Nutritional Supplements for Muscle Hypertrophy: Mechanisms and Morphology—Focused Evidence
Abstract
1. Introduction
- Q1. Morphology-direct endpoints (ultrasound/MRI thickness or cross-sectional area) are more specific indicators of hypertrophy in trained adults than lean-mass surrogates.
- Q2. Protein/EAA support hypertrophy primarily via MPS; consistent morphology-level benefits are most likely when baseline intake or per-meal leucine exposure is insufficient.
- Q3. Creatine contributes to hypertrophy predominantly indirectly through improved training volume/quality, with effects emerging over adequately long, progressive programs.
- Q4. HMB’s benefits, if present, are condition-dependent (e.g., high training load, energy deficit) and attenuated in eucaloric, well-trained conditions.
- Q5. Adjuncts (omega-3, citrulline/nitrates, collagen) are more likely to act as facilitators of training and recovery than as direct drivers of morphological change.
2. Materials and Methods
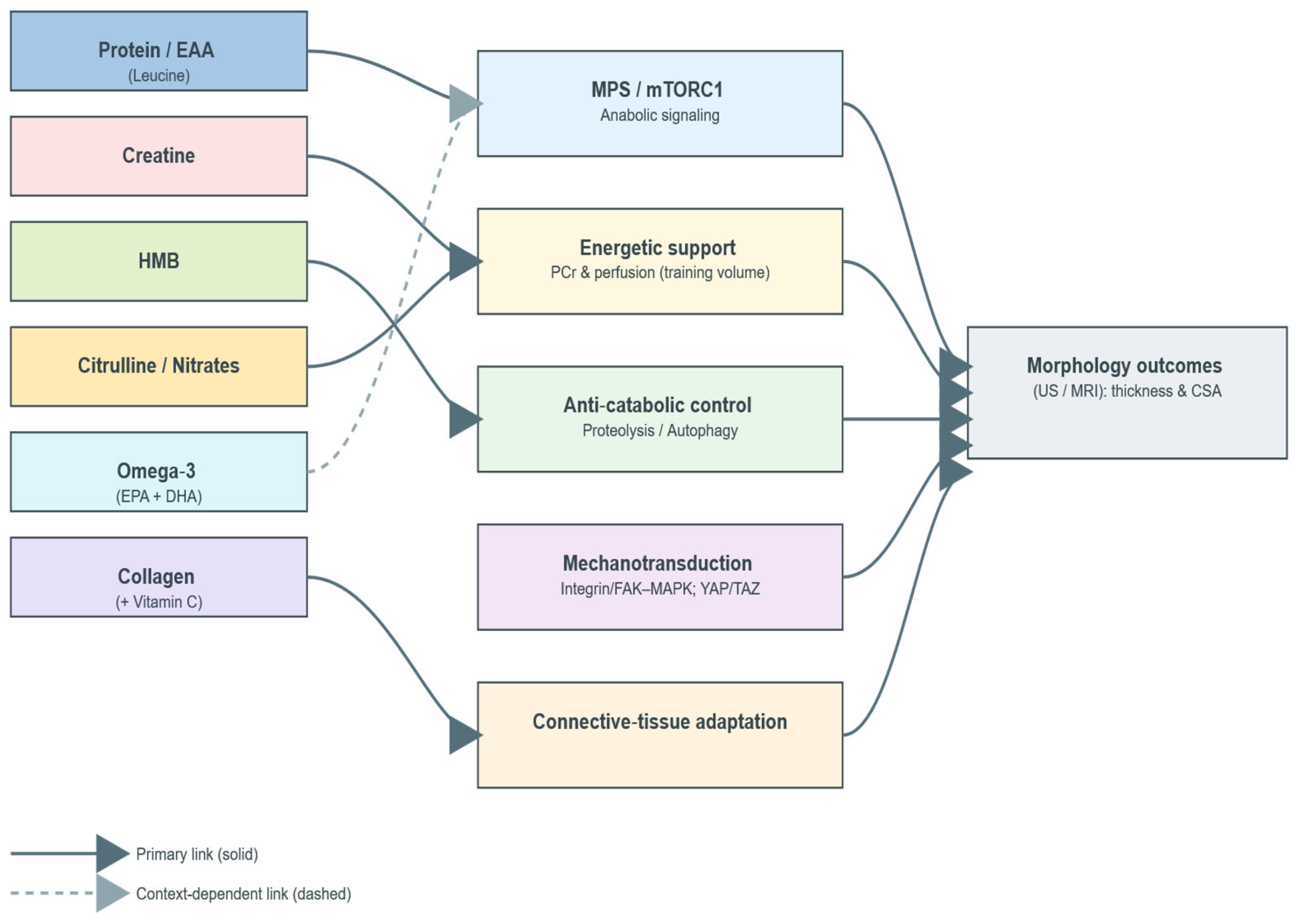
2.1. Conceptual Framework
2.2. Scope and Eligibility
2.2.1. Scope
2.2.2. Eligibility Criteria
2.2.3. Minimum Intervention Duration
2.2.4. Participants
2.2.5. Outcomes and Role of Surrogates
2.2.6. Rationale for Supplement Classes
- ▪ Protein/EAA (leucine)—direct stimulation of MPS via mTORC1-linked mechanisms (leucine “threshold”/per-meal distribution);
- ▪ Creatine monohydrate—energetic support (PCr buffering) enabling higher training volume/quality with downstream morphological accrual;
- ▪ β-hydroxy-β-methylbutyrate (HMB)—anti-catabolic candidate with putative benefit under high training stress or energy deficit;
- ▪ Adjuncts (omega-3, citrulline/nitrates, collagen)—plausible facilitators (anabolic sensitivity/recovery, perfusion/tolerance, connective-tissue adaptation), for which morphology-direct evidence remains limited or context-dependent.
2.2.7. Training Status (Operational Definitions)
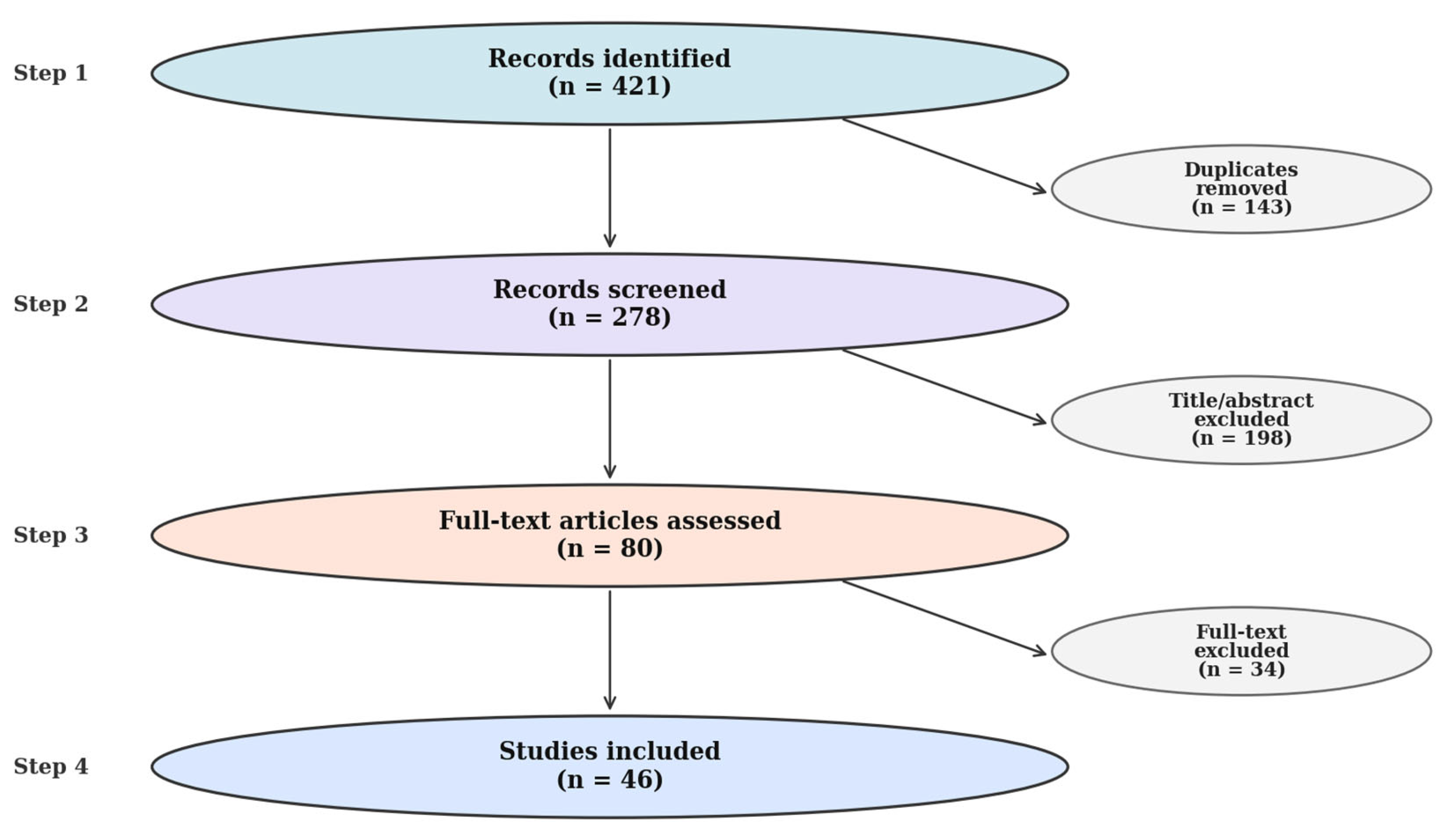
2.3. Information Sources and Search Strategy
- Trained ≥ 6 months of supervised resistance training or structured equivalent;
- Untrained < 3 months of resistance training or irregular/unstructured exposure.
