Nutrition-Based Paternal Influence on Gynecological Diseases in Female Offspring via Epigenetic Mechanisms
Abstract
1. Introduction
2. Mechanisms of Paternal Epigenetics and Its Role in Gynecological Cancers
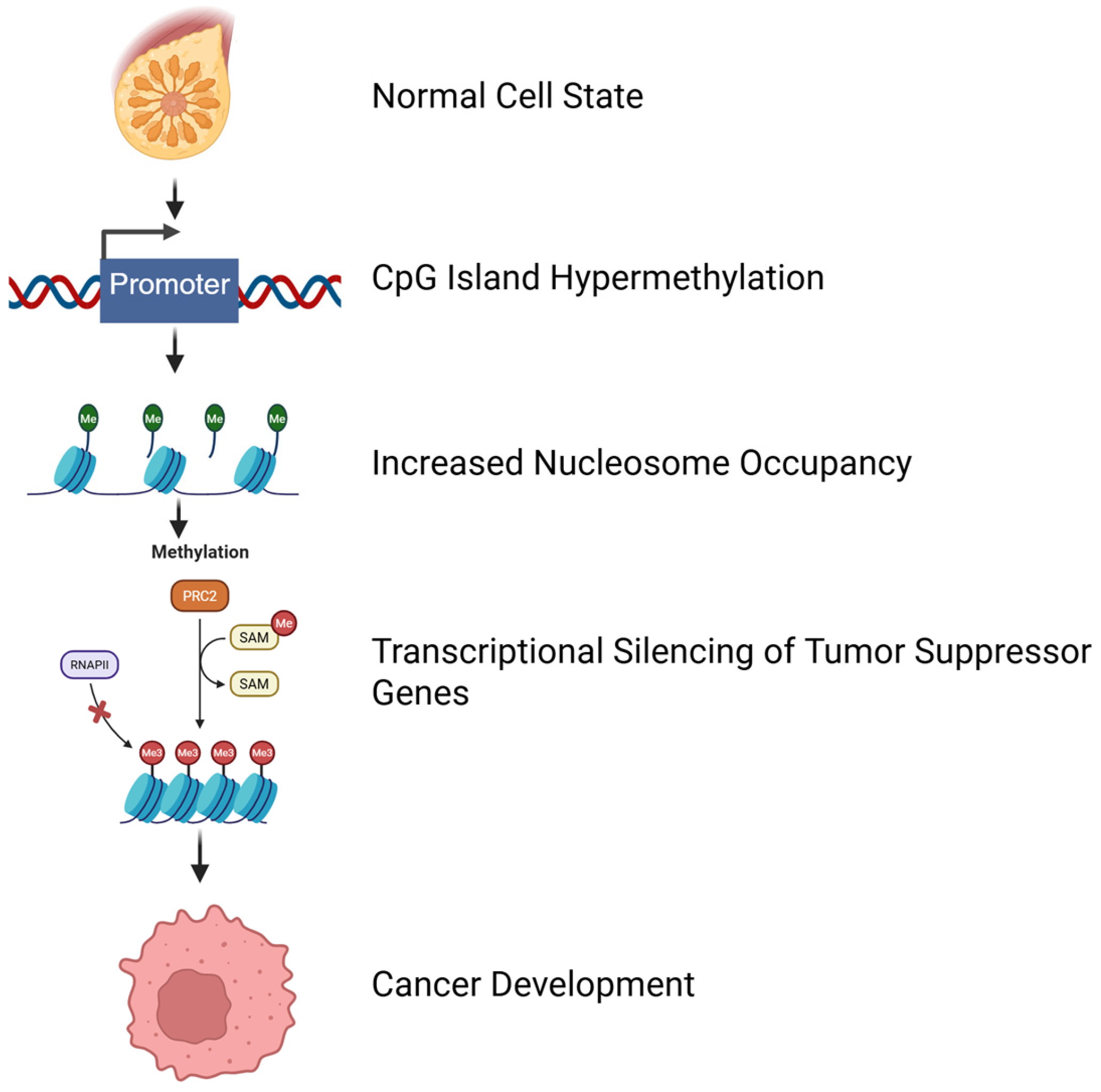
2.1. DNA Methylation, Paternal Transmission, and Nutritional Influence
| Type of Methylation | Function | Effect | Genes Affected | Reference |
|---|---|---|---|---|
| Hypomethylation | Occurs in tumor cells at the repetitive sequences residing in satellite regions | Chromosome breakage | Repetitive sequences in satellite or pericentromeric region | [47,48] |
| Hypomethylation | This leads to the activation of silenced genes and affects global DNA and specific genes | Gene activation | Silenced genes can activate proto-oncogenes and destabilize the genome promoting cancer progression | [47,49] |
| Hypermethylation | Often occurs at specific regulatory sites in the promoter regions or repetitive sequences | Tumor specificity | Genes involved in DNA repair and apoptosis, such as tumor suppressor genes | [50,51] |
| Hypermethylation | The heavy density of cytosine methylation in the CpG islands of the tumor suppressor gene promoters | Transcription block | Tumor suppressor genes | [50] |
| Aberrant Promoter Methylation | Leads to transcriptional silencing of tumor suppressors and metastasis inhibitor genes | Malignant and metastastic phenotype | Tumor suppressor | [51] |
2.2. Histone Modifications in Paternal Transmission
| Histone Modification | Description | Epigenetic Role | Location | Reference |
|---|---|---|---|---|
| H3 lysine 9 methylation (H3K9me3) | The addition of three methyl groups to the 9th lysine residue of histone H3 | Associated with transcriptional silencing by recruiting HP1 (heterochromatin protein 1) to initiate and maintain heterochromatin formation | Constitutive heterochromatin | [76] |
| H3 Lysine 4 methylation (H3K4me3) | The addition of three methyl groups to the 4th lysine residue of histone H3 | Associated with transcriptional activation and euchromatic regions | Promoter regions of active genes | [77] |
| H3 Lysine 27 methylation (H3K27me3) | The addition of three methyl groups to the 27th lysine residue of histone H3. | Associated with transcriptional repression particularly through Polycomb (Pc) group protein | Gene repressed by Polycomb group proteins | [78,79] |
| Histone acetylation | Histone acetyl transferases (HATs) add acetyl groups to histone tails | Facilitates gene transcription | [76] | |
| Deacetylation | Histone deacetylases (HDACs) remove acetyl groups from histone tails | Inhibits gene transcription | [80,81] | |
| Methylation | Histone methyltransferases add methyl groups to histone tails | Regulation of gene expression | [80,81] | |
| Phosphorylation | Kinases add phosphate groups to histone tails | Regulation of gene expression, DNA repair, and chromosome condensation | [81] |
2.3. Non-Coding RNAs (ncRNAs) in Paternal Transmission
| Differentially Regulated Paternal miRNA | Expression | Target Pathway/mRNA | Cause | Organism | Reference |
|---|---|---|---|---|---|
| miR-28a, miR-92a, miR-200c, miR-451a, miR-191, and miR-15b | ↑ | AMP-activated protein kinase pathway (Prkaa2, Cab39), mammalian target of rapamycin (mTOR) signaling pathway | Paternal malnutrition, low protein diet | Mouse | [87] |
| miRNA-1896, miRNA-874 and miRNA-296-5p | ↓ | Hypoxia signaling, insulin receptor signaling, NANOG pathway, CDK5 signaling, epithelial–mesenchymal transition pathway, ERK/MAPK pathway, SAPK/JNK signaling, estrogen receptor signaling, April mediated signaling, axonal guidance signaling | Paternal obesity | Mouse | [33] |
| miR-29c, miR-30a, miR-30c, miR-32, miR-193-5p, miR-204, miR-375, miR-5323p, and miR-698 | ↑ | Sirt1, Ube3a, Srsf2, IL6st, Ncl, Aara, Agfg1, and Ralbp1 ** | Chronic paternal stress | Mouse | [89] |
| miRNA- let-7d-5p, miR-10a-5p, miR-138-1-3p, miR-221-3p, miR-222-3p ‡ | Exposure to toxicants (herbicide- Vinclozolin, fungicide, jet fuel, pesticide-DDT) | Rat | [85] | ||
| miR-30c, miR-30e, miR-124, miR-145, miR-361,miR-762 ‡ | ↑ | Apoptosis, myogenesis, tumor suppression, immune response | Paternal exposure to radiation | Mouse | [98] |
| miR-29c, miR-134, miR-181a ‡ | ↓ | Bax, Bcl, PTEN, stem cell survival | |||
| Differentially regulated paternal tRF | Expression | Target pathway/mRNA | Cause | Organism | Reference |
| tRF-Gly-GCC, tRF-Gly-CCC, tRF-Val-CAC, tRF-Gly-TCC, tRF-Lys-CTT, and tRF-His-GTG | ↑ | Dub3, Ddr2, Tcstv3 | Paternal low protein diet | Mouse | [99,100,101] |
| tRF5-Gly-CCC, tRF5-Val-TAC, tRF5-Pro-AGG and tRF5-Ser-CGA | ↓ | Wnt/β-catenin | Paternal low protein diet | Mouse | [87] |
| tRF5-Ile-TAT, tRF5-Arg-ACG, and tRF5-SeC-TCA | ↑ | ||||
| tRNA-Pro-AGG-1-2, tRNA-Pro-TGG-1-4, tRNA-Pro-AGG-1-M8 | ↓ (Potentially) | Trim7, Ccdc136 | Exposure to toxicants (herbicide- Vinclozolin, fungicide, jet fuel, pesticide-DDT) | Rat | [85,102,103] |
2.4. RNA Modification
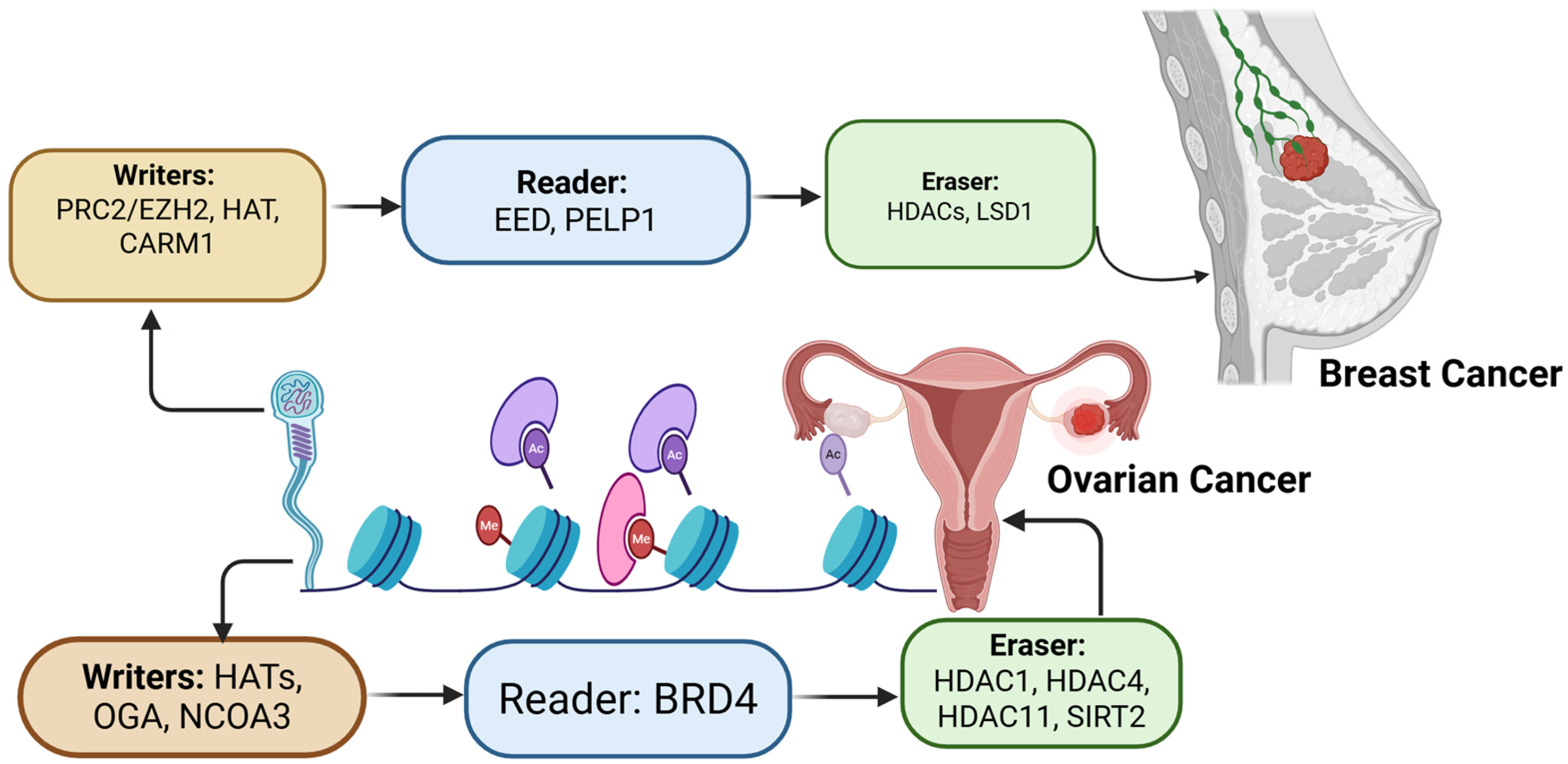
2.5. Epigenetics Regulations in Breast and Ovarian Cancer
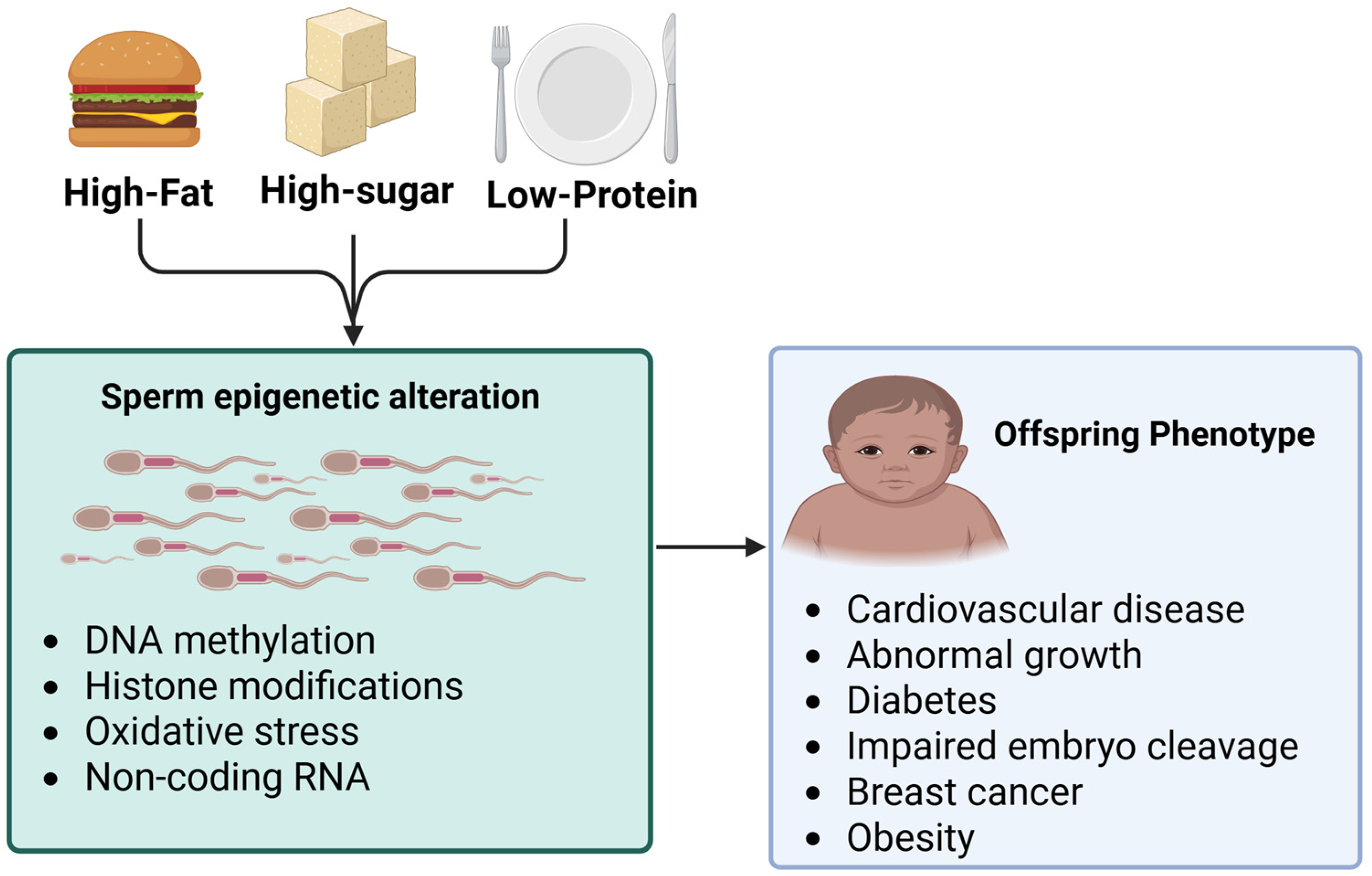
3. Nutrition Impacts on Sperm Epigenetics and Offspring’s Gynecological Cancers
3.1. Macronutrient on Sperm Epigenetics
3.2. Micronutrients and Sperm Epigenetics
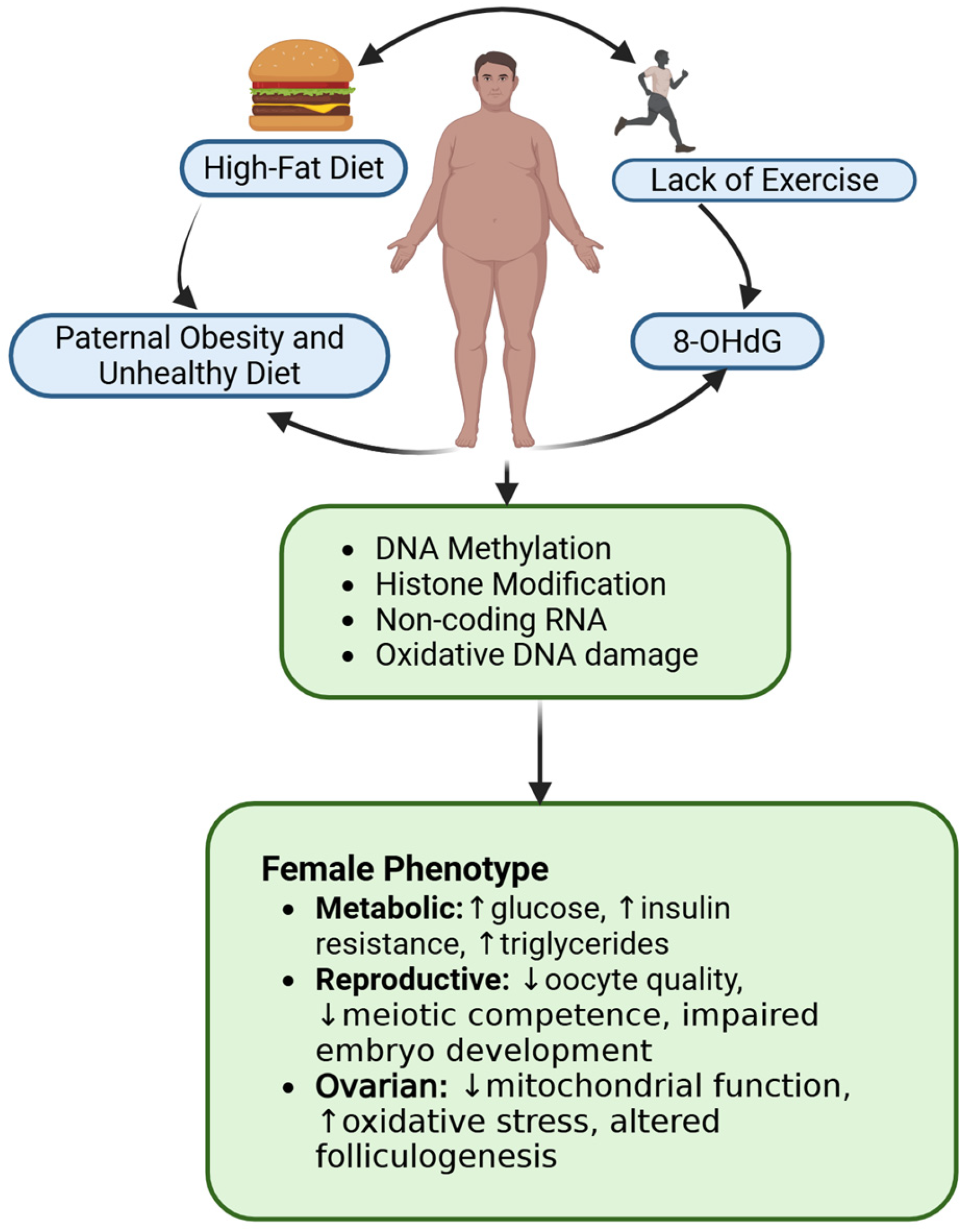
3.3. Influence of Paternal Obesity on Female Reproductive Health
3.4. Phytochemicals Influence Sperm Epigenetics
4. Conclusions
5. Limitation and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANKRD30A | Ankyrin Repeat Domain 30 |
| ACAA2 | Acetyl-CoA Acyltransferase |
| ACSL1 | Acyl-CoA Synthetase Long Chain Family Member 1 |
| Agfg1 | ArfGAP with FG repeats 1 |
| AMPK | Adenosine Monophosphate-activated Protein Kinase |
| BRCA1 and BRCA2 | Breast Cancer Susceptibility gene |
| Bax | BCL2-Associated X |
| Bcl | B Cell Lymphoma Protein |
