Folic Acid Combined with Melatonin Might Prevent Hepatic Steatosis by Alleviating Endoplasmic Reticulum Stress to Promote Lipid Droplet Lipolysis in High-Fat Diet-Fed Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Animals
2.3. Experimental Design
2.4. Serum Biochemical Analysis
2.5. Hepatic Biochemical Analysis
2.6. Histology Analysis
2.7. Immunohistochemistry
2.8. Western Blot
2.9. Statistical Analysis
3. Results
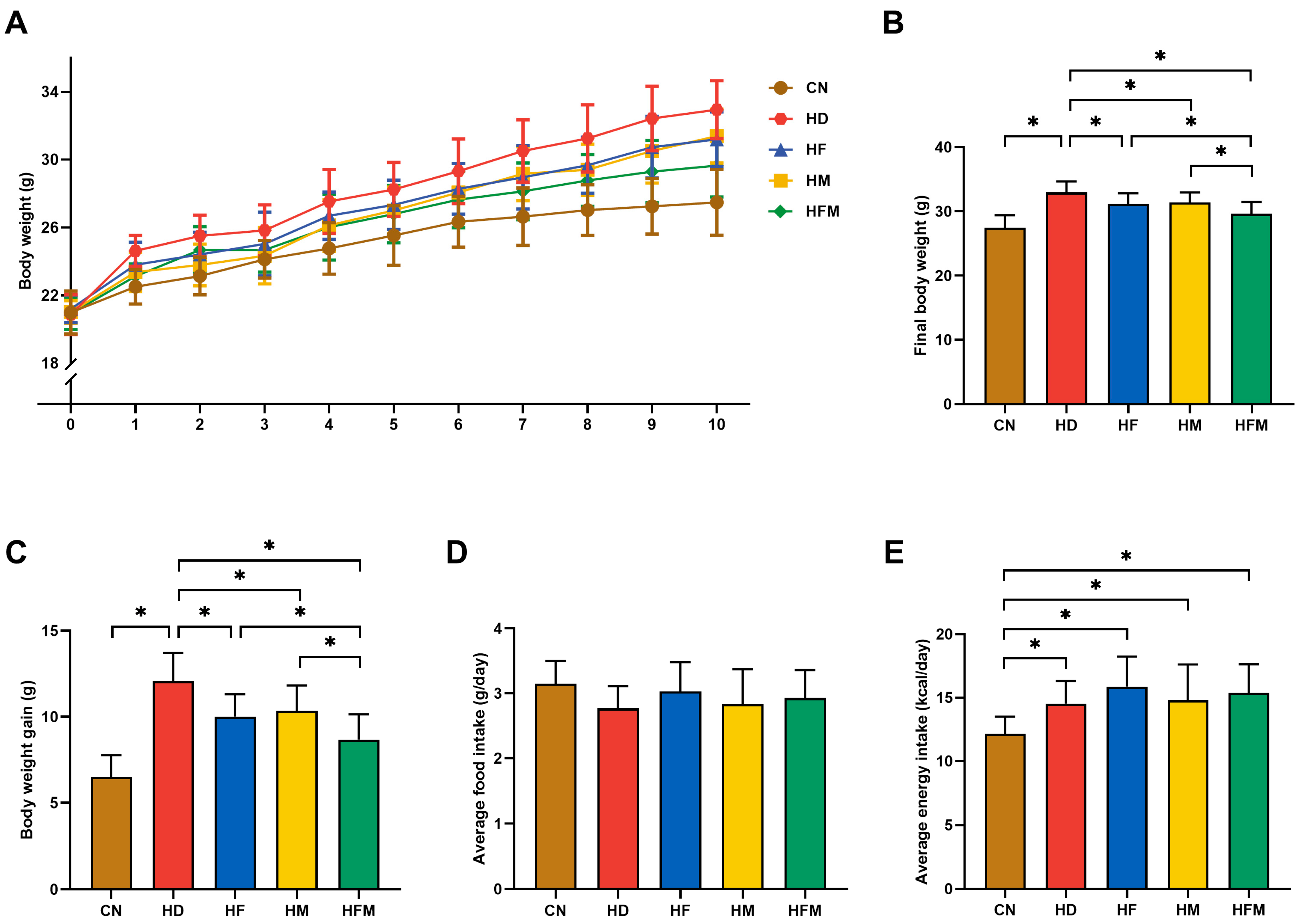
3.1. Effects of FA Combined with MLT on Body Weight and Food Intake
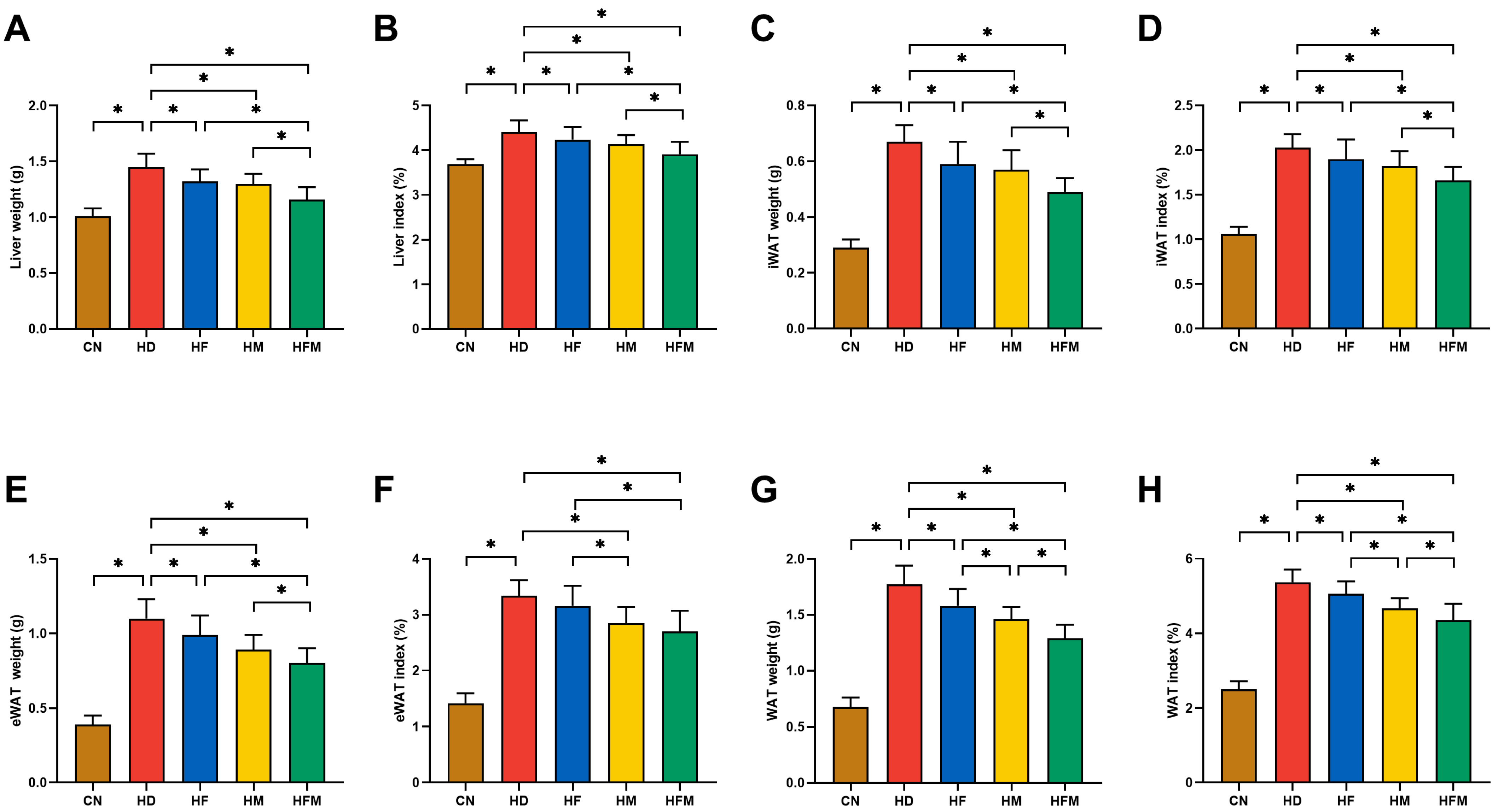
3.2. Effects of FA Combined with MLT on Liver Index and Adipose Index
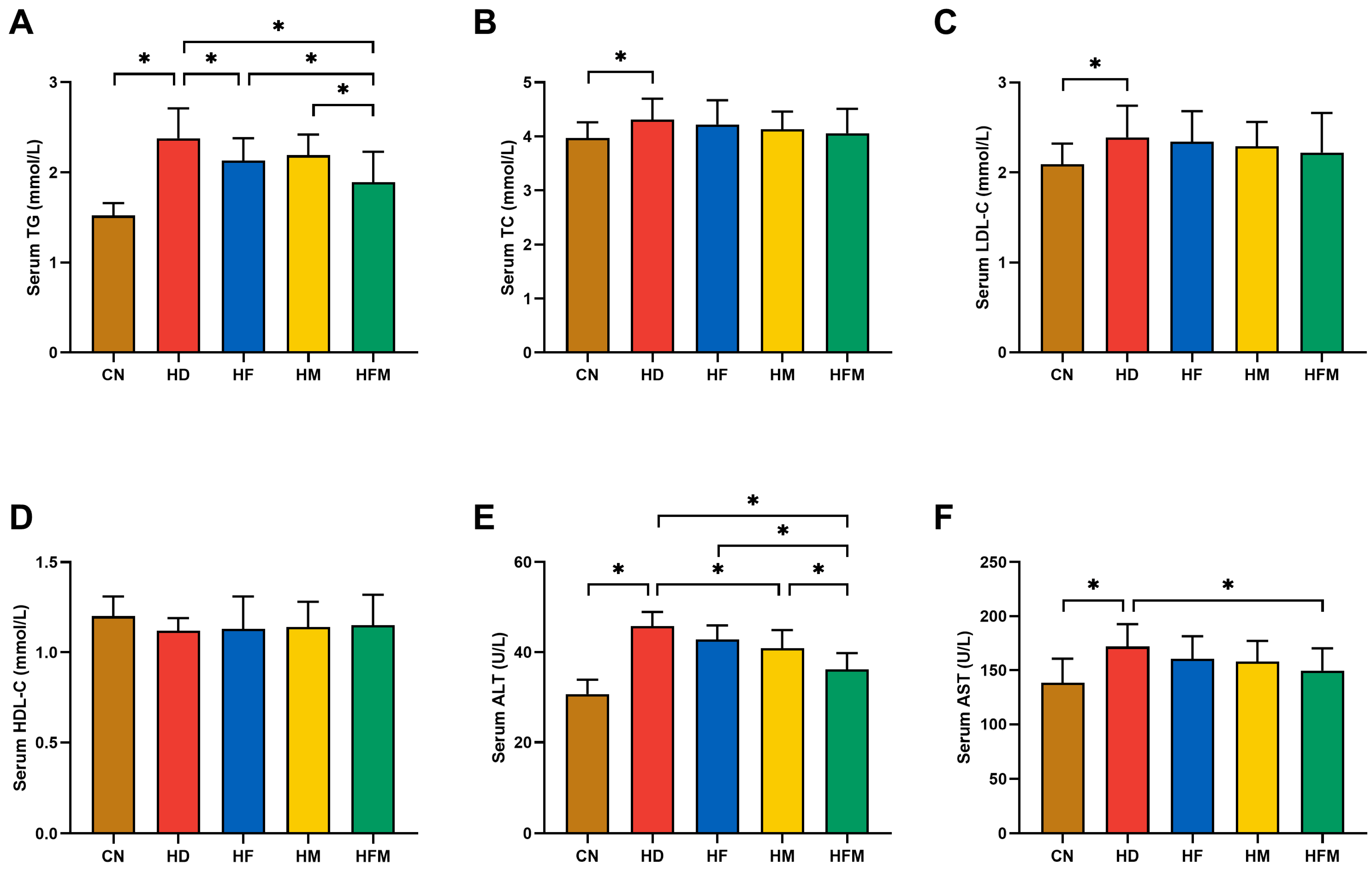
3.3. Effects of FA Combined with MLT on Serological Indicators
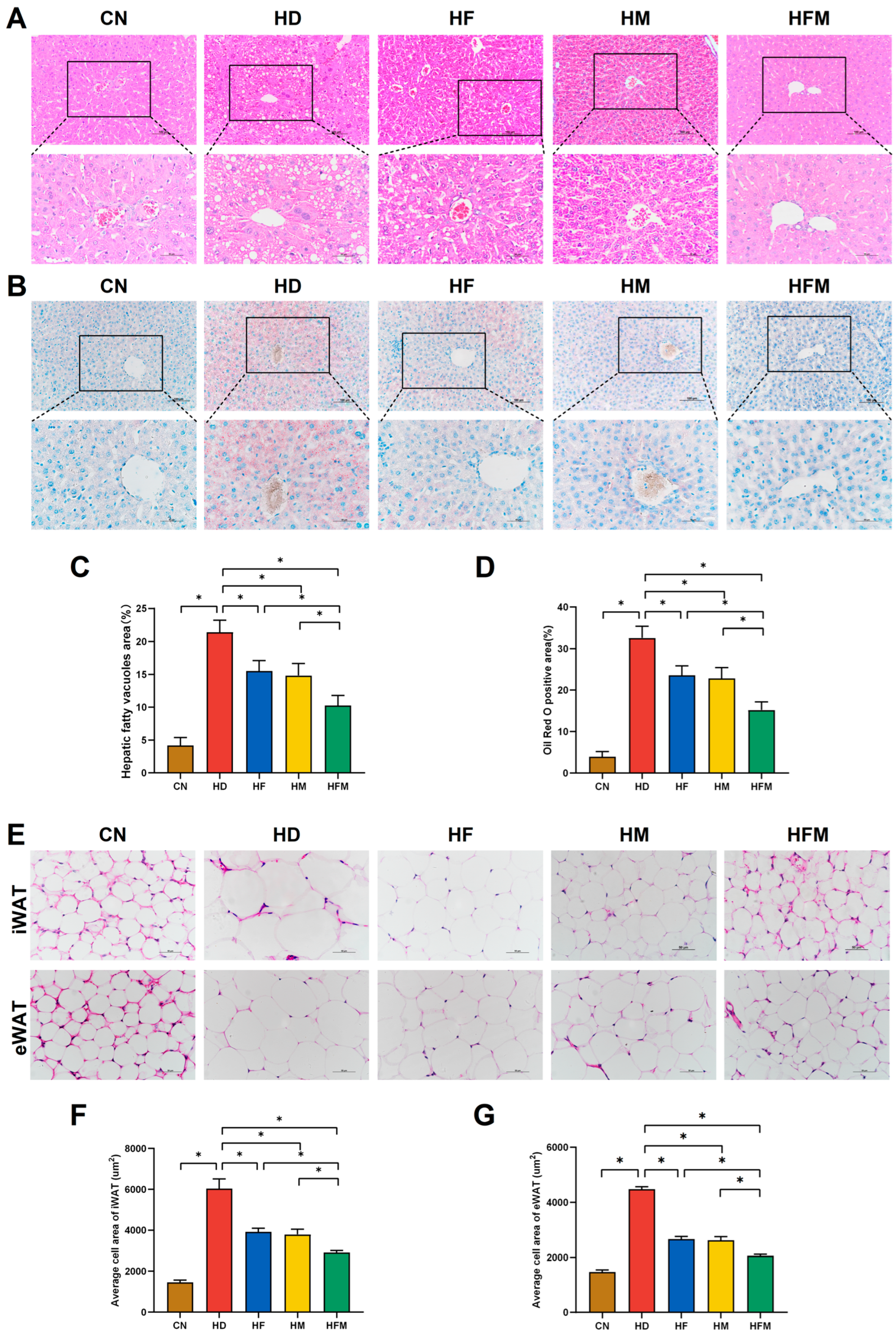
3.4. Effects of FA Combined with MLT on Pathological Changes of Hepatic and Adipose Tissues
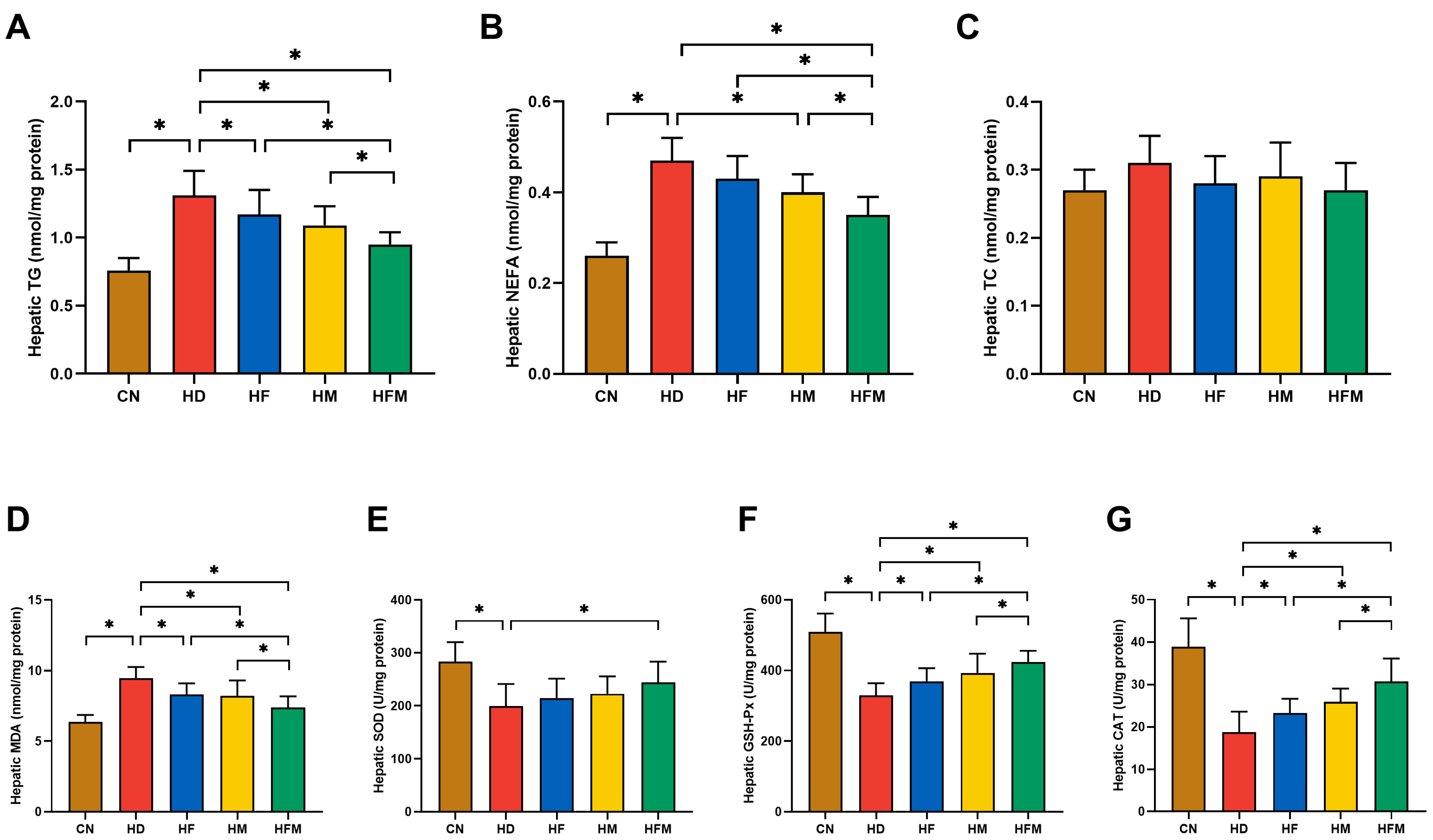
3.5. Effects of FA Combined with MLT on Hepatic Lipid Levels and Oxidative Stress Levels
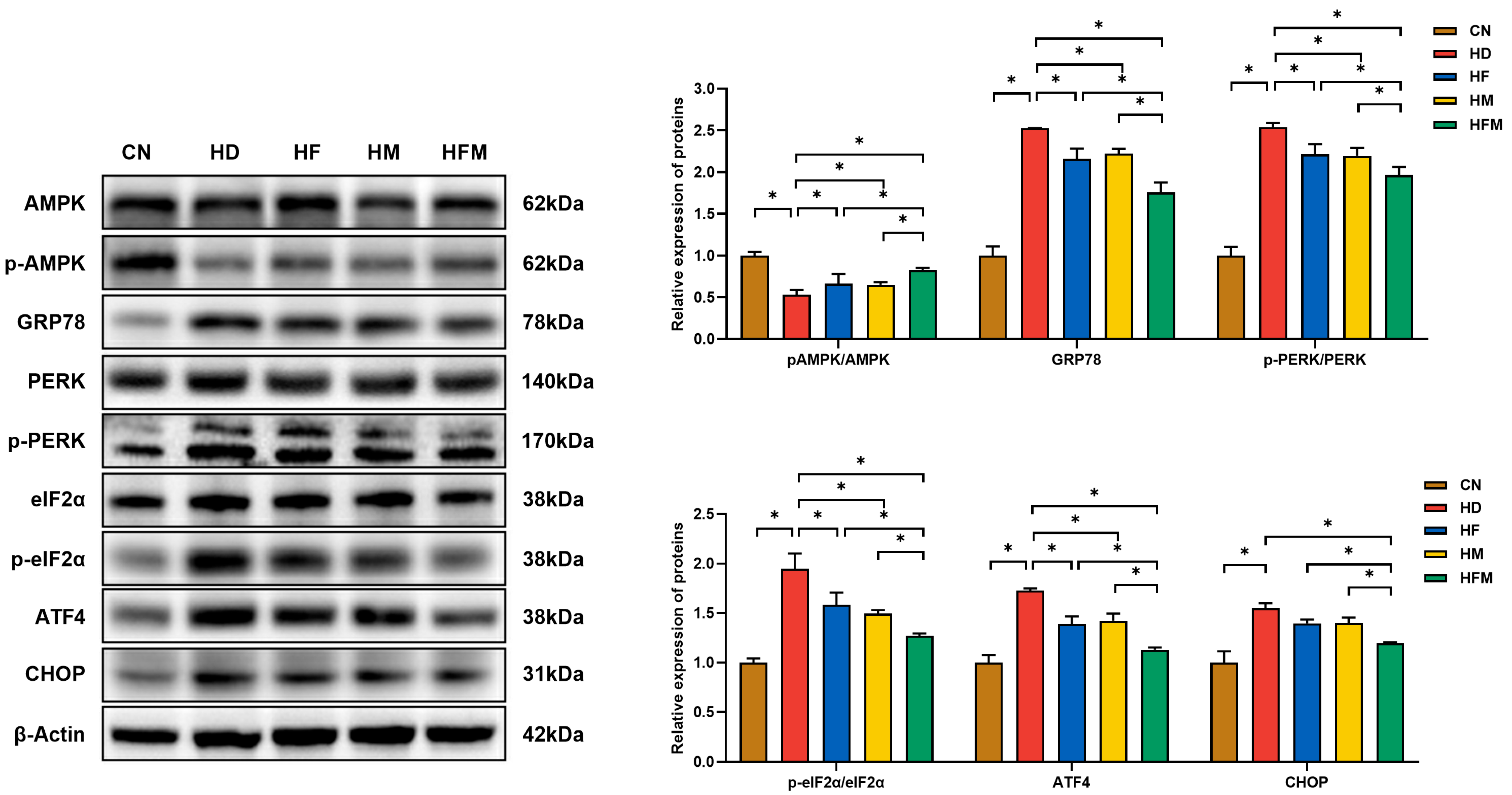
3.6. Effects of FA Combined with MLT on the Expression of Proteins Related to ERS
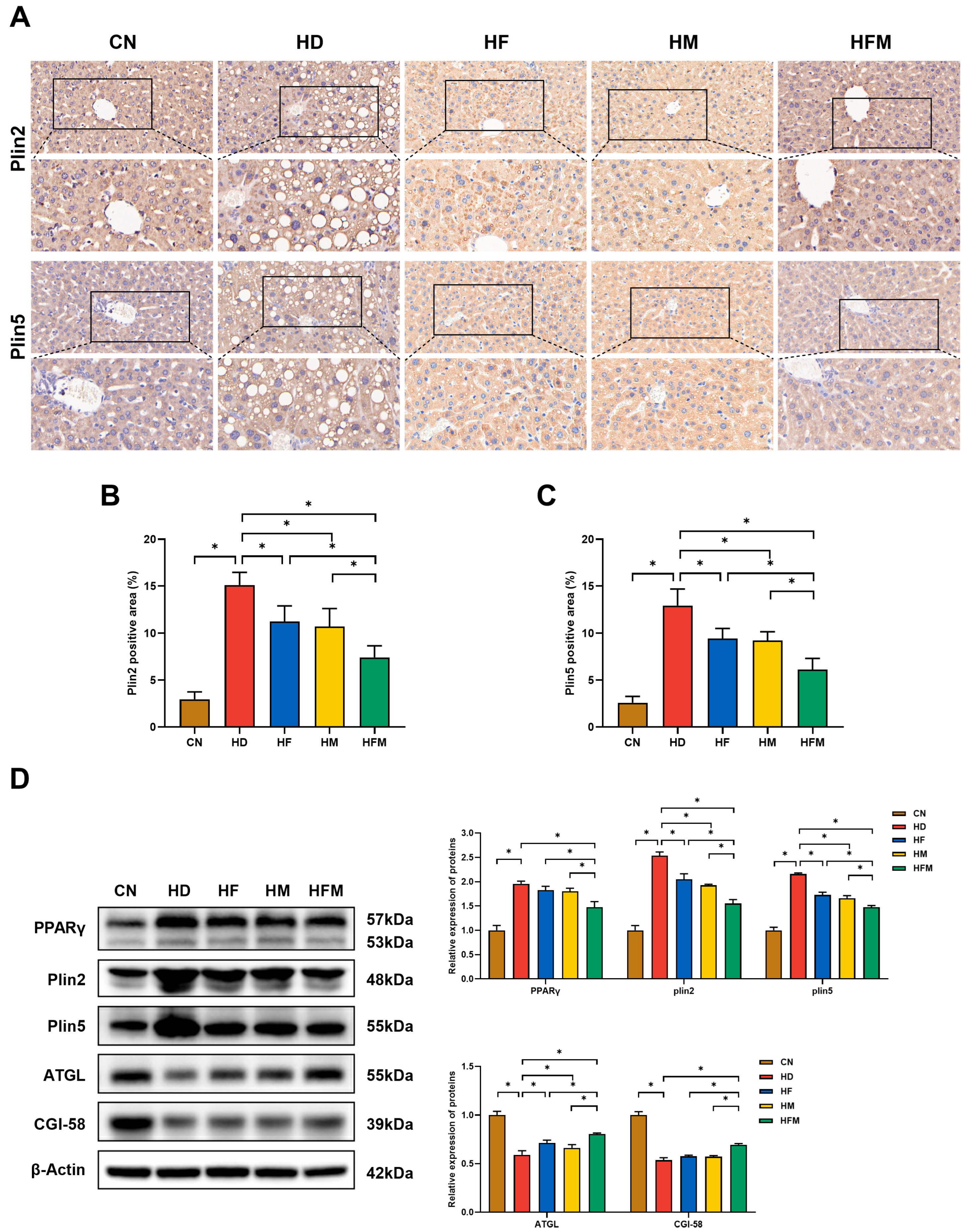
3.7. Effects of FA Combined with MLT on the Expression of Proteins Related to Hepatic LD Lipolysis
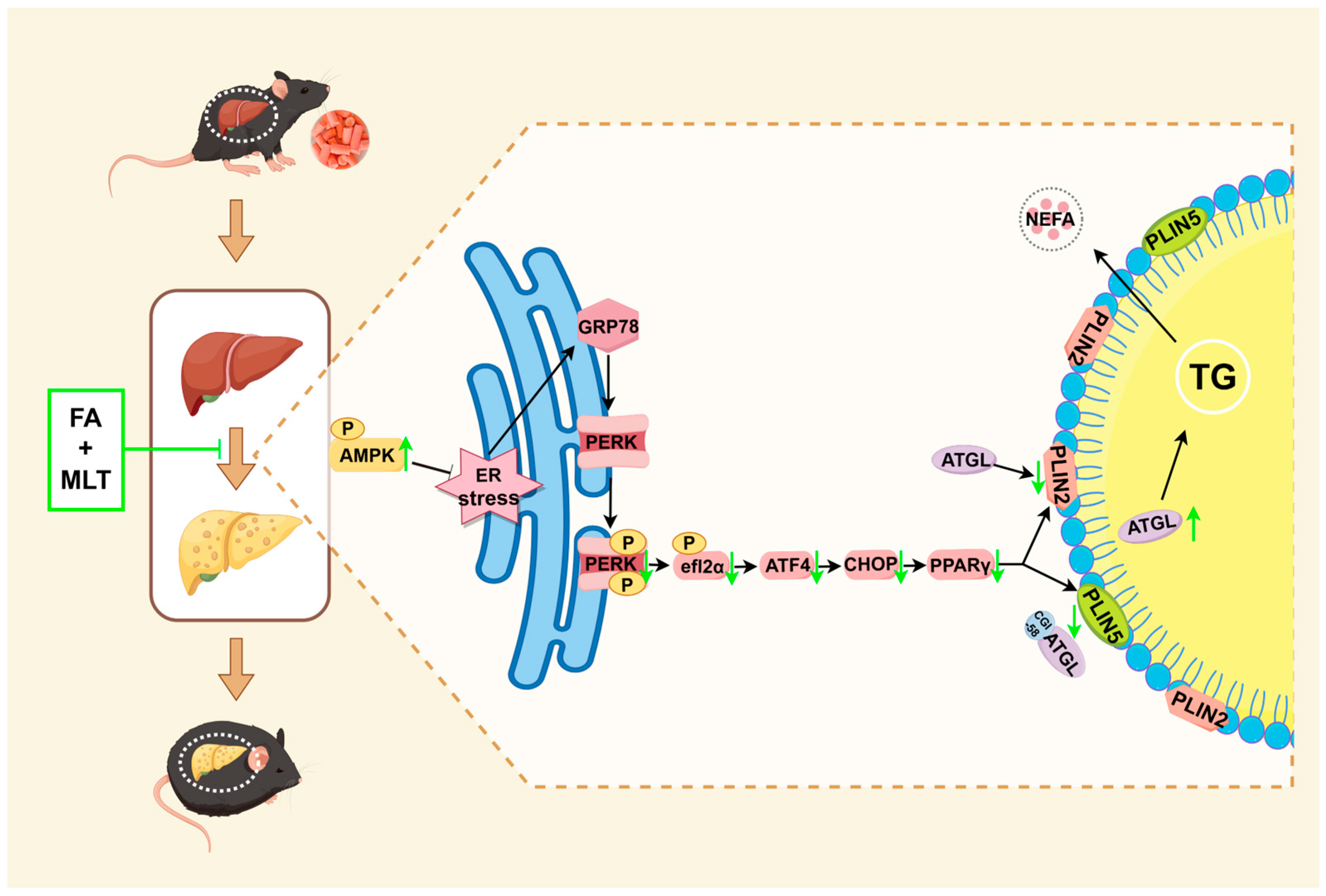
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Huang, X.; Yu, R.; Tan, X.; Guo, M.; Xia, Y.; Zou, H.; Liu, X.; Qin, C. Comparison of NAFLD, MAFLD, and MASLD Prevalence and Clinical Characteristics in Asia Adults. J. Clin. Exp. Hepatol. 2025, 15, 102420. [Google Scholar] [CrossRef]
- Le, M.H.; Yeo, Y.H.; Zou, B.; Barnet, S.; Henry, L.; Cheung, R.; Nguyen, M.H. Forecasted 2040 global prevalence of nonalcoholic fatty liver disease using hierarchical bayesian approach. Clin. Mol. Hepatol. 2022, 28, 841–850. [Google Scholar] [CrossRef]
- Israelsen, M.; Francque, S.; Tsochatzis, E.A.; Krag, A. Steatotic liver disease. Lancet 2024, 404, 1761–1778. [Google Scholar] [CrossRef]
- Mashek, D.G.; Khan, S.A.; Sathyanarayan, A.; Ploeger, J.M.; Franklin, M.P. Hepatic lipid droplet biology: Getting to the root of fatty liver. Hepatology 2015, 62, 964–967. [Google Scholar] [CrossRef]
