Schisandra chinensis Bee Pollen Extract Alleviates Obesity by Modulating Gut Microbiota-Driven Bile Acid Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of SCPE
2.2. Animals and Experimental Design
2.3. Biochemical Analysis
2.4. Histological Characterization
2.5. Oral Glucose Tolerance Test (OGTT)
2.6. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.7. Gut Microbiota Analysis
2.8. BA Metabolomic Analysis
2.9. Statistics Analysis
3. Results
3.1. SCPE Regulates the Gut Microbiota to Alleviate HFD-Induced Obesity and Metabolic Disturbances
3.2. SCPE Regulates the Gut Microbiota to Improve the Expression of BA Metabolism-Related Genes
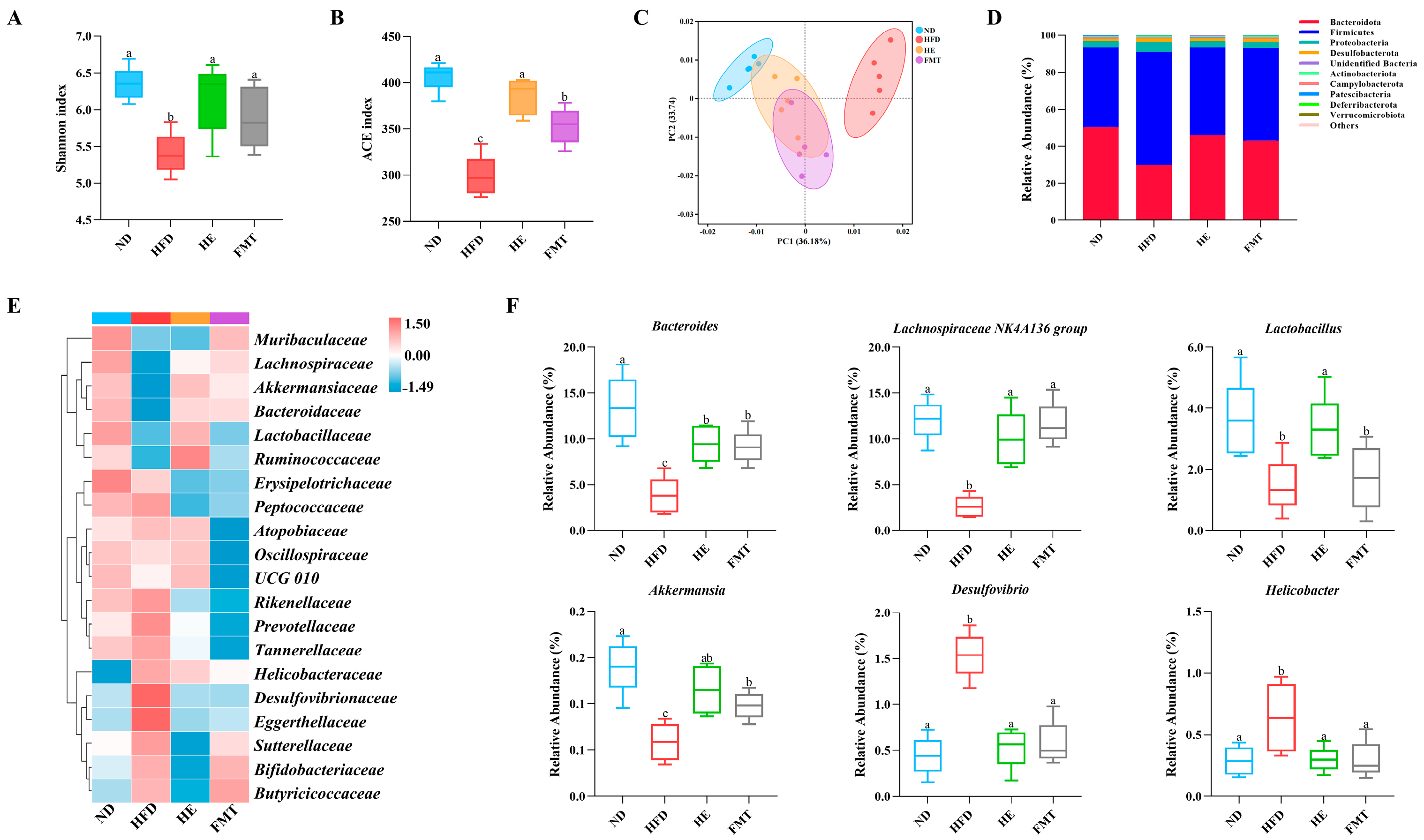
3.3. SCPE Reversed HFD-Induced Gut Microbiota Dysbiosis
3.4. SCPE Regulates the Gut Microbiota to Optimize BA Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zorena, K.; Jachimowicz-Duda, O.; Slezak, D.; Robakowska, M.; Mrugacz, M. Adipokines and obesity. potential link to metabolic disorders and chronic complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Ni, Q.; Sun, W.; Li, L.; Feng, X. The links between gut microbiota and obesity and obesity related diseases. Biomed. Pharmacother. 2022, 147, 37609. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Yacyshyn, B.R.; Yacyshyn, M.B. Gut microbiota and obesity: An opportunity to alter obesity through faecal microbiota transplant (FMT). Diabetes Obes. Metab. 2019, 21, 479–490. [Google Scholar] [CrossRef]
- Lai, Z.-L.; Tseng, C.-H.; Ho, H.J.; Cheung, C.K.; Lin, J.-Y.; Chen, Y.-J.; Cheng, F.-C.; Hsu, Y.-C.; Lin, J.-T.; El-Omar, E.M.; et al. Fecal microbiota transplantation confers beneficial metabolic effects of diet and exercise on diet-induced obese mice. Sci. Rep. 2018, 8, 15625. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clement, K. Metabolism and metabolic disorders and the microbiome: The intestinal microbiota associated with obesity, lipid metabolism, and metabolic health-pathophysiology and therapeutic strategies. Gastroenterology 2021, 160, 573–599. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 2020, 12, 1–19. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lin, C.-S.; Lu, C.-C.; Martel, J.; Ko, Y.-F.; Ojcius, D.M.; Tseng, S.-F.; Wu, T.-R.; Chen, Y.-Y.M.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef]
- Anhe, F.F.; Roy, D.; Pilon, G.; Dudonne, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef]
- Wahlstrom, A.; Sayin, S.I.; Marschall, H.-U.; Backhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- He, X.; Gao, X.; Hong, Y.; Zhong, J.; Li, Y.; Zhu, W.; Ma, J.; Huang, W.; Li, Y.; Li, Y.; et al. High fat diet and high sucrose intake divergently induce dysregulation of glucose homeostasis through distinct gut microbiota-derived bile acid metabolism in mice. J. Agric. Food Chem. 2023, 72, 230–244. [Google Scholar] [CrossRef]