| BMI1 | Polycomb Ring Finger 1 |
| BPA | Bisphenol A |
| BRD4 | Bromodomain Containing 4 |
| Ccdc136 | Coiled-Coil Domain Containing 136 |
| CARM1 | Coactivator-Associated Arginine Methyltransferase 1 |
| CpG | Cytosine Phosphate Guanine |
| CGI | CpG Island |
| CDK5 | Cyclin-Dependent Kinase 5 |
| CPT1A | Carnitine Palmitoyltransferase 1A |
| Cab39 | Calcium Binding Protein 39 |
| C12orf12 | Coiled-coil Glutamate Rich Protein 1 |
| DDT | Dichlorodiphenyltrichloroethane |
| DHRs | Differential Histone retention |
| DMR | Differentially Methylated Regions |
| DPPA | Developmental Pluripotency Associated gene |
| DNMT | DNA Methyltransferase protein |
| EGCG | Epigallocatechin Gallate |
| ELOVL5 | Fatty Acid Elongase 5 |
| ERK | Extracellular Regulated Kinase |
| ER | Estrogen Receptor |
| EZH2 | Enhancer of Zeste Homolog 2 |
| FLJ40201 | Ciliary Microtubule-associated Protein 2 |
| FTMT | Ferritin Mitochondrial gene |
| GLUT4 | Glucose Transporter 4 |
| GPx-1 | Glutathione Peroxidase 1 |
| HER2 | Human Epidermal Growth Factor 2 |
| HADH | 3-hydroxyacyl-CoA Dehydrogenase |
| HAT | Histone Acetyltransferase |
| HMT | Histone Acetyltransferase |
| HDAC | Histone Deacetylase |
| H3K4me2 | Histone 3 lysine 4 dimethylation |
| H3K18ac | Histone 3 lysine 18 acetylation |
| H3K27me3 | Histone 3 lysine 27 trimethylation |
| H3K9me | Histone 3 lysine 9 methylation |
| HFD | High Fat Diet |
| IGF2 | Insulin-like Growth Factor 2 |
| INSL6 | Insulin Like 6 gene |
| IL6st | Glycoprotein 130 (includes Interleukin 6) |
| IL8 | Interleukin 8 |
| JNK | c-Jun N-terminal Kinase Family Protein |
| KDM1/LSD1 | Lysine-specific Demethylase 1 |
| Kdm6a | Lysine-specific Demethylase 6A |
| lncRNA | Long non-coding RNAs |
| miRNAs | MicroRNA |
| mTOR | Mammalian Target of Rapamycin |
| MAPK | Mitogen Activated Kinase-like Protein |
| MEG3 | Maternally Expressed 3 (long non-coding RNA) |
| MEG3-IG | Maternally Expressed Gene 3-intergenic Differentially Methylated Region |
| m6A | N6-methyladenosine |
| MLL | Mixed-lineage Leukemia |
| MTHFR | Methylenetetrahydrofolate Reductase |
| NANOG | Homeobox Transcription Factor |
| NNAT | Neuronatin |
| Ncl | Nucleolin |
| NCOA3 | Nuclear Receptor Coactivator 3 |
| NRF1 | Nuclear Respiratory Factor 1 |
| Nfe2l2 | Nuclear Factor Erythroid 2-related Factor 2 |
| ncRNA | Non-coding RNA |
| OGA | O-GlcNAcase protein |
| OXSM | 3-oxoacyl-ACP Synthase |
| PRC2 | Polycomb Repressive Complex 2 |
| piRNA | PIWI-interacting |
| PECR | Peroxisomal Trans-2-Enoyl-CoA Reductase |
| PTEN | Phosphatase and Tensin Homolog |
| PI3K | Phosphoinositide 3-Kinase |
| PR | Progesterone Receptor |
| PR+ | Progesterone Receptor-Positive |
| Prkaa2 | Alpha-2 Catalytic Subunit of AMP-activated Protein Kinase |
| PELP1 | Proline-, Glutamic acid-, and Leucine-rich Protein 1 |
| PGC-1α | Peroxisome Proliferator-activated Receptor-gamma Coactivator 1-alpha |
| POHaD | Paternal Origins of Health and Disease |
| RNAPII | RNA Polymerase II |
| Ralbp1 | RalA Binding Protein 1 |
| ROS | Reactive Oxygen Species |
| SOHLH2 | Spermatogenesis and oogenesis specific basic helix-loop-helix 2 |
| SAM | S-adenosylmethionin |
| SAPK | Stress-Activated Protein Kinase |
| SCD | Stearoyl-CoA Desaturase |
| Sirt | Sirtuin |
| SFN | Sulforaphane |
| Srsf2 | Serine and arginine rich splicing factor 2 |
| TFAM | Mitochondrial Transcription Factor A |
| tRFs | tRNA-derived fragments |
| tsRNA | tRNA-derived small RNAs |
| tRNA | Transfer RNA |
| TAC | Total Antioxidant Capacity |
| Trim7 | Tripartite Motif Containing 7 |
| TMC | Total Motile Count |
| Ube3a | Ubiquitin Protein Ligase E3A (E6-AP) Protein |
| Utx | Ubiquitously Transcribed Tetratricopeptide Repeat on Chromosome X |
| Wnt | Wingless-related integration site |
References
- Bhat, S.A.; Majid, S.; Wani, H.A.; Rashid, S. Diagnostic utility of epigenetics in breast cancer—A review. Cancer Treat. Res. Commun. 2019, 19, 100125. [Google Scholar] [CrossRef]
- Chen, Q.W.; Zhu, X.Y.; Li, Y.Y.; Meng, Z.Q. Epigenetic regulation and cancer (Review). Oncol. Rep. 2014, 31, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Dawson, M.A.; Kouzarides, T. Cancer Epigenetics: From Mechanism to Therapy. Cell 2012, 150, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Youness, E. Overview on Epigenetics and Cancer. Clin. Med. Rev. Rep. 2020, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Muyrers-Chen, I.; Paro, R. Epigenetics: Unforeseen regulators in cancer. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2001, 1552, 15–26. [Google Scholar] [CrossRef]
- Larsson, L. Current Concepts of Epigenetics and Its Role in Periodontitis. Curr. Oral Health Rep. 2017, 4, 286–293. [Google Scholar] [CrossRef]
- Berger, S.L.; Kouzarides, T.; Shiekhattar, R.; Shilatifard, A. An operational definition of epigenetics: Figure 1. Genes Dev. 2009, 23, 781–783. [Google Scholar] [CrossRef]
- Guo, K.; Lu, M.; Bi, J.; Yao, T.; Gao, J.; Ren, F.; Zhu, L. Ferroptosis: Mechanism, immunotherapy and role in ovarian cancer. Front. Immunol. 2024, 15, 1410018. [Google Scholar] [CrossRef]
- Sharbatoghli, M.; Vafaei, S.; Aboulkheyr Es, H.; Asadi-Lari, M.; Totonchi, M.; Madjd, Z. Prediction of the treatment response in ovarian cancer: A ctDNA approach. J. Ovarian Res. 2020, 13, 124. [Google Scholar] [CrossRef]
- Ghoneum, A.; Afify, H.; Salih, Z.; Kelly, M.; Said, N. Role of tumor microenvironment in ovarian cancer pathobiology. Oncotarget 2018, 9, 22832–22849. [Google Scholar] [CrossRef]
- Gupta, V.; Yull, F.; Khabele, D. Bipolar Tumor-Associated Macrophages in Ovarian Cancer as Targets for Therapy. Cancers 2018, 10, 366. [Google Scholar] [CrossRef]
- Kozieł, M.J.; Piastowska-Ciesielska, A.W. Estrogens, Estrogen Receptors and Tumor Microenvironment in Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 14673. [Google Scholar] [CrossRef]