- Mashek, D.G. Hepatic lipid droplets: A balancing act between energy storage and metabolic dysfunction in NAFLD. Mol. Metab. 2021, 50, 101115. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Perilipins: A family of five fat-droplet storing proteins that play a significant role in fat homeostasis. J. Cell. Biochem. 2024, 125, e30579. [Google Scholar] [CrossRef] [PubMed]
- Straub, B.K.; Gyoengyoesi, B.; Koenig, M.; Hashani, M.; Pawella, L.M.; Herpel, E.; Mueller, W.; Macher-Goeppinger, S.; Heid, H.; Schirmacher, P. Adipophilin/perilipin-2 as a lipid droplet-specific marker for metabolically active cells and diseases associated with metabolic dysregulation. Histopathology 2013, 62, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Listenberger, L.L.; Ostermeyer-Fay, A.G.; Goldberg, E.B.; Brown, W.J.; Brown, D.A. Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J. Lipid Res. 2007, 48, 2751–2761. [Google Scholar] [CrossRef]
- Wang, H.; Sreenivasan, U.; Hu, H.; Saladino, A.; Polster, B.M.; Lund, L.M.; Gong, D.-W.; Stanley, W.C.; Sztalryd, C. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J. Lipid Res. 2011, 52, 2159–2168. [Google Scholar] [CrossRef]
- Granneman, J.G.; Moore, H.-P.H.; Mottillo, E.P.; Zhu, Z.; Zhou, L. Interactions of Perilipin-5 (Plin5) with Adipose Triglyceride Lipase. J. Biol. Chem. 2011, 286, 5126–5135. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Kaplowitz, N.; Lebeaupin, C.; Kroemer, G.; Kaufman, R.J.; Malhi, H.; Ren, J. Endoplasmic reticulum stress in liver diseases. Hepatology 2023, 77, 619–639. [Google Scholar] [CrossRef]
- Rutkowski, D.T.; Wu, J.; Back, S.-H.; Callaghan, M.U.; Ferris, S.P.; Iqbal, J.; Clark, R.; Miao, H.; Hassler, J.R.; Fornek, J.; et al. UPR Pathways Combine to Prevent Hepatic Steatosis Caused by ER Stress-Mediated Suppression of Transcriptional Master Regulators. Dev. Cell 2008, 15, 829–840. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, B.M.; Chung, K.W.; Choi, Y.J.; Yu, B.P.; Chung, H.Y. Interaction between CHOP and FoxO6 promotes hepatic lipid accumulation. Liver Int. 2020, 40, 2706–2718. [Google Scholar] [CrossRef]