2.4. Study Selection and Data Extraction
2.5. Quality Appraisal and Risk of Bias (RoB 2)
2.6. Evidence Appraisal and Synthesis Approach
3. Results
3.1. Proteins and Essential Amino Acids (EAA; Leucine)
3.2. Creatine Monohydrate
3.3. β-Hydroxy-β-Methylbutyrate (HMB)
3.4. Adjuncts: Omega-3 Fatty Acids, Citrulline/Nitrates, Collagen
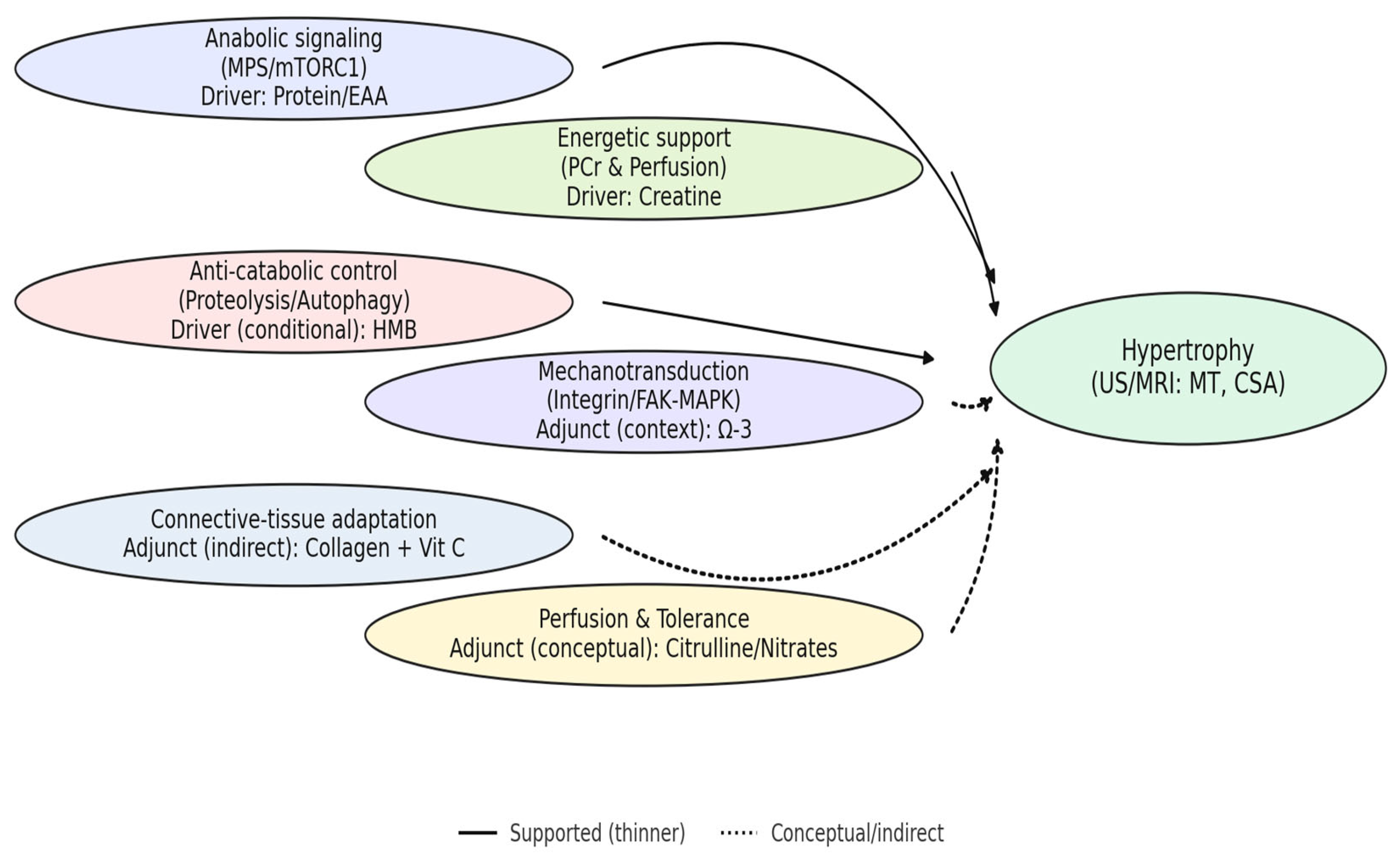
3.5. Evidence Synthesis Across Supplement Classes
3.6. Cross-Walk to Guiding Analytical Questions (Q1–Q5)
3.7. Quality and Risk-of-Bias Snapshot (Rolled-Up)
4. Discussion
4.1. Integrating Findings with the Guiding Analytical Questions (Q1–Q5)
4.2. Practical Translation for Resistance-Trained Populations
4.3. Safety and Ethical Considerations
4.4. Limitations, Measurement Issues, and Research Gaps
4.5. Standardizing Morphological Assessments (Recommendations)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iraki, J.; Fitschen, P.; Espinar, S.; Helms, E. Nutrition Recommendations for Bodybuilders in the Off-Season. Sports 2019, 7, 154. [Google Scholar] [CrossRef]
- Mazzilli, M.; Macaluso, F.; Zambelli, S.; Picerno, P.; Iuliano, E. The Use of Dietary Supplements in Fitness Practitioners: A Cross-Sectional Observation Study. Int. J. Environ. Res. Public Health 2021, 18, 5005. [Google Scholar] [CrossRef]
- Knapik, J.J.; Steelman, R.A.; Hoedebecke, S.S.; Austin, K.G.; Farina, E.K.; Lieberman, H.R. Prevalence of Dietary Supplement Use by Athletes: Systematic Review and Meta-Analysis. Sports Med. 2016, 46, 103–123. [Google Scholar] [CrossRef]
- Tidmas, V.; Turner, A.; Koropsak, D.; Tinsley, G.M. Nutritional and Non-Nutritional Strategies in Bodybuilding. Int. J. Environ. Res. Public Health 2022, 19, 4288. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis (EWGSOP2). Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Thurmond, D.C. Mechanisms by Which Skeletal Muscle Myokines Regulate Metabolism. Int. J. Mol. Sci. 2022, 23, 4636. [Google Scholar] [CrossRef]
- Manescu, D.C. Nutritional tips for muscular mass hypertrophy. Marathon 2016, 8, 79–83. [Google Scholar]
- McKay, M.J.; Weber, K.A., II; Wesselink, E.O.; Smith, Z.A.; Abbott, R.; Anderson, D.B.; Ashton-James, C.E.; Atyeo, J.; Beach, A.J.; Burns, J.; et al. MuscleMap: An Open-Source, Community-Supported Consortium for Whole-Body Quantitative MRI of Muscle. J. Imaging 2024, 10, 262. [Google Scholar] [CrossRef]
- Burke, R.; Piñero, A.; Coleman, M.; Mohan, A.; Sapuppo, M.; Augustin, F.; Aragon, A.A.; Candow, D.G.; Forbes, S.C.; Swinton, P.; et al. The Effects of Creatine Supplementation Combined with Resistance Training on Regional Measures of Muscle Hypertrophy: A Systematic Review with Meta-Analysis. Nutrients 2023, 15, 2116. [Google Scholar] [CrossRef]
- Gonzalez, A.M.; Townsend, J.R.; Pinzone, A.G.; Hoffman, J.R. Supplementation with Nitric Oxide Precursors for Strength Performance: A Review of the Current Literature. Nutrients 2023, 15, 660. [Google Scholar] [CrossRef]
- Hooijmans, M.T.; Schlaffke, L.; Bolsterlee, B.; Schlaeger, S.; Marty, B.; Mazzoli, V. Compositional and Functional MRI of Skeletal Muscle: A Review. J. Magn. Reson. Imaging 2024, 60, 860–877. [Google Scholar] [CrossRef]
- Sinha, U.; Sinha, S. Magnetic Resonance Imaging Biomarkers of Muscle. Tomography 2024, 10, 1411–1438. [Google Scholar] [CrossRef]
- Manescu, D.C. Alimentaţia în Fitness şi Bodybuilding; Editura ASE: Bucharest, Romania, 2010. [Google Scholar]
- Hirabara, S.M.; Marzuca-Nassr, G.N.; Cury-Boaventura, M.F. Nutrition and Exercise Interventions on Skeletal Muscle Physiology, Injury and Recovery: From Mechanisms to Therapy. Nutrients 2024, 16, 293. [Google Scholar] [CrossRef]
- Manescu, D.C. Nutriție Ergogenă, Suplimentație și Performanță; Editura Risoprint: Cluj, Romania, 2025. [Google Scholar]
- Kuikman, M.A.; Smith, E.; McKay, A.K.A.; McCormick, R.; Ackerman, K.E.; Harris, R.; Elliott-Sale, K.J.; Stellingwerff, T.; Burke, L.M. Impact of Acute Dietary and Exercise Manipulation on Next-Day RMR Measurements and DXA Body Composition Estimates. Med. Sci. Sports Exerc. 2025, 57, 285–295. [Google Scholar] [CrossRef]
- Kojima, C.; Namma-Motonaga, K.; Kamei, A.; Takahashi, Y.; Ishibashi, A.; Takahashi, H. Dynamics of Muscle Glycogen Increase in Brachial and Thigh Muscles with Carbohydrate Loading. Eur. J. Appl. Physiol. 2025, 125, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Tavoian, D.; Ampomah, K.; Amano, S.; Law, T.D.; Clark, B.C. Changes in DXA-Derived Lean Mass and MRI-Derived Cross-Sectional Area of the Thigh Are Modestly Associated. Sci. Rep. 2019, 9, 10028. [Google Scholar] [CrossRef] [PubMed]
- Stokes, T.; Tyler, C.; Goosey-Tolfrey, V.; Burniston, J.; English, C.; Phillips, B.E.; Wilkinson, D.J.; Atherton, P.J. Methodological Considerations for and Validation of the Use of B-Mode Ultrasonography to Estimate Changes in Muscle Size. Physiol. Rep. 2021, 9, e14683. [Google Scholar] [CrossRef] [PubMed]
- Ruple, B.A.; Smith, M.A.; Osburn, S.C.; Sexton, C.L.; Godwin, J.S.; Edison, J.L.; Poole, C.N.; Stock, M.S.; Fruge, A.D.; Young, K.C.; et al. Comparisons between skeletal muscle imaging techniques and histology in tracking midthigh hypertrophic adaptations following 10 weeks of resistance training. J. Appl. Physiol. 2022, 133, 416–425. [Google Scholar] [CrossRef]
- Engelke, K.; Chaudry, O.; Gast, L.; Ab Eldib, M.; Wang, L.; Laredo, J.-D.; Nagel, A.M. Magnetic Resonance Imaging Techniques for the Quantitative Analysis of Skeletal Muscle: State of the Art. J. Orthop. Translat. 2023, 42, 57–72. [Google Scholar] [CrossRef]
- Alic, L.; Griffin, J.F.; Eresen, A.; Kornegay, J.N.; Ji, J.X. Using MRI to quantify skeletal muscle pathology in Duchenne muscular dystrophy: A systematic mapping review. Muscle Nerve 2021, 64, 8–22. [Google Scholar] [CrossRef]
- Zamosteanu, D.; Filip, N.; Trandafir, L.M.; Ţarcă, E.; Pertea, M.; Bordeianu, G.; Bernic, J.; Heredea, A.M.; Cojocaru, E. Current Data on the Role of Amino Acids in the Management of Obesity in Children and Adolescents. Int. J. Mol. Sci. 2025, 26, 7129. [Google Scholar] [CrossRef]