- Zhang, W.; Torres-Rojas, C.; Yue, J.; Zhu, B.-M. Adipose-derived stem cells in ovarian cancer progression, metastasis, and chemoresistance. Exp. Biol. Med. 2021, 246, 1810–1815. [Google Scholar] [CrossRef]
- An, Y.; Yang, Q. Tumor-associated macrophage-targeted therapeutics in ovarian cancer. Int. J. Cancer 2020, 149, 21–30. [Google Scholar] [CrossRef]
- Fakhri, N.; Chad, M.A.; Lahkim, M.; Houari, A.; Dehbi, H.; Belmouden, A.; El Kadmiri, N. Risk factors for breast cancer in women: An update review. Med. Oncol. 2022, 39, 197. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.; Pattanayak, S.P. Strategic Developments & Future Perspective on Gene Therapy for Breast Cancer: Role of mTOR and Brk/ PTK6 as Molecular Targets. Curr. Gene Ther. 2020, 20, 237–258. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Gopinath, S.C.B. Molecular Mechanism of Breast Cancer and Predisposition of Mouse Mammary Tumor Virus Propagation Cycle. Curr. Med. Chem. 2025, 32, 2330–2348. [Google Scholar] [CrossRef] [PubMed]
- Soubry, A.; Hoyo, C.; Jirtle, R.L.; Murphy, S.K. A paternal environmental legacy: Evidence for epigenetic inheritance through the male germ line. BioEssays 2014, 36, 359–371. [Google Scholar] [CrossRef]
- Soubry, A. Epigenetics as a Driver of Developmental Origins of Health and Disease: Did We Forget the Fathers? BioEssays 2018, 40, 1700113. [Google Scholar] [CrossRef]
- Tabuchi, T.M.; Rechtsteiner, A.; Jeffers, T.E.; Egelhofer, T.A.; Murphy, C.T.; Strome, S. Caenorhabditis elegans sperm carry a histone-based epigenetic memory of both spermatogenesis and oogenesis. Nat. Commun. 2018, 9, 4310. [Google Scholar] [CrossRef]
- Champroux, A.; Cocquet, J.; Henry-Berger, J.; Drevet, J.R.; Kocer, A. A Decade of Exploring the Mammalian Sperm Epigenome: Paternal Epigenetic and Transgenerational Inheritance. Front. Cell Dev. Biol. 2018, 6, 50. [Google Scholar] [CrossRef]
- Curley, J.P.; Mashoodh, R.; Champagne, F.A. Epigenetics and the origins of paternal effects. Horm. Behav. 2011, 59, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Lismer, A.; Kimmins, S. Emerging evidence that the mammalian sperm epigenome serves as a template for embryo development. Nat. Commun. 2023, 14, 2142. [Google Scholar] [CrossRef] [PubMed]
- Pang, T.Y.; Short, A.K.; Bredy, T.W.; Hannan, A.J. Transgenerational paternal transmission of acquired traits: Stress-induced modification of the sperm regulatory transcriptome and offspring phenotypes. Curr. Opin. Behav. Sci. 2017, 14, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Kaneshiro, K.R.; Rechtsteiner, A.; Strome, S. Sperm-inherited H3K27me3 impacts offspring transcription and development in C. elegans. Nat. Commun. 2019, 10, 1271. [Google Scholar] [CrossRef]
- Feinberg, J.I.; Schrott, R.; Ladd-Acosta, C.; Newschaffer, C.J.; Hertz-Picciotto, I.; Croen, L.A.; Daniele Fallin, M.; Feinberg, A.P.; Volk, H.E. Epigenetic changes in sperm are associated with paternal and child quantitative autistic traits in an autism-enriched cohort. Mol. Psychiatry 2024, 29, 43–53. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S.; Liew, F.F.; Dhawan, V.; Das, B.; Mottola, F.; Slama, P.; Rocco, L.; Roychoudhury, S. Environmental and Genetic Traffic in the Journey from Sperm to Offspring. Biomolecules 2023, 13, 1759. [Google Scholar] [CrossRef]
- Hao, X.; Luo, H.; Krawczyk, M.; Wei, W.; Wang, W.; Wang, J.; Flagg, K.; Hou, J.; Zhang, H.; Yi, S.; et al. DNA methylation markers for diagnosis and prognosis of common cancers. Proc. Natl. Acad. Sci. USA 2017, 114, 7414–7419. [Google Scholar] [CrossRef]
- Mehrmohamadi, M.; Mentch, L.K.; Clark, A.G.; Locasale, J.W. Integrative modelling of tumour DNA methylation quantifies the contribution of metabolism. Nat. Commun. 2016, 7, 13666. [Google Scholar] [CrossRef]
- Aryee, M.J.; Liu, W.; Engelmann, J.C.; Nuhn, P.; Gurel, M.; Haffner, M.C.; Esopi, D.; Irizarry, R.A.; Getzenberg, R.H.; Nelson, W.G.; et al. DNA Methylation Alterations Exhibit Intraindividual Stability and Interindividual Heterogeneity in Prostate Cancer Metastases. Sci. Transl. Med. 2013, 5, ra110–ra169. [Google Scholar] [CrossRef]
- Brocato, J.; Costa, M. Basic mechanics of DNA methylation and the unique landscape of the DNA methylome in metal-induced carcinogenesis. Crit. Rev. Toxicol. 2013, 43, 493–514. [Google Scholar] [CrossRef] [PubMed]
- Klutstein, M.; Nejman, D.; Greenfield, R.; Cedar, H. DNA Methylation in Cancer and Aging. Cancer Res. 2016, 76, 3446–3450. [Google Scholar] [CrossRef] [PubMed]
- Fontelles, C.C.; Carney, E.; Clarke, J.; Nguyen, N.M.; Yin, C.; Jin, L.; Cruz, M.I.; Ong, T.P.; Hilakivi-Clarke, L.; De Assis, S. Paternal overweight is associated with increased breast cancer risk in daughters in a mouse model. Sci. Rep. 2016, 6, 28602. [Google Scholar] [CrossRef] [PubMed]
- Illum, L.R.H.; Bak, S.T.; Lund, S.; Nielsen, A.L. DNA methylation in epigenetic inheritance of metabolic diseases through the male germ line. J. Mol. Endocrinol. 2018, 60, R39–R56. [Google Scholar] [CrossRef]
- Wei, S.H.; Balch, C.; Paik, H.H.; Kim, Y.-S.; Baldwin, R.L.; Liyanarachchi, S.; Li, L.; Wang, Z.; Wan, J.C.; Davuluri, R.V.; et al. Prognostic DNA Methylation Biomarkers in Ovarian Cancer. Clin. Cancer Res. 2006, 12, 2788–2794. [Google Scholar] [CrossRef]
- Samudio-Ruiz, S.L.; Hudson, L.G. Increased DNA methyltransferase activity and DNA methylation following epidermal growth factor stimulation in ovarian cancer cells. Epigenetics 2012, 7, 216–224. [Google Scholar] [CrossRef]
- Fu, M.; Deng, F.; Chen, J.; Fu, L.; Lei, J.; Xu, T.; Chen, Y.; Zhou, J.; Gao, Q.; Ding, H. Current data and future perspectives on DNA methylation in ovarian cancer (Review). Int. J. Oncol. 2024, 64, 62. [Google Scholar] [CrossRef]