- Dozsa, A.; Dezso, B.; Toth, B.I.; Bacsi, A.; Poliska, S.; Camera, E.; Picardo, M.; Zouboulis, C.C.; Bíró, T.; Schmitz, G.; et al. PPARγ-Mediated and Arachidonic Acid–Dependent Signaling Is Involved in Differentiation and Lipid Production of Human Sebocytes. J. Investig. Dermatol. 2014, 134, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-Q.; Xu, S.-Q.; Lin, J.-G. Curcumin Recovers Intracellular Lipid Droplet Formation Through Increasing Perilipin 5 Gene Expression in Activated Hepatic Stellate Cells In Vitro. Curr. Med. Sci. 2019, 39, 766–777. [Google Scholar] [CrossRef]
- Asbaghi, O.; Ghanavati, M.; Ashtary-Larky, D.; Bagheri, R.; Rezaei Kelishadi, M.; Nazarian, B.; Nordvall, M.; Wong, A.; Dutheil, F.; Suzuki, K.; et al. Effects of Folic Acid Supplementation on Oxidative Stress Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antioxidants 2021, 10, 871. [Google Scholar] [CrossRef] [PubMed]
- Mokgalaboni, K.; Mashaba, G.R.; Phoswa, W.N.; Lebelo, S.L. Folic acid supplementation on inflammation and homocysteine in type 2 diabetes mellitus: Systematic review and meta-analysis of randomized controlled trials. Nutr. Diabetes 2024, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Rezaiian, F.; Saadati, S.; Naseri, K.; Ashtary-Larky, D.; Yousefi, M.; Golalipour, E.; Clark, C.C.T.; Rastgoo, S.; Asbaghi, O. The effects of folic acid supplementation on endothelial function in adults: A systematic review and dose-response meta-analysis of randomized controlled trials. Nutr. J. 2023, 22, 12. [Google Scholar] [CrossRef] [PubMed]
- Cipolla-Neto, J.; Amaral, F.G.D. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Reiter, R.J. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef] [PubMed]
- Ebaid, H.; Bashandy, S.A.E.; Abdel-Mageed, A.M.; Al-Tamimi, J.; Hassan, I.; Alhazza, I.M. Folic acid and melatonin mitigate diabetic nephropathy in rats via inhibition of oxidative stress. Nutr. Metab. 2020, 17, 6. [Google Scholar] [CrossRef]
- Ebaid, H.; Bashandy, S.A.; Alhazza, I.M.; Rady, A.; El-Shehry, S. Folic acid and melatonin ameliorate carbon tetrachloride-induced hepatic injury, oxidative stress and inflammation in rats. Nutr. Metab. 2013, 10, 20. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; D’Amico, R.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Melatonin Plus Folic Acid Treatment Ameliorates Reserpine-Induced Fibromyalgia: An Evaluation of Pain, Oxidative Stress, and Inflammation. Antioxidants 2019, 8, 628. [Google Scholar] [CrossRef]
