Migraine and Alcohol—Is It Really That Harmful?
Abstract
1. Introduction
2. Materials and Methods
3. Alcohol Effects on Non-Migraine Headache
3.1. Alcohol-Induced Headache
3.2. Tension-Type Headache
3.3. Cluster Headache
4. Alcohol and Migraine
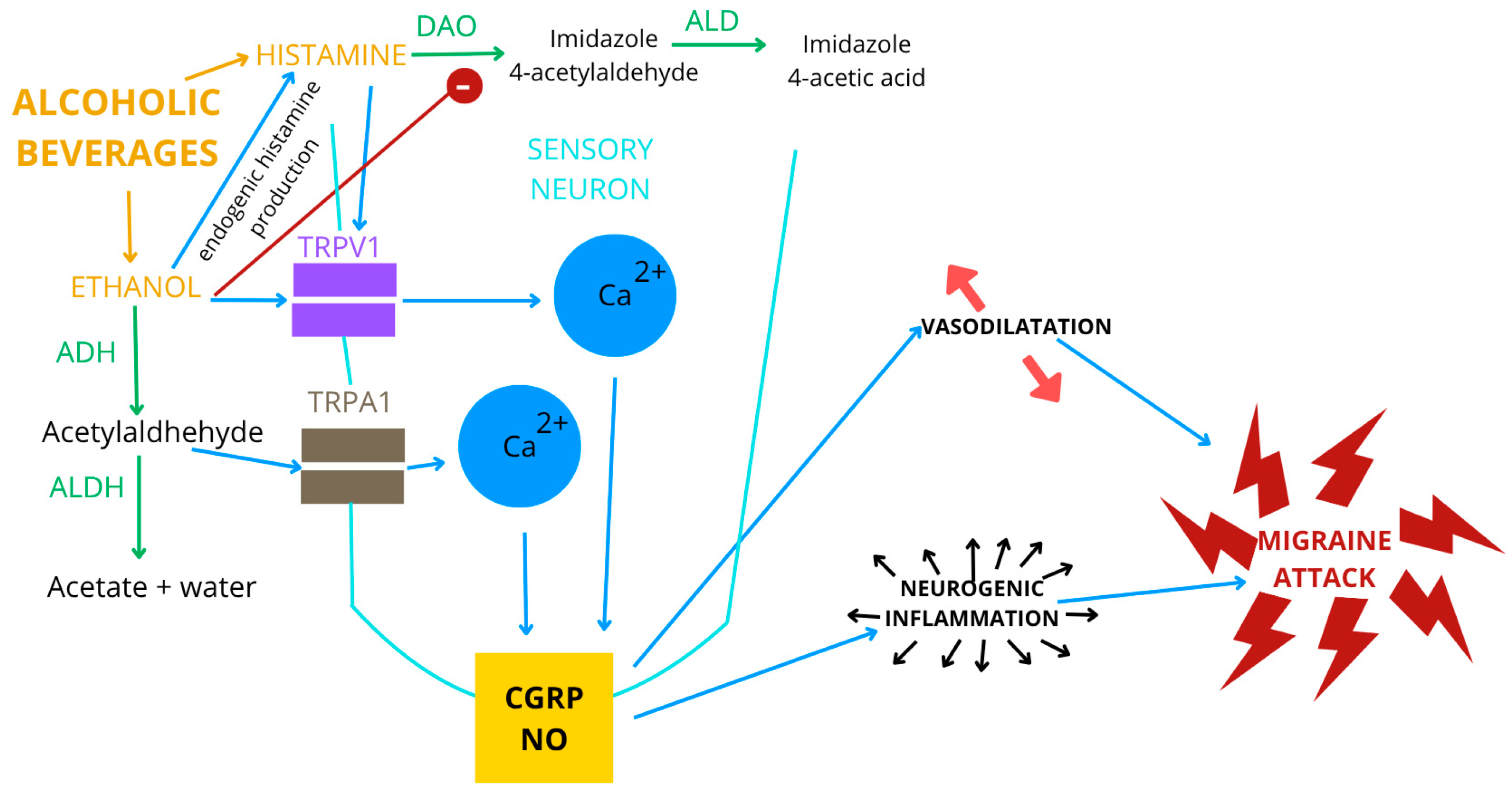
4.1. Potential Mechanisms
4.1.1. Ethanol
4.1.2. Wine and Other Alcoholic Beverages
Polyphenols
Biogenic Amines
Histamine
Tyramine
Sulphites
Nitrites/Nitrates
4.2. Alcohol as Migraine Trigger
4.3. Alcohol Consumption in Migraine Patients
4.4. Alcohol Consumption and the Risk of Migraine
4.5. Risk of Alcohol Use Disorders in Patients with Migraine
4.6. Genetic Predisposition
4.7. Recommendations for Migraine Patients Regarding Alcohol Use
| high-frequency migraine attack | trial of low-histamine diet | |
| with suspected histamine |  | + |
| intolerance | alcohol avoidance | |
| type/dose beverage | ||
| low-frequency migraine attack |  | + |
| diary-based monitoring |
5. Limitations
6. Conclusions
- AIH, classified as a secondary headache, is a pulsating, bilateral headache that worsens with physical activity and is provoked by alcohol consumption.
- TTH can be provoked by alcohol consumption, and some studies have reported that alcohol consumption by patients with TTH is similar or greater to that of migraineurs, and even TTH patients have more alcohol-related problems than migraineurs.
- CH is often provoked by alcohol, but strangely enough, many CH patients consume alcohol, even during attacks.
- There are no studies clearly indicating which alcohol components are responsible for migraine attacks; the following are taken into account: ethanol and components found in red wine, such as flavonoid phenols, serotonin, histamine, tyramine, sulfites and nitrites.
- Alcohol, especially red wine, is one of the most frequently mentioned factors provoking migraine attacks, which is not always confirmed by the few prospective studies.
- Migraineurs, especially those with active migraine, frequent attacks, and chronic migraine, avoid drinking alcohol.
- Alcohol consumption does not seem to affect the risk of migraine, and the risk of AUD is not increased in migraineurs.
- Further research is needed to assess the influence of genetic factors on the association between migraine and alcohol.