- Shen, L.; Kondo, Y.; Guo, Y.; Zhang, J.; Zhang, L.; Ahmed, S.; Shu, J.; Chen, X.; Waterland, R.A.; Issa, J.-P.J. Genome-Wide Profiling of DNA Methylation Reveals a Class of Normally Methylated CpG Island Promoters. PLoS Genet. 2007, 3, e181. [Google Scholar] [CrossRef]
- Uhm, K.-O.; Lee, E.S.; Lee, Y.M.; Kim, H.S.; Park, Y.-N.; Park, S.-H. Aberrant Promoter CpG Islands Methylation of Tumor Suppressor Genes in Cholangiocarcinoma. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2008, 17, 151–157. [Google Scholar] [CrossRef]
- Portela, A.; Liz, J.; Nogales, V.; Setién, F.; Villanueva, A.; Esteller, M. DNA methylation determines nucleosome occupancy in the 5′-CpG islands of tumor suppressor genes. Oncogene 2013, 32, 5421–5428. [Google Scholar] [CrossRef]
- Sharma, S.; De Carvalho, D.D.; Jeong, S.; Jones, P.A.; Liang, G. Nucleosomes Containing Methylated DNA Stabilize DNA Methyltransferases 3A/3B and Ensure Faithful Epigenetic Inheritance. PLoS Genet. 2011, 7, e1001286. [Google Scholar] [CrossRef]
- Shen, L.; Gao, G.; Zhang, Y.; Zhang, H.; Ye, Z.; Huang, S.; Huang, J.; Kang, J. A single amino acid substitution confers enhanced methylation activity of mammalian Dnmt3b on chromatin DNA. Nucleic Acids Res. 2010, 38, 6054–6064. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, M.; Sekiguchi, A.; Terry, M.J.; Oates, A.J.; Miyamoto, Y.; Chuu, Y.H.; Munakata, M.; Sekiya, T. A comprehensive catalog of CpG islands methylated in human lung adenocarcinomas for the identification of tumor suppressor genes. Oncogene 2002, 21, 3804–3813. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Serra, L.; Ballestar, E.; Fraga, M.F.; Alaminos, M.; Setien, F.; Esteller, M. A Profile of Methyl-CpG Binding Domain Protein Occupancy of Hypermethylated Promoter CpG Islands of Tumor Suppressor Genes in Human Cancer. Cancer Res. 2006, 66, 8342–8346. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.D.; Yavorski, J.M.; Mauro, J.A.; Blanck, G. Impact of SNPs on CpG Islands in the MYC and HRAS oncogenes and in a wide variety of tumor suppressor genes: A multi-cancer approach. Cell Cycle 2016, 15, 1572–1578. [Google Scholar] [CrossRef]
- Fukushima, N.; Sato, N.; Ueki, T.; Rosty, C.; Walter, K.M.; Wilentz, R.E.; Yeo, C.J.; Hruban, R.H.; Goggins, M. Aberrant Methylation of Preproenkephalin and p16 Genes in Pancreatic Intraepithelial Neoplasia and Pancreatic Ductal Adenocarcinoma. Am. J. Pathol. 2002, 160, 1573–1581. [Google Scholar] [CrossRef]
- Smith, G.; Carey, F.A.; Beattie, J.; Wilkie, M.J.V.; Lightfoot, T.J.; Coxhead, J.; Garner, R.C.; Steele, R.J.C.; Wolf, C.R. Mutations in APC, Kirsten-ras, and p53—Alternative genetic pathways to colorectal cancer. Proc. Natl. Acad. Sci. USA 2002, 99, 9433–9438. [Google Scholar] [CrossRef]
- Zheng, H.; Momeni, A.; Cedoz, P.-L.; Vogel, H.; Gevaert, O. Whole slide images reflect DNA methylation patterns of human tumors. NPJ Genom. Med. 2020, 5, 11. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, J.; Sidransky, D. DNA methylation markers in colorectal cancer. Cancer Metastasis Rev. 2010, 29, 181–206. [Google Scholar] [CrossRef]
- Jemal, A.; Murray, T.; Ward, E.; Samuels, A.; Tiwari, R.C.; Ghafoor, A.; Feuer, E.J.; Thun, M.J. Cancer Statistics, 2005. CA A Cancer J. Clin. 2005, 55, 10–30. [Google Scholar] [CrossRef]
- Parkin, D.M.; Pisani, P.; Ferlay, J. Global cancer statistics. CA A Cancer J. Clin. 1999, 49, 33–64. [Google Scholar] [CrossRef]
- Munshi, A.; Shafi, G.; Aliya, N.; Jyothy, A. Histone modifications dictate specific biological readouts. J. Genet. Genom. 2009, 36, 75–88. [Google Scholar] [CrossRef]
- Jenuwein, T.; Allis, C.D. Translating the Histone Code. Science 2001, 293, 1074–1080. [Google Scholar] [CrossRef]
- Antoniou, A.C.; Spurdle, A.B.; Sinilnikova, O.M.; Healey, S.; Pooley, K.A.; Schmutzler, R.K.; Versmold, B.; Engel, C.; Meindl, A.; Arnold, N.; et al. Common Breast Cancer-Predisposition Alleles Are Associated with Breast Cancer Risk in BRCA1 and BRCA2 Mutation Carriers. Am. J. Hum. Genet. 2008, 82, 937–948. [Google Scholar] [CrossRef]
- Sun, Y.-S.; Zhao, Z.; Yang, Z.-N.; Xu, F.; Lu, H.-J.; Zhu, Z.-Y.; Shi, W.; Jiang, J.; Yao, P.-P.; Zhu, H.-P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef]
- Pedroza, D.A.; Subramani, R.; Tiula, K.; Do, A.; Rashiraj, N.; Galvez, A.; Chatterjee, A.; Bencomo, A.; Rivera, S.; Lakshmanaswamy, R. Crosstalk between progesterone receptor membrane component 1 and estrogen receptor α promotes breast cancer cell proliferation. Lab. Investig. 2021, 101, 733–744. [Google Scholar] [CrossRef]
- Bernstein, B.E.; Mikkelsen, T.S.; Xie, X.; Kamal, M.; Huebert, D.J.; Cuff, J.; Fry, B.; Meissner, A.; Wernig, M.; Plath, K.; et al. A Bivalent Chromatin Structure Marks Key Developmental Genes in Embryonic Stem Cells. Cell 2006, 125, 315–326. [Google Scholar] [CrossRef]
- Lin, J.C.; Jeong, S.; Liang, G.; Takai, D.; Fatemi, M.; Tsai, Y.C.; Egger, G.; Gal-Yam, E.N.; Jones, P.A. Role of Nucleosomal Occupancy in the Epigenetic Silencing of the MLH1 CpG Island. Cancer Cell 2007, 12, 432–444. [Google Scholar] [CrossRef]
- Schotta, G.; Lachner, M.; Sarma, K.; Ebert, A.; Sengupta, R.; Reuter, G.; Reinberg, D.; Jenuwein, T. A silencing pathway to induce H3-K9 and H4-K20 trimethylation at constitutive heterochromatin. Genes Dev. 2004, 18, 1251–1262. [Google Scholar] [CrossRef]
- Li, B.; Carey, M.; Workman, J.L. The Role of Chromatin during Transcription. Cell 2007, 128, 707–719. [Google Scholar] [CrossRef]
- Heintzman, N.D.; Stuart, R.K.; Hon, G.; Fu, Y.; Ching, C.W.; Hawkins, R.D.; Barrera, L.O.; Van Calcar, S.; Qu, C.; Ching, K.A.; et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 2007, 39, 311–318. [Google Scholar] [CrossRef]
- Heintzman, N.D.; Hon, G.C.; Hawkins, R.D.; Kheradpour, P.; Stark, A.; Harp, L.F.; Ye, Z.; Lee, L.K.; Stuart, R.K.; Ching, C.W.; et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature 2009, 459, 108–112. [Google Scholar] [CrossRef]