- Jeong, D.; Park, K.; Lee, J.; Choi, J.; Du, H.; Jeong, H.; Li, L.; Sakai, K.; Kang, S. Effects of Resistance Exercise and Essential Amino Acid Intake on Muscle Quality, Myokine, and Inflammation Factors in Young Adult Males. Nutrients 2024, 16, 1688. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Breen, L.; Di Donato, D.M.; Hector, A.J.; Mitchell, C.J.; Moore, D.R.; Stellingwerff, T.; Breuille, D.; Offord, E.A.; Baker, S.K. Leucine Supplementation of a Low-Protein Meal Enhances Myofibrillar Protein Synthesis in Young Men: A Randomized Crossover Trial. Am. J. Clin. Nutr. 2014, 99, 276–286. [Google Scholar] [CrossRef]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and Exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef]
- Branch, J.D. Effect of creatine supplementation on body composition and performance: A meta-analysis. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 198–226. [Google Scholar] [CrossRef]
- Candow, D.G.; Chilibeck, P.D.; Burke, D.G.; Mueller, K.D.; Lewis, J.D. Effect of Different Frequencies of Creatine Supplementation on Muscle Size and Strength in Young Adults. J. Strength Cond. Res. 2011, 25, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Dinan, N.E.; Forbes, S.C.; Stout, J.R.; Cooke, M.; Taylor, K.-L.; Hannan, A.; Pulido, E.; Wilborn, C.; Ergogenic Adaptations Research Group. Effects of creatine monohydrate timing on resistance training adaptations and body composition. Front. Sports Act. Living 2022, 4, 1033842. [Google Scholar] [CrossRef] [PubMed]
- Roschel, H.; Gualano, B.; Ostojic, S.M.; Rawson, E.S. Creatine Supplementation and Brain Health. Nutrients 2021, 13, 586. [Google Scholar] [CrossRef] [PubMed]
- Rathmacher, J.A.; Pitchford, L.M.; Stout, J.R.; Townsend, J.R.; Jäger, R.; Kreider, R.B.; Campbell, B.I.; Kerksick, C.M.; Harty, P.S.; Candow, D.G.; et al. International Society of Sports Nutrition Position Stand: β-Hydroxy-β-Methylbutyrate (HMB). J. Int. Soc. Sports Nutr. 2025, 22, 2434734. [Google Scholar] [CrossRef] [PubMed]
- Bideshki, M.V.; Behzadi, M.; Jamali, M.; Jamilian, P.; Zarezadeh, M.; Pourghassem Gargari, B. Ergogenic Benefits of β-Hydroxy-β-Methyl Butyrate (HMB) Supplementation on Body Composition and Muscle Strength: An Umbrella Review of Meta-Analyses. J. Cachexia Sarcopenia Muscle 2025, 16, e13671. [Google Scholar] [CrossRef]
- Courel-Ibáñez, J.; Vetrovsky, T.; Dadova, K.; Pallarés, J.G.; Steffl, M. Health Benefits of β-Hydroxy-β-Methylbutyrate (HMB) Supplementation in Addition to Physical Exercise in Older Adults: A Systematic Review with Meta-Analysis. Nutrients 2019, 11, 2082. [Google Scholar] [CrossRef]
- Brown, K.; Persinger, A.; Pryke, A.; Lin, J.; Wallace, N.; Chizhikov, D.; Puppa, M. Effects of omega-3- and omega-6-rich high-fat diets on skeletal muscle protein degradation signaling in glucocorticoid-treated mice. Int. J. Funct. Nutr. 2025, 6, 4. [Google Scholar] [CrossRef]
- Son, W.; Brown, K.; Persinger, A.; Pryke, A.; Lin, J.; Powell, Z.; Wallace, N.; van der Merwe, M.; Puppa, M. Effect of Omega-3 Rich High-Fat Diet on Markers of Tissue Lipid Metabolism in Glucocorticoid-Treated Mice. Int. J. Mol. Sci. 2023, 24, 11492. [Google Scholar] [CrossRef] [PubMed]
- Da Boit, M.; Sibson, R.; Sivasubramaniam, S.; Meakin, J.R.; Greig, C.A.; Aspden, R.M.; Thies, F.; Jeromson, S.; Hamilton, D.L.; Speakman, J.R. Sex-Specific Effects of n-3 PUFA on Muscle Function and Quality in Older Adults: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2017, 105, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Alsharif, N.S.; Clifford, T.; Alhebshi, A.; Rowland, S.N.; Bailey, S.J. Effects of Dietary Nitrate Supplementation on Performance during Single and Repeated Bouts of Short-Duration High-Intensity Exercise: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Antioxidants 2023, 12, 1194. [Google Scholar] [CrossRef]
- Martínez-Puig, D.; Chevalier, X.; Manfredini, D.; Ornetti, P.; Henrotin, Y. Collagen Supplementation for Joint Health: Mechanisms and Clinical Evidence. Nutrients 2023, 15, 1332. [Google Scholar] [CrossRef]
- Hudson, J.L.; Bergia, R.E., III; Campbell, W.W. Protein Distribution and Muscle-Related Outcomes: Does the Evidence Support the Concept? Nutrients 2020, 12, 1441. [Google Scholar] [CrossRef]
- Layman, D.K. Impacts of Protein Quantity and Distribution on Body Composition. Front. Nutr. 2024, 11, 1388986. [Google Scholar] [CrossRef]
- Goldman, D.M.; Warbeck, C.B.; Karlsen, M.C. Completely Plant-Based Diets That Meet Energy Requirements for Resistance Training Can Supply Enough Protein and Leucine to Maximize Hypertrophy and Strength in Male Bodybuilders: A Modeling Study. Nutrients 2024, 16, 1122. [Google Scholar] [CrossRef]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohassel, P.; Rankin, D.; Mitchell, W.K.; Kumar, V.; Narayan, D.S.; Mittendorfer, B. Omega-3 Fatty Acids Increase the Rate of Muscle Protein Synthesis in Older Adults: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef]
- Amiri, E.; Sheikholeslami-Vatani, D. The role of resistance training and creatine supplementation on oxidative stress, antioxidant defense, muscle strength, and quality of life in older adults. Front. Public Health 2023, 11, 1062832. [Google Scholar] [CrossRef]
- Therdyothin, A.; Prokopidis, K.; Galli, F.; Witard, O.C.; Isanejad, M. The effects of omega-3 polyunsaturated fatty acids on muscle and whole-body protein synthesis: A systematic review and meta-analysis. Nutr. Rev. 2025, 83, e131–e143. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Silva, R.; Cholewa, J.M.; de Araújo Pessôa, K.; Xia, Z.; Lauver, J.D.; Rossi, F.E.; Zanchi, N.E. Creatine supplementation combined with blood flow restriction training enhances muscle thickness and performance: A randomized, placebo-controlled, and double-blind study. Appl. Physiol. Nutr. Metab. 2023, 48, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Lowery, R.P.; Joy, J.M.; Andersen, J.C.; Wilson, S.M.C.; Stout, J.R.; Duncan, N.; Fuller, J.C.; Baier, S.M.; Naimo, M.A.; et al. The Effects of 12 Weeks of Beta-Hydroxy-Beta-Methylbutyrate Free Acid Supplementation on Muscle Mass, Strength, and Power in Resistance-Trained Individuals: A Randomized, Double-Blind, Placebo-Controlled Study. Eur. J. Appl. Physiol. 2014, 114, 1217–1227. [Google Scholar] [CrossRef]
