Effects of Chronic Moderate Alcohol Intake on Metabolic Phenotypes and Gut Microbiota in Lean and Obese Mice with Distinct Dietary Structures
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Health Monitoring and Exclusion Criteria
2.3. Analysis of Biochemical Parameters
2.4. Histological Analysis
2.5. 16S rRNA Gene Sequencing
2.6. Targeted Metabolomics Analysis
2.7. RNA Extraction and Real-Time Quantitative PCR
2.8. Statistical Analyses
3. Results and Discussion
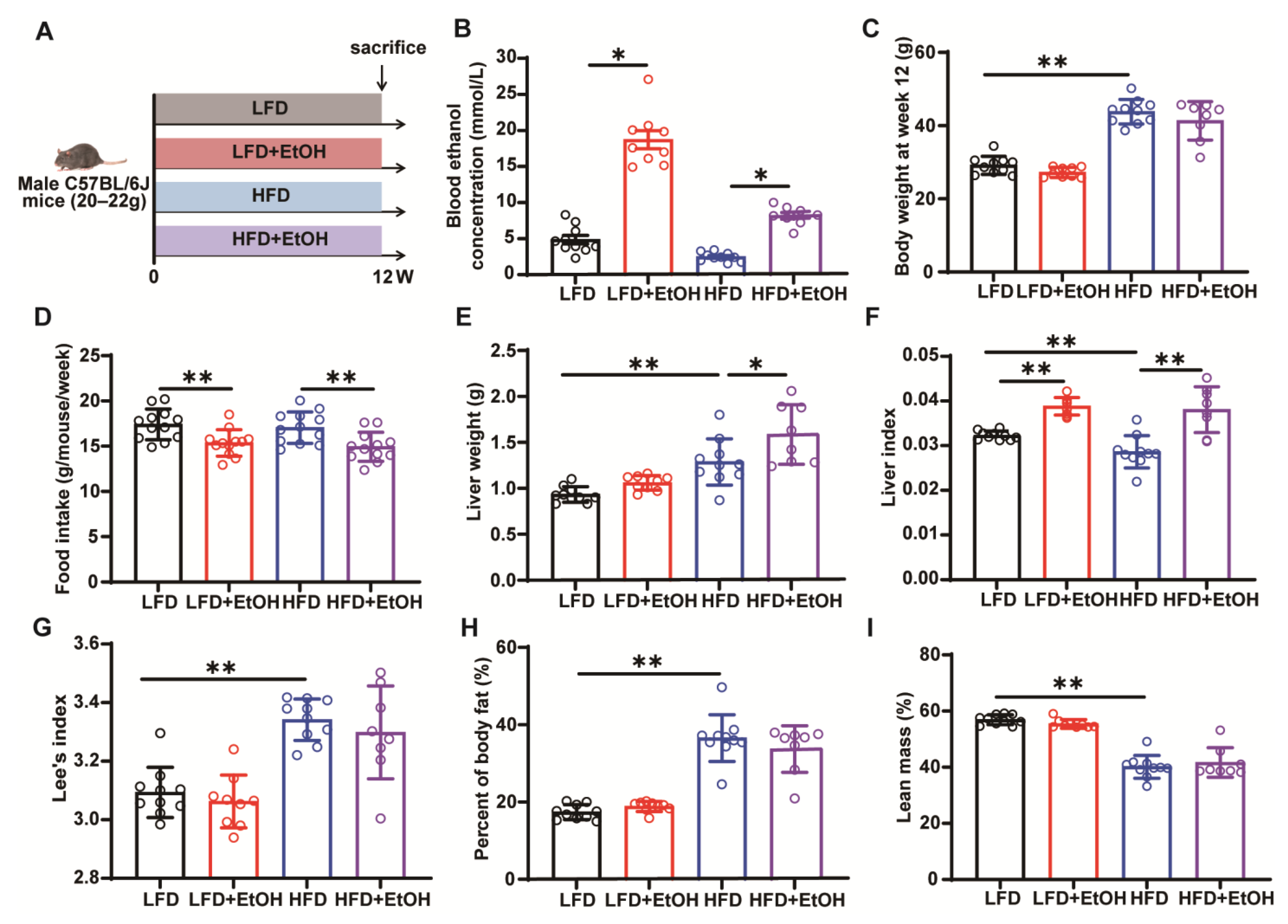
3.1. The Effect of Moderate Alcohol Intake on Basic Phenotypes in LFD or HFD-Fed Mice
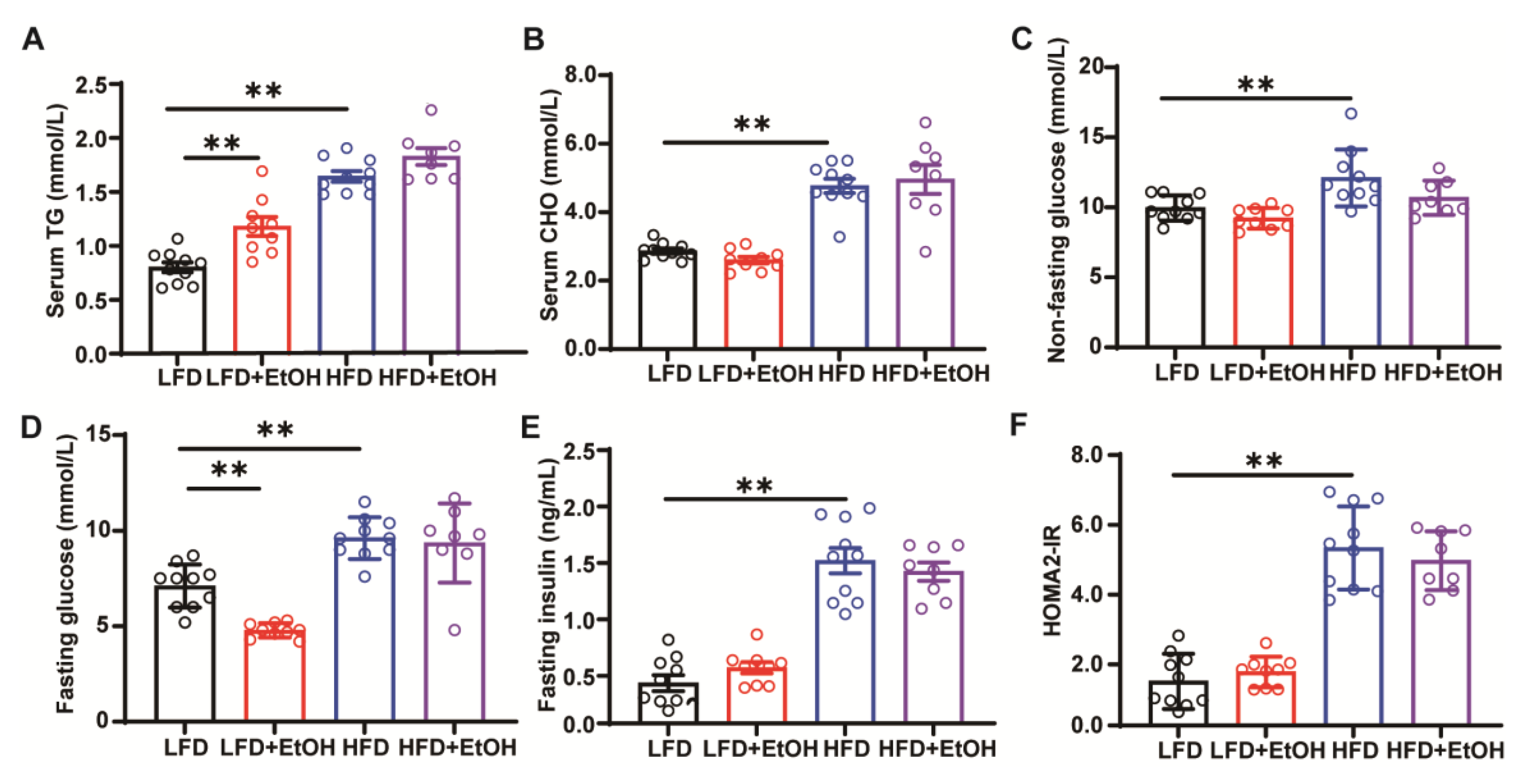
3.2. The Effect of Moderate Alcohol Intake on Hyperlipidemia and Insulin Resistance in LFD or HFD-Fed Mice
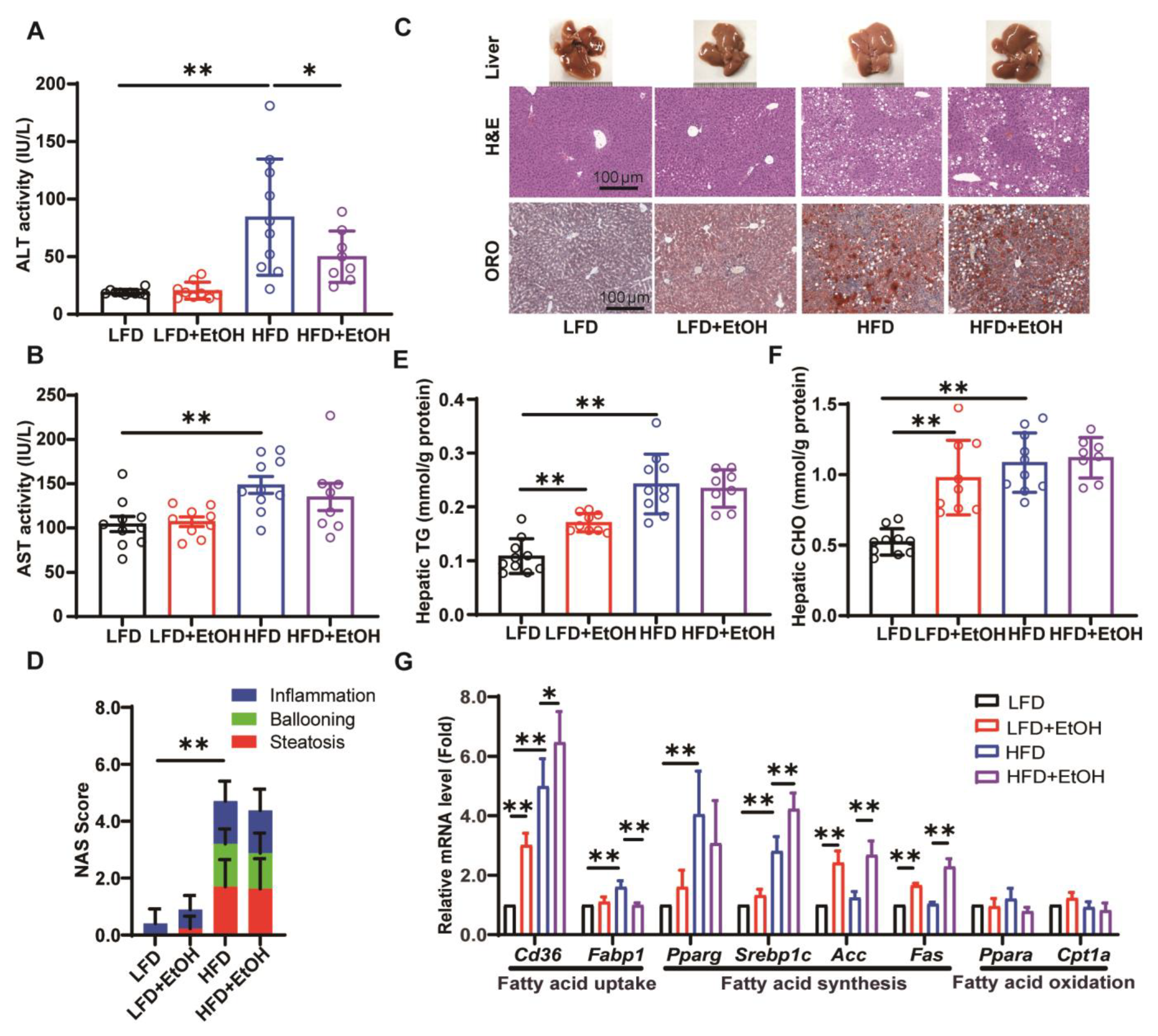
3.3. The Effects of Moderate Alcohol Intake on Hepatic Steatohepatitis and Liver Injury
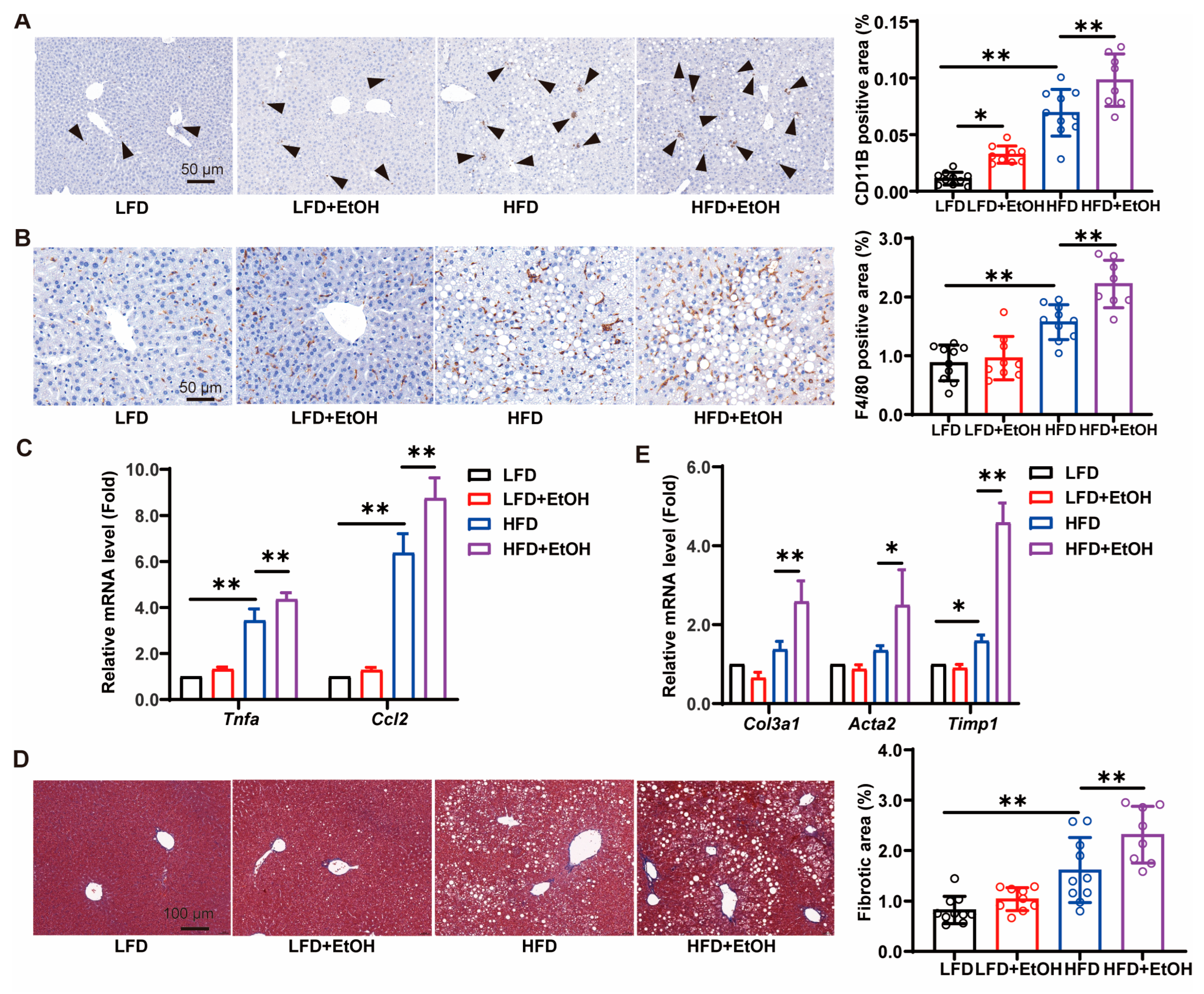
3.4. The Effects of Moderate Alcohol Intake on Inflammation and Fibrosis
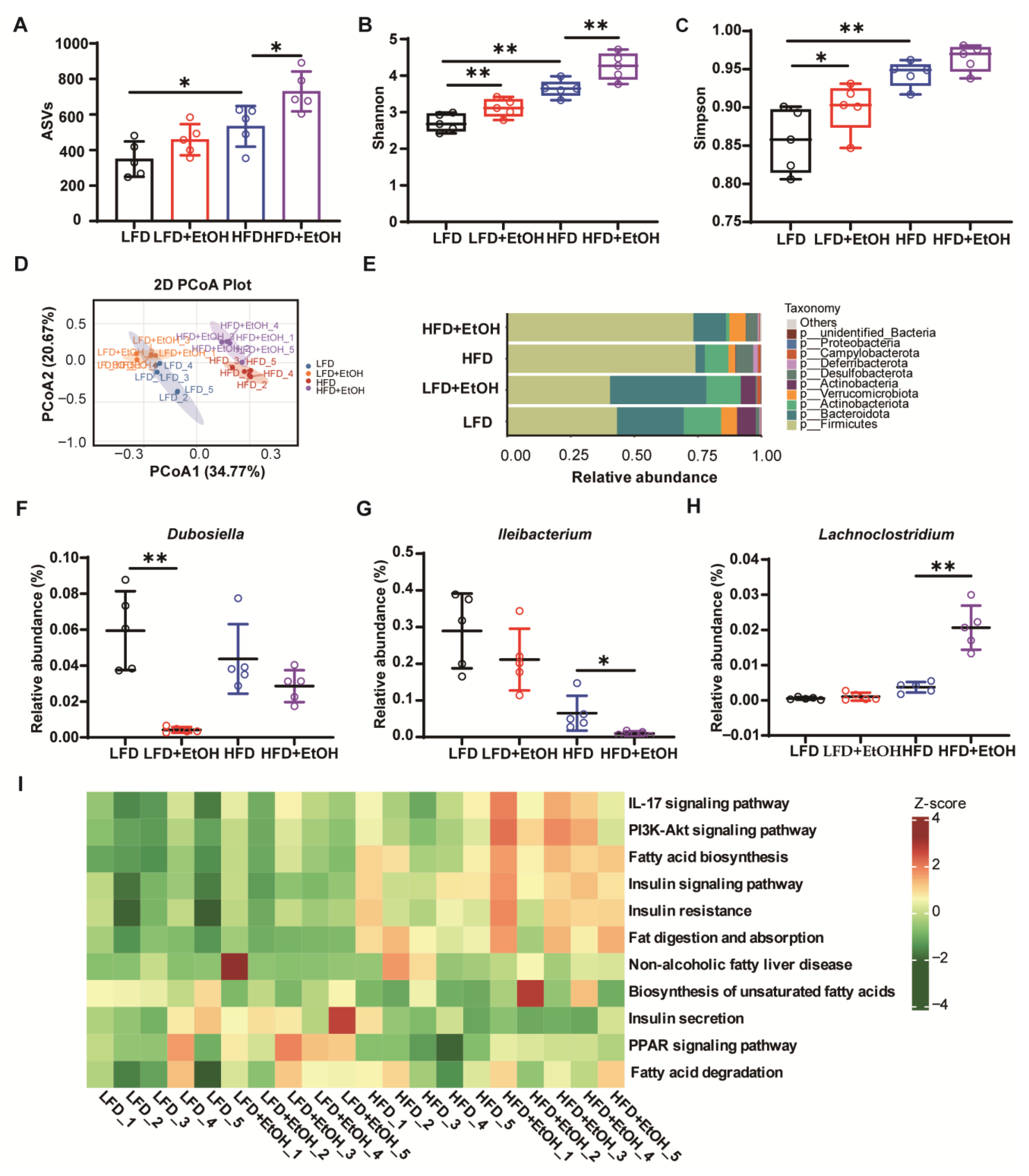
3.5. The Effects of Moderate Alcohol Intake on Gut Microbiota
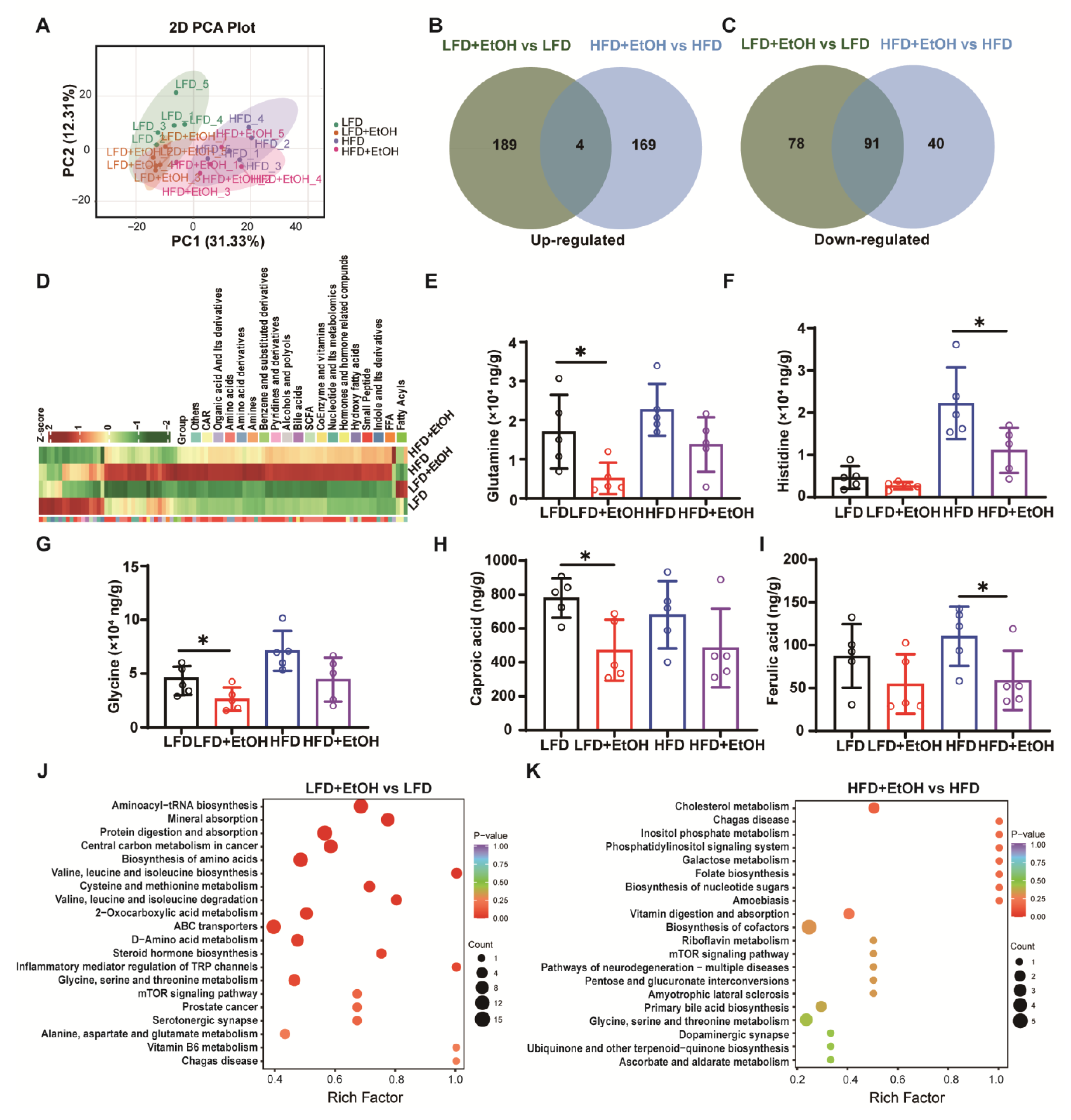
3.6. The Effect of Moderate Alcohol Intake on Gut Metabolites