- Jones, P.L.; Veenstra, G.J.C.; Wade, P.A.; Vermaak, D.; Kass, S.U.; Landsberger, N.; Strouboulis, J.; Wolffe, A.P. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 1998, 19, 187–191. [Google Scholar] [CrossRef]
- Cedar, H.; Bergman, Y. Linking DNA methylation and histone modification: Patterns and paradigms. Nat. Rev. Genet. 2009, 10, 295–304. [Google Scholar] [CrossRef]
- Sasaki, H.; Matsui, Y. Epigenetic events in mammalian germ-cell development: Reprogramming and beyond. Nat. Rev. Genet. 2008, 9, 129–140. [Google Scholar] [CrossRef]
- Kriaucionis, S.; Heintz, N. The Nuclear DNA Base 5-Hydroxymethylcytosine Is Present in Purkinje Neurons and the Brain. Science 2009, 324, 929–930. [Google Scholar] [CrossRef]
- Illingworth, R.S.; Bird, A.P. CpG islands—‘A rough guide’. FEBS Lett. 2009, 583, 1713–1720. [Google Scholar] [CrossRef]
- Kwon, M.J.; Kim, S.-S.; Choi, Y.-L.; Jung, H.S.; Balch, C.; Kim, S.-H.; Song, Y.-S.; Marquez, V.E.; Nephew, K.P.; Shin, Y.K. Derepression of CLDN3 and CLDN4 during ovarian tumorigenesis is associated with loss of repressive histone modifications. Carcinogenesis 2010, 31, 974–983. [Google Scholar] [CrossRef]
- Maldonado, L.; Hoque, M.O. Epigenomics and Ovarian Carcinoma. Biomark. Med. 2010, 4, 543–570. [Google Scholar] [CrossRef]
- Matei, D.; Nephew, K.P. Epigenetic Attire in Ovarian Cancer: The Emperor’s New Clothes. Cancer Res. 2020, 80, 3775–3785. [Google Scholar] [CrossRef]
- Siklenka, K.; Erkek, S.; Godmann, M.; Lambrot, R.; McGraw, S.; Lafleur, C.; Cohen, T.; Xia, J.G.; Suderman, M.; Hallett, M.; et al. Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science 2015, 350, aab2006. [Google Scholar] [CrossRef]
- Lismer, A.; Siklenka, K.; Lafleur, C.; Dumeaux, V.; Kimmins, S. Sperm histone H3 lysine 4 trimethylation is altered in a genetic mouse model of transgenerational epigenetic inheritance. Nucleic Acids Res. 2020, 48, 11380–11393. [Google Scholar] [CrossRef]
- Ben Maamar, M.; Sadler-Riggleman, I.; Beck, D.; Skinner, M.K. Epigenetic Transgenerational Inheritance of Altered Sperm Histone Retention Sites. Sci. Rep. 2018, 8, 5308. [Google Scholar] [CrossRef]
- Ben Maamar, M.; Sadler-Riggleman, I.; Beck, D.; McBirney, M.; Nilsson, E.; Klukovich, R.; Xie, Y.; Tang, C.; Yan, W.; Skinner, M.K. Alterations in sperm DNA methylation, non-coding RNA expression, and histone retention mediate vinclozolin-induced epigenetic transgenerational inheritance of disease. Environ. Epigenetics 2018, 4, dvy010. [Google Scholar] [CrossRef]
- Lesch, B.J.; Tothova, Z.; Morgan, E.A.; Liao, Z.C.; Bronson, R.T.; Ebert, B.L.; Page, D.C. Intergenerational epigenetic inheritance of cancer susceptibility in mammals. Elife 2019, 8, e39380. [Google Scholar] [CrossRef]
- Kondo, Y. Epigenetic Cross-Talk between DNA Methylation and Histone Modifications in Human Cancers. Yonsei Med. J. 2009, 50, 455. [Google Scholar] [CrossRef]
- Taberlay, P.C.; Jones, P.A. DNA Methylation and Cancer. In Epigenetics and Disease; Springer: Basel, Switzerland, 2011; pp. 1–23. [Google Scholar]
- Haun, W.J.; Springer, N.M. Maternal and paternal alleles exhibit differential histone methylation and acetylation at maize imprinted genes. Plant J. 2008, 56, 903–912. [Google Scholar] [CrossRef]
- Rahman, M.M.; Brane, A.C.; Tollefsbol, T.O. MicroRNAs and Epigenetics Strategies to Reverse Breast Cancer. Cells 2019, 8, 1214. [Google Scholar] [CrossRef]
- Kim, A.; Mo, K.; Kwon, H.; Choe, S.; Park, M.; Kwak, W.; Yoon, H. Epigenetic Regulation in Breast Cancer: Insights on Epidrugs. Epigenomes 2023, 7, 6. [Google Scholar] [CrossRef]
- Connolly, R.; Stearns, V. Epigenetics as a Therapeutic Target in Breast Cancer. J. Mammary Gland. Biol. Neoplasia 2012, 17, 191–204. [Google Scholar] [CrossRef]
- González-Rodríguez, P.; Füllgrabe, J.; Joseph, B. The hunger strikes back: An epigenetic memory for autophagy. Cell Death Differ. 2023, 30, 1404–1415. [Google Scholar] [CrossRef]
- Painter, R.C.; De Rooij, S.R.; Bossuyt, P.M.M.; Osmond, C.; Barker, D.J.P.; Bleker, O.P.; Roseboom, T.J. A possible link between prenatal exposure to famine and breast cancer: A preliminary study. Am. J. Hum. Biol. 2006, 18, 853–856. [Google Scholar] [CrossRef]
- Wilkinson, A.L.; Zorzan, I.; Rugg-Gunn, P.J. Epigenetic regulation of early human embryo development. Cell Stem Cell 2023, 30, 1569–1584. [Google Scholar] [CrossRef]
- McSwiggin, H.; Magalhães, R.; Nilsson, E.E.; Yan, W.; Skinner, M.K. Epigenetic transgenerational inheritance of toxicant exposure-specific non-coding RNA in sperm. Environ. Epigenetics 2024, 10, dvae014. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Z.; Wang, G.; Wang, H.; Zhou, Y.; Zhao, X.; Cheng, C.Y.; Qiao, Y.; Sun, F. Sperm epigenetic alterations contribute to inter- and transgenerational effects of paternal exposure to long-term psychological stress via evading offspring embryonic reprogramming. Cell Discov. 2021, 7, 101. [Google Scholar] [CrossRef]
- Da Cruz, R.S.; Carney, E.J.; Clarke, J.; Cao, H.; Cruz, M.I.; Benitez, C.; Jin, L.; Fu, Y.; Cheng, Z.; Wang, Y.; et al. Paternal malnutrition programs breast cancer risk and tumor metabolism in offspring. Breast Cancer Res. 2018, 20, 99. [Google Scholar] [CrossRef]
- Robles, V.; Valcarce, D.G.; Riesco, M.F. Non-coding RNA regulation in reproduction: Their potential use as biomarkers. Non-Coding RNA Res. 2019, 4, 54–62. [Google Scholar] [CrossRef]
- Rodgers, A.B.; Morgan, C.P.; Leu, N.A.; Bale, T.L. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl. Acad. Sci. USA 2015, 112, 13699–13704. [Google Scholar] [CrossRef]