- Alcohol leads to many health problems, including some cancers and road accidents, and the WHO states that there is no safe dose of alcohol, so all patients, including those with migraine, should be advised to avoid drinking alcohol.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borończyk, M.; Zduńska, A.; Węgrzynek-Gallina, J.; Grodzka, O.; Lasek-Bal, A.; Domitrz, I. Migraine and stroke: Correlation, coexistence, dependence—A modern perspective. J. Headache Pain 2025, 26, 39. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, K.; Zhang, Y.; Jia, X.; Li, J.; Hu, J.; He, X.; Chen, X.; Wu, J. Association Between Dietary Alcohol Intake and Migraine or Severe Headache Miscellaneous Pain: The NHANES 1999–2004. Brain Behav. 2025, 15, e70400. [Google Scholar] [CrossRef]
- Maisch, B. Alcohol consumption-none is better than a little. Herz 2024, 49, 409–419. [Google Scholar] [CrossRef]
- Schnedl, W.J.; Queissner, R. Migraines appear more likely to be caused by histamine than ethanol. Eur. J. Neurol. 2019, 26, e79. [Google Scholar] [CrossRef]
- Panconesi, A. Alcohol-induced headaches: Evidence for a central mechanism? J. Neurosci. Rural. Pr. 2016, 7, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Panconesi, A.; Bartolozzi, M.L.; Guidi, L. Alcohol and migraine: What should we tell patients? Curr. Pain. Headache Rep. 2011, 15, 177–184. [Google Scholar] [CrossRef]
- Kuster, G.W.; da Silva, A.L.; Aquino, C.H.; Ziviani, L.F.; Domingues, R.B. Frequency and features of delayed alcohol-induced headache among university students. Headache 2006, 46, 688–691. [Google Scholar] [CrossRef]
- García-Azorín, D.; Aparicio-Cordero, L.; Talavera, B.; Johnson, A.; Schytz, H.W.; Guerrero-Peral, Á.L. Clinical characterization of delayed alcohol-induced headache: A study of 1,108 participants. Neurology 2020, 95, e2161–e2169. [Google Scholar] [CrossRef]
- Mostofsky, E.; Bertisch, S.M.; Vgontzas, A.; Buettner, C.; Li, W.; Rueschman, M.; Mittleman, M.A. Prospective cohort study of daily alcoholic beverage intake as a potential trigger of headaches among adults with episodic migraine. Ann. Med. 2020, 52, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.W.; Sun, C.; Lay, C. Alcohol hangover headache. Headache 2007, 47, 277–279. [Google Scholar] [CrossRef]
- Sun-Edelstein, C.; Mauskop, A. Foods and supplements in the management of migraine headaches. Clin. J. Pain. 2009, 25, 446–452. [Google Scholar] [CrossRef]
- Krymchantowski, A.V.; da Cunha Jevoux, C. Wine and headache. Headache 2014, 54, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Panconesi, A.; Franchini, M.; Bartolozzi, M.L.; Mugnai, S.; Guidi, L. Alcoholic drinks as triggers in primary headaches. Pain. Med. 2013, 14, 1254–1259. [Google Scholar] [CrossRef]
- Lebedeva, E.R.; Kobzeva, N.R.; Gilev, D.V.; Olesen, J. Factors Associated with Primary Headache According to Diagnosis, Sex, and Social Group. Headache 2016, 56, 341–356. [Google Scholar] [CrossRef]
- Schramm, S.H.; Obermann, M.; Katsarava, Z.; Diener, H.C.; Moebus, S.; Yoon, M.S. Epidemiological profiles of patients with chronic migraine and chronic tension-type headache. J. Headache Pain. 2013, 14, 40. [Google Scholar] [CrossRef]
- Domingues, R.B.; Domingues, S.A. Headache is associated with lower alcohol consumption among medical students. Arq. Neuropsiquiatr. 2011, 69, 620–623. [Google Scholar] [CrossRef]
- Hagen, K.; Åsberg, A.N.; Stovner, L.; Linde, M.; Zwart, J.A.; Winsvold, B.S.; Heuch, I. Lifestyle factors and risk of migraine and tension-type headache. Follow-up data from the Nord-Trøndelag Health Surveys 1995–1997 and 2006–2008. Cephalalgia 2018, 38, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, T.; Ishizaki, K.; Fukuhara, Y.; Ijiri, T.; Kusumi, M.; Wakutani, Y.; Mori, M.; Kawashima, M.; Kowa, H.; Adachi, Y.; et al. Population-based door-to-door survey of migraine in Japan: The Daisen study. Headache 2004, 44, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Spierings, E.L.; Ranke, A.H.; Honkoop, P.C. Precipitating and aggravating factors of migraine versus tension-type headache. Headache 2001, 41, 554–558. [Google Scholar] [CrossRef]
- Wöber, C.; Holzhammer, J.; Zeitlhofer, J.; Wessely, P.; Wöber-Bingöl, C. Trigger factors of migraine and tension-type headache: Experience and knowledge of the patients. J. Headache Pain. 2006, 7, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Karli, N.; Zarifoglu, M.; Calisir, N.; Akgoz, S. Comparison of pre-headache phases and trigger factors of migraine and episodic tension-type headache: Do they share similar clinical pathophysiology? Cephalalgia 2005, 25, 444–451. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Q.; Li, N.; Tan, G.; Chen, L.; Zhou, J. Triggers of migraine and tension-type headache in China: A clinic-based survey. Eur. J. Neurol. 2013, 20, 689–696. [Google Scholar] [CrossRef]
- Kim, S.A.; Choi, S.Y.; Youn, M.S.; Pozo-Rosich, P.; Lee, M.J. Epidemiology, burden and clinical spectrum of cluster headache: A global update. Cephalalgia 2023, 43, 3331024231201577. [Google Scholar] [CrossRef]
- Lund, N.; Barloese, M.; Petersen, A.; Haddock, B.; Jensen, R. Chronobiology differs between men and women with cluster headache, clinical phenotype does not. Neurology 2017, 88, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, A.; Fourier, C.; Ran, C.; Waldenlind, E.; Sjöstrand, C.; Belin, A.C. Cluster headache-clinical pattern and a new severity scale in a Swedish cohort. Cephalalgia 2018, 38, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Rozen, T.D.; Fishman, R.S. Cluster headache in the United States of America: Demographics, clinical characteristics, triggers, suicidality, and personal burden. Headache 2012, 52, 99–113. [Google Scholar] [CrossRef]
- Lund, N.; Petersen, A.; Snoer, A.; Jensen, R.H.; Barloese, M. Cluster headache is associated with unhealthy lifestyle and lifestyle-related comorbid diseases: Results from the Danish Cluster Headache Survey. Cephalalgia 2019, 39, 254–263. [Google Scholar] [CrossRef]