- Jakubowski, J.S.; Wong, E.P.T.; Nunes, E.A.; Noguchi, K.S.; Vandeweerd, J.K.; Murphy, K.T.; Morton, R.W.; McGlory, C.; Phillips, S.M. Equivalent Hypertrophy and Strength Gains in β-Hydroxy-β-Methylbutyrate- or Leucine-Supplemented Men. Med. Sci. Sports Exerc. 2019, 51, 65–74. [Google Scholar] [CrossRef]
- Hu, Y.-G.; Shi, J.-H.; Yu, D.-X.; Huang, H.-B. The Effects of β-Hydroxy-β-Methyl Butyrate Supplementation in Surgical Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2025, 12, 1621206. [Google Scholar] [CrossRef]
- Tomczyk, M. Omega-3 Fatty Acids and Muscle Strength—Current State of Knowledge and Future Perspectives. Nutrients 2024, 16, 4075. [Google Scholar] [CrossRef]
- Shaw, G.; Lee-Barthel, A.; Ross, M.L.; Wang, B.; Baar, K. Vitamin C-Enriched Gelatin Supplementation before Intermittent Activity Augments Collagen Synthesis. Am. J. Clin. Nutr. 2017, 105, 136–143. [Google Scholar] [CrossRef]
- Manescu, D.C. Fitness; Editura Risoprint: Cluj, Romania, 2025. [Google Scholar]
- Inacio, P.A.Q.; Gomes, Y.S.M.; de Aguiar, A.J.N.; Lopes-Martins, P.S.L.; Aimbire, F.; Leonardo, P.S.; Sá Filho, A.S.; Lopes-Martins, R.A.B. The Effects of Collagen Peptides as a Dietary Supplement on Muscle Damage Recovery and Fatigue Responses: An Integrative Review. Nutrients 2024, 16, 3403. [Google Scholar] [CrossRef]
- Forsting, J.; Forsting, M.; Froeling, M.; Güttsches, A.-K.; Südkamp, N.; Roos, A.; Vorgerd, M.; Schlaffke, L.; Rehmann, R. Quantitative muscle MRI captures early fat infiltration in leg muscles: A study on calpainopathy. Sci. Rep. 2022, 12, 19934. [Google Scholar] [CrossRef]
- De Mello, R.; Ma, Y.; Ji, Y.; Du, J.; Chang, E.Y. Quantitative MRI Musculoskeletal Techniques: An Update. AJR Am. J. Roentgenol. 2019, 213, 524–533. [Google Scholar] [CrossRef]
- Chianca, V.; Vincenzo, B.; Cuocolo, R.; Zappia, M.; Guarino, S.; Di Pietto, F.; Del Grande, F. MRI Quantitative Evaluation of Muscle Fatty Infiltration. Magnetochemistry 2023, 9, 111. [Google Scholar] [CrossRef]
- Eck, B.L.; Yang, M.; Elias, J.J.; Winalski, C.S.; Altahawi, F.; Subhas, N.; Li, X. Quantitative MRI for Evaluation of Musculoskeletal Disease: Cartilage and Muscle Composition, Joint Inflammation, and Biomechanics in Osteoarthritis. Investig. Radiol. 2023, 58, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Emanuelsson, E.B.; Berry, D.B.; Reitzner, S.M.; Arif, M.; Mardinoglu, A.; Gustafsson, T.; Ward, S.R.; Sundberg, C.J.; Chapman, M.A. MRI Characterization of Skeletal Muscle Size and Fatty Infiltration in Long-Term Trained and Untrained Individuals. Physiol. Rep. 2022, 10, e15398. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.N.; Ducker, K.J.; Furzer, B.J.; Dymock, M.; Landers, G.J. Acute exercise affects dual-energy X-ray absorptiometry body composition estimates but not standardised ultrasound measurements of subcutaneous adipose tissue. Clin. Physiol. Funct. Imaging 2023, 43, 345–353. [Google Scholar] [CrossRef]
- Kondo, E.; Takai, E.; Sagayama, H.; Takahashi, H. Comparison of three type(s) of muscle glycogen loading interventions using a very-high-carbohydrate diet in an elite male racewalker: A case report. Phys. Act. Nutr. 2023, 27, 47–54. [Google Scholar] [CrossRef]
- Högelin, E.R.; Thulin, K.; von Walden, F.; Fornander, L.; Michno, P.; Alkner, B. Reliability and validity of an ultrasound-based protocol for measurement of quadriceps muscle thickness in children. Front. Physiol. 2022, 13, 830216. [Google Scholar] [CrossRef]
- Thanaj, M.; Basty, N.; Whitcher, B.; Sorokin, E.P.; Liu, Y.; Srinivasan, R.; Cule, M.; Thomas, E.L.; Bell, J.D. Precision MRI phenotyping of muscle volume and quality at a population scale. Front. Physiol. 2024, 15, 1288657. [Google Scholar] [CrossRef]
- Areta, J.L.; Burke, L.M.; Ross, M.L.; Camera, D.M.; West, D.W.D.; Broad, E.M.; Jeacocke, N.A.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M.; et al. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J. Physiol. 2013, 591, 2319–2331. [Google Scholar] [CrossRef]
- Helms, E.R.; Aragon, A.A.; Fitschen, P.J. Evidence-Based Recommendations for Natural Bodybuilding Contest Preparation: Nutrition and Supplementation. J. Int. Soc. Sports Nutr. 2014, 11, 20. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition Position Stand: Safety and Efficacy of Creatine Supplementation in Exercise, Sport, and Medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef]
- Holeček, M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J. Cachexia Sarcopenia Muscle 2017, 8, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.J.; Matias, C.N.; Monteiro, C.P.; Valamatos, M.J.; Reis, J.F.; Batista, A.; Oliveira, A.C.; Alves, F.; Sardinha, L.B.; Phillips, S.M. No Effect of HMB or α-HICA Supplementation on Training-Induced Changes in Body Composition. Eur. J. Sport Sci. 2019, 19, 802–810. [Google Scholar] [CrossRef]
- Deutz, N.E.P.; Pereira, S.L.; Hays, N.P.; Oliver, J.S.; Edens, N.K.; Evans, C.M.; Wolfe, R.R. Effect of β-hydroxy-β-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin. Nutr. 2013, 32, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-R.; Jo, E.; Khamoui, A.V. Chronic Fish Oil Consumption with Resistance Training Improves Grip Strength, Physical Function, and Blood Pressure in Community-Dwelling Older Adults. Sports 2019, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Baráth, A.; Annár, D.; Györe, I.; Szmodis, M. The Effects of L-Citrulline and Malic Acid on Substrate Utilisation and Lactate Elimination. Appl. Sci. 2024, 14, 8055. [Google Scholar] [CrossRef]
- Zdzieblik, D.; Oesser, S.; Baumstark, M.W.; Gollhofer, A.; König, D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: A randomized controlled trial. Br. J. Nutr. 2015, 114, 1237–1245. [Google Scholar] [CrossRef]
- Reidy, P.T.; Borack, M.S.; Markofski, M.M.; Dickinson, J.M.; Deer, R.R.; Husaini, S.H.; Walker, D.K.; Igbinigie, S.; Robertson, S.M.; Cope, M.B.; et al. Protein Supplementation Has Minimal Effects on Muscle Adaptations during Resistance Exercise Training in Young Men: A Double-Blind Randomized Clinical Trial. J. Nutr. 2016, 146, 1660–1669. [Google Scholar] [CrossRef]