4. Discussion
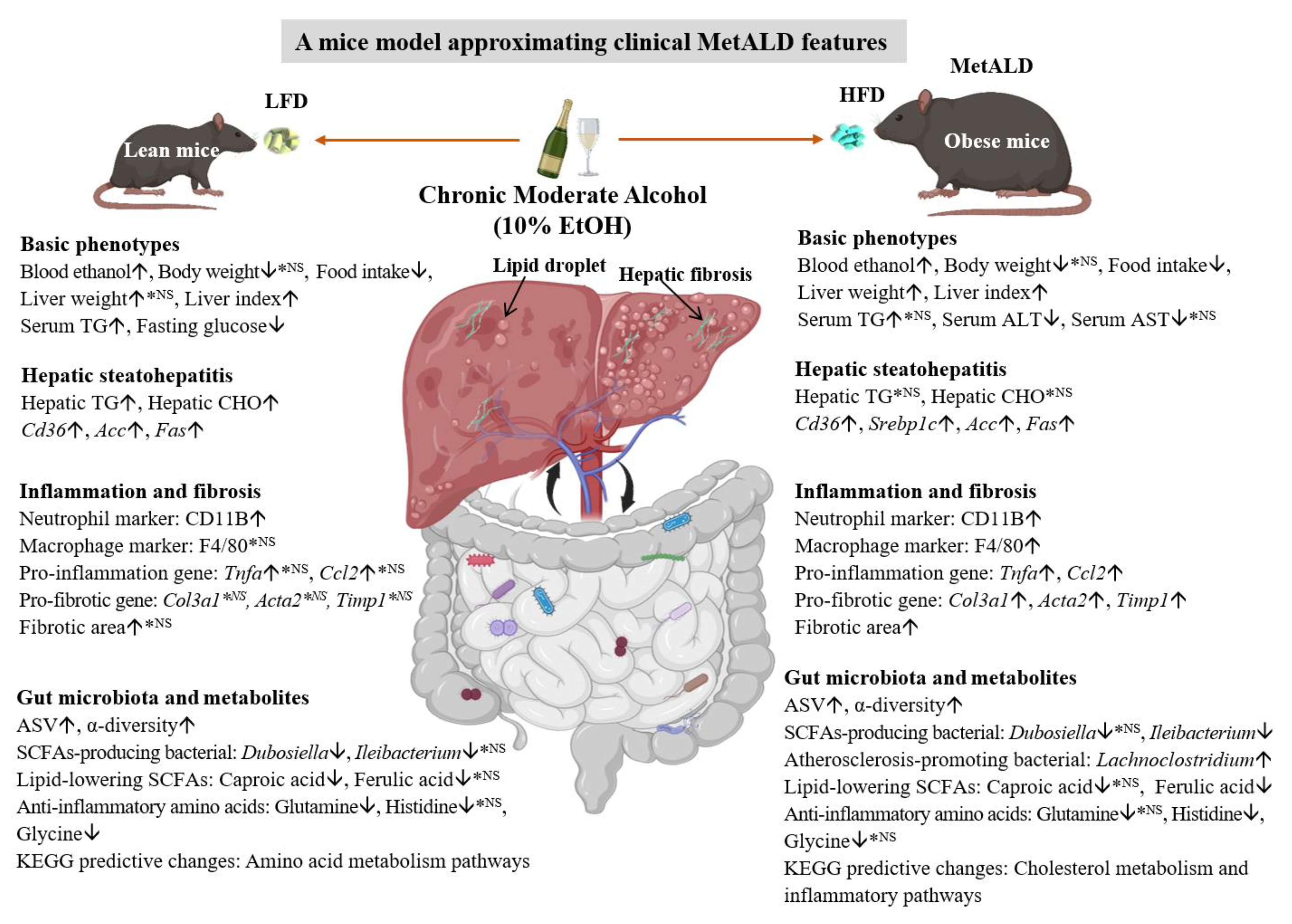
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALD | Alcohol-associated liver disease |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| ASV | Amplicon Sequence Variant |
| CHO | Cholesterol |
| EtOH | Ethanol |
| HFD | High-fat diet |
| HOMA2-IR | Homeostatic Model Assessment 2 of Insulin Resistance |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LFD | Low-fat diet |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MetALD | Metabolic and alcohol-associated liver disease |
| NAFLD | Nonalcoholic fatty liver disease |
| NAS | NAFLD activity score |
| PCA | Principal component analysis |
| PCoA | Principal coordinates analysis |
| SCFA | Short-chain fatty acid |
| SLD | Steatotic liver disease |
| TG | Triglyceride |
References
- Gripshover, T.C.; Treves, R.S.; Hardesty, J.E. Identification of Preclinical Biomarkers of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) versus MASLD and Increased Alcohol Intake and the Impact of Diet. Am. J. Pathol. 2025, in press. [Google Scholar] [CrossRef]
- Sikhwal, S.; Gripshover, T.C.; Treves, R.S.; Hardesty, J.E. Alcohol Preference Impacts Multi-Organ Transcriptome in MetALD. Genes 2025, 16, 1121. [Google Scholar] [CrossRef]