- Zheng, J.; Ye, C.; Hu, B.; Yang, H.; Yao, Q.; Ma, J.; Liu, Y.; Liu, H. Bile acid profiles in bile and feces of obese mice by a high-performance liquid chromatography-tandem mass spectrometry. Biotechnol. Appl. Biochem. 2021, 68, 1332–1341. [Google Scholar] [CrossRef]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Jian, O.-y.; Li, X.-p.; Liu, C.-w.; Jie, O.-y.; Tang, J.-y.; Liu, Q.; Huang, J.-a.; Liu, Z. Moringa-Fu brick tea extract attenuated high-fat diet-induced obesity via modulating bile acid metabolism and gut microbiota in rats. J. Funct. Foods 2023, 109, 105766. [Google Scholar] [CrossRef]
- Mo, W.; Zou, J.; Wu, M.; Peng, Z.; He, W.; Li, W.; Wu, X. Noni (Morinda citrifolia L.) fruit polysaccharide ameliorated high-fat diet-induced obesity by modulating gut microbiota and improving bile acid metabolism. J. Funct. Foods 2023, 101, 105408. [Google Scholar] [CrossRef]
- Shen, Z.; Geng, Q.; Huang, H.; Yao, H.; Du, T.; Chen, L.; Wu, Z.; Miao, X.; Shi, P. Antioxidative and cardioprotective effects of Schisandra chinensis bee pollen extract on isoprenaline-induced myocardial infarction in rats. Molecules 2019, 24, 1090. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Sun, Y.; Han, S.; Gong, J.; Zhang, Y.; Feng, Z.; Yao, H.; Shi, P. Schisandra chinensis bee pollen extract inhibits proliferation and migration of hepatocellular carcinoma hepG2 cells via ferroptosis-, Wnt-, and focal adhesion-signaling pathways. Drug Des. Dev. Ther. 2024, 18, 2745–2760. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, X.; Zhou, Y.; Zhao, X.; Chen, M.; Zhao, H.; Cao, W. Schisandra chinensis bee pollen ameliorates colitis in mice by modulating gut microbiota and regulating Treg/Th17 balance. Foods 2024, 13, 585. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Chen, S.; Liu, X.; Zhao, H.; Cao, W. Impact of Schisandra chinensis bee pollen on nonalcoholic fatty liver disease and gut microbiota in high fat diet induced obese mice. Nutrients 2019, 11, 346. [Google Scholar] [CrossRef]
- An, X.; Sun, S.; Sun, J.; Liao, R.; Ma, R.; Zhao, H.; Liu, Q. Different regulatory effects of Lycium barbarum polysaccharide components on gut microbiota in vivo and in vitro. Food Biosci. 2024, 61, 104643. [Google Scholar] [CrossRef]
- Li, D.; Cui, Y.; Wang, X.; Liu, F.; Li, X. Apple polyphenol extract improves high-fat diet-induced hepatic steatosis by regulating bile acid synthesis and gut microbiota in C57BL/6 male mice. J. Agric. Food Chem. 2021, 69, 6829–6841. [Google Scholar] [CrossRef]