- Vaz, C.; Kermack, A.J.; Burton, M.; Tan, P.F.; Huan, J.; Yoo, T.P.X.; Donnelly, K.; Wellstead, S.J.; Fisk, H.L.; Houghton, F.D.; et al. Short-term diet intervention alters the small non-coding RNA (sncRNA) landscape of human sperm. bioRxiv 2021. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Deng, L.; Jiang, X.; Zhang, Y.; Lee, L.T.O.; Zhang, H. ACSL1-induced ferroptosis and platinum resistance in ovarian cancer by increasing FSP1 N-myristylation and stability. Cell Death Discov. 2023, 9, 83. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, S.; Su, H.; Meng, Y.; Zang, C.; Ning, P.; Hu, L.; Shao, H. CPT1A-mediated MFF succinylation promotes stemness maintenance in ovarian cancer stem cells. Commun. Biol. 2025, 8, 250. [Google Scholar] [CrossRef]
- Zhao, G.; Tan, Y.; Cardenas, H.; Vayngart, D.; Wang, Y.; Huang, H.; Keathley, R.; Wei, J.-J.; Ferreira, C.R.; Orsulic, S.; et al. Ovarian cancer cell fate regulation by the dynamics between saturated and unsaturated fatty acids. Proc. Natl. Acad. Sci. USA 2022, 119, e2203480119. [Google Scholar] [CrossRef]
- Thomas, R.; Al-Rashed, F.; Akhter, N.; Al-Mulla, F.; Ahmad, R. ACSL1 Regulates TNFα-Induced GM-CSF Production by Breast Cancer MDA-MB-231 Cells. Biomolecules 2019, 9, 555. [Google Scholar] [CrossRef]
- Das, M.; Giannoudis, A.; Sharma, V. The role of CPT1A as a biomarker of breast cancer progression: A bioinformatic approach. Sci. Rep. 2022, 12, 16441. [Google Scholar] [CrossRef]
- Kieu, T.-L.-V.; Pierre, L.; Derangère, V.; Perrey, S.; Truntzer, C.; Jalil, A.; Causse, S.; Groetz, E.; Dumont, A.; Guyard, L.; et al. Downregulation of Elovl5 promotes breast cancer metastasis through a lipid-droplet accumulation-mediated induction of TGF-β receptors. Cell Death Dis. 2022, 13, 758. [Google Scholar] [CrossRef]
- Nikulin, S.; Zakharova, G.; Poloznikov, A.; Raigorodskaya, M.; Wicklein, D.; Schumacher, U.; Nersisyan, S.; Bergquist, J.; Bakalkin, G.; Astakhova, L.; et al. Effect of the Expression of ELOVL5 and IGFBP6 Genes on the Metastatic Potential of Breast Cancer Cells. Front. Genet. 2021, 12, 662843. [Google Scholar] [CrossRef]
- Paris, L.; Giardullo, P.; Leonardi, S.; Tanno, B.; Meschini, R.; Cordelli, E.; Benassi, B.; Longobardi, M.G.; Izzotti, A.; Pulliero, A.; et al. Transgenerational inheritance of enhanced susceptibility to radiation-induced medulloblastoma in newborn Ptch1+/− mice after paternal irradiation. Oncotarget 2015, 6, 36098–36112. [Google Scholar] [CrossRef]
- Sharma, U.; Conine, C.C.; Shea, J.M.; Boskovic, A.; Derr, A.G.; Bing, X.Y.; Belleannee, C.; Kucukural, A.; Serra, R.W.; Sun, F.; et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 2016, 351, 391–396. [Google Scholar] [CrossRef]
- Liu, D.; Wu, C.; Wang, J.; Zhang, L.; Sun, Z.; Chen, S.; Ding, Y.; Wang, W. Transfer RNA-derived fragment 5′tRF-Gly promotes the development of hepatocellular carcinoma by direct targeting of carcinoembryonic antigen-related cell adhesion molecule 1. Cancer Sci. 2022, 113, 3476–3488. [Google Scholar] [CrossRef]
- Chen, F.; Song, C.; Meng, F.; Zhu, Y.; Chen, X.; Fang, X.; Ma, D.; Wang, Y.; Zhang, C. 5′-tRF-GlyGCC promotes breast cancer metastasis by increasing fat mass and obesity-associated protein demethylase activity. Int. J. Biol. Macromol. 2023, 226, 397–409. [Google Scholar] [CrossRef]
- Hernandez-Alias, X.; Benisty, H.; Schaefer, M.H.; Serrano, L. Translational efficiency across healthy and tumor tissues is proliferation-related. Mol. Syst. Biol. 2020, 16, e9275. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Mo, Y.; Ren, D.; Liu, S.; Zeng, Z.; Xiong, W. Transfer RNA-derived small RNAs in tumor microenvironment. Mol. Cancer 2023, 22, 32. [Google Scholar] [CrossRef] [PubMed]
- Schreier, J.; Dietz, S.; Boermel, M.; Oorschot, V.; Seistrup, A.-S.; de Jesus Domingues, A.M.; Bronkhorst, A.W.; Nguyen, D.A.H.; Phillis, S.; Gleason, E.J.; et al. Membrane-associated cytoplasmic granules carrying the Argonaute protein WAGO-3 enable paternal epigenetic inheritance in Caenorhabditis elegans. Nat. Cell Biol. 2022, 24, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Petri, B.J.; Klinge, C.M. m6A readers, writers, erasers, and the m6A epitranscriptome in breast cancer. J. Mol. Endocrinol. 2023, 70, e220110. [Google Scholar] [CrossRef]
- Kumari, K.; Groza, P.; Aguilo, F. Regulatory roles of RNA modifications in breast cancer. NAR Cancer 2021, 3, zcab036. [Google Scholar] [CrossRef]
- Benak, D.; Benakova, S.; Plecita-Hlavata, L.; Hlavackova, M. The role of m6A and m6Am RNA modifications in the pathogenesis of diabetes mellitus. Front. Endocrinol. 2023, 14, 1223583. [Google Scholar] [CrossRef]
- Dai, Q.; Ye, Y. Development and Validation of a Novel Histone Acetylation-Related Gene Signature for Predicting the Prognosis of Ovarian Cancer. Front. Cell Dev. Biol. 2022, 10, 793425. [Google Scholar] [CrossRef]
- Gao, Y.; Tollefsbol, T.O. Impact of Epigenetic Dietary Components on Cancer through Histone Modifications. Curr. Med. Chem. 2015, 22, 2051–2064. [Google Scholar] [CrossRef]
- Lapierre, M.; Linares, A.; Dalvai, M.; Duraffourd, C.; Bonnet, S.; Boulahtouf, A.; Rodriguez, C.; Jalaguier, S.; Assou, S.; Orsetti, B.; et al. Histone deacetylase 9 regulates breast cancer cell proliferation and the response to histone deacetylase inhibitors. Oncotarget 2016, 7, 19693–19708. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, S.; Liao, L.; Chen, X.; Meistrich, M.; Xu, J. Jmjd1a Demethylase-regulated Histone Modification Is Essential for cAMP-response Element Modulator-regulated Gene Expression and Spermatogenesis. J. Biol. Chem. 2010, 285, 2758–2770. [Google Scholar] [CrossRef]
- Brykczynska, U.; Hisano, M.; Erkek, S.; Ramos, L.; Oakeley, E.J.; Roloff, T.C.; Beisel, C.; Schübeler, D.; Stadler, M.B.; Peters, A.H.F.M. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat. Struct. Mol. Biol. 2010, 17, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.; Cortez, V.; Vadlamudi, R. PELP1 oncogenic functions involve CARM1 regulation. Carcinogenesis 2013, 34, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Messier, T.L.; Gordon, J.A.R.; Boyd, J.R.; Tye, C.E.; Browne, G.; Stein, J.L.; Lian, J.B.; Stein, G.S. Histone H3 lysine 4 acetylation and methylation dynamics define breast cancer subtypes. Oncotarget 2016, 7, 5094–5109. [Google Scholar] [CrossRef]