- Targher, G.; Valenti, L.; Byrne, C.D. Metabolic Dysfunction-Associated Steatotic Liver Disease. N. Engl. J. Med. 2025, 393, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Desalegn, H.; Farias, R.; Hudson, D.; Idalsoaga, F.; Cabrera, D.; Diaz, L.A.; Arab, J.P. Prevention and control of risk factors in metabolic and alcohol-associated steatotic liver disease. Metab. Target Organ Damage 2024, 4, 25. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- Basa, M.L.; Cha, D.S.; Mitchell, D.P.; Chan, D.L. Metabolic bariatric surgery, alcohol misuse and liver cirrhosis: A narrative review. Metab. Target Organ Damage 2024, 4, 29. [Google Scholar] [CrossRef]
- Bucher, S.; Begriche, K.; Catheline, D.; Trak-Smayra, V.; Tiaho, F.; Coulouarn, C.; Pinon, G.; Lagadic-Gossmann, D.; Rioux, V.; Fromenty, B. Moderate chronic ethanol consumption exerts beneficial effects on nonalcoholic fatty liver in mice fed a high-fat diet: Possible role of higher formation of triglycerides enriched in monounsaturated fatty acids. Eur. J. Nutr. 2020, 59, 1619–1632. [Google Scholar] [CrossRef]