- Collins, S.L.; Stine, J.C.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef]
- Sheth, S.G.; Flamm, S.L.; Gordon, F.D.; Chopra, S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am. J. Gastroenterol. 1998, 93, 44–48. [Google Scholar] [CrossRef]
- Thomas, C.; Pellicciari, R.; Pruzanski, M.; Auwerx, J.; Schoonjans, K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 2008, 7, 678–693. [Google Scholar] [CrossRef] [PubMed]
- Aguchem, R.N.; Okagu, I.U.; Okorigwe, E.M.; Uzoechina, J.O.; Nnemolisa, S.C.; Ezeorba, T.P.C. Role of CETP, PCSK-9, and CYP7-alpha in cholesterol metabolism: Potential targets for natural products in managing hypercholesterolemia. Life Sci. 2024, 351, 122823. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Up to date on cholesterol 7 alpha-hydroxylase (CYP7A1) in bile acid synthesis. Liver Res. 2020, 4, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Yan, S.; Sun, W.; Yan, J.; Teng, M.; Jia, M.; Tian, S.; Zhou, Z.; Zhu, W. Chlorothalonil induces obesity in mice by regulating host gut microbiota and bile acids metabolism via FXR pathways. J. Hazard. Mater. 2023, 452, 131310. [Google Scholar] [CrossRef]
- Xu, M.; Shen, Y.; Cen, M.; Zhu, Y.; Cheng, F.; Tang, L.; Zheng, X.; Kim, J.J.; Dai, N.; Hu, W. Modulation of the gut microbiota-farnesoid X receptor axis improves deoxycholic acid-induced intestinal inflammation in mice. J. Crohns Colitis 2021, 15, 1197–1210. [Google Scholar] [CrossRef]
- Akinrotimi, O.; Riessen, R.; VanDuyne, P.; Park, J.E.; Lee, Y.K.; Wong, L.-J.; Zavacki, A.M.; Schoonjans, K.; Anakk, S. Small heterodimer partner deletion prevents hepatic steatosis and when combined with farnesoid X receptor loss protects against type 2 diabetes in mice. Hepatology 2017, 66, 1854–1865. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xu, W.; He, W.; Ding, M.; Xia, T.; Tan, X. Simiao Wan attenuates high-fat diet-induced hyperlipidemia in mice by modulating the gut microbiota-bile acid axis. J. Ethnopharmacol. 2025, 337, 118868. [Google Scholar] [CrossRef]
- Rao, A.; Kosters, A.; Mells, J.E.; Zhang, W.; Setchell, K.D.R.; Amanso, A.M.; Wynn, G.M.; Xu, T.; Keller, B.T.; Yin, H.; et al. Inhibition of ileal bile acid uptake protects against nonalcoholic fatty liver disease in high-fat diet-fed mice. Sci. Transl. Med. 2016, 8, 122. [Google Scholar] [CrossRef]