- Falahi, F.; van Kruchten, M.; Martinet, N.; Hospers, G.; Rots, M.G. Current and upcoming approaches to exploit the reversibility of epigenetic mutations in breast cancer. Breast Cancer Res. 2014, 16, 412. [Google Scholar] [CrossRef]
- Fontelles, C.C.; Da Cruz, R.S.; Gonsiewski, A.K.; Barin, E.; Tekmen, V.; Jin, L.; Cruz, M.I.; Loudig, O.; Warri, A.; De Assis, S. Paternal obesity and epigenetic inheritance of breast cancer: The role of systemic effects and transmission to the second generation. bioRxiv 2020. [Google Scholar] [CrossRef]
- Soubry, A.; Murphy, S.K.; Vansant, G.; He, Y.; Price, T.M.; Hoyo, C. Opposing Epigenetic Signatures in Human Sperm by Intake of Fast Food Versus Healthy Food. Front. Endocrinol. 2021, 12, 625204. [Google Scholar] [CrossRef]
- Akhatova, A.; Jones, C.; Coward, K.; Yeste, M. How do lifestyle and environmental factors influence the sperm epigenome? Effects on sperm fertilising ability, embryo development, and offspring health. Clin. Epigenetics 2025, 17, 7. [Google Scholar] [CrossRef]
- Pascoal, G.F.L.; Geraldi, M.V.; Maróstica, M.R., Jr.; Ong, T.P. Effect of Paternal Diet on Spermatogenesis and Offspring Health: Focus on Epigenetics and Interventions with Food Bioactive Compounds. Nutrients 2022, 14, 2150. [Google Scholar] [CrossRef]
- Lambrot, R.; Xu, C.; Saint-Phar, S.; Chountalos, G.; Cohen, T.; Paquet, M.; Suderman, M.; Hallett, M.; Kimmins, S. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat. Commun. 2013, 4, 2889. [Google Scholar] [CrossRef]
- Skoracka, K.; Eder, P.; Łykowska-Szuber, L.; Dobrowolska, A.; Krela-Kaźmierczak, I. Diet and Nutritional Factors in Male (In)fertility-Underestimated Factors. J. Clin. Med. 2020, 9, 1400. [Google Scholar] [CrossRef]
- Bodden, C.; Hannan, A.J.; Reichelt, A.C. Diet-Induced Modification of the Sperm Epigenome Programs Metabolism and Behavior. Trends Endocrinol. Metab. 2020, 31, 131–149. [Google Scholar] [CrossRef]
- Eid, N. Defining the Links Between Paternal Diet, Metabolic Health, and Reproductive Fitness in Mice. Ph.D. Thesis, University of Nottingham (United Kingdom), Nottingham, UK, 2023. [Google Scholar]
- Crisóstomo, L.; Jarak, I.; Rato, L.P.; Raposo, J.F.; Batterham, R.L.; Oliveira, P.F.; Alves, M.G. Inheritable testicular metabolic memory of high-fat diet causes transgenerational sperm defects in mice. Sci. Rep. 2021, 11, 9444. [Google Scholar] [CrossRef] [PubMed]
- Jahan-Mihan, A.; Leftwich, J.; Berg, K.; Labyak, C.; Nodarse, R.R.; Allen, S.; Griggs, J. The Impact of Parental Preconception Nutrition, Body Weight, and Exercise Habits on Offspring Health Outcomes: A Narrative Review. Nutrients 2024, 16, 4276. [Google Scholar] [CrossRef] [PubMed]
- Almujaydil, M.S. The Role of Dietary Nutrients in Male Infertility: A Review. Life 2023, 13, 519. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Sikeli, P.; Lukáčová, J.; Massányi, P.; Lukáč, N. Mineral Nutrients and Male Fertility. J. Microbiol. Biotechnol. Food Sci. 2013, 3, 1–14. [Google Scholar]
- Hoek, J.; Koster, M.P.H.; Schoenmakers, S.; Willemsen, S.P.; Koning, A.H.J.; Steegers, E.A.P.; Steegers-Theunissen, R.P.M. Does the father matter? The association between the periconceptional paternal folate status and embryonic growth. Fertil. Steril. 2019, 111, 270–279. [Google Scholar] [CrossRef]
- Ames, B.N. Micronutrient deficiencies. A major cause of DNA damage. Ann. N. Y. Acad. Sci. 1999, 889, 87–106. [Google Scholar] [CrossRef]
- Billah, M.M.; Khatiwada, S.; Morris, M.J.; Maloney, C.A. Effects of paternal overnutrition and interventions on future generations. Int. J. Obes. 2022, 46, 901–917. [Google Scholar] [CrossRef]
- Du Toit, E.W. Impact of Micro-Nutrient Supplementation on Semen Parameters. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2016. [Google Scholar]
- Erdoğan, K.; Sanlier, N.T.; Sanlier, N. Are epigenetic mechanisms and nutrition effective in male and female infertility? J. Nutr. Sci. 2023, 12, e103. [Google Scholar] [CrossRef]
- Singh, K.; Jaiswal, D. One-carbon metabolism, spermatogenesis, and male infertility. Reprod. Sci. 2013, 20, 622–630. [Google Scholar] [CrossRef]
- Ames, B.N.; Wakimoto, P. Are vitamin and mineral deficiencies a major cancer risk? Nat. Rev. Cancer 2002, 2, 694–704. [Google Scholar] [CrossRef]
- McPherson, N.O.; Shehadeh, H.; Fullston, T.; Zander-Fox, D.L.; Lane, M. Dietary Micronutrient Supplementation for 12 Days in Obese Male Mice Restores Sperm Oxidative Stress. Nutrients 2019, 11, 2196. [Google Scholar] [CrossRef]
- Palmer, N.O.; Bakos, H.W.; Fullston, T.; Lane, M. Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis 2012, 2, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Raad, G.; Hazzouri, M.; Bottini, S.; Trabucchi, M.; Azoury, J.; Grandjean, V. Paternal obesity: How bad is it for sperm quality and progeny health? Basic Clin. Androl. 2017, 27, 20. [Google Scholar] [CrossRef]
- Larqué, C.; Lugo-Martínez, H.; Mendoza, X.; Nochebuena, M.; Novo, L.; Vilchis, R.; Sánchez-Bringas, G.; Ubaldo, L.; Velasco, M.; Escalona, R. Paternal Obesity Induced by High-Fat Diet Impairs the Metabolic and Reproductive Health of Progeny in Rats. Metabolites 2023, 13, 1098. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Yang, L.; Guo, Z.; Yao, N.; Zhang, S.; Pu, P. Obesity and its impact on female reproductive health: Unraveling the connections. Front. Endocrinol. 2024, 14, 1326546. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.R.; Jenkins, T.G.; Carrell, D.T.; Hotaling, J.M. Obesity, male infertility, and the sperm epigenome. Fertil. Steril. 2017, 107, 848–859. [Google Scholar] [CrossRef]
- Fullston, T.; Palmer, N.O.; Owens, J.A.; Mitchell, M.; Bakos, H.W.; Lane, M. Diet-induced paternal obesity in the absence of diabetes diminishes the reproductive health of two subsequent generations of mice. Hum. Reprod. 2012, 27, 1391–1400. [Google Scholar] [CrossRef]