- Vornoli, A.; Souid, A.; Lazzari, B.; Turri, F.; Pizzi, F.; Bramanti, E.; Campanella, B.; Trouki, C.; Raffaelli, A.; Wojcik, M.; et al. A Moderate Intake of Beer Improves Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) in a High-Fat Diet (HFD)-Induced Mouse Model. Molecules 2024, 29, 5954. [Google Scholar] [CrossRef] [PubMed]
- Buyco, D.G.; Martin, J.; Jeon, S.; Hooks, R.; Lin, C.; Carr, R. Experimental models of metabolic and alcoholic fatty liver disease. World J. Gastroenterol. 2021, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Andaloro, S.; Mancuso, F.; Miele, L.; Addolorato, G.; Gasbarrini, A.; Ponziani, F.R. Effect of Low-Dose Alcohol Consumption on Chronic Liver Disease. Nutrients 2024, 16, 613. [Google Scholar] [CrossRef]
- Boyle, M.; Masson, S.; Anstee, Q.M. The bidirectional impacts of alcohol consumption and the metabolic syndrome: Cofactors for progressive fatty liver disease. J. Hepatol. 2018, 68, 251–267. [Google Scholar] [CrossRef]
- Babuta, M.; Morel, C.; de Carvalho Ribeiro, M.; Datta, A.A.; Calenda, C.; Copeland, C.; Nasser, I.; Szabo, G. A novel experimental model of MetALD in male mice recapitulates key features of severe alcohol-associated hepatitis. Hepatol. Commun. 2024, 8, e0450. [Google Scholar] [CrossRef]
- Chang, B.; Xu, M.J.; Zhou, Z.; Cai, Y.; Li, M.; Wang, W.; Feng, D.; Bertola, A.; Wang, H.; Kunos, G.; et al. Short- or long-term high-fat diet feeding plus acute ethanol binge synergistically induce acute liver injury in mice: An important role for CXCL1. Hepatology 2015, 62, 1070–1085. [Google Scholar] [CrossRef]
- Hwang, S.; Ren, T.; Gao, B. Obesity and binge alcohol intake are deadly combination to induce steatohepatitis: A model of high-fat diet and binge ethanol intake. Clin. Mol. Hepatol. 2020, 26, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Chao, X.; Ni, H.M.; Ding, W.X. An Update on Animal Models of Alcohol-Associated Liver Disease. Am. J. Pathol. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Briand, F.; Dubroca, C.; Wettstein, G.; Grasset, E.; Breyner, N.; Bigot, C.; Assaly, R.; Broqua, P.; Sulpice, T. Lanifibranor and semaglutide demonstrate multiple metabolic benefits in free-choice diet induced obese hamster models of MASH and MetALD. Eur. J. Pharmacol. 2025, 1003, 177945. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2005. Available online: https://www.fda.gov/media/72309/download (accessed on 30 May 2025).