- Lund, N.L.T.; Snoer, A.H.; Petersen, A.S.; Beske, R.P.; Jennum, P.J.; Jensen, R.H.; Barloese, M.C.J. Disturbed sleep in cluster headache is not the result of transient processes associated with the cluster period. Eur. J. Neurol. 2019, 26, 290–298. [Google Scholar] [CrossRef]
- Sjöstrand, C.; Russell, M.B.; Ekbom, K.; Waldenlind, E. Familial cluster headache: Demographic patterns in affected and nonaffected. Headache 2010, 50, 374–382. [Google Scholar] [CrossRef]
- Jensen, R.M.; Lyngberg, A.; Jensen, R.H. Burden of cluster headache. Cephalalgia 2007, 27, 535–541. [Google Scholar] [CrossRef]
- Lambru, G.; Castellini, P.; Manzoni, G.C.; Torelli, P. Mode of occurrence of traumatic head injuries in male patients with cluster headache or migraine: Is there a connection with lifestyle? Cephalalgia 2010, 30, 1502–1508. [Google Scholar] [CrossRef]
- Schürks, M.; Kurth, T.; Knorn, P.; Pageler, L.; Diener, H.C. Predictors of hazardous alcohol consumption among patients with cluster headache. Cephalalgia 2006, 26, 623–627. [Google Scholar] [CrossRef]
- Davis-Martin, R.E.; Polk, A.N.; Smitherman, T.A. Alcohol Use as a Comorbidity and Precipitant of Primary Headache: Review and Meta-analysis. Curr. Pain. Headache Rep. 2017, 21, 42. [Google Scholar] [CrossRef]
- Deng, X.S.; Deitrich, R.A. Ethanol metabolism and effects: Nitric oxide and its interaction. Curr. Clin. Pharmacol. 2007, 2, 145–153. [Google Scholar] [CrossRef]
- Gazzieri, D.; Trevisani, M.; Tarantini, F.; Bechi, P.; Masotti, G.; Gensini, G.F.; Castellani, S.; Marchionni, N.; Geppetti, P.; Harrison, S. Ethanol dilates coronary arteries and increases coronary flow via transient receptor potential vanilloid 1 and calcitonin gene-related peptide. Cardiovasc. Res. 2006, 70, 589–599. [Google Scholar] [CrossRef]
- Kamm, K. CGRP and Migraine: What Have We Learned from Measuring CGRP in Migraine Patients So Far? Front. Neurol. 2022, 13, 930383. [Google Scholar] [CrossRef] [PubMed]
- Saloman, J.L.; Chung, M.K.; Ro, J.Y. P2X3 and TRPV1 functionally interact and mediate sensitization of trigeminal sensory neurons. Neuroscience 2013, 232, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Tozzi, A.; Rainero, I.; Cupini, L.M.; Calabresi, P.; Ayata, C.; Sarchielli, P. Cortical spreading depression as a target for anti-migraine agents. J. Headache Pain. 2013, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Baliño, P.; Alfonso-Loeches, S.; Aragón, C.M.; Guerri, C. Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav. Immun. 2011, 25 (Suppl. S1), S80–S91. [Google Scholar] [CrossRef]
- Cohen, C.F.; Roh, J.; Lee, S.H.; Park, C.K.; Berta, T. Targeting Nociceptive Neurons and Transient Receptor Potential Channels for the Treatment of Migraine. Int. J. Mol. Sci. 2023, 24, 7897. [Google Scholar] [CrossRef] [PubMed]
- Iannone, L.F.; De Logu, F.; Geppetti, P.; De Cesaris, F. The role of TRP ion channels in migraine and headache. Neurosci. Lett. 2022, 768, 136380. [Google Scholar] [CrossRef]
- Borkum, J.M. Migraine Triggers and Oxidative Stress: A Narrative Review and Synthesis. Headache 2016, 56, 12–35. [Google Scholar] [CrossRef] [PubMed]
- Fila, M.; Chojnacki, C.; Chojnacki, J.; Blasiak, J. Nutrients to Improve Mitochondrial Function to Reduce Brain Energy Deficit and Oxidative Stress in Migraine. Nutrients 2021, 13, 4433. [Google Scholar] [CrossRef]
- Do, N.; Zuo, D.; Kim, M.; Kim, M.; Ha, H.J.; Blumberg, P.M.; Ann, J.; Hwang, S.W.; Lee, J. Discovery of Dual TRPA1 and TRPV1 Antagonists as Novel Therapeutic Agents for Pain. Pharmaceuticals 2024, 17, 1209. [Google Scholar] [CrossRef]
- García-Martín, E.; Martínez, C.; Serrador, M.; Alonso-Navarro, H.; Navacerrada, F.; Agúndez, J.A.; Jiménez-Jiménez, F.J. Alcohol dehydrogenase 2 genotype and risk for migraine. Headache 2010, 50, 85–91. [Google Scholar] [CrossRef]
- Paungarttner, J.; Quartana, M.; Patti, L.; Sklenárová, B.; Farham, F.; Jiménez, I.H.; Soylu, M.G.; Vlad, I.M.; Tasdelen, S.; Mateu, T.; et al. Migraine—A borderland disease to epilepsy: Near it but not of it. J. Headache Pain. 2024, 25, 11. [Google Scholar] [CrossRef]
- Mathew, A.A.; Panonnummal, R. Cortical spreading depression: Culprits and mechanisms. Exp. Brain Res. 2022, 240, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Guedes, R.C.A.; Abadie-Guedes, R. Brain Aging and Electrophysiological Signaling: Revisiting the Spreading Depression Model. Front. Aging Neurosci. 2019, 11, 136. [Google Scholar] [CrossRef]
- Panconesi, A. Alcohol and migraine: Trigger factor, consumption, mechanisms. A review. J. Headache Pain. 2008, 9, 19–27. [Google Scholar] [CrossRef]
- Pashek, R.E.; Nkambule, B.B.; Chan, M.V.; Thibord, F.; Lachapelle, A.R.; Cunha, J.; Chen, M.H.; Johnson, A.D. Alcohol intake including wine drinking is associated with decreased platelet reactivity in a large population sample. Int. J. Epidemiol. 2023, 52, 1939–1950. [Google Scholar] [CrossRef] [PubMed]
- Mansvelt, E.P.; van Velden, D.P.; Fourie, E.; Rossouw, M.; van Rensburg, S.J.; Smuts, C.M. The in vivo antithrombotic effect of wine consumption on human blood platelets and hemostatic factors. Ann. N. Y. Acad. Sci. 2002, 957, 329–332. [Google Scholar] [CrossRef]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Beneficial Effects of Red Wine Polyphenols on Human Health: Comprehensive Review. Curr. Issues Mol. Biol. 2023, 45, 782–798. [Google Scholar] [CrossRef]
- Radonjić, S.; Maraš, V.; Raičević, J.; Košmerl, T. Wine or Beer? Comparison, Changes and Improvement of Polyphenolic Compounds during Technological Phases. Molecules 2020, 25, 4960. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Garaguso, I. Effect of Sulfites on Antioxidant Activity, Total Polyphenols, and Flavonoid Measurements in White Wine. Foods 2018, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- El Rayess, Y.; Nehme, N.; Azzi-Achkouty, S.; Julien, S.G. Wine Phenolic Compounds: Chemistry, Functionality and Health Benefits. Antioxidants 2024, 13, 1312. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Kontaxakis, E.; Trantas, E.; Ververidis, F. Resveratrol: A Fair Race Towards Replacing Sulfites in Wines. Molecules 2020, 25, 2378. [Google Scholar] [CrossRef]
