↓ Bifidobacterium | Inconsistent evidence; Risk of nutritional deficiencies [98] |
| Low-FODMAP | ↓ Prevotella, ↓ Bacteroides | Improvement of GI symptoms; Inconclusive behavioral impact [107] |
| Low-GI diet | ↑ Anti-inflammatory metabolites; ↑ Diversity (preclinical) | ↓ oxidative stress and neuroinflammation (animal) |
| Ketogenic diet (KD) | ↓ Actinobacteria, Akkermansia, B. fragilis, Bilophila | ↓ inflammatory cytokines, ↑ BDNF; Possible behavioral benefits [101] |
| Prebiotics (inulin, GOS, FOS) | ↑ Bifidobacterium, ↑ SCFAs, ↑ Lactobacillus | Barrier improvement, ↓ inflammation [132] |
| Probiotics | Increased beneficial strains; ↓ Enterobacteriaceae | GI improvement; Variable behavioral effects [136] |
| Postbiotics | ↑ beneficial metabolites (e.g., butyrate) | Barrier improvement and immunomodulation (preclinical) [137] |
| FMT | ↑ Diversity ↑ Bifidobacterium, ↑ Prevotella, ↓ Desulfovibrio | Persistent GI and behavioral improvement for up to 2 years [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biagioli, V.; Matera, M.; Cavecchia, I.; Di Pierro, F.; Zerbinati, N.; Striano, P. Gut Microbiota and Autism: Unlocking Connections. Nutrients 2025, 17, 3706. https://doi.org/10.3390/nu17233706
Biagioli V, Matera M, Cavecchia I, Di Pierro F, Zerbinati N, Striano P. Gut Microbiota and Autism: Unlocking Connections. Nutrients. 2025; 17(23):3706. https://doi.org/10.3390/nu17233706
Chicago/Turabian StyleBiagioli, Valentina, Mariarosaria Matera, Ilaria Cavecchia, Francesco Di Pierro, Nicola Zerbinati, and Pasquale Striano. 2025. "Gut Microbiota and Autism: Unlocking Connections" Nutrients 17, no. 23: 3706. https://doi.org/10.3390/nu17233706
APA StyleBiagioli, V., Matera, M., Cavecchia, I., Di Pierro, F., Zerbinati, N., & Striano, P. (2025). Gut Microbiota and Autism: Unlocking Connections. Nutrients, 17(23), 3706. https://doi.org/10.3390/nu17233706