- Sanchez-Garrido, M.A.; Ruiz-Pino, F.; Velasco, I.; Barroso, A.; Fernandois, D.; Heras, V.; Manfredi-Lozano, M.; Vazquez, M.J.; Castellano, J.M.; Roa, J.; et al. Intergenerational Influence of Paternal Obesity on Metabolic and Reproductive Health Parameters of the Offspring: Male-Preferential Impact and Involvement of Kiss1-Mediated Pathways. Endocrinology 2017, 159, 1005–1018. [Google Scholar] [CrossRef]
- Sultan, S.; Patel, A.G.; El-Hassani, S.; Whitelaw, B.; Leca, B.M.; Vincent, R.P.; le Roux, C.W.; Rubino, F.; Aywlin, S.J.B.; Dimitriadis, G.K. Male Obesity Associated Gonadal Dysfunction and the Role of Bariatric Surgery. Front. Endocrinol. 2020, 11, 408. [Google Scholar] [CrossRef]
- Venigalla, G.; Ila, V.; Dornbush, J.; Bernstein, A.; Loloi, J.; Pozzi, E.; Miller, D.; Ramasamy, R. Male obesity: Associated effects on fertility and the outcomes of offspring. Andrology 2025, 13, 64–71. [Google Scholar] [CrossRef]
- Barbouni, K.; Jotautis, V.; Metallinou, D.; Diamanti, A.; Orovou, E.; Liepinaitienė, A.; Nikolaidis, P.; Karampas, G.; Sarantaki, A. When Weight Matters: How Obesity Impacts Reproductive Health and Pregnancy-A Systematic Review. Curr. Obes. Rep. 2025, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Fullston, T.; McPherson, N.O.; Zander-Fox, D.; Lane, M. The most common vices of men can damage fertility and the health of the next generation. J. Endocrinol. 2017, 234, F1–F6. [Google Scholar] [CrossRef] [PubMed]
- McPherson, N.O.; Owens, J.A.; Fullston, T.; Lane, M. Preconception diet or exercise intervention in obese fathers normalizes sperm microRNA profile and metabolic syndrome in female offspring. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E805–E821. [Google Scholar] [CrossRef]
- Ramadan, A.G.; Abdel-Rehim, W.M.; El-Tahan, R.A.; Elblehi, S.S.; Kamel, M.A.; Shaker, S.A. Maternal and paternal obesity differentially reprogram the ovarian mitochondrial biogenesis of F1 female rats. Sci. Rep. 2023, 13, 15480. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, H.; Chen, M.; Tollefsbol, T.O. Paternal Combined Botanicals Contribute to the Prevention of Estrogen Receptor-Negative Mammary Cancer in Transgenic Mice. J. Nutr. 2023, 153, 1959–1973. [Google Scholar] [CrossRef]
- D’Angelo, S.; Scafuro, M.; Meccariello, R. BPA and Nutraceuticals, Simultaneous Effects on Endocrine Functions. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 594–604. [Google Scholar] [CrossRef]
- Divella, R.; Daniele, A.; Savino, E.; Paradiso, A. Anticancer Effects of Nutraceuticals in the Mediterranean Diet: An Epigenetic Diet Model. Cancer Genom. Proteom. 2020, 17, 335–350. [Google Scholar] [CrossRef]
- Montano, L.; Maugeri, A.; Volpe, M.G.; Micali, S.; Mirone, V.; Mantovani, A.; Navarra, M.; Piscopo, M. Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids. Int. J. Mol. Sci. 2022, 23, 1568. [Google Scholar] [CrossRef]

| Micronutrient | Role in Sperm | Deficiency Effects on Sperm | Offspring/Health Consequences | Organism | References |
|---|---|---|---|---|---|
| Folate (B9) | One-carbon metabolism, DNA and histone methylation, nucleotide synthesis | Low sperm count, increase DNA damage | Infertility, congenital malformations | Mice, rat | [119] |
| Vitamin B6/B12 | DNA synthesis, cofactors in homocysteine metabolism | Chromosomal instability, hypomethylation | Altered DNA methylation | Human, mice, rat | [133,134] |
| Vitamin C | Testosterone regulation, antioxidant protection | Low motility and sperm count, DNA oxidation | Protects against smoking-related sperm DNA damage | Human, mice, rat | [132] |
| Vitamin D | Vitamin D receptor in sperm/testis, calcium transfer | Altered morphology, low sperm count | Infertility, interacts with epigenetic regulation | Human, mice, rat | [132] |
| Vitamin E | Protects DNA from ROS, antioxidant | Oxidative DNA damage | Human, mice, rat | [132] | |
| Iron (Fe) | Integral to Heme proteins and support DNA/RNA structure | Impaired Spermatogenesis | Developmental and metabolic risk | Rat | [127] |
| Iodine | Thyroid-dependent spermatogenesis | Testicular atrophy with hypothyroxinemia, decrease motility and sperm count | Rat, goat | [127] | |
| Zinc | Chromatin stability, protamine cross-linking; antioxidant enzymes; transcriptional cofactors | Oxidative damage, decrease motility/morphology, poor chromatin integrity | Offspring cancer risk through germline DNA damage | Rat | [134,135] |
| Selenium | Sperm maturation, Selonoproteins (GPx-1 cofactor) | ROS accumulation, reduces motility | Altered mammary development and increases breast cancer risk in daughters | Mice | [135] |
| Magnesium | Glutathione synthesis | Oxidative DNA damage, energy dysregulation | Rat, goat | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durojaye, T.J.; Ganguly, S.; Li, Y.; Tollefsbol, T.O. Nutrition-Based Paternal Influence on Gynecological Diseases in Female Offspring via Epigenetic Mechanisms. Nutrients 2025, 17, 3690. https://doi.org/10.3390/nu17233690
Durojaye TJ, Ganguly S, Li Y, Tollefsbol TO. Nutrition-Based Paternal Influence on Gynecological Diseases in Female Offspring via Epigenetic Mechanisms. Nutrients. 2025; 17(23):3690. https://doi.org/10.3390/nu17233690
Chicago/Turabian StyleDurojaye, Titilayomi J., Sebanti Ganguly, Yuanyuan Li, and Trygve O. Tollefsbol. 2025. "Nutrition-Based Paternal Influence on Gynecological Diseases in Female Offspring via Epigenetic Mechanisms" Nutrients 17, no. 23: 3690. https://doi.org/10.3390/nu17233690
APA StyleDurojaye, T. J., Ganguly, S., Li, Y., & Tollefsbol, T. O. (2025). Nutrition-Based Paternal Influence on Gynecological Diseases in Female Offspring via Epigenetic Mechanisms. Nutrients, 17(23), 3690. https://doi.org/10.3390/nu17233690