- Miraldi, E.; Baini, G.; Biagi, M.; Cappellucci, G.; Giordano, A.; Vaccaro, F.; Bertelli, A.A.E. Wine, Polyphenols, and the Matrix Effect: Is Alcohol Always the Same? Int. J. Mol. Sci. 2024, 25, 9796. [Google Scholar] [CrossRef]
- Boccardi, V.; Tagliafico, L.; Persia, A.; Page, E.; Ottaviani, S.; Cremonini, A.L.; Borgarelli, C.; Pisciotta, L.; Mecocci, P.; Nencioni, A.; et al. The Potential Effects of Red Wine and Its Components on Neurocognitive Disorders: A Narrative Review. Nutrients 2024, 16, 3431. [Google Scholar] [CrossRef]
- Nalazek-Rudnicka, K.; Wojnowski, W.; Wasik, A. Occurrence and Levels of Biogenic Amines in Beers Produced by Different Methods. Foods 2021, 10, 2902. [Google Scholar] [CrossRef]
- Ordóñez, J.L.; Troncoso, A.M.; García-Parrilla, M.D.C.; Callejón, R.M. Recent trends in the determination of biogenic amines in fermented beverages—A review. Anal. Chim. Acta 2016, 939, 10–25. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M. Update on Biogenic Amines in Fermented and Non-Fermented Beverages. Foods 2022, 11, 353. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Domínguez-Perles, R.; Medina, S. Winery By-Products as Sources of Bioactive Tryptophan, Serotonin, and Melatonin: Contributions to the Antioxidant Power. Foods 2023, 12, 1571. [Google Scholar] [CrossRef]
- Marques, C.; Dinis, L.T.; Santos, M.J.; Mota, J.; Vilela, A. Beyond the Bottle: Exploring Health-Promoting Compounds in Wine and Wine-Related Products-Extraction, Detection, Quantification, Aroma Properties, and Terroir Effects. Foods 2023, 12, 4277. [Google Scholar] [CrossRef] [PubMed]
- Hamel, E. Serotonin and migraine: Biology and clinical implications. Cephalalgia 2007, 27, 1293–1300. [Google Scholar] [CrossRef]
- Zhang, X.C.; Strassman, A.M.; Burstein, R.; Levy, D. Sensitization and activation of intracranial meningeal nociceptors by mast cell mediators. J. Pharmacol. Exp. Ther. 2007, 322, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L.L.; Olesen, J. Nitric oxide in primary headaches. Curr. Opin. Neurol. 2001, 14, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Karatzi, K.; Papamichael, C.; Aznaouridis, K.; Karatzis, E.; Lekakis, J.; Matsouka, C.; Boskou, G.; Chiou, A.; Sitara, M.; Feliou, G.; et al. Constituents of red wine other than alcohol improve endothelial function in patients with coronary artery disease. Coron. Artery Dis. 2004, 15, 485–490. [Google Scholar] [CrossRef]
- Kanny, G.; Gerbaux, V.; Olszewski, A.; Frémont, S.; Empereur, F.; Nabet, F.; Cabanis, J.C.; Moneret-Vautrin, D.A. No correlation between wine intolerance and histamine content of wine. J. Allergy Clin. Immunol. 2001, 107, 375–378. [Google Scholar] [CrossRef]
- Hrubisko, M.; Danis, R.; Huorka, M.; Wawruch, M. Histamine Intolerance-The More We Know the Less We Know. A Review. Nutrients 2021, 13, 2228. [Google Scholar] [CrossRef]
- Jochum, C. Histamine Intolerance: Symptoms, Diagnosis, and Beyond. Nutrients 2024, 16, 1219. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Jin, H.; Chen, L.; Ji, J.; Zhang, Z. Histamine Intolerance-A Kind of Pseudoallergic Reaction. Biomolecules 2022, 12, 454. [Google Scholar] [CrossRef] [PubMed]
- Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.; Vidal-Carou, M.D.C. Histamine Intolerance: The Current State of the Art. Biomolecules 2020, 10, 1181. [Google Scholar] [CrossRef]
- Schirone, M.; Visciano, P.; Tofalo, R.; Suzzi, G. Histamine Food Poisoning. Handb. Exp. Pharmacol. 2017, 241, 217–235. [Google Scholar] [CrossRef]
- Schnedl, W.J.; Enko, D. Histamine Intolerance Originates in the Gut. Nutrients 2021, 13, 1262. [Google Scholar] [CrossRef]
- San Mauro Martin, I.; Brachero, S.; Garicano Vilar, E. Histamine intolerance and dietary management: A complete review. Allergol. Immunopathol. 2016, 44, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Schnedl, W.J.; Schenk, M.; Lackner, S.; Enko, D.; Mangge, H.; Forster, F. Diamine oxidase supplementation improves symptoms in patients with histamine intolerance. Food Sci. Biotechnol. 2019, 28, 1779–1784. [Google Scholar] [CrossRef]
- Izquierdo-Casas, J.; Comas-Basté, O.; Latorre-Moratalla, M.L.; Lorente-Gascón, M.; Duelo, A.; Soler-Singla, L.; Vidal-Carou, M.C. Diamine oxidase (DAO) supplement reduces headache in episodic migraine patients with DAO deficiency: A randomized double-blind trial. Clin. Nutr. 2019, 38, 152–158. [Google Scholar] [CrossRef]
- García-Martín, E.; Martínez, C.; Serrador, M.; Alonso-Navarro, H.; Ayuso, P.; Navacerrada, F.; Agúndez, J.A.; Jiménez-Jiménez, F.J. Diamine oxidase rs10156191 and rs2052129 variants are associated with the risk for migraine. Headache 2015, 55, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Casas, J.; Comas-Basté, O.; Latorre-Moratalla, M.L.; Lorente-Gascón, M.; Duelo, A.; Vidal-Carou, M.C.; Soler-Singla, L. Low serum diamine oxidase (DAO) activity levels in patients with migraine. J. Physiol. Biochem. 2018, 74, 93–99. [Google Scholar] [CrossRef]
- Lackner, S.; Malcher, V.; Enko, D.; Mangge, H.; Holasek, S.J.; Schnedl, W.J. Histamine-reduced diet and increase of serum diamine oxidase correlating to diet compliance in histamine intolerance. Eur. J. Clin. Nutr. 2019, 73, 102–104. [Google Scholar] [CrossRef]
- Iannone, L.F.; Nassini, R.; Patacchini, R.; Geppetti, P.; De Logu, F. Neuronal and non-neuronal TRPA1 as therapeutic targets for pain and headache relief. Temperature 2023, 10, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Zaeem, Z.; Zhou, L.; Dilli, E. Headaches: A Review of the Role of Dietary Factors. Curr. Neurol. Neurosci. Rep. 2016, 16, 101. [Google Scholar] [CrossRef]
- Mercanti, N.; Macaluso, M.; Pieracci, Y.; Flamini, G.; Scappaticci, G.; Marianelli, A.; Zinnai, A. Towards Sulphite-Free Winemaking: A New Horizon of Vinification and Maturation. Foods 2024, 13, 1108. [Google Scholar] [CrossRef]