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Jiang, J.; Li, H.; Tang, M.; Lei, L.; Li, H.Y.; Dong, B.; Li, J.R.; Wang, X.K.; Sun, H.; Li, J.Y.; et al. Upregulation of Hepatic Glutathione S-Transferase Alpha 1 Ameliorates Metabolic Dysfunction-Associated Steatosis by Degrading Fatty Acid Binding Protein 1. Int. J. Mol. Sci. 2024, 25, 5086. [Google Scholar] [CrossRef]
- Li, H.; Liu, N.N.; Li, J.R.; Dong, B.; Wang, M.X.; Tan, J.L.; Wang, X.K.; Jiang, J.; Lei, L.; Li, H.Y.; et al. Combined Use of Bicyclol and Berberine Alleviates Mouse Nonalcoholic Fatty Liver Disease. Front. Pharmacol. 2022, 13, 843872. [Google Scholar] [CrossRef]
- Lei, L.; Li, H.; Wang, X.K.; Li, J.R.; Sun, H.; Li, H.Y.; Li, J.Y.; Tang, M.; Xu, J.C.; Dong, B.; et al. Tubulointerstitial nephritis antigen-like 1 promotes the progression of liver fibrosis after HCV eradication with direct-acting antivirals. Int. J. Biol. Sci. 2025, 21, 802–822. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Li, H.; Dong, B.; Jiang, J.; Liu, N.; Tan, J.; Wang, X.; Lei, L.; Li, H.; et al. Down-Regulating the High Level of 17-Beta-Hydroxysteroid Dehydrogenase 13 Plays a Therapeutic Role for Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 5544. [Google Scholar] [CrossRef]
- Kanuri, G.; Landmann, M.; Priebs, J.; Spruss, A.; Loscher, M.; Ziegenhardt, D.; Rohl, C.; Degen, C.; Bergheim, I. Moderate alcohol consumption diminishes the development of non-alcoholic fatty liver disease (NAFLD) in ob/ob mice. Eur. J. Nutr. 2016, 55, 1153–1164. [Google Scholar] [CrossRef]
- Osaki, A.; Okazaki, Y.; Kimoto, A.; Izu, H.; Kato, N. Beneficial effect of a low dose of ethanol on liver function and serum urate in rats fed a high-fat diet. J. Nutr. Sci. Vitaminol. 2014, 60, 408–412. [Google Scholar] [CrossRef]
- Huang, M.; Kim, H.G.; Zhong, X.; Dong, C.; Zhang, B.; Fang, Z.; Zhang, Y.; Lu, X.; Saxena, R.; Liu, Y.; et al. Sestrin 3 Protects Against Diet-Induced Nonalcoholic Steatohepatitis in Mice Through Suppression of Transforming Growth Factor beta Signal Transduction. Hepatology 2020, 71, 76–92. [Google Scholar] [CrossRef]
- Agus, A.; Clement, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Tillotson, G.; MacKenzie, T.N.; Warren, C.A.; Wexler, H.M.; Goldstein, E.J.C. Bacteroides and related species: The keystone taxa of the human gut microbiota. Anaerobe 2024, 85, 102819. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef]
- Chavoya-Guardado, M.A.; Vasquez-Garibay, E.M.; Ruiz-Quezada, S.L.; Ramirez-Cordero, M.I.; Larrosa-Haro, A.; Castro-Albarran, J. Firmicutes, Bacteroidetes and Actinobacteria in Human Milk and Maternal Adiposity. Nutrients 2022, 14, 2887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tu, S.; Ji, X.; Wu, J.; Meng, J.; Gao, J.; Shao, X.; Shi, S.; Wang, G.; Qiu, J.; et al. Dubosiella newyorkensis modulates immune tolerance in colitis via the L-lysine-activated AhR-IDO1-Kyn pathway. Nat. Commun. 2024, 15, 1333. [Google Scholar] [CrossRef]
- Qiu, J.; Cheng, Y.; Deng, Y.; Ren, G.; Wang, J. Composition of gut microbiota involved in alleviation of dexamethasone-induced muscle atrophy by whey protein. NPJ Sci. Food 2023, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cao, X.; Fang, X.; Guo, A.; Li, E. Inhibitory effects of fermented Ougan (Citrus reticulata cv. Suavissima) juice on high-fat diet-induced obesity associated with white adipose tissue browning and gut microbiota modulation in mice. Food Funct. 2021, 12, 9300–9314. [Google Scholar] [CrossRef]
- Wu, C.; Fei, J.; Xu, Q.; Tao, Y.; Zhou, Z.; Wang, Y.; Wu, J.; Gu, H.F. Interaction between Plasma Metabolomics and Intestinal Microbiome in db/db Mouse, an Animal Model for Study of Type 2 Diabetes and Diabetic Kidney Disease. Metabolites 2022, 12, 775. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.Y.; Huang, F.Q.; Lao, X.; Lu, Y.; Gao, X.; Alolga, R.N.; Yin, K.; Zhou, X.; Wang, Y.; Liu, B.; et al. Integrated metagenomics identifies a crucial role for trimethylamine-producing Lachnoclostridium in promoting atherosclerosis. NPJ Biofilms Microbiomes 2022, 8, 11. [Google Scholar] [CrossRef]
- Petrus, P.; Lecoutre, S.; Dollet, L.; Wiel, C.; Sulen, A.; Gao, H.; Tavira, B.; Laurencikiene, J.; Rooyackers, O.; Checa, A.; et al. Glutamine Links Obesity to Inflammation in Human White Adipose Tissue. Cell Metab. 2020, 31, 375–390.e11. [Google Scholar] [CrossRef] [PubMed]