- Chen, L.; Yao, X.; Young, A.; McNulty, J.; Anderson, D.; Liu, Y.; Nystrom, C.; Croom, D.; Ross, S.; Collins, J.; et al. Inhibition of apical sodium-dependent bile acid transporter as a novel treatment for diabetes. Am. J. Physiol.-Endocrinol. Metab. 2012, 302, E68–E76. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.P.; Wang, H.H.; Ohashi, A.; Wang, D.Q.H. Role of intestinal sterol transporters Abcg5, Abcg8, and Npc111 in cholesterol absorption in mice: Gender and age effects. Am. J. Physiol.-Gastrointest. Liver Physiol. 2006, 290, G269–G276. [Google Scholar] [CrossRef]
- Hu, H.; Shao, W.; Liu, Q.; Liu, N.; Wang, Q.; Xu, J.; Zhang, X.; Weng, Z.; Lu, Q.; Jiao, L.; et al. Gut microbiota promotes cholesterol gallstone formation by modulating bile acid composition and biliary cholesterol secretion. Nat. Commun. 2022, 13, 84–92. [Google Scholar] [CrossRef]
- Barrasa, J.I.; Olmo, N.; Antonia Lizarbe, M.; Turnay, J. Bile acids in the colon, from healthy to cytotoxic molecules. Toxicol. Vitr. 2013, 27, 964–977. [Google Scholar] [CrossRef]
- Xu, M.; Cen, M.; Shen, Y.; Zhu, Y.; Cheng, F.; Tang, L.; Hu, W.; Dai, N. Deoxycholic acid-induced gut dysbiosis disrupts bile acid enterohepatic circulation and promotes intestinal inflammation. Dig. Dis. Sci. 2021, 66, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dong, W.; Liu, L.; Xu, M.; Wang, Y.; Liu, T.; Zhang, Y.; Wang, B.; Cao, H. Interplay between bile acids and the gut microbiota promotes intestinal carcinogenesis. Mol. Carcinog. 2019, 58, 1155–1167. [Google Scholar] [CrossRef]
- Islam, K.B.M.S.; Fukiya, S.; Hagio, M.; Fujii, N.; Ishizuka, S.; Ooka, T.; Ogura, Y.; Hayashi, T.; Yokota, A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011, 141, 1773–1781. [Google Scholar] [CrossRef]
- Mouzaki, M.; Wang, A.Y.; Bandsma, R.; Comelli, E.M.; Arendt, B.M.; Zhang, L.; Fung, S.; Fischer, S.E.; McGilvray, I.G.; Allard, J.P. Bile acids and dysbiosis in non-alcoholic fatty liver disease. PLoS ONE 2016, 11, 0151829. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlstrom, A.; Felin, J.; Jantti, S.; Marschall, H.-U.; Bamberg, K.; Angelin, B.; Hyotylainen, T.; Oresic, M.; Backhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, H.; Duan, M.; Liu, R.; Zhu, Q.; Zhang, K.; Wang, L. Cinnamaldehyde improves metabolic functions in streptozotocin-induced diabetic mice by regulating gut microbiota. Drug Des. Dev. Ther. 2021, 15, 2339–2355. [Google Scholar] [CrossRef]
- Kuang, J.; Wang, J.; Li, Y.; Li, M.; Zhao, M.; Ge, K.; Zheng, D.; Cheung, K.C.P.; Liao, B.; Wang, S.; et al. Hyodeoxycholic acid alleviates non-alcoholic fatty liver disease through modulating the gut-liver axis. Cell Metab. 2023, 35, 1752–1766. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; An, Y.; Tang, H.; Wang, Y. Alterations of bile acids and gut microbiota in obesity induced by high fat diet in rat model. J. Agric. Food Chem. 2019, 67, 3624–3632. [Google Scholar] [CrossRef] [PubMed]