- Karampatea, A.; Skendi, A.; Irakli, M.; Bouloumpasi, E. Replacement of Sulfur Dioxide in White, Rosé, and Red Wines by a Blend of Tannins Extracted from Multiple Plant Materials. Beverages 2024, 10, 110. [Google Scholar] [CrossRef]
- Kesserwani, H. Migraine Triggers: An Overview of the Pharmacology, Biochemistry, Atmospherics, and Their Effects on Neural Networks. Cureus 2021, 13, e14243. [Google Scholar] [CrossRef]
- Karwowska, M.; Kononiuk, A. Nitrates/Nitrites in Food-Risk for Nitrosative Stress and Benefits. Antioxidants 2020, 9, 241. [Google Scholar] [CrossRef]
- Griesenbeck, J.S.; Steck, M.D.; Huber, J.C., Jr.; Sharkey, J.R.; Rene, A.A.; Brender, J.D. Development of estimates of dietary nitrates, nitrites, and nitrosamines for use with the Short Willet Food Frequency Questionnaire. Nutr. J. 2009, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nemzer, B.; Kalita, D.; Yashin, A.Y.; Yashin, Y.I. Chemical Composition and Polyphenolic Compounds of Red Wines: Their Antioxidant Activities and Effects on Human Health—A Review. Beverages 2022, 8, 1. [Google Scholar] [CrossRef]
- López-Lira, C.; Valencia, P.; Urtubia, A.; Landaeta, E.; Tapia, R.A.; Franco, W. Influence of Organic Nitrogen Derived from Recycled Wine Lees and Inorganic Nitrogen on the Chemical Composition of Cabernet Sauvignon Wines Fermented in the Presence of Non-Saccharomyces Yeasts Candida boidinii, C. oleophila, and C. zemplinina. Foods 2024, 13, 4166. [Google Scholar] [CrossRef]
- van den Brand, A.D.; Beukers, M.; Niekerk, M.; van Donkersgoed, G.; van der Aa, M.; van de Ven, B.; Bulder, A.; van der Voet, H.; Sprong, C.R. Assessment of the combined nitrate and nitrite exposure from food and drinking water: Application of uncertainty around the nitrate to nitrite conversion factor. Food Addit. Contam. Part A 2020, 37, 568–582. [Google Scholar] [CrossRef]
- Olesen, J. The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol. Ther. 2008, 120, 157–171. [Google Scholar] [CrossRef]
- Neri, M.; Frustaci, A.; Milic, M.; Valdiglesias, V.; Fini, M.; Bonassi, S.; Barbanti, P. A meta-analysis of biomarkers related to oxidative stress and nitric oxide pathway in migraine. Cephalalgia 2015, 35, 931–937. [Google Scholar] [CrossRef]
- Tietjen, G.E.; Herial, N.A.; White, L.; Utley, C.; Kosmyna, J.M.; Khuder, S.A. Migraine and biomarkers of endothelial activation in young women. Stroke 2009, 40, 2977–2982. [Google Scholar] [CrossRef]
- Heshmat-Ghahdarijani, K.; Javanmard, S.H.; Sonbolestan, S.A.; Saadatnia, M.; Sonbolestan, S.A. Endothelial Function in Patients with Migraine without Aura during the Interictal Period. Int. J. Prev. Med. 2015, 6, 2. [Google Scholar] [CrossRef]
- Yilmaz Avci, A.; Akkucuk, M.H.; Torun, E.; Arikan, S.; Can, U.; Tekindal, M.A. Migraine and subclinical atherosclerosis: Endothelial dysfunction biomarkers and carotid intima-media thickness: A case-control study. Neurol. Sci. 2019, 40, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.A.; Rueda-Clausen, C.F.; Silva, S.Y.; Zarruk, J.G.; Guzmán, J.C.; Morillo, C.A.; Vesga, B.; Pradilla, G.; Flórez, M.; López-Jaramillo, P. Endothelial function in patients with migraine during the interictal period. Headache 2007, 47, 45–51. [Google Scholar] [CrossRef]
- Barbanti, P.; Egeo, G.; Aurilia, C.; Fofi, L.; Della-Morte, D. Drugs targeting nitric oxide synthase for migraine treatment. Expert. Opin. Investig. Drugs 2014, 23, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Milheiro, J.; Ferreira, L.C.; Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. A simple dispersive solid phase extraction clean-up/concentration method for selective and sensitive quantification of biogenic amines in wines using benzoyl chloride derivatisation. Food Chem. 2019, 274, 110–117. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Serreli, G.; Montoro, P.; D’Urso, G.; Congiu, F.; Kowalczyk, A. Biogenic amines and other polar compounds in long aged oxidized Vernaccia di Oristano white wines. Food Res. Int. 2018, 111, 97–103. [Google Scholar] [CrossRef]
- Martuscelli, M.; Arfelli, G.; Manetta, A.C.; Suzzi, G. Biogenic amines content as a measure of the quality of wines of Abruzzo (Italy). Food Chem. 2013, 140, 590–597. [Google Scholar] [CrossRef]
- Redruello, B.; Ladero, V.; Del Rio, B.; Fernández, M.; Martin, M.C.; Alvarez, M.A. A UHPLC method for the simultaneous analysis of biogenic amines, amino acids and ammonium ions in beer. Food Chem. 2017, 217, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, A.B.W.; Davis-Martin, R.E.; Houle, T.T.; Turner, D.P.; Smitherman, T.A. Perceived triggers of primary headache disorders: A meta-analysis. Cephalalgia 2018, 38, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Onderwater, G.L.J.; van Oosterhout, W.P.J.; Schoonman, G.G.; Ferrari, M.D.; Terwindt, G.M. Alcoholic beverages as trigger factor and the effect on alcohol consumption behavior in patients with migraine. Eur. J. Neurol. 2019, 26, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Kelman, L. The triggers or precipitants of the acute migraine attack. Cephalalgia 2007, 27, 394–402. [Google Scholar] [CrossRef]
- Fukui, P.T.; Gonçalves, T.R.; Strabelli, C.G.; Lucchino, N.M.; Matos, F.C.; Santos, J.P.; Zukerman, E.; Zukerman-Guendler, V.; Mercante, J.P.; Masruha, M.R.; et al. Trigger factors in migraine patients. Arq. Neuropsiquiatr. 2008, 66, 494–499. [Google Scholar] [CrossRef]
- Andress-Rothrock, D.; King, W.; Rothrock, J. An analysis of migraine triggers in a clinic-based population. Headache 2010, 50, 1366–1370. [Google Scholar] [CrossRef]
- Finocchi, C.; Sivori, G. Food as trigger and aggravating factor of migraine. Neurol. Sci. 2012, 33 (Suppl. S1), 77–80. [Google Scholar] [CrossRef]
- Hauge, A.W.; Kirchmann, M.; Olesen, J. Trigger factors in migraine with aura. Cephalalgia 2010, 30, 346–353. [Google Scholar] [CrossRef]
- Hauge, A.W.; Kirchmann, M.; Olesen, J. Characterization of consistent triggers of migraine with aura. Cephalalgia 2011, 31, 416–438. [Google Scholar] [CrossRef]