- Mobley, C.B.; Haun, C.T.; Roberson, P.A.; Mumford, P.W.; Kephart, W.C.; Romero, M.A.; Osburn, S.C.; Vann, C.G.; Young, K.C.; Beck, D.T.; et al. Effects of Whey, Soy or Leucine Supplementation with 12 Weeks of Resistance Training on Strength, Body Composition, and Skeletal Muscle and Adipose Tissue Histological Attributes in College-Aged Males. Nutrients 2017, 9, 972. [Google Scholar] [CrossRef]
- Joy, J.M.; Lowery, R.P.; Wilson, J.M.; Purpura, M.; De Souza, E.O.; Wilson, S.M.; Kalman, D.S.; Dudeck, J.E.; Jäger, R. The Effects of 8 Weeks of Whey or Rice Protein Supplementation on Body Composition and Exercise Performance. Nutr. J. 2013, 12, 86. [Google Scholar] [CrossRef]
- Lynch, H.M.; Wharton, C.; Johnston, C.S.; Kris-Etherton, P.M.; Post, R.E.; Parkinson, A.L.; Little, R.B.; Most, M.; West, S.G.; Armamento-Villareal, R.; et al. No Significant Differences in Muscle Growth and Strength Development When Consuming Soy and Whey Protein Supplements Matched for Leucine Following a 12-Week Resistance Training Program in Men and Women: A Randomized Trial. Int. J. Environ. Res. Public Health 2020, 17, 3871. [Google Scholar] [CrossRef]
- Babault, N.; Païzis, C.; Deley, G.; Guérin-Deremaux, L.; Saniez, M.H.; Lefranc-Millot, C.; Allaert, F.A. Pea Proteins Oral Supplementation Promotes Muscle Thickness Gains during Resistance Training: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial vs. Whey Protein. J. Int. Soc. Sports Nutr. 2015, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Bridge, A.; Brown, J.; Snider, H.; Nasato, M.; Prapavessis, H. Greek Yogurt and 12 Weeks of Exercise Training on Strength, Muscle Thickness, and Body Composition in Lean, Untrained, University-Aged Males. Front. Nutr. 2019, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Sharp, M.H.; Lowery, R.P.; Shields, K.A.; Lane, J.R.; Gray, J.L.; Partl, J.M.; Hayes, D.W.; Wilson, G.J.; Hollmer, C.A.; Minivich, J.R.; et al. The Effects of Beef, Chicken, or Whey Protein after Workout on Body Composition and Muscle Performance. J. Strength Cond. Res. 2018, 32, 2233–2242. [Google Scholar] [CrossRef] [PubMed]
- Naclerio, F.; Seijo, M.; Larumbe-Zabala, E.; Earnest, C.P. Carbohydrates Alone or Mixing with Beef or Whey Protein Promote Similar Training Outcomes in Resistance-Training Males: A Double-Blind, Randomized Controlled Clinical Trial. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 408–420. [Google Scholar] [CrossRef]
- Farup, J.; Rahbek, S.K.; Vendelbo, M.H.; Matzon, A.; Hindhede, J.; Bejder, A.; Ringgaard, S.; Vissing, K. High-Leucine Whey Protein Hydrolysate Augments Muscle and Tendon Hypertrophy Following 12 Weeks of Resistance Training—Irrespective of Contraction Mode. Scand. J. Med. Sci. Sports 2014, 24, 788–798. [Google Scholar] [CrossRef]
- Vieillevoye, S.; Poortmans, J.R.; Duchateau, J.; Carpentier, A. Effects of a Combined Essential Amino Acids/Carbohydrate Supplementation on Muscle Mass, Architecture and Maximal Strength Following Heavy-Load Training. Eur. J. Appl. Physiol. 2010, 110, 479–488. [Google Scholar] [CrossRef]
- Banaszek, A.; Townsend, J.R.; Bender, D.; Vantrease, W.C.; Marshall, A.C.; Johnson, K.D. The Effects of Whey vs. Pea Protein on Physical Adaptations Following 8-Weeks of High-Intensity Functional Training (HIFT): A Pilot Study. Sports 2019, 7, 12. [Google Scholar] [CrossRef]
- Jacinto, J.L.; Nunes, J.P.; Gorissen, S.H.M.; Capel, D.M.G.; Bernardes, A.G.; Ribeiro, A.S.; Cyrino, E.S.; Phillips, S.M.; Aguiar, A.F. Whey Protein Supplementation Is Superior to Leucine-Matched Collagen Peptides to Increase Muscle Thickness During a 10-Week Resistance Training Program in Untrained Young Adults. Int. J. Sport Nutr. Exerc. Metab. 2022, 32, 133–143. [Google Scholar] [CrossRef]
- Hartman, J.W.; Tang, J.E.; Wilkinson, S.B.; Tarnopolsky, M.A.; Lawrence, R.L.; Fullerton, A.V.; Phillips, S.M. Consumption of Fat-Free Fluid Milk after Resistance Exercise Promotes Greater Lean Mass Accretion than Does Consumption of Soy or Carbohydrate in Young, Novice, Male Weightlifters. Am. J. Clin. Nutr. 2007, 86, 373–381. [Google Scholar] [CrossRef]
- Chilibeck, P.D.; Stride, D.; Farthing, J.P.; Burke, D.G. Effect of Creatine Ingestion after Exercise on Muscle Thickness in Males and Females. Med. Sci. Sports Exerc. 2004, 36, 1781–1788. [Google Scholar] [CrossRef]
- Mills, S.; Candow, D.G.; Forbes, S.C.; Neary, J.P.; Ormsbee, M.J.; Antonio, J. Effects of Creatine Supplementation during Resistance Training Sessions in Physically Active Young Adults. Nutrients 2020, 12, 1880. [Google Scholar] [CrossRef]
- Pakulak, A.; Muddle, T.W.D.; Rollo, I.; Galloway, S.D.R.; Pritchard, H.J.; Tallis, J. Effects of Creatine and Caffeine Supplementation during Resistance Training on Body Composition, Strength, Endurance, RPE and Fatigue in Trained Young Adults. J. Diet. Suppl. 2022, 19, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Aragon, A.A.; Wilborn, C.; Urbina, S.; Hayward, S.; Krieger, J. Pre- versus Post-Exercise Protein Intake Has Similar Effects on Muscular Adaptations. PeerJ 2017, 5, e2825. [Google Scholar] [CrossRef] [PubMed]
- Joy, J.M.; Lowery, R.P.; Wilson, J.M.; Purpura, M.; De Souza, E.O.; McDonnell, E.; Wilson, S.M.C.; Kalman, D.S.; Dudeck, J.E.; Jäger, R. Phosphatidic Acid Enhances mTOR Signaling and Resistance Exercise Training Adaptations in Human Skeletal Muscle. Nutr. Metab. 2014, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.M.; Sell, K.M.; Ghigiarelli, J.J.; Kelly, C.F.; Shone, E.W.; Accetta, M.R.; Baum, J.B.; Mangine, G.T. Effects of Phosphatidic Acid Supplementation on Muscle Thickness and Strength in Resistance-Trained Men. Appl. Physiol. Nutr. Metab. 2017, 42, 443–448. [Google Scholar] [CrossRef]
- Andre, T.L.; Gann, J.J.; McKinley-Barnard, S.K.; Song, J.J.; Willoughby, D.S. Eight Weeks of Phosphatidic Acid Supplementation in Conjunction with Resistance Training Does Not Differentially Affect Body Composition and Muscle Strength in Resistance-Trained Men. J. Sports Sci. Med. 2016, 15, 532–539. [Google Scholar]
- Escalante, G.; Alencar, M.; Haddock, B.; Harvey, P. The Effects of Phosphatidic Acid Supplementation on Strength, Body Composition, Muscular Endurance, Power, Agility, and Vertical Jump in Resistance Trained Men. J. Int. Soc. Sports Nutr. 2016, 13, 24. [Google Scholar] [CrossRef]