- Grau, K.R.; Zhu, S.; Peterson, S.T.; Helm, E.W.; Philip, D.; Phillips, M.; Hernandez, A.; Turula, H.; Frasse, P.; Graziano, V.R.; et al. The intestinal regionalization of acute norovirus infection is regulated by the microbiota via bile acid-mediated priming of type III interferon. Nat. Microbiol. 2020, 5, 84–92. [Google Scholar] [CrossRef]
- Prete, R.; Long, S.L.; Gallardo, A.L.; Gahan, C.G.; Corsetti, A.; Joyce, S.A. Beneficial bile acid metabolism from Lactobacillus plantarum of food origin. Sci. Rep. 2020, 10, 1165. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Liao, C.; Su, D.; Mula, T.; Gegen, Z.; Li, Z.; Tu, Y. Wuwei Qingzhuo San ameliorates hyperlipidemia in mice fed with HFD by regulating metabolomics and intestinal flora composition. Front. Pharmacol. 2022, 13, 842671. [Google Scholar] [CrossRef]
- He, K.; Hu, Y.; Ma, H.; Zou, Z.; Xiao, Y.; Yang, Y.; Feng, M.; Li, X.; Ye, X. Rhizoma coptidis alkaloids alleviate hyperlipidemia in B6 mice by modulating gut microbiota and bile acid pathways. Biochim. Biophys. Acta-Mol. Basis Dis. 2016, 1862, 1696–1709. [Google Scholar] [CrossRef]
- Chen, W.; Wei, Y.; Xiong, A.; Li, Y.; Guan, H.; Wang, Q.; Miao, Q.; Bian, Z.; Xiao, X.; Lian, M.; et al. Comprehensive analysis of serum and fecal bile acid profiles and interaction with gut microbiota in primary biliary cholangitis. Clin. Rev. Allergy Immunol. 2020, 58, 25–38. [Google Scholar] [CrossRef]
- Song, Z.; Cai, Y.; Lao, X.; Wang, X.; Lin, X.; Cui, Y.; Kalavagunta, P.K.; Liao, J.; Jin, L.; Shang, J.; et al. Taxonomic profiling and populational patterns of bacterial bile salt hydrolase (BSH) genes based on worldwide human gut microbiome. Microbiome 2019, 7, 9. [Google Scholar] [CrossRef]
- Anhe, F.F.; Nachbar, R.T.; Varin, T.V.; Trottier, J.; Dudonne, S.; Le Barz, M.; Feutry, P.; Pilon, G.; Barbier, O.; Desjardins, Y.; et al. Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. Gut 2019, 68, 453–464. [Google Scholar] [CrossRef]
- Yang, J.Y.; Lee, Y.S.; Kim, Y.; Lee, S.H.; Ryu, S.; Fukuda, S.; Hase, K.; Yang, C.S.; Lim, H.S.; Kim, M.S.; et al. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017, 10, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Bindels, L.B. Microbiome metabolomics reveals new drivers of human liver steatosis. Nat. Med. 2018, 24, 906–907. [Google Scholar] [CrossRef]