- Asbaghi, O.; Ashtary-Larky, D.; Bagheri, R.; Nazarian, B.; Pourmirzaei Olyaei, H.; Rezaei Kelishadi, M.; Nordvall, M.; Wong, A.; Dutheil, F.; Naeini, A.A. Beneficial effects of folic acid supplementation on lipid markers in adults: A GRADE-assessed systematic review and dose-response meta-analysis of data from 21,787 participants in 34 randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2022, 62, 8435–8453. [Google Scholar] [CrossRef]
- Pan, M.; Song, Y.L.; Xu, J.M.; Gan, H.Z. Melatonin ameliorates nonalcoholic fatty liver induced by high-fat diet in rats. J. Pineal Res. 2006, 41, 79–84. [Google Scholar] [CrossRef]
- Bahrami, M.; Cheraghpour, M.; Jafarirad, S.; Alavinejad, P.; Asadi, F.; Hekmatdoost, A.; Mohammadi, M.; Yari, Z. The effect of melatonin on treatment of patients with non-alcoholic fatty liver disease: A randomized double blind clinical trial. Complement. Ther. Med. 2020, 52, 102452. [Google Scholar] [CrossRef]
- Tobar Leitão, S.A.; Soares, D.D.S.; Carvas Junior, N.; Zimmer, R.; Ludwig, N.F.; Andrades, M. Study of anesthetics for euthanasia in rats and mice: A systematic review and meta-analysis on the impact upon biological outcomes (SAFE-RM). Life Sci. 2021, 284, 119916. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, X.; Zhang, L.; Sun, D.; Ma, Y.; Bai, Y.; Bai, X.; Liang, X.; Liang, H. Nicotinamide Riboside Ameliorates Fructose-Induced Lipid Metabolism Disorders in Mice by Activating Browning of WAT, and May Be Also Related to the Regulation of Gut Microbiota. Nutrients 2024, 16, 3920. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Wang, Y.; Zhao, X.; Zhang, L.; Li, J.; Zhang, Y.; Wang, P.; Liang, H. Dietary Folic Acid Supplementation Attenuates Maternal High-Fat Diet-Induced Fetal Intrauterine Growth Retarded via Ameliorating Placental Inflammation and Oxidative Stress in Rats. Nutrients 2023, 15, 3263. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, M.; Ma, Y.; Du, R.; Wang, B.; Lan, T.; Zhang, H.; Xue, M.; Liang, H. Folic acid intervention ameliorates hepatic steatosis after long-term alcohol exposure by alleviating endoplasmic reticulum stress. J. Nutr. Biochem. 2025, 141, 109896. [Google Scholar] [CrossRef]