- Lowery, R.P.; Joy, J.M.; Rathmacher, J.A.; Baier, S.M.; Fuller, J.C., Jr.; Shelley, M.C., II; Jäger, R.; Purpura, M.; Wilson, S.M.C.; Wilson, J.M. Interaction of Beta-Hydroxy-Beta-Methylbutyrate Free Acid and Adenosine Triphosphate on Muscle Mass, Strength, and Power in Resistance-Trained Individuals. J. Strength Cond. Res. 2016, 30, 1843–1854. [Google Scholar] [CrossRef]
- Townsend, J.R.; Hart, T.L.; Haynes, J.T.; Woods, C.A.; Toy, A.M.; Pihera, B.C. Influence of Dietary Nitrate Supplementation on Physical Performance and Body Composition Following Offseason Training in Division I Athletes. J. Diet. Suppl. 2022, 19, 534–549. [Google Scholar] [CrossRef]
- Jerger, S.; Bohm, S.; Marzilger, R.; Mersmann, F.; Arampatzis, A. Effects of Specific Collagen Peptide Supplementation on Tendon and Muscle Adaptations to High-Load Resistance Training: A Randomized Controlled Trial. Scand. J. Med. Sci. Sports 2022, 32, 1212–1226. [Google Scholar] [CrossRef]
- Balshaw, T.G.; Funnell, M.P.; McDermott, E.; Maden-Wilkinson, T.M.; Abela, S.; Quteishat, B.; Edsey, M.; James, L.J.; Folland, J.P. The effect of specific bioactive collagen peptides on function and muscle remodeling during human resistance training. Acta Physiol. 2023, 237, e13903. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Bridge, J.E.; Clark, D.R.; Stewart, C.E.; Erskine, R.M. Collagen Supplementation Augments Changes in Patellar Tendon Properties in Female Soccer Players. Front. Physiol. 2023, 14, 1089971. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.M.; Lievense, K.K.; Norton, S.C.; Costa, J.V.; Alphin, K.H.; Bailey, L.A.; Miller, G.D. The Effects of Graded Protein Intake in Conjunction with Resistance Training on Muscle Mass, Strength, and Physical Function in Older Adults: A Randomized Trial. Nutrients 2022, 14, 2739. [Google Scholar] [CrossRef]
- Fraschetti, E.C.; Abdul-Sater, A.A.; Perry, C.G.R.; Josse, A.R. R. Resistance Exercise Training and Greek Yogurt Consumption Modulate Markers of Systemic Inflammation in Healthy Young Males—A Secondary Analysis of a Randomized Controlled Trial. Nutrients 2025, 17, 2816. [Google Scholar] [CrossRef]
- Joy, J.M.; Vogel, R.M.; Shane Broughton, K.; Kudla, U.; Kerr, N.Y.; Davison, J.M. Daytime and Nighttime Casein Supplements Similarly Increase Muscle Size and Strength in Response to Resistance Training. J. Int. Soc. Sports Nutr. 2018, 15, 24. [Google Scholar] [CrossRef]
- Babault, N.; Deley, G.; Le Ruyet, P.; Morgan, F.; Allaert, F.A. Effects of Soluble Milk Protein or Casein Supplementation on Muscle Fatigue Following Resistance Training Program: A Randomized, Double-Blind, Placebo-Controlled Study. J. Int. Soc. Sports Nutr. 2014, 11, 36. [Google Scholar] [CrossRef]
- Snijders, T.; Res, P.T.; Smeets, J.S.J.; van Vliet, S.; van Kranenburg, J.; Maase, K.; Verdijk, L.B.; van Loon, L.J.C. Protein Ingestion before Sleep Increases Muscle Mass and Strength Gains during Prolonged Resistance-Type Exercise Training in Healthy Young Men. J. Nutr. 2015, 145, 1178–1184. [Google Scholar] [CrossRef]
- Souza-Junior, T.P.; Willardson, J.M.; Bloomer, R.; Leite, R.D.; Fleck, S.J.; Oliveira, P.R.; Simão, R. Strength and Hypertrophy Responses to Constant and Decreasing Rest Intervals in Trained Men Using Creatine Supplementation. J. Int. Soc. Sports Nutr. 2011, 8, 17. [Google Scholar] [CrossRef]
- Cribb, P.J.; Williams, A.D.; Hayes, A. Effects of Whey Isolate, Creatine, and Resistance Training on Muscle Hypertrophy. Med. Sci. Sports Exerc. 2007, 39, 1960–1968. [Google Scholar] [CrossRef]
- Tritto, A.C.; Bueno, S.; Rodrigues, R.M.P.; Gualano, B.; Roschel, H.; Artioli, G.G. Negligible Effects of β-Hydroxy-β-Methylbutyrate Free Acid and Calcium Salt on Strength and Hypertrophic Responses to Resistance Training: A Randomized, Placebo-Controlled Study. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 505–511. [Google Scholar] [CrossRef]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A Systematic Review, Meta-Analysis and Meta-Regression of the Effect of Protein Supplementation on Resistance Training-Induced Gains in Muscle Mass and Strength in Healthy Adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Ellerbroek, A.; Silver, T.; Vargas, L.; Peacock, C. The Effects of Consuming a High Protein Diet (4.4 g/kg/d) on Body Composition in Resistance-Trained Individuals. J. Int. Soc. Sports Nutr. 2022, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Ellerbroek, A.; Silver, T.; Orris, S.; Scheiner, M.; Gonzalez, A.; Peacock, C. A High Protein Diet (3.4 g/kg/d) Combined with a Heavy Resistance Training Program Improves Body Composition in Healthy Trained Men and Women—A Follow-Up Investigation. J. Int. Soc. Sports Nutr. 2022, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Candow, D.G.; Vogt, E.; Johannsmeyer, S.; Forbes, S.C.; Farthing, J.P. Strategic Creatine Supplementation and Resistance Training in Healthy Older Adults. Appl. Physiol. Nutr. Metab. 2015, 40, 689–694. [Google Scholar] [CrossRef]
- Antonio, J.; Ellerbroek, A.; Silver, T.; Vargas, L.; Tamayo, A.; Buehn, R.; Peacock, C.A. A High-Protein Diet Has No Harmful Effects: A One-Year Crossover Study in Resistance-Trained Men. J. Nutr. Metab. 2016, 2016, 9104792. [Google Scholar] [CrossRef]
- Haun, C.T.; Vann, C.G.; Mobley, C.B.; Roberson, P.A.; Osburn, S.C.; Holmes, H.M.; Mumford, P.M.; Romero, M.A.; Young, K.C.; Moon, J.R.; et al. Effects of Graded Whey Supplementation During Extreme-Volume Resistance Training. Front. Nutr. 2018, 5, 84. [Google Scholar] [CrossRef]
- Trommelen, J.; Kouw, I.W.K.; Holwerda, A.M.; Snijders, T.; Halson, S.L.; Rollo, I. Presleep Dietary Protein-Derived Amino Acids Are Utilized for De Novo Myofibrillar Protein Synthesis During Overnight Recovery from Exercise in Healthy Older Men. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E457–E467. [Google Scholar] [CrossRef]
- Haun, C.T.; Vann, C.G.; Osburn, S.C.; Mumford, P.W.; Roberson, P.A.; Romero, M.A.; Fox, C.D.; Johnson, C.A.; Parry, H.A.; Kavazis, A.N.; et al. Muscle Fiber Hypertrophy in Response to 6 Weeks of High-Volume Resistance Training in Trained Young Men Is Largely Attributed to Sarcoplasmic Hypertrophy. PLoS ONE 2019, 14, e0215267. [Google Scholar] [CrossRef]