- Yadav, R.K.; Kalita, J.; Misra, U.K. A study of triggers of migraine in India. Pain. Med. 2010, 11, 44–47. [Google Scholar] [CrossRef]
- Sulena; Singla, M.; Brar, J.; Kale, R.; Kale, S. Clinical Profile of Migraine in a Rural Population Presenting to Tertiary Care Hospital in North India. Ann. Indian. Acad. Neurol. 2020, 23, 781–786. [Google Scholar] [CrossRef]
- Mollaoğlu, M. Trigger factors in migraine patients. J. Health Psychol. 2013, 18, 984–994. [Google Scholar] [CrossRef]
- Özcan, R.K.; Özmen, S.G. The Association Between Migraine, Metabolic Syndrome, Insulin Resistance, and Obesity in Women: A Case-Control Study. Şişli Etfal Hastan. Tip Bülteni 2019, 53, 395–402. [Google Scholar] [CrossRef]
- Baldacci, F.; Vedovello, M.; Ulivi, M.; Vergallo, A.; Poletti, M.; Borelli, P.; Nuti, A.; Bonuccelli, U. How aware are migraineurs of their triggers? Headache 2013, 53, 834–837. [Google Scholar] [CrossRef]
- Park, J.W.; Chu, M.K.; Kim, J.M.; Park, S.G.; Cho, S.J. Analysis of Trigger Factors in Episodic Migraineurs Using a Smartphone Headache Diary Applications. PLoS ONE 2016, 11, e0149577. [Google Scholar] [CrossRef] [PubMed]
- Wöber, C.; Brannath, W.; Schmidt, K.; Kapitan, M.; Rudel, E.; Wessely, P.; Wöber-Bingöl, C. Prospective analysis of factors related to migraine attacks: The PAMINA study. Cephalalgia 2007, 27, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Vila, C.; McGown, C. Influence of trigger factors on the efficacy of almotriptan as early intervention for the treatment of acute migraine in a primary care setting: The START study. Expert. Rev. Neurother. 2010, 10, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Casanova, A.; Vives-Mestres, M.; Donoghue, S.; Mian, A.; Wöber, C. The role of avoiding known triggers, embracing protectors, and adhering to healthy lifestyle recommendations in migraine prophylaxis: Insights from a prospective cohort of 1125 people with episodic migraine. Headache 2023, 63, 51–61. [Google Scholar] [CrossRef]
- Lisicki, M.; Schoenen, J. Old Habits Die Hard: Dietary Habits of Migraine Patients Challenge our Understanding of Dietary Triggers. Front. Neurol. 2021, 12, 748419. [Google Scholar] [CrossRef]
- Hindiyeh, N.A.; Zhang, N.; Farrar, M.; Banerjee, P.; Lombard, L.; Aurora, S.K. The Role of Diet and Nutrition in Migraine Triggers and Treatment: A Systematic Literature Review. Headache 2020, 60, 1300–1316. [Google Scholar] [CrossRef]
- Marmura, M.J. Triggers, Protectors, and Predictors in Episodic Migraine. Curr. Pain. Headache Rep. 2018, 22, 81. [Google Scholar] [CrossRef]
- Tu, Y.H.; Chang, C.M.; Yang, C.C.; Tsai, I.J.; Chou, Y.C.; Yang, C.P. Dietary Patterns and Migraine: Insights and Impact. Nutrients 2025, 17, 669. [Google Scholar] [CrossRef]
- van den Hoek, T.C.; Verhagen, I.E.; de Boer, I.; Terwindt, G.M. Substance use in a Dutch migraine cohort compared with the general population. Headache 2024, 64, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Zlotnik, Y.; Plakht, Y.; Aven, A.; Engel, Y.; Am, N.B.; Ifergane, G. Alcohol consumption and hangover patterns among migraine sufferers. J. Neurosci. Rural. Pr. 2014, 5, 128–134. [Google Scholar] [CrossRef]
- Schramm, S.; Tenhagen, I.; Schmidt, B.; Holle-Lee, D.; Naegel, S.; Katsarava, Z.; Jöckel, K.H.; Moebus, S. Prevalence and risk factors of migraine and non-migraine headache in older people—Results of the Heinz Nixdorf Recall study. Cephalalgia 2021, 41, 649–664. [Google Scholar] [CrossRef]
- Schramm, S.H.; Moebus, S.; Lehmann, N.; Galli, U.; Obermann, M.; Bock, E.; Yoon, M.S.; Diener, H.C.; Katsarava, Z. The association between stress and headache: A longitudinal population-based study. Cephalalgia 2015, 35, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Luo, J. Association between migraine and anxiety symptoms: Results from the study of women’s health across the nation. J. Affect. Disord. 2021, 295, 1229–1233. [Google Scholar] [CrossRef]
- Le, H.; Tfelt-Hansen, P.; Skytthe, A.; Kyvik, K.O.; Olesen, J. Association between migraine, lifestyle and socioeconomic factors: A population-based cross-sectional study. J. Headache Pain. 2011, 12, 157–172. [Google Scholar] [CrossRef]
- Kim, B.S.; Chung, C.S.; Lee, C.B.; Rhee, P.L. Migraineurs Initially Visiting the Gastroenterology Department. Headache 2016, 56, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Scher, A.I.; Terwindt, G.M.; Picavet, H.S.; Verschuren, W.M.; Ferrari, M.D.; Launer, L.J. Cardiovascular risk factors and migraine: The GEM population-based study. Neurology 2005, 64, 614–620. [Google Scholar] [CrossRef]
- Rist, P.M.; Buring, J.E.; Kurth, T. Dietary patterns according to headache and migraine status: A cross-sectional study. Cephalalgia 2015, 35, 767–775. [Google Scholar] [CrossRef]
- Aamodt, A.H.; Stovner, L.J.; Hagen, K.; Bråthen, G.; Zwart, J. Headache prevalence related to smoking and alcohol use. The Head-HUNT Study. Eur. J. Neurol. 2006, 13, 1233–1238. [Google Scholar] [CrossRef]
- Geisler, C.; Pankoke, J.; Schlicht, K.; Knappe, C.; Rohmann, N.; Hartmann, K.; Settgast, U.; Türk, K.; Seoudy, A.K.; Franke, A.; et al. BMI, Alcohol Consumption and Gut Microbiome Species Richness Are Related to Structural and Functional Neurological Abnormalities. Nutrients 2021, 13, 3743. [Google Scholar] [CrossRef]
- Pellegrino Baena, C.; Goulart, A.C.; Santos, I.S.; Suemoto, C.K.; Lotufo, P.A.; Bensenor, I.J. Migraine and cognitive function: Baseline findings from the Brazilian Longitudinal Study of Adult Health: ELSA-Brasil. Cephalalgia 2018, 38, 1525–1534. [Google Scholar] [CrossRef]
- McMurtray, A.M.; Saito, E.K.; Diaz, N.; Mehta, B.; Nakamoto, B. Greater frequency of depression associated with chronic primary headaches than chronic post-traumatic headaches. Int. J. Psychiatry Med. 2013, 45, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Vives-Mestres, M.; Casanova, A.; Puig, X.; Ginebra, J.; Rosen, N. Alcohol as a trigger of migraine attacks in people with migraine. Results from a large prospective cohort study in English-speaking countries. Headache 2022, 62, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.T.; Vij, B. Diet and Headache: Part 1. Headache 2016, 56, 1543–1552. [Google Scholar] [CrossRef]