- Ali, S.M.J.; Lai, M. Metabolic Dysfunction–Associated Steatotic Liver Disease. Ann. Intern. Med. 2025, 178, ITC1–ITC16. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Yu, Y.; Cheng, S.; Wu, J. Advances in research on metabolic dysfunction-associated steatotic liver disease. Life Sci. 2025, 362, 123362. [Google Scholar] [CrossRef]
- Gluchowski, N.L.; Becuwe, M.; Walther, T.C.; Farese, R.V. Lipid droplets and liver disease: From basic biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 343–355. [Google Scholar] [CrossRef]
- Recena Aydos, L.; Aparecida Do Amaral, L.; Serafim De Souza, R.; Jacobowski, A.C.; Freitas Dos Santos, E.; Rodrigues Macedo, M.L. Nonalcoholic Fatty Liver Disease Induced by High-Fat Diet in C57bl/6 Models. Nutrients 2019, 11, 3067. [Google Scholar] [CrossRef]
- Kim, H.; Min, H. Folic acid supplementation prevents high fructose-induced non-alcoholic fatty liver disease by activating the AMPK and LKB1 signaling pathways. Nutr. Res. Pract. 2020, 14, 309. [Google Scholar] [CrossRef]
- Genario, R.; Cipolla-Neto, J.; Bueno, A.A.; Santos, H.O. Melatonin supplementation in the management of obesity and obesity-associated disorders: A review of physiological mechanisms and clinical applications. Pharmacol. Res. 2021, 163, 105254. [Google Scholar] [CrossRef]
- Aleliunas, R.E.; Aljaadi, A.M.; Laher, I.; Glier, M.B.; Green, T.J.; Murphy, M.; Miller, J.W.; Devlin, A.M. Folic Acid Supplementation of Female Mice, with or without Vitamin B-12, before and during Pregnancy and Lactation Programs Adiposity and Vascular Health in Adult Male Offspring. J. Nutr. 2016, 146, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, L.; Zhao, X.; Ma, Y.; Sun, D.; Bai, Y.; Liu, W.; Liang, X.; Liang, H. Folic Acid Prevents High-Fat Diet-Induced Postpartum Weight Retention in Rats, Which Is Associated with a Reduction in Endoplasmic Reticulum Stress-Mediated Hepatic Lipogenesis. Nutrients 2024, 16, 4377. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Zhang, X.; Zhang, L.; Zhao, X.; Xu, Y.; Wang, P.; Liang, X.; Xue, M.; Liang, H. Maternal Folic Acid Supplementation during Pregnancy Prevents Hepatic Steatosis in Male Offspring of Rat Dams Fed High-Fat Diet, Which Is Associated with the Regulation of Gut Microbiota. Nutrients 2023, 15, 4726. [Google Scholar] [CrossRef]