- Willoughby, D.S.; Stout, J.R.; Wilborn, C.D. Effects of Resistance Training and Protein Plus Amino Acid Supplementation on Muscle Anabolism, Mass, and Strength. Amino Acids 2007, 32, 467–477. [Google Scholar] [CrossRef]

| Supplement & Comparator | Population (N/Sex/Age/Training Status) | RT Program & Duration | Imaging (Site) | Dose & Timing | ΔMT/CSA (Primary Outcome) |
|---|---|---|---|---|---|
| Creatine vs. placebo (ID 14) | 30 M; 22 ± 2 y; Trained | Split 5 d/ wk supervised; 6 wks | US multisite (upper/lower) | ~0.1 g·kg−1·d−1 intra-workout | ↑ MT over time in both; no between-group difference; strength ↑ with creatine |
| Creatine vs. caffeine vs. combo vs. placebo (ID 16) | 32 M/F; 24 ± 3 y; Trained | Total-body 4 d/wk; 6 wks | US (knee extensors; elbow flexors/extensors) | ~0.1 g·kg−1 pre-workout; caffeine 3 mg·kg−1 | ↑ MT knee extensors with creatine; others ≈ NS |
| HMB-FA vs. placebo (ID 23) | 40 M; 23 ± 2 y; Trained | Periodized RT with overreaching; 12 wks | US (quadriceps MT) | 3 g·d−1 (split doses) | ↑ MT and ↑ LBM vs. placebo; strength/power ↑ |
| Whey/Soy/Leucine vs. Placebo (ID 2) | 28 M; 21 ± 3 y; Untrained | Supervised RT; 12 wks | US (VL thickness) | 2×/day; ~3 g leucine/serving | Time effect on VL; no supplement effect; satellite cells ↑ with whey |
| Whey vs. placebo (ID 11) | 26 M/F; 25 ± 4 y; Mixed | ≥3 mo RT; supervised; 12 wks | US (biceps; VL/VI/RF) | Post-exercise; daily dose | Local VI ↑ with whey; otherwise NS |
| Pea protein vs. Whey vs. placebo (ID 5) | 24 M; 20 ± 2 y; Untrained | Upper-body focus; 12 wks | US (biceps MT) | 25 g ×2/day (incl. post-session) | ↑ biceps all groups; Pea > placebo in weakest subgroup; Pea ≈ Whey |
| Hydrolyzed whey vs. CHO (ID 9) | 18 M; 24 ± 3 y; Trained | Concentric vs. eccentric emphasis; 12 wks | MRI (quadriceps CSA; tendon) | ~0.3 g·kg−1 post-session | Whey H > CHO for muscle & tendon hypertrophy |
| Greek yogurt vs. CHO (ID 6) | 20 M; 19 ± 2 y; Untrained | Supervised RT; 12 wks | US (biceps MT) | ~20 g protein/serving peri-workout & snacks | ↑ biceps MT > CHO; strength ↑ |
| Whey vs. collagen (leucine-equated) (ID 12) | 22 M/F; 21 ± 2 y; Untrained | Supervised RT; 10 wks | US (arm/thigh MT) | Iso-leucine doses | Whey > collagen for ↑ MT during RT |
| Study | Imaging (Site[s]) | Δ Treat (Summary) | Δ Control (Summary) | Between-Group Outcome (ΔΔ) | Effect Size (Approx.) | Reliability (ICC/TE) | Assessor Blinded | Overall RoB2 |
|---|---|---|---|---|---|---|---|---|
| Creatine vs. placebo | US multisite (upper/lower) | ↑ MT both groups | ↑ MT both groups | n.s. | small, n.s. | NR | Y | Some concerns |
| Creatine vs. caffeine vs. placebo | US knee extensors + EF | ↑ MT (knee extensors) | ≈NS | small ΔΔ | small | ICC NR | NR | Some concerns |
| HMB-FA vs. placebo | US quadriceps | ↑ MT, ↑ LBM, ↑ strength | slight ↑MT | significant ΔΔ (p < 0.05) | medium | ICC NR | NR | High |
| Whey/Soy/ Leucine vs. placebo | US VL | ↑ VL | ↑ VL | n.s. | small, n.s. | Single-site US | Y | Some concerns |
| Whey vs. placebo | US biceps + VL/VI/RF | ↑ VI thickness | ≈NS | small ΔΔ (VI) | small | Variable reliability | NR | Some concerns |
| Pea vs. whey vs. placebo | US biceps | ↑ biceps MT; Pea > placebo | ↑ biceps MT | Pea > placebo; Pea ≈ whey | small | Reported consistent | Y | Low |
| Hydrolyzed whey vs. CHO | MRI quadriceps CSA + tendon | ↑ CSA, tendon hypertrophy | smaller ↑ | Whey > CHO | medium | Standardized MRI | Y | Low |
| Greek yogurt vs. CHO | US biceps | ↑ biceps MT; ↑ strength | ↑ biceps MT (less) | Yogurt > CHO | small–medium | NR | NR | Some concerns |
| Whey vs. collagen | US arm + thigh | ↑ MT (whey) | smaller ↑ MT | Whey > collagen | small–medium | ICC NR | NR | Some concerns |
| Supplement | Primary Mechanistic Node(s) | Typical Dosing (Range) | Typical Timing | Morphology (US/MRI) Evidence in Trained Adults | Consistency | Key Caveats |
|---|---|---|---|---|---|---|
| Protein/EAA (Leucine) | MPS/mTORC1; translational efficiency; leucine threshold | ~0.3 g·kg−1· meal−1 or 2–3 g leucine/meal; 3–5 meals/day | Pre/ Post RT; distributed | Positive when baseline intake/distribution is inadequate; diminishing returns when already optimized | Moderate to High | Endpoint choice; per-meal distribution; baseline intake; ceiling effect at high protein |
| Creatine Monohydrate | PCr buffering → set quality & training volume | 3–5 g/day (±short loading ~0.3 g·kg−1·day−1 × 5–7 days) | Daily; align with RT blocks | Small–moderate gains with long, progressive RT; neutral when blocks are short or under-progressed | Moderate | Program duration; early water shifts; inter-individual variability; imaging site |
| HMB (FA or Ca-salt) | Anti-catabolic (proteolysis/ autophagy); membrane stability | ~3 g /day (FA or Ca-salt) | Daily; consider high-stress or deficit | Context-dependent; mixed/neutral in eucaloric, highly trained settings | Low to Moderate | Context-dependent; formulation differences; energy status; industry sponsorship |
| Omega-3 Fatty Acids | Membrane/ inflammation; anabolic sensitivity | ~1–3 g /day EPA + DHA | Daily | Limited/heterogeneous direct morphology outcomes; may facilitate training/recovery | Low | Dependent on adequate protein and overload; small samples; heterogeneous dosing |
| Citrulline/ Nitrates | NO pathway; perfusion/ tolerance | 6–8 g L-citrulline; dietary nitrates | Pre - exercise | Inconsistent morphology; performance effects more common | Low | Large inter-study variation; acute vs. chronic effects; weak morphology linkage |
| Collagen (+Vitamin C) | Connective-tissue support | 10–15 g /day | Daily; ± pre-rehab/RT | Limited direct CSA gains; indirect support to training continuity | Low | Outcome alignment; Vit C timing; often inferior to whey comparators; targeted use-cases |
| Protein/EAA (Leucine) | MPS/mTORC1; translational efficiency; leucine threshold | ~0.3 g·kg−1· meal−1 or 2–3 g leucine/meal; 3–5 meals/day | Pre/ Post RT; distributed | Positive when baseline intake/distribution is inadequate; diminishing returns when already optimized | Moderate to High | Endpoint choice; per-meal distribution; baseline intake; ceiling effect at high protein |