- Evans, E.W.; Lipton, R.B.; Peterlin, B.L.; Raynor, H.A.; Thomas, J.G.; O’Leary, K.C.; Pavlovic, J.; Wing, R.R.; Bond, D.S. Dietary intake patterns and diet quality in a nationally representative sample of women with and without severe headache or migraine. Headache 2015, 55, 550–561. [Google Scholar] [CrossRef]
- Sarker, M.A.; Rahman, M.; Harun-Or-Rashid, M.; Hossain, S.; Kasuya, H.; Sakamoto, J.; Hamajima, N. Association of smoked and smokeless tobacco use with migraine: A hospital-based case-control study in Dhaka, Bangladesh. Tob. Induc. Dis. 2013, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Gür-Özmen, S.; Karahan-Özcan, R. Iron Deficiency Anemia Is Associated with Menstrual Migraine: A Case-Control Study. Pain. Med. 2016, 17, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Papasavva, M.; Vikelis, M.; Siokas, V.; Katsarou, M.S.; Dermitzakis, E.V.; Raptis, A.; Kalliantasi, A.; Dardiotis, E.; Drakoulis, N. Variability in oxidative stress-related genes (SOD2, CAT, GPX1, GSTP1, NOS3, NFE2L2, and UCP2) and susceptibility to migraine clinical phenotypes and features. Front. Neurol. 2022, 13, 1054333. [Google Scholar] [CrossRef]
- Błaszczyk, B.; Straburzyński, M.; Więckiewicz, M.; Budrewicz, S.; Niemiec, P.; Staszkiewicz, M.; Waliszewska-Prosół, M. Relationship between alcohol and primary headaches: A systematic review and meta-analysis. J. Headache Pain. 2023, 24, 116. [Google Scholar] [CrossRef]
- Holsteen, K.K.; Hittle, M.; Barad, M.; Nelson, L.M. Development and Internal Validation of a Multivariable Prediction Model for Individual Episodic Migraine Attacks Based on Daily Trigger Exposures. Headache 2020, 60, 2364–2379. [Google Scholar] [CrossRef]
- Carr, T.; Kilian, C.; Llamosas-Falcón, L.; Zhu, Y.; Lasserre, A.M.; Puka, K.; Probst, C. The risk relationships between alcohol consumption, alcohol use disorder and alcohol use disorder mortality: A systematic review and meta-analysis. Addiction 2024, 119, 1174–1187. [Google Scholar] [CrossRef]
- Dueland, A.N. Headache and Alcohol. Headache 2015, 55, 1045–1049. [Google Scholar] [CrossRef]
- Domingues, R.B.; Domingues, S.A.; Lacerda, C.B.; Machado, T.V.; Duarte, H.; Teixeira, A.L. Alcohol use problems in migraine and tension-type headache. Arq. Neuropsiquiatr. 2014, 72, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, M.; Vaingankar, J.A.; Abdin, E.; Chong, S.A. Psychiatric morbidity in pain conditions: Results from the Singapore Mental Health Study. Pain. Res. Manag. 2013, 18, 185–190. [Google Scholar] [CrossRef]
- Yuan, S.; Daghlas, I.; Larsson, S.C. Alcohol, coffee consumption, and smoking in relation to migraine: A bidirectional Mendelian randomization study. Pain 2022, 163, e342–e348. [Google Scholar] [CrossRef]
- Liu, X.; Yu, Y.; Hou, L.; Yu, Y.; Wu, Y.; Wu, S.; He, Y.; Ge, Y.; Wei, Y.; Luo, Q.; et al. Association between dietary habits and the risk of migraine: A Mendelian randomization study. Front. Nutr. 2023, 10, 1123657. [Google Scholar] [CrossRef]
- Rehm, J.; Rovira, P.; Llamosas-Falcón, L.; Shield, K.D. Dose-Response Relationships between Levels of Alcohol Use and Risks of Mortality or Disease, for All People, by Age, Sex, and Specific Risk Factors. Nutrients 2021, 13, 2652. [Google Scholar] [CrossRef]
- Tadokoro, T.; Oura, K.; Nakahara, M.; Fujita, K.; Tani, J.; Morishita, A.; Kobara, H. Genetic Polymorphisms of ALDH2 and ADH1B in Alcohol-Induced Liver Injury: Molecular Mechanisms of Inflammation and Disease Progression in East Asian Populations. Int. J. Mol. Sci. 2025, 26, 8328. [Google Scholar] [CrossRef]
- Succi, M.; Coppola, F.; Testa, B.; Pellegrini, M.; Iorizzo, M. Alcohol or No Alcohol in Wine: Half a Century of Debate. Foods 2025, 14, 1854. [Google Scholar] [CrossRef] [PubMed]
- Hay, S.I.; Ong, K.L.; Santomauro, D.F.; Aalipour, M.A.; Aalruz, H.; Ababneh, H.S.; Abaraogu, U.O.; Abate, B.B.; Abbafati, C.; Abbas, N.; et al. Burden of 375 diseases and injuries, risk-attributable burden of 88 risk factors, and healthy life expectancy in 204 countries and territories, including 660 subnational locations, 1990-2023: A systematic analysis for the Global Burden of Disease Study 2023. Lancet 2025, 406, 1873–1922. [Google Scholar] [CrossRef]
- Bramness, J.G.; Lid, T.G.; Bolstad, I.; Lien, L.; Abebe, D.S. Predictors of alcohol use disorder in patients with hypertension: A national registry-based cohort study. BMC Public. Health 2025, 25, 2352. [Google Scholar] [CrossRef] [PubMed]
- Buse, D.C.; McGinley, J.S.; Lipton, R.B. Predicting the Future of Migraine Attack Prediction. Headache 2020, 60, 2125–2128. [Google Scholar] [CrossRef]
- Peres, M.F.; Mercante, J.P.; Guendler, V.Z.; Corchs, F.; Bernik, M.A.; Zukerman, E.; Silberstein, S.D. Cephalalgiaphobia: A possible specific phobia of illness. J. Headache Pain. 2007, 8, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Sohi, I.; Franklin, A.; Chrystoja, B.; Wettlaufer, A.; Rehm, J.; Shield, K. The Global Impact of Alcohol Consumption on Premature Mortality and Health in 2016. Nutrients 2021, 13, 3145. [Google Scholar] [CrossRef] [PubMed]
- Rumgay, H.; Shield, K.; Charvat, H.; Ferrari, P.; Sornpaisarn, B.; Obot, I.; Islami, F.; Lemmens, V.; Rehm, J.; Soerjomataram, I. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021, 22, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Rumgay, H.; Murphy, N.; Ferrari, P.; Soerjomataram, I. Alcohol and Cancer: Epidemiology and Biological Mechanisms. Nutrients 2021, 13, 3173. [Google Scholar] [CrossRef] [PubMed]

| Reference | Country | Method of Study | Patients No | Diagnosis | % of Patients Reporting Alcohol As a Trigger | Trigger Factor |
|---|---|---|---|---|---|---|
| Spierings 2001 [20] | USA | Questionnaire | 17/38 | TTH/M | 29/42 | Alcohol |
| Wöber 2006 [21] | Austria | Cross-sectional study | 22/66 | TTH/MO | 31.8/40.9 | Alcohol |