- Schemitt, E.G.; Hartmann, R.M.; Colares, J.R.; Licks, F.; Salvi, J.O.; Marroni, C.A.; Marroni, N.P. Protective action of glutamine in rats with severe acute liver failure. World J. Hepatol. 2019, 11, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, K.K.; Shukla, P.K.; Mir, H.; Manda, B.; Gangwar, R.; Yadav, N.; McMullen, M.; Nagy, L.E.; Rao, R. Glutamine supplementation attenuates ethanol-induced disruption of apical junctional complexes in colonic epithelium and ameliorates gut barrier dysfunction and fatty liver in mice. J. Nutr. Biochem. 2016, 27, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Chen, Y.H.; Pratama, S.A.; Chen, Y.L.; Shirakawa, H.; Peng, H.C.; Yang, S.C. The Prophylactic Effects of Glutamine on Muscle Protein Synthesis and Degradation in Rats with Ethanol-Induced Liver Damage. Nutrients 2021, 13, 2788. [Google Scholar] [CrossRef]
- Hasegawa, S.; Ichiyama, T.; Sonaka, I.; Ohsaki, A.; Okada, S.; Wakiguchi, H.; Kudo, K.; Kittaka, S.; Hara, M.; Furukawa, S. Cysteine, histidine and glycine exhibit anti-inflammatory effects in human coronary arterial endothelial cells. Clin. Exp. Immunol. 2012, 167, 269–274. [Google Scholar] [CrossRef]
- Ikeda, T.; Takii, K.; Omichi, Y.; Nishimoto, Y.; Ichikawa, D.; Matsunaga, T.; Kawauchi, A.; Kimura, I. Hexanoic Acid Improves Metabolic Health in Mice Fed High-Fat Diet. Nutrients 2025, 17, 2868. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, T.; Fu, Y.; Yu, T.; Ding, Y.; Nie, H. Ferulic Acid: A Review of Pharmacology, Toxicology, and Therapeutic Effects on Pulmonary Diseases. Int. J. Mol. Sci. 2023, 24, 8011. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, Y.; Li, M.; Huang, Z.; Jiang, J.; Chen, Y.; Chen, J.; Jia, Y.; Zhang, L.; Zhou, F. Ferulic Acid Ameliorates Atherosclerotic Injury by Modulating Gut Microbiota and Lipid Metabolism. Front. Pharmacol. 2021, 12, 621339. [Google Scholar] [CrossRef]
- Llamoza-Torres, C.J.; Fuentes-Pardo, M.; Ramos-Molina, B. Metabolic dysfunction-associated steatotic liver disease: A key factor in hepatocellular carcinoma therapy response. Metab. Target Organ Damage 2024, 4, 40. [Google Scholar] [CrossRef]
- Sanal, M.G.; Gish, R.G.; Méndez-Sánchez, N.; Yu, M.-L.; Chan, W.-K.; Wei, L.; Grønbæk, H.; Zheng, M.; George, J. NAFLD to MAFLD: Collaboration, not confusion—Rethinking the naming of fatty liver disease. Metab. Target Organ Damage 2024, 4, 45. [Google Scholar] [CrossRef]
- Ciardullo, S.; Perseghin, G. From NAFLD to MAFLD and MASLD: A tale of alcohol, stigma and metabolic dysfunction. Metab. Target Organ Damage 2024, 4, 30. [Google Scholar] [CrossRef]
- Nasr, P.; Jönsson, C.; Ekstedt, M.; Kechagias, S. Non-metabolic causes of steatotic liver disease. Metab. Target Organ Damage 2023, 3, 19. [Google Scholar] [CrossRef]
- Ting, P.S.; Lin, W.T.; Liangpunsakul, S.; Novack, M.; Huang, C.K.; Lin, H.Y.; Tseng, T.S.; Chen, P.H. Convergence of Alcohol Consumption and Dietary Quality in US Adults Who Currently Drink Alcohol: An Analysis of Two Core Risk Factors of Liver Disease. Nutrients 2024, 16, 3866. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, S.; Lee, Y.; Kwon, Y.J.; Lee, J.W. Higher Adherence to the Mediterranean Diet Is Associated with a Lower Risk of Steatotic, Alcohol-Related, and Metabolic Dysfunction-Associated Steatotic Liver Disease: A Retrospective Analysis. Nutrients 2024, 16, 3551. [Google Scholar] [CrossRef]
- Coker, C.R.; Aguilar, E.A.; Snyder, A.E.; Bingaman, S.S.; Graziane, N.M.; Browning, K.N.; Arnold, A.C.; Silberman, Y. Access schedules mediate the impact of high fat diet on ethanol intake and insulin and glucose function in mice. Alcohol 2020, 86, 45–56. [Google Scholar] [CrossRef]
- Lin, C.Y.; Omoscharka, E.; Liu, Y.; Cheng, K. Establishment of a Rat Model of Alcoholic Liver Fibrosis with Simulated Human Drinking Patterns and Low-Dose Chemical Stimulation. Biomolecules 2023, 13, 1293. [Google Scholar] [CrossRef] [PubMed]
- Huby, T.; Gautier, E.L. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat. Rev. Immunol. 2022, 22, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Szabo, G. Two Faces of Neutrophils in Liver Disease Development and Progression. Hepatology 2021, 74, 503–512. [Google Scholar] [CrossRef]
- McClain, C.J.; Song, Z.; Barve, S.S.; Hill, D.B.; Deaciuc, I. Recent advances in alcoholic liver disease. IV. Dysregulated cytokine metabolism in alcoholic liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G497–G502. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek, E.; Goluch, Z.; Bochniak, M. Vaccinium spp. Berries in the Prevention and Treatment of Non-Alcoholic Fatty Liver Disease: A Comprehensive Update of Preclinical and Clinical Research. Nutrients 2024, 16, 2940. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.K.; Tang, M.; Lei, L.; Li, J.R.; Sun, H.; Jiang, J.; Dong, B.; Li, H.Y.; Jiang, J.D.; et al. Bacteroides thetaiotaomicron ameliorates mouse hepatic steatosis through regulating gut microbial composition, gut-liver folate and unsaturated fatty acids metabolism. Gut Microbes 2024, 16, 2304159. [Google Scholar] [CrossRef]