- Wang, L.; McFadden, J.W.; Yang, G.; Zhu, H.; Lian, H.; Fu, T.; Sun, Y.; Gao, T.; Li, M. Effect of melatonin on visceral fat deposition, lipid metabolism and hepatic lipo-metabolic gene expression in male rats. J. Anim. Physiol. Anim. Nutr. 2021, 105, 787–796. [Google Scholar] [CrossRef]
- Jin, X.; Liu, X.; Wang, Y.; Li, X.; Zhang, T.; Li, J.; Lei, Z.; Yang, Y. The Mechanism by Which Melatonin Improves the Dysregulation of Glucose and Lipid Metabolism in Castrated Female Mice. J. Pineal Res. 2025, 77, e70082. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Song, M.J.; Malhi, H. The unfolded protein response and hepatic lipid metabolism in non alcoholic fatty liver disease. Pharmacol. Ther. 2019, 203, 107401. [Google Scholar] [CrossRef]
- Lee, A.S. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 2005, 35, 373–381. [Google Scholar] [CrossRef]
- Hetz, C.; Chevet, E.; Oakes, S.A. Proteostasis control by the unfolded protein response. Nat. Cell Biol. 2015, 17, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhou, D.-L.; Wei, X.-H.; Zhong, R.-Y.; Xu, J.; Sun, L. Astragaloside IV attenuates free fatty acid-induced ER stress and lipid accumulation in hepatocytes via AMPK activation. Acta Pharmacol. Sin. 2017, 38, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.J.; Choi, H.-E.; Cheon, H.G. 5-LO inhibition ameliorates palmitic acid-induced ER stress, oxidative stress and insulin resistance via AMPK activation in murine myotubes. Sci. Rep. 2017, 7, 5025. [Google Scholar] [CrossRef] [PubMed]
- Sid, V.; Wu, N.; Sarna, L.K.; Siow, Y.L.; House, J.D.; O, K. Folic acid supplementation during high-fat diet feeding restores AMPK activation via an AMP-LKB1-dependent mechanism. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 309, R1215–R1225. [Google Scholar] [CrossRef]