- Bustos, A.Y.; Font de Valdez, G.; Fadda, S.; Taranto, M.P. New insights into bacterial bile resistance mechanisms: The role of bile salt hydrolase and its impact on human health. Food Res. Int. 2018, 112, 250–262. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.-y.; Wei, Y.-l.; Hao, J.-y.; Lei, Y.-q.; Zhao, W.-b.; Xiao, Y.-h.; Sun, A.-d. The polyphenol-rich extract from chokeberry (Aronia melanocarpa L.) modulates gut microbiota and improves lipid metabolism in diet-induced obese rats. Nutr. Metab. 2020, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Hou, T. Modulatory effects of Lactarius hatsudake on obesity and gut microbiota in high-fat diet-fed C57BL/6 mice. Foods 2024, 13, 948. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hao, W.; He, Z.; Kwek, E.; Zhu, H.; Ma, N.; Ma, K.Y.; Chen, Z.-Y. Blueberry and cranberry anthocyanin extracts reduce bodyweight and modulate gut microbiota in C57BL/6 J mice fed with a high-fat diet. Eur. J. Nutr. 2021, 60, 2735–2746. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, F.; Wu, D.; Zhu, X.; Gao, X.; Hu, X.; Xu, F.; Ma, T.; Zhao, H.; Cao, W. Fu loose tea administration ameliorates obesity in high-fat diet-fed C57BL/6J mice: A comparison with fu brick tea and orlistat. Foods 2024, 13, 206. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Lin, Y.-H.; Chen, H.-J.; Chou, S.-C.; Cheng, A.-C.; Kalyanam, N.; Ho, C.-T.; Pan, M.-H. Piceatannol exerts anti-obesity effects in C57BL/6 mice through modulating adipogenic proteins and gut microbiota. Molecules 2016, 21, 1419. [Google Scholar] [CrossRef]




| Group | TBA Content (μmol/L) | ||
|---|---|---|---|
| Serum | Liver | Feces | |
| ND | 5.50 ± 0.33 a | 36.91 ± 1.71 a | 46.20 ± 2.14 a |
| HFD | 13.05 ± 1.00 b | 43.43 ± 2.39 a | 116.77 ± 3.05 d |
| HE | 4.81 ± 0.55 a | 36.68 ± 2.46 a | 78.84 ± 2.90 b |
| FMT | 6.16 ± 0.32 a | 41.27 ± 1.97 a | 99.36 ± 2.49 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, X.; Zhang, J.; Chou, R.; Zhao, C.; Zhao, H.; Cao, W.; Cheng, N. Schisandra chinensis Bee Pollen Extract Alleviates Obesity by Modulating Gut Microbiota-Driven Bile Acid Metabolism. Nutrients 2025, 17, 3597. https://doi.org/10.3390/nu17223597
An X, Zhang J, Chou R, Zhao C, Zhao H, Cao W, Cheng N. Schisandra chinensis Bee Pollen Extract Alleviates Obesity by Modulating Gut Microbiota-Driven Bile Acid Metabolism. Nutrients. 2025; 17(22):3597. https://doi.org/10.3390/nu17223597
Chicago/Turabian StyleAn, Xin, Jingxuan Zhang, Runwen Chou, Cheng Zhao, Haoan Zhao, Wei Cao, and Ni Cheng. 2025. "Schisandra chinensis Bee Pollen Extract Alleviates Obesity by Modulating Gut Microbiota-Driven Bile Acid Metabolism" Nutrients 17, no. 22: 3597. https://doi.org/10.3390/nu17223597
APA StyleAn, X., Zhang, J., Chou, R., Zhao, C., Zhao, H., Cao, W., & Cheng, N. (2025). Schisandra chinensis Bee Pollen Extract Alleviates Obesity by Modulating Gut Microbiota-Driven Bile Acid Metabolism. Nutrients, 17(22), 3597. https://doi.org/10.3390/nu17223597