- Mamun, M.A.A.; Rakib, A.; Mandal, M.; Singh, U.P. Impact of a High-Fat Diet on the Gut Microbiome: A Comprehensive Study of Microbial and Metabolite Shifts During Obesity. Cells 2025, 14, 463. [Google Scholar] [CrossRef]
- Xiao, L.; Sonne, S.B.; Feng, Q.; Chen, N.; Xia, Z.; Li, X.; Fang, Z.; Zhang, D.; Fjaere, E.; Midtbo, L.K.; et al. High-fat feeding rather than obesity drives taxonomical and functional changes in the gut microbiota in mice. Microbiome 2017, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Kosnicki, K.L.; Penprase, J.C.; Cintora, P.; Torres, P.J.; Harris, G.L.; Brasser, S.M.; Kelley, S.T. Effects of moderate, voluntary ethanol consumption on the rat and human gut microbiome. Addict. Biol. 2019, 24, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Brigagao Pacheco da Silva, C.; Nascimento-Silva, E.A.; Zaramela, L.S.; da Costa, B.R.B.; Rodrigues, V.F.; De Martinis, B.S.; Carlos, D.; Tostes, R.C. Drinking pattern and sex modulate the impact of ethanol consumption on the mouse gut microbiome. Physiol. Genom. 2025, 57, 179–194. [Google Scholar] [CrossRef]
- Peterson, V.L.; Jury, N.J.; Cabrera-Rubio, R.; Draper, L.A.; Crispie, F.; Cotter, P.D.; Dinan, T.G.; Holmes, A.; Cryan, J.F. Drunk bugs: Chronic vapour alcohol exposure induces marked changes in the gut microbiome in mice. Behav. Brain Res. 2017, 323, 172–176. [Google Scholar] [CrossRef]
- Ciocan, D.; Voican, C.S.; Wrzosek, L.; Hugot, C.; Rainteau, D.; Humbert, L.; Cassard, A.M.; Perlemuter, G. Bile acid homeostasis and intestinal dysbiosis in alcoholic hepatitis. Aliment. Pharmacol. Ther. 2018, 48, 961–974. [Google Scholar] [CrossRef]
- Walter, J.; Britton, R.A.; Roos, S. Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4645–4652. [Google Scholar] [CrossRef]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef]
- Aguiar, A.S.; Da-Silva, V.A.; Boaventura, G.T. Can calories from ethanol contribute to body weight preservation by malnourished rats? Braz. J. Med. Biol. Res. 2004, 37, 841–846. [Google Scholar] [CrossRef]
- Yeomans, M.R. Effects of alcohol on food and energy intake in human subjects: Evidence for passive and active over-consumption of energy. Br. J. Nutr. 2004, 92 (Suppl. 1), S31–S34. [Google Scholar] [CrossRef] [PubMed]
- Badaoui, A.; De Saeger, C.; Duchemin, J.; Gihousse, D.; de Timary, P.; Starkel, P. Alcohol dependence is associated with reduced plasma and fundic ghrelin levels. Eur. J. Clin. Investig. 2008, 38, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Wren, A.M.; Seal, L.J.; Cohen, M.A.; Brynes, A.E.; Frost, G.S.; Murphy, K.G.; Dhillo, W.S.; Ghatei, M.A.; Bloom, S.R. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 2001, 86, 5992. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.M.; Wang, R.Q.; Fu, N. Peroxisome proliferator-activated receptor alpha, a potential therapeutic target for alcoholic liver disease. World J. Gastroenterol. 2014, 20, 8055–8060. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.-J.; Nian, Z.-D.; Li, N.; Wang, T.; Sun, H.; Tang, M.; Li, J.-R.; Dong, B.; Xu, J.-C.; Gong, Y.; et al. Effects of Chronic Moderate Alcohol Intake on Metabolic Phenotypes and Gut Microbiota in Lean and Obese Mice with Distinct Dietary Structures. Nutrients 2025, 17, 3658. https://doi.org/10.3390/nu17233658
Gao J-J, Nian Z-D, Li N, Wang T, Sun H, Tang M, Li J-R, Dong B, Xu J-C, Gong Y, et al. Effects of Chronic Moderate Alcohol Intake on Metabolic Phenotypes and Gut Microbiota in Lean and Obese Mice with Distinct Dietary Structures. Nutrients. 2025; 17(23):3658. https://doi.org/10.3390/nu17233658
Chicago/Turabian StyleGao, Jiu-Jiao, Zi-Die Nian, Ning Li, Tong Wang, Han Sun, Mei Tang, Jian-Rui Li, Biao Dong, Jing-Chen Xu, Yue Gong, and et al. 2025. "Effects of Chronic Moderate Alcohol Intake on Metabolic Phenotypes and Gut Microbiota in Lean and Obese Mice with Distinct Dietary Structures" Nutrients 17, no. 23: 3658. https://doi.org/10.3390/nu17233658
APA StyleGao, J.-J., Nian, Z.-D., Li, N., Wang, T., Sun, H., Tang, M., Li, J.-R., Dong, B., Xu, J.-C., Gong, Y., Liu, X.-Y., Jiang, J.-D., Li, H., & Peng, Z.-G. (2025). Effects of Chronic Moderate Alcohol Intake on Metabolic Phenotypes and Gut Microbiota in Lean and Obese Mice with Distinct Dietary Structures. Nutrients, 17(23), 3658. https://doi.org/10.3390/nu17233658