- Fan, C.; Feng, J.; Tang, C.; Zhang, Z.; Feng, Y.; Duan, W.; Zhai, M.; Yan, Z.; Zhu, L.; Feng, L.; et al. Melatonin suppresses ER stress-dependent proapoptotic effects via AMPK in bone mesenchymal stem cells during mitochondrial oxidative damage. Stem Cell Res. Ther. 2020, 11, 442. [Google Scholar] [CrossRef]
- De La Rosa Rodriguez, M.A.; Kersten, S. Regulation of lipid droplet-associated proteins by peroxisome proliferator-activated receptors. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2017, 1862, 1212–1220. [Google Scholar] [CrossRef]
- Carr, R.M.; Ahima, R.S. Pathophysiology of lipid droplet proteins in liver diseases. Exp. Cell Res. 2016, 340, 187–192. [Google Scholar] [CrossRef]
- Langhi, C.; Marquart, T.J.; Allen, R.M.; Baldán, Á. Perilipin-5 is regulated by statins and controls triglyceride contents in the hepatocyte. J. Hepatol. 2014, 61, 358–365. [Google Scholar] [CrossRef]
- Conte, M.; Franceschi, C.; Sandri, M.; Salvioli, S. Perilipin 2 and Age-Related Metabolic Diseases: A New Perspective. Trends Endocrinol. Metab. 2016, 27, 893–903. [Google Scholar] [CrossRef]
- Imamura, M.; Inoguchi, T.; Ikuyama, S.; Taniguchi, S.; Kobayashi, K.; Nakashima, N.; Nawata, H. ADRP stimulates lipid accumulation and lipid droplet formation in murine fibroblasts. Am. J. Physiol.-Endocrinol. Metab. 2002, 283, E775–E783. [Google Scholar] [CrossRef]
- Ma, S.Y.; Sun, K.S.; Zhang, M.; Zhou, X.; Zheng, X.H.; Tian, S.Y.; Liu, Y.S.; Chen, L.; Gao, X.; Ye, J.; et al. Disruption of Plin5 degradation by CMA causes lipid homeostasis imbalance in NAFLD. Liver Int. 2020, 40, 2427–2438. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Gao, X.; Li, L.; Yuan, Y.; Liu, F.; Zhang, L.; Wu, J.; Hu, P.; Zhang, X.; et al. Perilipin 5 improves hepatic lipotoxicity by inhibiting lipolysis. Hepatology 2015, 61, 870–882. [Google Scholar] [CrossRef]
- Najt, C.P.; Senthivinayagam, S.; Aljazi, M.B.; Fader, K.A.; Olenic, S.D.; Brock, J.R.L.; Lydic, T.A.; Jones, A.D.; Atshaves, B.P. Liver-specific loss of Perilipin 2 alleviates diet-induced hepatic steatosis, inflammation, and fibrosis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2016, 310, G726–G738. [Google Scholar] [CrossRef]