| Takeshima 2004 [19] | Japan | Questionnare | 1125/122/ 301/41 | ETTH/CTTH/ MO/MA | 1.9/0.0/ 1.4/0.0 | Wine |
| Karli 2005 [22] | Turkey | Questionnaire | 31/23/33 | ETTH/MA/MO | 6.5/0/6.1 | Alcohol |
| Panconesi 2013 [14] | Italy | Questionnaire | 47/401 | TH/M | 0/4.9 | Alcohol |
| Wang 2013 [23] | China | Cross-sectional survey | 344/394 | TH/M | 7.6/11.4 | Alcohol |
| Nutrients | Alcoholic Beverages | References | |||
|---|---|---|---|---|---|
| Red Wine | White Wine | Beer | Spirits | ||
| Polyphenols [mg/100 mL] | 108.38 | 59.91 | 71.48 | 1.25 | [101] |
| Flavonoids [mg/100 mL] | 82.5 | 34.83 | 45.9 | 0 | [101] |
| Resveratrol [mg/100 mL] | 1.83 | 0.59 | 0 | 0 | [101] |
| Serotonin [µg/l] | <LOD − 2.28 | No data available | No data available | [64] | |
| Histamine [mg/L] | <LOD − 28.1 | <LOD – 16.6 | 0.1–5.0 | No data available | [61,102,103] |
| Tyramine [mg/L] | 0.5–37.5 | 0–6.8 | 0.1–58.3 | No data available | [104,105] |
| Nitrates [mg/L] | - | 0.21–54.1 | - | [89,93] | |
| Nitrites [mg/serving] | - | < LOD | - | ||
| Nitrosamines [μg/serving] | 0.019 μg/136 g | 0.53 μg/357 g | 0.02 μg/41 g | ||
| Reference | Country | Method of Study | Patients No | Diagnosis | % of Patients Reporting Alcohol As a Trigger | Type of Alcohol |
|---|---|---|---|---|---|---|
| Onderwater 2019 [107] | The Netherlands | Cross-sectional questionnaire study | 2197 | MO | 35.6 | Alcohol |
| 77.8 | Red wine | |||||
| Wöber 2006 [21] | Austria | Cross-sectional study | 66 | MO | 40.9 | Alcohol |
| Kelman 2007 [108] | USA | Observational retrospective questionnaire study | 1750 | MO | 37.8 | Alcohol |
| Fukui 2008 [109] | Brazil | Observational retrospective questionnaire study | 200 | MO | 34 | Alcohol |
| 1.5 | Red wine | |||||
| 10.5 | White wine | |||||
| 1.5 | Soft drink | |||||
| Andress-Rothrock 2010 [110] | USA | Questionnaire survey | 200 | MO | 20.5 | Alcohol |
| Finocchi 2012 [111] | Italy | Observational retrospective questionnaire study | 100 | MO | 20 | Wine |
| Hauge 2010 [112] | Denmark | Observational retrospective questionnaire study | 347 | MA | 34.5 | Alcohol |
| Hauge 2011 [113] | Denmark | Questionnaire survey | 126 | MA | 22 20.91 11.5 9.41 5.23 18 | Alcohol Red wine Liquor Champagne or sparkling wine White wine Beer |
| Yadav 2010 [114] | India | Observational retrospectivequestionnaire study | 182 | MO | 0 | Alcohol |
| Sulena 2020 [115] | India | Observational retrospective questionnaire study | 1065/180 | MO/MA | 2.9 | Food items including alcohol |
| Takeshima 2004 [19] | Japan | Questionnaire survey | 213/31 | MO/MA | 1.4/0 | Wine |
| Karli 2005 [22] | Turkey | Observational retrospective questionnaire study | 33/23 | MO/MA | 6.1/0 | Alcohol |
| Mollaoğlu 2013 [116] | Turkey | Prospective cohort study | 126 | MO | 3.9 | Alcohol |
| Özcan 2019 [117] | Turkey | Questionnaire | 142 | MO + MA | 2 | Wine |
| Panconesi 2013 [14] | Italy | Cross-sectional study | 401 | MO + MA | 4.9 | Alcohol |
| 2.9 | Wine |
| Reference | Country | Method of Study | Migraine Patients No | Control Group (No Headache) No | % Migraine Drinkers | % Control Group Drinkers | Type of Alcohol and/or Method of Consumption |
|---|---|---|---|---|---|---|---|
| Van den Hoek 2024 [127] | The Netherlands | Questionnaire | 5176 | 8370 | 63 | 78 | Current alcohol consumption |
| Lebedeva 2016 [15] | International | Interview | 496 | 1014 | 26 | 27.8 | Light alcoholic drinks |
| 9.5 | 9.3 | Strong alcoholic drinks | |||||
| Zlotnik 2014 [128] | Israel | Questionnaire | 95 | 597 | 78.95 | 81.41 | Alcohol |
| Lisicki 2021 [123] | International | Questionnaire | 59 | 77 | 71 40 37 2 | 75 33 35 8 | Wine consumption Once a month Once a week Everyday |
| Schramm 2021 [129] | Germany | Questionnaire | 584 MA 168 MO 416 | 634 | 13.7 11.8 | 13.6 | Alcohol abuse |
| Schramm 2015 [130] | Germany | Questionnaire | 724 | 1074 | 0.3 | 1 | Alcohol use (daily or almost daily drinking of AD) |
| Hagen 2018 [18] | Norway | Questionnaire | 644 | 12,815 | 9.8 | 7 | Never |
| 65.5 | 51.2 | <4/mth | |||||
| 17.6 | 15.8 | 4–7/mth | |||||
| 3.6 | 6.5 | >8/mth | |||||
| Luo 2021 [131] | USA | Questionnaire | 517 | 2683 | 10.02 | 13.61 | Had any alcohol in the last 24 h |
| Le 2011 [132] | Denmark | Questionnaire | 8044 MA-3086 MO-4958 | 23,821 | 27.7 (MA-29.8, MO-26.3) | 18.9 | Alcohol |
| 21.8 (MA-21.0, MO-22.4) | 20.5 | Never/seldom Monthly | |||||
| 50.5 (MA-49.2, MO-51.3) | 60.6 | Weekly | |||||
| Kim 2016 [133] | Korea | Cross-sectional study | 51 | 102 | 5.9 | 22.5 | Alcohol drinking once a week |
| Scher 2005 [134] | Netherland | Questionnaire | 620 MA-192 MO-396 | 5135 | MA-48, MO-51 MA-26, MO-24 MA-23, MO-23 MA-3.3, MO-2.4 | 37 23 32 7.3 | 0 drinks/day <1 drinks/day, 1–3 drinks/day >4 drinks/day |
| Rist 2015 [135] | USA | Cross-sectional study | 7042 | 25,755 | 1.17 1.19 1.13 1.22 | 39.3 80.4 59.1 78.1 | Total alcohol consumption Beer White wine Red wine |
| Aamodt 2006 [136] | Norway | A population-based cross-sectional study | 6209 | 13,873 | 35 | 31.6 | Abstainers |
| 36.5 | 34.6 | 1–4 glasses/2 weeks | |||||
| 16.7 | 19.8 | 4–14 glasses/2 weeks | |||||
| 1.5 | 2.2 | >14 glasses/2 weeks | |||||
| Geisler 2021 [137] | Germany | Questionnaire | 135 | 612 | 1.1 | 2.56 | Alcohol consumption as a % of energy requirement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zduńska, A.; Cegielska, J.; Zduński, S.; Domitrz, I. Migraine and Alcohol—Is It Really That Harmful? Nutrients 2025, 17, 3620. https://doi.org/10.3390/nu17223620
Zduńska A, Cegielska J, Zduński S, Domitrz I. Migraine and Alcohol—Is It Really That Harmful? Nutrients. 2025; 17(22):3620. https://doi.org/10.3390/nu17223620
Chicago/Turabian StyleZduńska, Anna, Joanna Cegielska, Sebastian Zduński, and Izabela Domitrz. 2025. "Migraine and Alcohol—Is It Really That Harmful?" Nutrients 17, no. 22: 3620. https://doi.org/10.3390/nu17223620
APA StyleZduńska, A., Cegielska, J., Zduński, S., & Domitrz, I. (2025). Migraine and Alcohol—Is It Really That Harmful? Nutrients, 17(22), 3620. https://doi.org/10.3390/nu17223620