| Creatine Monohydrate | PCr buffering → set quality & training volume | 3–5 g/day (±short loading ~0.3 g·kg−1·day−1 × 5–7 days) | Daily; align with RT blocks | Small–moderate gains with long, progressive RT; neutral when blocks are short or under-progressed | Moderate | Program duration; early water shifts; inter-individual variability; imaging site |
| HMB (FA or Ca-salt) | Anti-catabolic (proteolysis/ autophagy); membrane stability | ~3 g /day (FA or Ca-salt) | Daily; consider high-stress or deficit | Context-dependent; mixed/neutral in eucaloric, highly trained settings | Low to Moderate | Context-dependent; formulation differences; energy status; industry sponsorship |
| Omega-3 Fatty Acids | Membrane/ inflammation; anabolic sensitivity | ~1–3 g /day EPA + DHA | Daily | Limited/heterogeneous direct morphology outcomes; may facilitate training/recovery | Low | Dependent on adequate protein and overload; small samples; heterogeneous dosing |
| Citrulline/ Nitrates | NO pathway; perfusion/ tolerance | 6–8 g L-citrulline; dietary nitrates | Pre - exercise | Inconsistent morphology; performance effects more common | Low | Large inter-study variation; acute vs. chronic effects; weak morphology linkage |
| Collagen (+Vitamin C) | Connective-tissue support | 10–15 g /day | Daily; ± pre-rehab/RT | Limited direct CSA gains; indirect support to training continuity | Low | Outcome alignment; Vit C timing; often inferior to whey comparators; targeted use-cases |
| Supplement Class | Imaging RCTs (n) | Median Duration (wk) | Typical ΔΔ (US/MRI) | Typical Δ% Hypertrophy | Signal Strength |
|---|---|---|---|---|---|
| Protein/EAA (Leucine) | ~9 | 10–12 | +1–2 mm MT (vastus lateralis, biceps) | +3–5% vs. controls (when baseline intake <1.6 g·kg−1·day−1) | Strong, but conditional |
| Creatine monohydrate | ~7 | 8–12 | +1.5–2.0 mm MT or +0.3–0.5 cm2 CSA (KE, EF) | +5–7% hypertrophy over controls in long programs | Moderate-to-strong |
| HMB (FA/Ca-salt) | ~3 | 12 | FA trial: +2–3 mm quadriceps MT; others neutral | +4–6% in stressed/deficit states; ≈0% in eucaloric trained | Weak-to-moderate, context-dependent |
| Omega-3 fatty acids | 2 | 8–12 | ≈n.s. for MT/CSA in young trained adults | ≤1% Δ vs. controls | Weak |
| Citrulline/Nitrates | 1–2 | 6–8 | No reproducible MT/CSA effect | 0% | Very weak |
| Collagen (+Vit C) | 2 | 8–10 | Neutral for MT; ↑ tendon CSA ~5–10% | 0% muscle; +5–10% tendon | Weak (connective tissue support) |
| Trial | Randomization | Outcome Measurement | Reporting Bias | Notes (Sponsorship/Blinding) | Overall Risk |
|---|---|---|---|---|---|
| Creatine vs. placebo | Low | Some concerns (single-site US) | Low | Independent; assessor blinded | Some concerns |
| Creatine vs. caffeine vs. combo vs. placebo | Some concerns | Some concerns (limited sites; ICC NR) | Low | Independent; blinding NR | Some concerns |
| HMB-FA vs. placebo | Some concerns | Some concerns (industry involvement; ICC NR) | High | Industry-sponsored; assessor NR | High |
| Whey/Soy/Leucine vs. placebo | Low | Some concerns (single-muscle US) | Low | Independent; assessor blinded | Some concerns |
| Whey vs. placebo | Low | Some concerns (multi-site US; variable reliability) | Low | Independent; blinding NR | Some concerns |
| Pea protein vs. Whey vs. placebo | Low | Low (US; biceps thickness; consistent reporting) | Low | Independent | Low |
| Hydrolyzed whey vs. CHO | Low | Low (MRI standardized, tendon + muscle CSA) | Low | Independent; assessor blinded | Low |
| Greek yogurt vs. CHO | Low | Some concerns (single-site US) | Low | Independent; blinding NR | Some concerns |
| Whey vs. collagen (leucine-equated) | Low | Some concerns (US; variable sites; ICC NR) | Low | Independent; blinding NR | Some concerns |
| Domain | Minimum Reporting Requirement |
|---|---|
| Anatomical site | Exact landmark (e.g., vastus lateralis at 50% femur length); specify side and limb dominance. |
| Probe handling (US) | Angle of insonation, applied pressure, coupling medium (gel, stand-off pad). |
| Timing/participant state | Standardize time of day, hydration, prior exercise, and nutrition before scans. |
| Repetition/averaging | Acquire ≥3 images per site recommended; report procedure for averaging values. |
| Reliability metrics | Provide intra- and inter-rater ICC; Typical Error (TE) or coefficient of variation (CV). |
| Assessor blinding | State explicitly whether imaging assessor was blinded to allocation and time point. |
| Data reporting | Report baseline and post values (mean ± SD), within-group Δ, between-group ΔΔ with 95% CI. |
| Units & transparency | Use standardized units (mm for MT; cm2 for CSA); pre-register measurement protocol; report device make/model. |
| Domain | Reporting Requirement |
|---|---|
| Training load & volume | Total sets, reps, and load lifted per week (absolute and relative to baseline). |
| Intensity & effort | %1RM or RIR (repetitions in reserve) for each session. |
| Session density | Rest intervals and total session duration. |
| Progression | Week-to-week changes in load, volume, or intensity. |
| Performance outcomes | Strength (1RM, isometric MVC), endurance, power (jump, sprint). |
| Morphological outcomes | US/MRI: MT, CSA with exact site and reliability indices. |
| Linking variables | Paired reporting of workload and morphology to permit mediation analyses. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mănescu, A.M.; Hangu, S.Ș.; Mănescu, D.C. Nutritional Supplements for Muscle Hypertrophy: Mechanisms and Morphology—Focused Evidence. Nutrients 2025, 17, 3603. https://doi.org/10.3390/nu17223603
Mănescu AM, Hangu SȘ, Mănescu DC. Nutritional Supplements for Muscle Hypertrophy: Mechanisms and Morphology—Focused Evidence. Nutrients. 2025; 17(22):3603. https://doi.org/10.3390/nu17223603
Chicago/Turabian StyleMănescu, Andreea Maria, Simona Ștefania Hangu, and Dan Cristian Mănescu. 2025. "Nutritional Supplements for Muscle Hypertrophy: Mechanisms and Morphology—Focused Evidence" Nutrients 17, no. 22: 3603. https://doi.org/10.3390/nu17223603
APA StyleMănescu, A. M., Hangu, S. Ș., & Mănescu, D. C. (2025). Nutritional Supplements for Muscle Hypertrophy: Mechanisms and Morphology—Focused Evidence. Nutrients, 17(22), 3603. https://doi.org/10.3390/nu17223603