- Mass-Sanchez, P.B.; Krizanac, M.; Štancl, P.; Leopold, M.; Engel, K.M.; Buhl, E.M.; Van Helden, J.; Gassler, N.; Schiller, J.; Karlić, R.; et al. Perilipin 5 deletion protects against nonalcoholic fatty liver disease and hepatocellular carcinoma by modulating lipid metabolism and inflammatory responses. Cell Death Discov. 2024, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Zhou, D.; Ye, T.; Zhou, P.; Liu, Z.; Liu, X.; Wang, Z.; Hua, T.; Zhang, Z.; et al. Swimming exercise ameliorates insulin resistance and nonalcoholic fatty liver by negatively regulating PPARγ transcriptional network in mice fed high fat diet. Mol. Med. 2023, 29, 150. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Lei, P.; Teng, C.; Sun, Y.; Song, X.; Li, B.; Shan, Y. Targeting PLIN2/PLIN5-PPARγ: Sulforaphane Disturbs the Maturation of Lipid Droplets. Mol. Nutr. Food Res. 2019, 63, 1900183. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.; Ma, Y.; Bai, Y.; Bai, X.; Liu, W.; Du, L.; Wang, P.; Liang, X.; Liang, H.; Zhang, H. Folic Acid Combined with Melatonin Might Prevent Hepatic Steatosis by Alleviating Endoplasmic Reticulum Stress to Promote Lipid Droplet Lipolysis in High-Fat Diet-Fed Mice. Nutrients 2025, 17, 3641. https://doi.org/10.3390/nu17233641
Sun D, Ma Y, Bai Y, Bai X, Liu W, Du L, Wang P, Liang X, Liang H, Zhang H. Folic Acid Combined with Melatonin Might Prevent Hepatic Steatosis by Alleviating Endoplasmic Reticulum Stress to Promote Lipid Droplet Lipolysis in High-Fat Diet-Fed Mice. Nutrients. 2025; 17(23):3641. https://doi.org/10.3390/nu17233641
Chicago/Turabian StyleSun, Dan, Yanzhen Ma, Yixian Bai, Xue Bai, Weiheng Liu, Lin Du, Peng Wang, Xi Liang, Hui Liang, and Huaqi Zhang. 2025. "Folic Acid Combined with Melatonin Might Prevent Hepatic Steatosis by Alleviating Endoplasmic Reticulum Stress to Promote Lipid Droplet Lipolysis in High-Fat Diet-Fed Mice" Nutrients 17, no. 23: 3641. https://doi.org/10.3390/nu17233641
APA StyleSun, D., Ma, Y., Bai, Y., Bai, X., Liu, W., Du, L., Wang, P., Liang, X., Liang, H., & Zhang, H. (2025). Folic Acid Combined with Melatonin Might Prevent Hepatic Steatosis by Alleviating Endoplasmic Reticulum Stress to Promote Lipid Droplet Lipolysis in High-Fat Diet-Fed Mice. Nutrients, 17(23), 3641. https://doi.org/10.3390/nu17233641




